Chapter 102 Injuries to the liver and biliary tract
Evolution of the Approach to Hepatic Trauma
More than a century ago, Pringle (1908) described hepatic vascular inflow occlusion in a trauma patient by manual compression of the hepatoduodenal ligament, a maneuver that bears his name. Although less often appreciated, Pringle also described exsanguinating hemorrhage from the liver following hematoma decompression during laparotomy and discussed the use of packing to control bleeding from the liver. Packing is therefore not a new concept. Between the turn of the twentieth century and World War II, surgeons packed deep liver lacerations and sometimes added sutures over the pack to achieve tamponade (Schroeder, 1906).
As techniques for selective hemostasis of liver injuries evolved during World War II and later in Vietnam, packing acquired a bad reputation and was abandoned in favor of alternatives such as deep liver sutures and hepatic artery ligation (Aaron et al, 1975; Lewis et al, 1974; Mays, 1967). The report of anatomic right hepatectomy by Lortat-Jacob and Robert in 1952 and the subsequent dissemination of the technical aspects of the operation (Quattlebaum & Quattlebaum, 1959) was rapidly followed by enthusiastic reports of the successful application of these formal resection techniques to trauma (Ackroyd et al, 1969; McClelland et al, 1964).
In the late 1970s, packing reemerged as a valid hemostatic technique for major liver injuries. In 1976, Lucas reported 3 patients in a series of 637 who survived packing (Lucas & Ledgerwood, 1976), and 3 years later, Calne reported 4 additional patients transferred to their hospital with liver packs in place (Calne et al, 1979). These and other reports (Malhotra et al, 2000) coincided with a growing realization among surgeons that major formal hepatic resection in critically injured patients is ill advised, because the combination of severe trauma and extensive surgery often leads to an irreversible physiologic insult and death (Fang et al, 2000).
The reintroduction of packing in the 1990s was the harbinger of a major paradigm shift in trauma surgery, from long and complex definitive procedures to a staged approach that was aptly referred to as damage control (Rotondo et al, 1993). In this new paradigm, the goals of the initial operation are limited to rapid control of bleeding and spillage of intestinal contents. This is followed by resuscitation of the patient in the surgical ICU followed by a planned reoperation for definitive reconstruction of the anatomy several days later. By the beginning of the new millennium, this new approach had become the standard of care for major abdominal trauma (Johnson et al, 2001; Shapiro et al, 2000), thus packing and temporary abdominal closure replaced hepatectomy as the dominant approach for major hepatic wounds (Johnson et al, 2001; Knudson et al, 1990; Malhotra et al, 2000; Pachter et al, 1996).
The application of nonoperative management to liver injuries in the last 15 years represents a similarly dramatic revolution. Computed tomography (CT) permitted precise delineation of the anatomy of injured abdominal solid organs. With better appreciation of the natural history of these injuries came an increasingly conservative approach. Nonoperative management was initially implemented with striking success for pediatric splenic trauma (Aronson et al, 1977) and was then successfully applied to the pediatric liver (Karp et al, 1983) and subsequently to the adult spleen and liver (Meyer et al, 1985).
Since the early 1990s, nonoperative management has evolved into the standard of care for blunt hepatic trauma in hemodynamically stable patients (Stein & Scalea, 2006). However, in recent years, it has also become apparent that the conservative approach requires more than close monitoring. In fact, these patients often require adjunctive imaging and interventional techniques to manage the sequelae of major liver trauma (see Section II and Chapters 27 and 28; Carrillo et al, 1999). The most important of these adjuncts is selective angiographic embolization of hepatic artery branches, which has largely replaced operative ligation of the right or left hepatic artery at the hilum (see Chapter 28; Wahl et al, 2002).
Surgical Anatomy of the Liver from the Trauma Surgeon’s Perspective (See Chapters 1A and 1B)
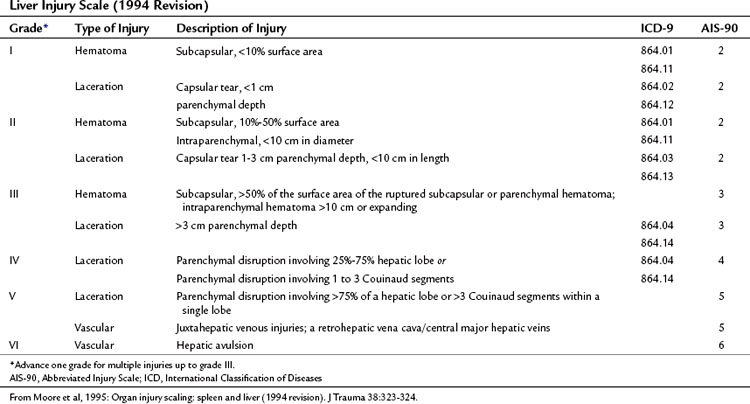
The American Association for the Surgery of Trauma (AAST) published a liver injury scale that creates a unified framework for describing the severity of hepatic trauma (Table 102.1, Fig. 102.1; Moore et al, 1995). This scale is useful not only as a descriptive tool but also as an outcome predictor that facilitates comparisons across groups of patients.
