Inhaled Toxins
Many airborne toxins produce local noxious effects on the airways and lungs as irritants. The respiratory tract can also serve as a portal of entry for systemic poisons as simple or systemic asphyxiants. Inhalational exposure can be covert and indolent (as in occupational exposure to asbestos or urban exposure to photochemical smog) or fulminant and obvious. The circumstances and location of the exposure, the presence of combustion or odors, and the number and condition of victims assist in the diagnosis. Despite the array of possible toxic inhalants, identification of a specific inhalant is generally unnecessary because therapy is based primarily on the clinical manifestations (Table 159-1).
Table 159-1
| INHALANT | SOURCE OR USE | PREDOMINANT CLASS |
| Acrolein | Combustion | Irritant, highly soluble |
| Ammonia | Fertilizer, combustion | Irritant, highly soluble |
| Carbon dioxide | Fermentation, complete combustion, fire extinguisher | Simple asphyxiant; systemic effects |
| Carbon monoxide | Incomplete combustion, methylene chloride | Chemical asphyxiant |
| Chloramine | Mixed cleaning products (e.g., hypochlorite bleach and ammonia) | Irritant, highly soluble |
| Chlorine | Swimming pool disinfectant, cleaning products | Irritant, intermediate solubility |
| Chlorobenzylidene malononitrile (CS), chloroacetophenone (CN) | Tear gas (Mace) | Pharmacologic irritant |
| Hydrogen chloride | Tanning and electroplating industry | Irritant, highly soluble |
| Hydrogen cyanide | Combustion of plastics, acidification of cyanide salts | Chemical asphyxiant |
| Hydrogen fluoride | Hydrofluoric acid | Irritant, highly soluble; systemic effects |
| Hydrogen sulfide | Decaying organic matter, oil industry, mines, asphalt | Chemical asphyxiant; irritant, highly soluble |
| Methane | Natural gas, swamp gas | Simple asphyxiant |
| Methylbromide | Fumigant | Chemical asphyxiant |
| Nitrogen | Mines, scuba diving (nitrogen narcosis, decompression sickness) | Simple asphyxiant; systemic effects |
| Nitrous oxide | Inhalant of abuse, whipping cream, racing fuel booster | Simple asphyxiant |
| Noble gases (e.g., helium) | Industry, laboratories | Simple asphyxiant |
| Oxides of nitrogen | Silos, anesthetics, combustion | Irritant, intermediate solubility |
| Oxygen | Medical use, hyperbaric conditions | Irritant, free radical; systemic effects |
| Ozone | Electrostatic energy | Irritant, free radical |
| Phosgene | Combustion of chlorinated hydrocarbons | Irritant, poorly soluble |
| Phosphine | Hydration of aluminum or zinc phosphide (fumigants) | Chemical asphyxiant |
| Smoke (varying composition) | Combustion | Variable, but may include all classes |
| Sulfur dioxide | Photochemical smog (fossil fuels) | Irritant, highly soluble |
Simple Asphyxiants
Most simple asphyxiations are workplace related and usually occur during the use of liquefied gas while the employee is breathing through an airline respirator or working in a confined space.1 Since the advent of catalytic converters, most deaths from the intentional inhalation of automotive exhaust result from simple asphyxiation, due to hypoxia, and not from carbon monoxide (CO) poisoning.2
Clinical Features
Acute effects occur within minutes of onset of hypoxia and are the manifestations of ischemia. A fall in the FIO2 from normal, 0.21 (i.e., 21%), to 0.15 results in autonomic stimulation (e.g., tachycardia, tachypnea, and dyspnea) and cerebral hypoxia (e.g., ataxia, dizziness, incoordination, and confusion). Dyspnea is not an early finding because hypoxemia is not as potent a stimulus for this sensation, or for breathing, as are hypercarbia and acidosis. Lethargy from cerebral edema is expected as the FIO2 falls below 0.1 (10%), and life is difficult to sustain at an FIO2 below 0.06 (6%).3 Because removal from exposure terminates the simple asphyxiation and allows restoration of oxygenation and clinical improvement, most patients present with resolving symptoms. However, failure to improve suggests complications of ischemia (e.g., seizures, coma, and cardiac arrest) and is associated with a poor prognosis.
Pulmonary Irritants
Principles of Disease
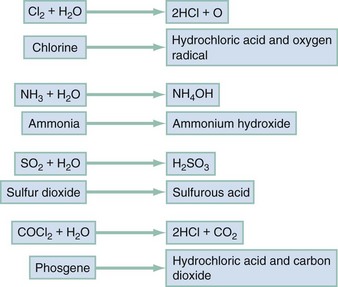
Irritant gases dissolve in the respiratory tract mucus and alter the air-lung interface by invoking an irritant or inflammatory response. When these gases are dissolved, most of them produce an acid or alkaline product, but several generate oxygen-derived free radicals that produce direct cellular toxicity (Fig. 159-1). The clinical effects of pulmonary irritants can be predicted by their water solubility (see Table 159-1).
Clinical Features
Highly water-soluble gases have their greatest impact on the mucous membranes of the eyes and upper airway. Exposure results in immediate irritation, with lacrimation, nasal burning, and cough. Although their pungent odors and rapid symptom onset tend to limit significant exposure, massive or prolonged exposure can result in life-threatening laryngeal edema, laryngospasm, bronchospasm, or acute respiratory distress syndrome (ARDS) (formerly known as noncardiogenic pulmonary edema).4 Poorly water-soluble gases do not readily irritate the mucous membranes at low concentrations, and some have pleasant odors (e.g., phosgene’s odor is similar to that of hay). Because there are no immediate symptoms, prolonged breathing in the toxic environment allows time for the gas to reach the alveoli. Even moderate exposure causes irritation of the lower airway, alveoli, and parenchyma and causes pulmonary endothelial injury after a 2- to 24-hour delay. Initial symptoms consistent with acute respiratory distress syndrome may be mild, only to progress to overt respiratory failure and acute respiratory distress syndrome during the ensuing 24 to 36 hours.5
Gases with intermediate water solubility tend to produce syndromes that are a composite of the clinical features manifested with the other gases, depending on the extent of exposure. Massive exposure is most often associated with rapid onset of upper airway irritation and more moderate exposure with delayed onset of lower airway symptoms.6
Diagnostic Strategies and Differential Considerations
Bronchospasm, cough, chest tightness, and acute conjunctival irritation frequently follow allergen exposure, but the history generally suggests the diagnosis. ARDS occurs after many physiologic insults, including trauma and sepsis, highlighting the need for accurate history taking.5
Management
Bronchospasm generally responds to inhaled beta-adrenergic agonists; the role of ipratropium is not yet defined. Other than as a standard treatment of a comorbid condition, such as asthma, there is no clear indication for corticosteroids.7
Patients exposed to chlorine or hydrogen chloride gas receive symptomatic relief from nebulized 2% sodium bicarbonate solution.6 Because the inflammatory cascade is not altered, however, the component of lung injury mediated by free radicals probably continues and causes delayed deterioration. Patients receiving inhalational bicarbonate therapy require extensive discharge instructions for signs and symptoms of pulmonary irritation or admission to the hospital.
Smoke Inhalation
Clinical Features
Most smoke-associated morbidity and mortality relate to respiratory tract damage. Thermal and irritant-induced laryngeal injury may produce cough or stridor, but these findings are often delayed. Soot and irritant toxins in the airways can produce early cough, dyspnea, and bronchospasm. Subsequently, a cascade of airway inflammation results in acute lung injury with failure of pulmonary gas exchange. The time between smoke exposure and the onset of clinical symptoms is highly variable and dependent on the degree and nature of the exposure. Singed nasal hairs and soot in the sputum suggest substantial exposure but are neither sufficiently sensitive nor specific to be practical.8
Diagnostic Strategies and Differential Considerations
Early death is caused by asphyxia, airway compromise, or metabolic poisoning (e.g., CO). Airway patency should be evaluated early. If evidence of significant airway exposure is present, such as carbonaceous sputum or hoarse voice, the airway should be examined by direct or fiberoptic laryngoscopy. Simply observing the patient for deterioration can result in airway compromise requiring rapid and, by then, very difficult airway intervention. Signs of alveolar filling or hyperinflation on chest radiography, abnormal flow-volume loop or diffusing capacity for CO on pulmonary function testing, or abnormal distribution and clearance of radiolabeled gas on ventilation scans can help predict lower airway injury.9
Metabolic acidosis, particularly when it is associated with a serum lactate level greater than 10 mmol/L, suggests concomitant cyanide poisoning.10 Oxygenation should be assessed by co-oximetry because blood gas analysis and pulse oximetry may be inaccurate in CO-poisoned patients (see later).