1 Superficial parenchymal wounds that require only a simple suture or use of a local hemostatic technique
2 Deep parenchymal injury or extensive parenchymal disruption, which can be packed
3 Deep injury involving major vascular structures, which requires special maneuvers to achieve hemostasis and often carries a poor prognosis
The Stable Patient with Blunt Hepatic Injury
Blunt hepatic trauma in a hemodynamically stable patient is typically discovered on CT scan. If the patient is stable with no signs of peritoneal irritation, presumably indicating a hollow viscus injury, the current standard of care is nonoperative management, regardless of the grade or extent of the injury (McVay et al, 2008). Although the overall success rate of nonoperative management of hepatic trauma ranges between 85% and 98% (Croce et al, 1995; Malhotra et al, 2000; Pachter et al, 1996; Velmahos et al, 2003), high grades of injury carry a greater risk of failure and often require adjunctive measures and a multidisciplinary approach as described below (Carrillo et al, 1999; Sriussadaporn et al, 2002).
Practical Aspects of Nonoperative Management
Stable patients with high-grade liver injuries (grade III and above) are admitted to the intensive care unit for close hemodynamic monitoring and serial abdominal examination. This must include careful palpation of the liver to assess for new tenderness or any increase in liver size; both are indicative of intrahepatic hemorrhage. Many of these patients have associated injuries, such as traumatic brain injury or lung contusions, which also require intensive care. Monitoring bladder pressures for early recognition of intraabdominal hypertension is indicated in patients who have received massive fluid resuscitation and in those who have a firm, distended abdomen (Fusco et al, 2001; Yang et al, 2002).
The question of routine follow-up imaging is controversial (Alonso et al, 2003). Although routine follow-up imaging is not indicated, most patients with high-grade injuries undergo subsequent CT scans in search of complications such as biloma or hepatic necrosis or because of associated injuries. Fever, leukocytosis, unexplained drops in hematocrit, and a rising bilirubin are obvious indications for repeat imaging (Pachter et al, 1996).
Faliure of Nonoperative Management
Failure of nonoperative management is an uncommon but potentially life-threatening complication that should be recognized early and addressed aggressively (Galvan & Peitzman, 2006). Ongoing bleeding is the major cause of failure. Evidence of ongoing hemorrhage includes hypotension, falling hematocrit, an increase in liver size, or the onset of new or increased pain in the right upper quadrant. If the patient is stable, a repeat CT scan will demonstrate the bleeding, which can then be controlled by angiographic embolization (Misselbeck et al, 2009). The patient in shock is urgently taken to the operating room for laparotomy. The complications of nonoperative management are listed in Box 102.1.
Adjuncts to Nonoperative Management
Adjunctive modalities have a crucial role in the nonoperative management of hepatic trauma. These modalities include angiography, image-guided drainage, endoscopic retrograde cholangiopancreatography (ERCP), and laparoscopy (see Chapters 18, 19, 21, and 28; Carrillo et al, 2010).