Management
The acute management of smoke inhalation is identical to that of other irritant inhalational injuries. Rapid assessment of the airway and early intubation, as indicated, are critical because deterioration may be occult and rapid. Inhaled beta-adrenergic agonists are widely used but without evidence supporting their benefit. Optimal supportive care and maintenance of adequate oxygenation (e.g., suctioning and pulmonary toilet) are the most important aspects of care. Bronchoscopy with bronchoalveolar lavage is frequently recommended to clear debris and toxins from the distal airways. Corticosteroids, whether they are inhaled or administered systemically, are not demonstrated to be helpful and are potentially harmful in patients with cutaneous burns.11 Ibuprofen, antioxidants, exogenous surfactant, and high-frequency ventilation yield variably improved survival in experimental and clinical trials; none is considered standard care. Antibiotics should be used only in patients with suspected infection.
Cyanide and Hydrogen Sulfide
Hydrogen sulfide poisoning most often occurs in petroleum refinery and sewage storage tank workers. An Internet-derived means of suicide involves generation of hydrogen sulfide from sulfur-containing products, such as detergent, mixed with acids in an enclosed space, such as an automobile. On occasion, well-intentioned but hasty would-be rescuers become victims, emphasizing the need for proper training and equipment. Hydrogen sulfide has a noxious odor similar to rotten eggs, which becomes unnoticeable with extremely high concentrations or prolonged exposure (olfactory fatigue).12
Principles of Disease
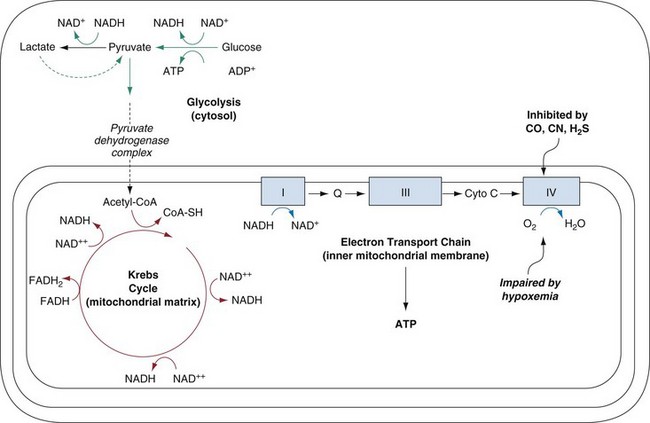
Gaseous cyanide is rapidly absorbed after inhalation and is immediately distributed to the oxygen-using body tissues. Inhibition of oxidative metabolism by binding to complex IV of the electron transport chain within mitochondria occurs within seconds. The poisoned tissue rapidly depletes its adenosine triphosphate reserves and ceases to function (Fig. 159-2). Cyanide has no evident effect on other oxygen-binding enzyme systems, most notably hemoglobin. This is probably explained by the oxidation state of its iron moiety; cyanide binds only to oxidized iron (Fe3+), whereas deoxyhemoglobin contains reduced iron (Fe2+).
Hydrogen sulfide exerts its toxic effects both as a pulmonary irritant and as a cellular poison.12 Its deadly metabolic effects are produced by a mechanism identical to that for cyanide poisoning. However, hydrogen sulfide’s spontaneous dissociation from the mitochondrial cytochrome complex IV is rapid, allowing many patients to survive after brief exposure.
Clinical Features
Because cyanide and hydrogen sulfide prevent tissue extraction of oxygen from the blood, the oxygen content of venous blood remains high, approaching that of arterial blood. Clinically, this may appear as the “arterialization” or brightening of venous blood to resemble arterial blood. A comparison of the measured venous (ideally but impractically mixed venous) and arterial oxygen contents may assist in the diagnosis of cyanide poisoning.13 A low arterial-venous oxygen difference is suggestive of cyanide poisoning but is neither exclusionary nor pathognomonic for the diagnosis.
Patients surviving cyanide or hydrogen sulfide poisoning may have persistent or delayed-onset neurologic syndromes identical to those noted in patients with CO poisoning or cardiac arrest.14
Diagnostic Strategies and Differential Considerations
In practice, the diagnosis is based on the circumstances of exposure and a corroborative physical examination. Rapid cardiovascular collapse, hypotension, bradycardia, ventricular dysrhythmias, and seizures in a fire victim should suggest cyanide poisoning but are also consistent with severe CO poisoning.10 An elevated carboxyhemoglobin concentration in a fire victim may also suggest concomitant cyanide poisoning. However, the presence of these dramatic clinical findings with a low carboxyhemoglobin level is also concerning for cyanide poisoning. Pulse oximetry and ABG analysis are accurate in cases of isolated cyanide or hydrogen sulfide poisoning. An increased anion gap metabolic acidosis and elevated serum lactate concentration are usually present. A lactate concentration greater than 10 mmol/L in a fire victim is highly predictive of cyanide poisoning.10 However, the carboxyhemoglobin level and other laboratory test results may take too long to be obtained, and the delay has important implications for the treatment of cyanide poisoning.
Management
Hydrogen Cyanide
Hydroxocobalamin is another antidotal therapy that takes advantage of the high affinity of cobalt for cyanide. On binding of cyanide, cyanocobalamin, or vitamin B12, is formed. The initial dose is 5 g IV during 15 minutes for adults and 70 mg/kg IV for children, up to an adult dose.15 Thiosulfate, 12.5 g, can be coadministered if it is available. The known adverse effects of hydroxocobalamin are mild and include hypertension in those not cyanide poisoned and a bright red discoloration of the patient’s skin. The drug’s red color can interfere with certain spectrophotometric laboratory tests, including carboxyhemoglobin and possibly serum lactate, and blood samples should be obtained before the administration of the first dose of hydroxocobalamin.
There are insufficient clinical data to fully support the use of one cyanide antidote over the other.16 However, hydroxocobalamin has been used for years and is largely replacing the cyanide antidote kit in the United States because of its ease of use and theoretic safety in CO-poisoned fire victims. Direct comparison to thiosulfate alone in this population has not been and likely never will be performed.17
Hydrogen Sulfide
Because the bond between hydrogen sulfide and cytochrome oxidase is rapidly reversible, removal from exposure and standard resuscitative techniques are usually sufficient to reverse hydrogen sulfide toxicity. Use of the nitrite portion of the cyanide antidote kit is suggested to create MetHb for patients with severe or prolonged toxicity.12 Sodium thiosulfate is unnecessary because hydrogen sulfide is not detoxified by rhodanese. There is no defined role for hyperbaric oxygen therapy in cases of hydrogen sulfide toxicity.
Carbon Monoxide
CO is the most common cause of acute poisoning death in developed nations and the most common cause of fire-related death.18 CO is generated through incomplete combustion of virtually all carbon-containing products. Structure fires (e.g., wood), clogged vents for home heating units (e.g., methane), and use of gasoline-powered generators indoors are examples of the myriad means through which patients are poisoned by CO. Appropriate public health authorities (e.g., fire department and Department of Health officials) should be informed immediately about any potential public health risks that are identified during the care of a CO-exposed patient.
Principles of Disease
Delayed-onset neurologic complications may be a manifestation of the hypoxic insult, although reperfusion injury and lipid peroxidation related to platelet-induced nitric oxide release may play a significant role.19 By alteration of the platelet-associated nitric oxide cycle, the microvascular endothelium of the central nervous system undergoes free radical–mediated injury, resulting in localized inflammation and dysfunction. Animal models and human reports suggest that loss of consciousness during CO exposure may be necessary and is certainly a risk factor for the development of delayed neurologic sequelae.20
Clinical Features
Delayed neurologic sequelae are a well-documented phenomenon after CO exposure, although the frequency varies from 12 to 50%, depending on the definition and the sensitivity of the test used for their detection.21 Patients have a variety of neurologic abnormalities after an asymptomatic period ranging from 2 to 40 days.20 The delayed neurologic effects can be divided into those with readily identifiable neurologic syndromes (e.g., focal deficits and seizures) and those with primarily psychiatric or cognitive findings (e.g., apathy and memory deficits). Although the delayed neuropsychiatric sequelae require formal neuropsychiatric testing to be detected, the impact of these abnormalities on the patient’s daily function may be significant. Risk factors that predict the development of delayed neurologic sequelae include age and loss of consciousness. Because most CO-poisoned patients reaching the emergency department survive, prevention of delayed neurologic and neuropsychiatric sequelae is the major goal of therapy.