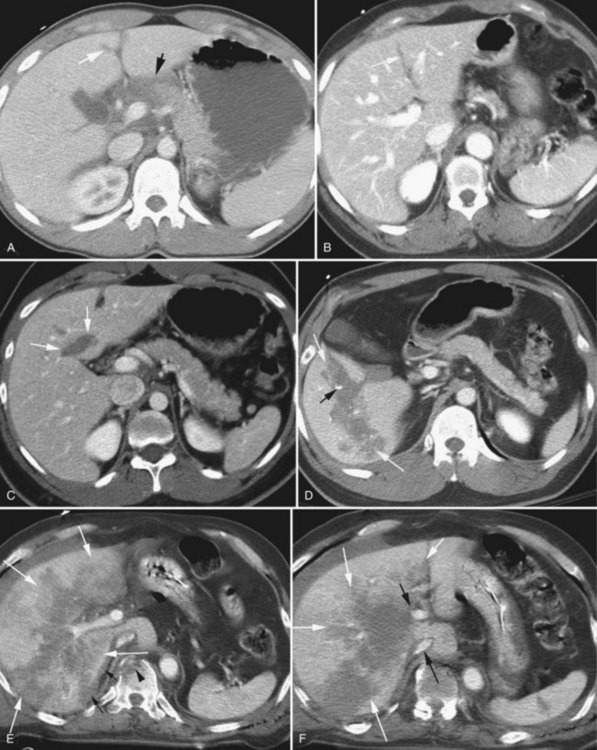
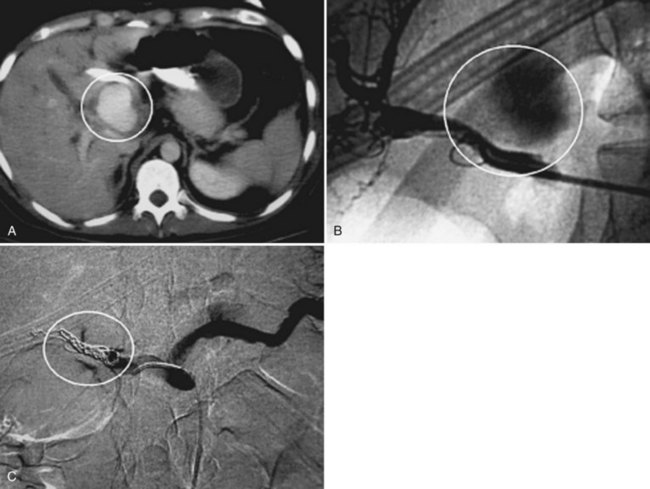
Angiography is by far the most important adjunct. A “blush” of intravenous contrast on CT scan (i.e., active extravasation) typically results from injury to a branch of the hepatic artery (Fig. 102.2). This blush is an indication for selective angiographic embolization of the bleeding vessel to prevent pseudoaneurysm formation and delayed hemorrhage (Demetriades et al, 2006; Eubanks et al, 2003).
A blush on CT scan is by no means the only indication for angiography. Evidence suggests that liberal use of angiography for any high-grade liver injury is safe and effective, because significant injury to lobar and segmental vessels may occur without a blush, and if left untreated, this may lead to delayed hemorrhage (Mohr et al, 2003).
CT-guided percutaneous drainage plays a key role in nonoperative management, because bile collections are common around the injured liver (Castagnetti et al, 2006). Such drainage obviates the need for laparotomy in the majority of cases. Persistent high-output bile drainage from a percutaneously placed drain indicates injury to a segmental or lobar bile duct. In these patients, ERCP may be used to delineate the site of injury and to decompress the biliary tree by endoscopic papillotomy and stenting (Lubezky et al, 2006); however, in the absence of ductal isolation, adequate external drainage should be sufficient for fistula closure. Image-guided percutaneous drainage is also useful in addressing perihepatic or intrahepatic abscesses.
Delayed laparoscopic lavage is emerging as another useful adjunct to conservative management (Carrillo et al, 2001; Franklin et al, 2007). This minimally invasive procedure is indicated when the patient fails to thrive, with persistent low-grade fever and other signs of ongoing systemic inflammatory response. Port placement is similar to a laparoscopic cholecystectomy with a single subhepatic lateral port. The injured liver is inspected, perihepatic collections are drained and cultured under direct vision, and the infected “rind” of clotted blood and bile on the surface of the liver is gently and bluntly teased off the capsule. The area of injury is typically obvious and is carefully avoided. The liver is irrigated with the suction irrigator, and drains are placed under direct vision in the relevant spaces above or below it.
Other Complications of Nonoperative Management
Rarely, a false aneurysm of a branch of the hepatic artery (Schouten van der Velden et al, 2009) will decompress into an injured biliary radical in a delayed fashion, causing hemobilia (Green et al, 2001; see Chapters 104 and 105). The patient presents weeks or months after the injury with upper gastrointestinal bleeding, jaundice, and colicky abdominal pain as a result of blood and clots in the biliary tree that are subsequently passed through the ampulla of Vater. Angiographic embolization (see Chapters 28 and 83) of the source of bleeding is the treatment of choice (Mohr et al, 2003).
The most devastating complication of nonoperative management is missed hollow viscus injury. This complication is very uncommon (Miller et al, 2002), but when it occurs, the mortality rate is high. The diagnosis is particularly difficult, because there is often free fluid in the peritoneal cavity as a result of the liver injury. The abdomen may be firm and difficult to examine, because the patient is intubated and sedated. The signs of a missed hollow viscus injury are therefore subtle, and timely diagnosis requires a high index of suspicion. The decision not to operate should be critically reevaluated with any signs of hyperdynamic physiology. Any adverse change in the abdominal findings or in the expected clinical trajectory should prompt a repeat CT scan or laparotomy in search of a missed injury. Failure to achieve timely source control results in the patient’s death.
Nonoperative Management of Penetrating Hepatic Trauma?
It was only natural that following the dramatic success of nonoperative management for blunt hepatic trauma in the 1990s, attempts would be made to extend the concept to penetrating injuries. Although conservative management of stab wounds to the abdomen, including the liver, has been practiced for years, application of this approach to gunshot wounds is controversial. In 1994, Renz and Feliciano reported 32 patients with right thoracoabdominal gunshot wounds, of whom 7 had CT-documented liver injuries that were successfully treated nonoperatively. Five years later, Demetriades and colleagues (1999) reported 16 patients with penetrating liver injuries, 5 of whom needed a delayed laparotomy; 1 patient died. Clearly, nonoperative management of hepatic gunshot wounds must be applied carefully and selectively and is not currently the standard of care. Success hinges on complete CT delineation of the entire bullet trajectory within the liver and the ability to reliably exclude injury to adjacent hollow organs, especially the right colon (Demetriades et al, 2006).
The Unstable Patient with Hepatic Trauma
The patient in shock with hemoperitoneum requires immediate laparotomy to control the bleeding regardless of the injury mechanism. In this stressful situation, the keys to success are careful forethought and orderly conduct of the operation to achieve rapid hemostasis. Immediate availability of type-specific blood is mandatory, and early activation of a transfusion protocol providing for fresh frozen plasma and platelets reduces the risk of coagulopathy in the patient with massive hemorrhage (Dente et al, 2009). A full set of vascular instruments, a rapid infusion device, an autotransfuser, and competent assistance are other essential adjuncts.
Packing of Deep Liver Lacerations
Packing is the workhorse of surgery for hepatic trauma and is especially useful for high-grade lacerations or parenchymal disruptions that are not amenable to the local hemostatic measures described above (Nicol et al, 2007). The technique of packing is often poorly executed, but the goal is to effect hemostasis by compressing the bleeding parenchyma between packs placed above and below it. Packing only over the injury without creating an opposing pressure vector often fails.
It is important to note that packing is most effective for venous injuries. For this reason, we advocate immediate postoperative angiography with selective embolization for high-grade liver injuries with arterial hemorrhage that cannot be directly addressed by suture-ligation of the bleeders (Gaarder et al, 2007).
Although assertive packing with laparotomy pads is essential to achieve hemostasis, sometimes overenthusiasm results in overpacking, which can result in hypotension as a result of vena caval compression (Meldrum et al, 1995). The problem should be recognized immediately, and the packs removed gradually, to identify the degree of packing that achieves hemostasis without the adverse effects.
Other Techniques for Hemostasis of Deep Liver Lacerations
If packing fails to control the bleeding, inflow occlusion is the next step. The index finger is placed through the foramen of Winslow posterior to the hepatoduodenal ligament, and a window is created in the gastrohepatic ligament. An umbilical tape is then passed through the window in the gastrohepatic ligament and out through the foramen of Winslow, thereby surrounding the portal triad, which is then occluded en masse with either a Rummel tourniquet or a vascular clamp. The occlusion time should be kept to a minimum: 15 minutes of hepatic inflow occlusion is safe in elective hepatic resection (Tralhão et al, 2009). Intermittent hepatic inflow occlusion has been used safely with a total inflow occlusion time in excess of 120 minutes (Ishizaki et al, 2006), although no level 1 data are available to define the safe duration of hepatic inflow occlusion in trauma patients.
If the bullet or knife tract is superficial, a tractotomy can expose the injured vessel for direct suture ligation (Badger et al, 2009). Tractotomy can be performed using the Endo GIA (gastrointestinal anastomosis) stapler (Covidien, Boulder, CO) with a vascular stapler load, gradually dividing the roof of the tract in a piecemeal fashion so that the deep bleeders in the tract can be individually suture ligated or doubly clipped with a hemoclip. If the injury involves the left lateral segment, a stapled segmentectomy, using either the Endo GIA or a TA (thoracoabdominal; Covidien) stapler, is an expedient solution (Schemmer et al, 2007).