Diagnostic Strategies and Differential Considerations
The ABG analysis is a poor screening test for CO poisoning other than to identify the presence of a metabolic acidosis and a normal partial pressure of oxygen (PO2). CO decreases oxygen bound to hemoglobin but does not affect the amount of oxygen dissolved in blood. Because the PO2, a measure of dissolved oxygen, is normal in patients with CO poisoning, the calculated oxygen saturation will be normal even in the presence of significant CO poisoning. Most pulse oximeters are unable to detect CO poisoning because COHb essentially is misinterpreted as oxyhemoglobin. Newer pulse co-oximeters are capable of noninvasively detecting COHb as well as methemoglobinemia, but the exact clinical utility of this test is not yet fully defined.22
Management
There is controversy regarding the benefit of HBO because the effect is not immediate (as with life and death) but requires close follow-up and sophisticated testing. Several evidence-based reviews have suggested a limited role for HBO, although this conclusion is disputed.23–25 A decrease of delayed neurologic sequelae from approximately 12% to less than 1% is associated with HBO.21 When HBO administration is delayed more than 6 hours after exposure, its efficacy appears to decrease,26 suggesting the need for rapid decision-making. Evidence also suggests that HBO positively affects the development of the delayed neuropsychiatric and delayed neurologic sequelae after CO poisoning.27–29 A randomized, double-blind study found that HBO therapy was superior to normobaric oxygen therapy at reducing the incidence of delayed neurologic sequelae at both 6 weeks and 1 year after poisoning.27 However, another found no benefit of HBO on the development of delayed neurologic sequelae compared with extensive normobaric oxygen.28 In this study, however, the majority of patients were suicidal and possibly depressed, which would interfere with performance on the neuropsychiatric testing needed to differentiate the two groups of patients. A recent trial found that in comatose patients, one HBO session was superior to two sessions; however, in patients with transient loss of consciousness (i.e., syncope), outcome after HBO therapy was equivalent to 6 hours of normobaric oxygen therapy.30
In addition to use of HBO in patients with obvious signs of tissue hypoxia, some institutions have set a conservative COHb level of 25% at which asymptomatic or minimally symptomatic patients will be referred for HBO therapy. Some institutions use COHb levels of 40%, and others refrain from specifying a number. The decision to perform HBO therapy should be considered in light of the transport and other medical requirements. Special consideration is given to pregnant women because of the relative hypoxia of the fetus. Because fetal CO poisoning is associated with dysfunction and death and HBO therapy appears to be safe in pregnancy, many institutions initiate HBO therapy in a pregnant woman to a COHb level of 15%.31
Further study is still needed to define the optimal duration, pressure, and frequency as well as the cost-benefit and risk-benefit relationships of HBO therapy. At this time, discussion with a regional HBO center or poison control center is advisable. Patients with elevated COHb levels who do not require HBO should be treated with normobaric oxygen delivered by a tight-fitting non-rebreather face mask until the symptoms resolve and the COHb levels fall. The total duration of such therapy is undefined, and although 3 days was suggested in one study,28 most mildly CO-poisoned patients probably require no more than 6 hours of therapy.32
Simultaneous Carbon Monoxide and Cyanide Poisoning (Fire Victim)
Concurrent toxicity from CO and cyanide is widely reported and a major factor in the mortality associated with smoke.10,33 Smoke inhalation victims who present with coma and metabolic acidosis can have severe CO poisoning, cyanide poisoning, or both. Nitrite-induced methemoglobinemia, which further reduces the tissue oxygen delivery, may be detrimental to patients with elevated COHb levels or otherwise impaired oxygen delivery.
Sodium thiosulfate, administered without nitrites,34 or hydroxocobalamin should be given to all smoke inhalation victims with coma, hypotension, severe acidosis, or cardiovascular collapse in whom cyanide poisoning cannot be rapidly excluded. If the COHb level is known to be low and the patient has persistent acidosis or hemodynamic instability, the complete cyanide antidote kit, including the nitrites, can be administered. Patients with high COHb levels undergoing HBO therapy can also receive nitrite therapy while pressurized with little concern of decreasing the oxygen-carrying capacity. Alternatively, hydroxocobalamin, with or without sodium thiosulfate, can be administered in either of these last two situations.
Disposition
The decision to transfer a patient to an HBO facility must consider the time delay to therapy, patient issues (e.g., burns and age), and potential transport-related complications.35 At a minimum, prolonged normobaric oxygen therapy should be administered, although the benefit of this remains undefined. Admission decisions should be based on the patient’s clinical condition. All patients exposed to CO require close follow-up for delayed neurologic sequelae.
References
1. Gill, JR, Ely, SF, Hua, S. Environmental gas displacement: Three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002;23:26.
2. Studdert, DM, Gurrin, LC, Jatkar, U, Pirkis, J. Relationship between vehicle emissions laws and incidence of suicide by motor vehicle exhaust gas in Australia, 2001-06: An ecological analysis. PLoS Med. 7, 2010.