More often, the problem arises from a deep tract or through-and-through injury to the main mass of the right lobe. Filling the tract with omentum, Flowseal (Baxter Healthcare, Zurich, Switzerland), or Avitene (Davol, Warwick, RI) will often achieve hemostasis. Balloon tamponade is another useful technique (Poggetti et al, 1992), wherein a balloon device is improvised from a red rubber (Robinson) catheter inserted into a 1-inch Penrose drain. The distal end of the drain is tied closed, and the proximal end is secured snugly around the catheter with a pair of heavy silk ties. The balloon device is inserted through an appropriate stab incision in the right upper quadrant abdominal wall and into the tract and is inflated with saline injected through the catheter. A closed suction drain is placed in the subhepatic space. The balloon is deflated and removed either at planned relaparotomy, 48 to 72 hours after the original operation, or percutaneously if the abdomen was closed.
Sometimes a bleeding vessel can be exposed and suture ligated at the base of a deep liver laceration, using the technique of finger-fracture hepatotomy with selective vascular ligation under inflow occlusion (Hirshberg & Mattox, 2006); however, attempts to reach a deep vessel are often ill advised, as they lead to continued blood loss and physiologic deterioration and may even aggravate the bleeding. Packing, when successful, is the best approach, and immediate postoperative angiographic embolization (see Chapters 28 and 83), if available, can be a lifesaving adjunct.
Bleeding from the Hepatic Veins and Juxtahepatic Vena Cava
The large retrohepatic veins are a low-pressure system; when injured, they are often contained by the triangular ligaments of the liver. An injury to the retrohepatic cava or the major hepatic veins can therefore manifest either as bleeding through a hole in the liver, if the suspensory ligaments remain intact, or as bleeding around the liver, if they are disrupted (Buckman et al, 2000); bleeding through the liver stops with effective packing of the hole in the liver, but bleeding around the liver does not.
Vascular isolation of the injured liver is a formidable surgical challenge (see Chapter 91A; Yellin et al, 1971), not only because of the technical complexity involved but also because cross-clamping the suprahepatic and infrahepatic vena cavae (Krige et al, 1990) is poorly tolerated by the hypovolemic patient. Furthermore, although creating a sling to occlude the infrahepatic vena cava can usually be accomplished relatively quickly after a Kocher maneuver, dissection of the infradiaphragmatic suprahepatic cava is difficult, particularly for the emergency surgeon who operates infrequently in this area.
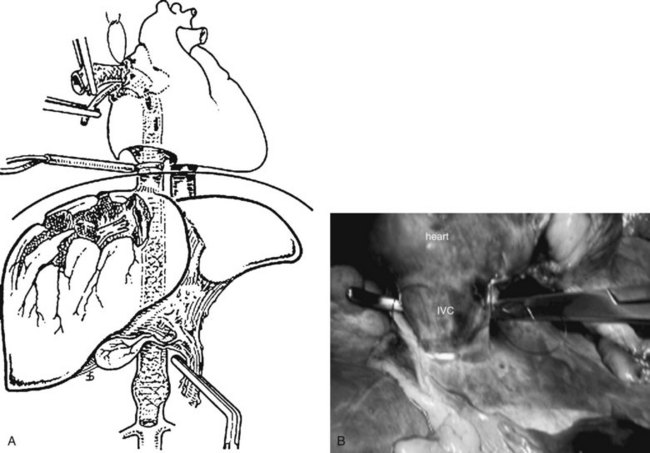
Several options are available for achieving outflow control, and by far the most well-known is the atriocaval shunt (Fig. 102.3; Schrock et al, 1968). After performing a median sternotomy, a No. 32 chest tube is inserted through the purse-stringed right atrial appendage and threaded through the right atrium and juxtahepatic vena cava to a position just above the renal veins. At the same time, a second team isolates the inferior vena cava (IVC) in its suprarenal infrahepatic segment, and a side hole is fashioned at the level of the right atrium. Rummel tourniquets are tightened around the vena cava and the stent within the pericardium and just above the renal veins. Using a 9.5-mm endotracheal tube with a side hole 17 cm from the tip obviates the need to isolate the suprarenal cava, because inflating the balloon of the tube occludes the caval lumen. This maneuver shunts venous return to the heart around the injured segment and controls most of the bleeding, enabling the surgeon to visualize and repair the hole in the vein by completely mobilizing the right lobe of the liver and rotating it medially. This is a difficult and uncommonly used technique, as evidenced by the popular adage that “the number of articles published about atriocaval shunt far exceeds the number of patients who survived it” (Fig. 102.3).
Another option for outflow control is complete hepatic vascular isolation (see Chapter 91A) by clamping the inferior vena cava above and below the liver, in conjunction with simultaneous aortic cross-clamping (Khaneja et al, 1997), or use of a centrifugal pump to return blood to the heart (Baumgartner et al, 1995; Horwitz et al, 1995) to reduce the risk of hypovolemic arrest after caval occlusion. Even hepatic explantation and back-bench repair of hepatic vein avulsion followed by hepatic autotransplantation has been reported (Boggi et al, 2006). Despite all of these elegant technical concepts, which are of very limited utility in the real clinical situation, the mortality rate for unstable patients with juxtahepatic vena caval injury unresponsive to packing remains extremely high (Phelan et al, 2001).
Urgent Reoperation
The most common causes of bleeding found at urgent relaparotomy for trauma are inadequate hemostasis of the original injury, another source of bleeding in the vicinity, iatrogenic trauma, or diffuse coagulopathy (Hirshberg et al, 1993). In cases of rebleeding, mortality is extremely high, and the prognosis is especially bleak, if no discrete source of hemorrhage is found.
Planned Reoperation
Planned reoperation is an integral part of the damage-control sequence for treatment of severe liver trauma. In general, the patient is reexplored 36 to 48 hours after the initial operation, once hemodynamic stability has been achieved. Although such a practice is not supported by data, trauma surgeons are reluctant to remove liver packs sooner than 36 hours after the initial operation, because earlier removal often results in rebleeding and a need to repack the liver. Packs should rarely, if ever, be left in any patient for more than 72 hours (Scott et al, 2006), because the risk of infection increases sharply. If the packed liver injury is associated with damage control of a bowel injury, intestinal reconstruction should be performed, and the packs should be removed last.
Extrahepatic Biliary Injury
Most extrahepatic biliary injuries result from penetrating trauma (Posner & Moore, 1985) and are discovered at exploratory laparotomy. The gallbladder is the most common structure injured, and cholecystectomy definitively solves the problem (Posner & Moore, 1985); however, it may be unwise in an unstable coagulopathic patient with an associated liver injury, because it creates another source of hemorrhage from the liver bed. A tube cholecystostomy or closure with an absorbable stitch and drainage of a small hole in the gallbladder are therefore better options in a damage-control situation.
Small stab wounds to the extrahepatic biliary ducts can be managed by primary closure with absorbable suture and external drainage (Bade et al, 1989). The use of a T-tube for a common bile duct (CBD) injury is rarely a practical option in a healthy young adult with a normal CBD. Major injuries to the extrahepatic bile ducts require Roux-en-Y choledochojejunostomy or hepaticojejunostomy for definitive repair (Degiannis et al, 2001).
Penetrating trauma to the biliary tree is often associated with injuries to vascular structures such as the portal vein, hepatic artery, and vena cava (Jurkovich et al, 1995). Injuries to adjacent structures—such as the duodenum, pancreas, stomach, and liver—are also common. The hemodynamic stability of the patient and the complexity of the other injuries will govern management of the bile duct injury.
Blunt injury to the gallbladder as a result of a “blowout” (Zellweger et al, 2005) or adhesive tear is uncommon, but the treatment principles are similar to those for penetrating injury. Rarely, the surgeon may encounter avulsion of the common bile duct from the duodenum. If this is an isolated injury, the duodenum can be oversewn, and a Roux-en-Y choledochojejunostomy can be constructed (Melton et al, 2003). Regardless of the type of biliary management, wide drainage is essential.
Aaron S, et al. Selective ligation of the hepatic artery for trauma of the liver. Surg Gynecol Obstet. 1975;141:187-189.
Ackroyd FW, et al. Massive hepatic resection in the treatment of severe liver trauma. Am J Surg. 1969;117:442-448.
Alonso M, et al. Practice Management Guidelines for the Nonoperative Management of Blunt Injury to the Liver and Spleen. Available at http://www.east.org/tpg/livspleen, 2003.
Aronson DZ, et al. Nonoperative management of splenic trauma in children: a report of six consecutive cases. Pediatrics. 1977;60:482-485.
Bade PG, et al. Surgical options in traumatic injury to the extrahepatic biliary tract. Br J Surg. 1989;76:256-258.
Badger SA, et al. Management of liver trauma. World J Surg. 2009;33:2522-2537.
Baumgartner F, et al. Venovenous bypass for major hepatic and caval trauma. J Trauma. 1995;39:671-673.