3. DeBehnke, DJ, et al. The hemodynamic and arterial blood gas response to asphyxiation: A canine model of pulseless electrical activity. Resuscitation. 1995;30:169.
4. White, CW, Martin, JG. Chlorine gas inhalation: Human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010;7:257–263.
5. Tsushima, K, et al. Acute lung injury review. Intern Med. 2009;48:621–630.
6. Traub, SJ, Hoffman, RS, Nelson, LS. Case report and literature review of chlorine gas toxicity. Vet Hum Toxicol. 2002;44:235.
7. Peter, JV, et al. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: Meta-analysis. BMJ. 2008;336:1006.
8. Clark, WR, Bonaventura, M, Myers, W. Smoke inhalation and airway management at a regional burn unit: 1974-1983. Part I: Diagnosis and consequences of smoke inhalation. J Burn Care Rehabil. 1989;10:52.
9. Lin, WY, Kao, CH, Wang, SJ. Detection of acute inhalation injury in fire victims by means of technetium-99m DTPA radioaerosol inhalation lung scintigraphy. Eur J Nucl Med. 1997;24:125.
10. Baud, FJ, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325:1761.
11. Greenhalgh, DG. Steroids in the treatment of smoke inhalation injury. J Burn Care Res. 2009;30:165–169.
12. Reiffenstein, RJ, Hulbert, WC, Roth, SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109.
13. Johnson, RP, Mellors, JW. Arteriolization of venous blood gases: A clue to the diagnosis of cyanide poisoning. J Emerg Med. 1988;6:401.
14. Snyder, JW, et al. Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am J Emerg Med. 1995;13:199.
15. Borron, SW, et al. Hydroxocobalamin for severe acute cyanide poisoning by ingestion or inhalation. Am J Emerg Med. 2007;25:551.
16. Rodgers, GC, Jr., Condurache, CT. Antidotes and treatments for chemical warfare/terrorism agents: An evidence-based review. Clin Pharmacol Ther. 2010;88:318–327.
17. Erdman, AR. Is hydroxocobalamin safe and effective for smoke inhalation? Searching for guidance in the haze. Ann Emerg Med. 2007;49:814.
18. King, M, Bailey, C. Carbon monoxide–related deaths, United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56:1309–1312.
19. Weaver, LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217–1225.
20. Choi, IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433.
21. Thom, SR, et al. Delayed neuropsychiatric sequelae following CO poisoning. Ann Emerg Med. 1994;23:612.
22. Touger, M, et al. Performance of the RAD-57 pulse CO-oximeter compared with standard laboratory carboxyhemoglobin measurement. Ann Emerg Med. 2010;56:382–388.
23. Juurlink, DN, Stanbrook, MB, McGuigan, MA. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. (1):2005.
24. Wolf, SJ, Lavonas, EJ, Sloan, EP, Jagoda, AS, American College of Emergency Physicians. Clinical policy: Critical issues in the management of adult patients presenting to the emergency department with acute carbon monoxide poisoning. Ann Emerg Med. 2008;51:138.
25. Logue, CJ. An inconvenient truth? Ann Emerg Med. 2008;51:339.
26. Goulon, M, et al. Intoxication oxy carbonee et anoxic aique par inalation de gaz de charbon et d’hydvocarbure. Ann Med Interne (Paris). 1969;120:335.
27. Weaver, LK, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057.
28. Scheinkestel, CD, et al. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: A randomized controlled clinical trial. Med J Aust. 1999;170:203.
29. Hardy, KR, Thom, SR. Pathophysiology and treatment of carbon monoxide poisoning. J Toxicol Clin Toxicol. 1994;32:613.
30. Annane, D, et al. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: Two randomized controlled trials. Intensive Care Med. 2011;37:486–492.
31. Elkharrat, D, et al. Acute carbon monoxide intoxication and hyperbaric oxygen in pregnancy. Intensive Care Med. 1991;17:289.
32. Raphael, JC, et al. Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication. Lancet. 1989;2:414.
33. Alarie, Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32:259.
34. Ivankovich, AD, et al. Cyanide antidotes and methods of their administration in dogs: A comparative study. Anesthesiology. 1980;52:210.
35. Sloan, EP, et al. Complications and protocol considerations in carbon monoxide–poisoned patients who require hyperbaric oxygen therapy: Report from a ten-year experience. Ann Emerg Med. 1989;18:629.