Boggi U, et al. Extracorporeal repair and liver autotransplantation after total avulsion of hepatic veins and retrohepatic inferior vena cava injury secondary to blunt abdominal trauma. J Trauma. 2006;60:405-406.
Buckman RFJr, et al. Juxtahepatic venous injuries: a critical review of reported management strategies. J Trauma. 2000;48:978-984.
Calne RY, et al. The treatment of major liver trauma by primary packing with transfer of the patient for definitive treatment. Br J Surg. 1979;66:338-339.
Carrillo EH, et al. Interventional techniques are useful adjuncts in nonoperative management of hepatic injuries. J Trauma. 1999;46:619-622. discussion 622-614
Carrillo EH, et al. Delayed laparoscopy facilitates the management of biliary peritonitis in patients with complex liver injuries. Surg Endosc. 2001;15:319-322.
Castagnetti M, et al. Minimally invasive management of bile leaks after blunt liver trauma in children. J Pediatr Surg. 2006;41:1539-1544.
Croce MA, et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients: results of a prospective trial. Ann Surg. 1995;221:744-753. discussion 753-745
Degiannis E, et al. Gunshot injuries of the extrahepatic biliary ducts. Eur J Surg. 2001;167:618-621.
Demetriades D, et al. Gunshot injuries to the liver: the role of selective nonoperative management. J Am Coll Surg. 1999;188:343-348.
Demetriades D, et al. Selective nonoperative management of penetrating abdominal solid organ injuries. Ann Surg. 2006;244:620-628.
Dente CJ, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616-1624.
Eubanks JW3rd, et al. Significance of “blush” on computed tomography scan in children with liver injury. J Pediatr Surg. 2003;38:363-366. discussion 363-366
Fang JF, et al. Blunt hepatic injury: minimal intervention is the policy of treatment. J Trauma. 2000;49:722-728.
Franklin GA, et al. Prevention of bile peritonitis by laparoscopic evacuation and lavage after nonoperative treatment of liver injuries. Am Surg. 2007;73:611-616. discussion 616-617
Fusco MA, et al. Estimation of intra-abdominal pressure by bladder pressure measurement: validity and methodology. J Trauma. 2001;50:297-302.
Gaarder C, et al. Liver injuries—improved results with a formal protocol including angiography. Injury. 2007;38:1075-1083.
Galvan DA, Peitzman AB. Failure of nonoperative management of abdominal solid organ injuries. Curr Opin Crit Care. 2006;12:590-594.
Green MH, et al. Haemobilia. Br J Surg. 2001;88:773-786.
Hirshberg A, Mattox KL. Top Knife: The Art and Craft of Trauma Surgery. New York: Springer-Verlag; 2006. p 127
Hirshberg A, et al. Reoperation for bleeding in trauma. Arch Surg. 1993;128:1163-1167.
Horwitz JR, et al. Venovenous bypass as an adjunct for the management of a retrohepatic venous injury in a child. J Trauma. 1995;39:584-585.
Ishizaki Y, et al. Safety of prolonged intermittent Pringle maneuver during hepatic resection. Arch Surg. 2006;141:649-653. discussion 654
Johnson JW, et al. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51:261-269. discussion 269-271
Jurkovich GJ, et al. Portal triad injuries. J Trauma. 1995;39:426-434.
Karp MP, et al. The nonoperative management of pediatric hepatic trauma. J Pediatr Surg. 1983;18:512-518.
Khaneja SC, et al. Management of penetrating juxtahepatic inferior vena cava injuries under total vascular occlusion. J Am Coll Surg. 1997;184:469-474.
Knudson MM, et al. Nonoperative management of blunt liver injuries in adults: the need for continued surveillance. J Trauma. 1990;30:1494-1500.
Krige JE, et al. Severe juxtahepatic venous injury: survival after prolonged hepatic vascular isolation without shunting. HPB Surg. 1990;3:39-43. discussion 43-35
Lewis FR, et al. Hepatic artery ligation: adjunct in the management of massive hemorrhage from the liver. J Trauma. 1974;14:743-755.
Lortat-Jacob JL, Robert HG. [Well-defined technic for right hepatectomy]. Presse Med (in French). 1952;60:549-551.
Lubezky N, et al. Endoscopic sphincterotomy and temporary internal stenting for bile leaks following complex hepatic trauma. Br J Surg. 2006;93:78-81.
Lucas CE, Ledgerwood AM. Prospective evaluation of hemostatic techniques for liver injuries. J Trauma. 1976;16:442-451.
Malhotra AK, et al. Blunt hepatic injury: a paradigm shift from operative to nonoperative management in the 1990s. Ann Surg. 2000;231:804-813.
Mays ET. Observations and management after hepatic artery ligation. Surg Gynecol Obstet. 1967;124:801-807.
McClelland R, et al. Hepatic resection for massive trauma. J Trauma. 1964;4:282-291.
McVay MR, et al. Throwing out the “grade” book: management of isolated spleen and liver injury based on hemodynamic status. J Pediatr Surg. 2008;43:1072-1076.
Meldrum DR, et al. Jack A. Barney Resident Research Award: cardiopulmonary hazards of perihepatic packing for major liver injuries. Am J Surg. 1995;170:537-540. discussion 540-532
Melton SM, et al. Common bile duct transection in blunt abdominal trauma: case report emphasizing mechanism of injury and therapeutic management. J Trauma. 2003;54:781-785.
Meyer AA, et al. Selective nonoperative management of blunt liver injury using computed tomography. Arch Surg. 1985;120:550-554.
Miller PR, et al. Associated injuries in blunt solid organ trauma: implications for missed injury in nonoperative management. J Trauma. 2002;53:238-242. discussion 242-234
Misselbeck TS, et al. Hepatic angioembolization in trauma patients: indications and complications. J Trauma. 2009;67:769-773.
Mohr AM, et al. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma. 2003;55:1077-1081. discussion 1081-1072
Moore EE, et al. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38:323-324.
Nicol AJ, et al. Packing for control of hemorrhage in major liver trauma. World J Surg. 2007;31:569-574.
Pachter HL, et al. Status of nonoperative management of blunt hepatic injuries in 1995: a multicenter experience with 404 patients. J Trauma. 1996;40:31-38.
Phelan H, et al. Retrohepatic vena cava and juxtahepatic venous injuries. South Med J. 2001;94:728-731.
Poggetti RS, et al. Balloon tamponade for bilobar transfixing hepatic gunshot wounds. J Trauma. 1992;33:694-697.
Posner MC, Moore EE. Extrahepatic biliary tract injury: operative management plan. J Trauma. 1985;25:833-837.
Pringle JH. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:541-549.
Quattlebaum JK, Quattlebaum JKJr. Technic of hepatic lobectomy. Ann Surg. 1959;149:648-651.
Renz BM, Feliciano DV. Gunshot wounds to the right thoracoabdomen: a prospective study of nonoperative management. J Trauma. 1994;37:737-744.
Rotondo MF, et al. “Damage control”: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375-382. discussion 382-373
Schemmer P, et al. The use of Endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg. 2007;24:300-305.
Schouten van der Velden AP, et al. Hemobilia as a late complication after blunt abdominal trauma: a case report and review of the literature. J Emerg Med. 2010;39:592-595.
Schrock T, et al. Management of blunt trauma to the liver and hepatic veins. Arch Surg. 1968;96:698-704.
Schroeder W. The progress of liver hemostasis: reports of cases (resection, sutures, etc). Surg Gynecol Obstet. 1906;2:52-61.
Scott BG, et al. Early aggressive closure of the open abdomen. J Trauma. 2006;60:17-22.
Shapiro MB, et al. Damage control: collective review. J Trauma. 2000;49:969-978.
Sriussadaporn S, et al. A multidisciplinary approach in the management of hepatic injuries. Injury. 2002;33:309-315.
Stein DM, Scalea TM. Nonoperative management of spleen and liver injuries. J Intensive Care Med. 2006;21:296-304.
Tralhão JG, et al. Intermittent Pringle maneuver and hepatic function: perioperative monitoring by noninvasive ICG-clearance. World J Surg. 2009;33:2627-2634.
Velmahos GC, et al. High success with nonoperative management of blunt hepatic trauma: the liver is a sturdy organ. Arch Surg. 2003;138:475-480. discussion 480-471
Wahl WL, et al. The need for early angiographic embolization in blunt liver injuries. J Trauma. 2002;52:1097-1101.
Yang EY, et al. The abdominal compartment syndrome complicating nonoperative management of major blunt liver injuries: recognition and treatment using multimodality therapy. J Trauma. 2002;52:982-986.
Yellin AE, et al. Vascular isolation in treatment of juxtahepatic venous injuries. Arch Surg. 1971;102:566-573.
Zellweger R, et al. Gall bladder injuries as part of the spectrum of civilian abdominal trauma in South Africa. Aust N Z J Surg. 2005;75:559-561.