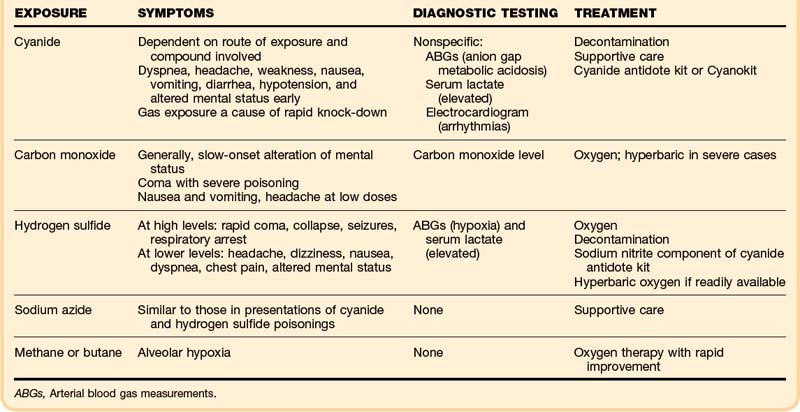
153 Inhaled Toxins
• Cyanide gas and hydrogen sulfide are rapid knockdown agents.
• The key characteristic of cyanide is profound metabolic acidosis with a wide anion gap and elevation of serum lactate.
• Clinical suspicion is paramount to the diagnosis of cyanide toxicity; treatment may be required before the diagnosis is confirmed.
• Hydroxycobalamin (Cyanokit) has fewer adverse events than the cyanide kit; it has one component instead of three.
• Rescuers must wear adequate personal protective equipment, including a self-contained breathing apparatus, to remove a victim from the source of exposure to hydrogen sulfide.
• Patients who present alert, without altered mental status and with normal respiratory effort after exposure to hydrogen sulfide, should have a good outcome.
• If administered shortly after severe hydrogen sulfide exposures, sodium nitrite may improve outcome.
• The mainstays of treatment for hydrogen sulfide toxicity are 100% oxygen and meticulous supportive care.
Cyanide
Epidemiology
Cyanide exists in several forms. The gaseous form is hydrogen cyanide (HCN); the salt forms are potassium cyanide and sodium cyanide. The inorganic salts release HCN gas when they are dissolved in water. Cyanogens are compounds that are metabolized to cyanide in vivo. The two clinically important cyanogens are amygdalin and acetonitrile. Amygdalin is a naturally occurring cyanogen found in the seeds of plants in the Prunus species and in the pits of other fruits (Box 153.1). Acetonitrile is found in some artificial nail removal products. Unlike other cyanide products, cyanogens may produce delayed cyanide toxicity because of the time required for their biotransformation. Cyanide gas is also released during combustion of certain natural and synthetic materials (Box 153.2). Consequently, cyanide poisoning can occur in victims of structure fires.
Pathophysiology
Cyanide is a potent cellular poison. The primary clinical effect occurs through the inhibition of oxidative phosphorylation. Cyanide binds to the ferric (Fe3+) moiety of cytochrome oxidase aa3, the last enzyme of the electron transport chain.1 The net effects of cyanide poisoning are reduced oxygen consumption and conversion of aerobic to anaerobic metabolism; these effects lead to profound metabolic acidosis and an elevated serum lactate concentration.
Differential Diagnosis and Medical Decision Making
Without a history of cyanide exposure, the diagnosis is often difficult to make (Table 153.1). All medical causes of altered mental status, hypotension, and acidosis must be considered. The differential diagnosis of cyanide poisoning is listed in Box 153.3.
Treatment
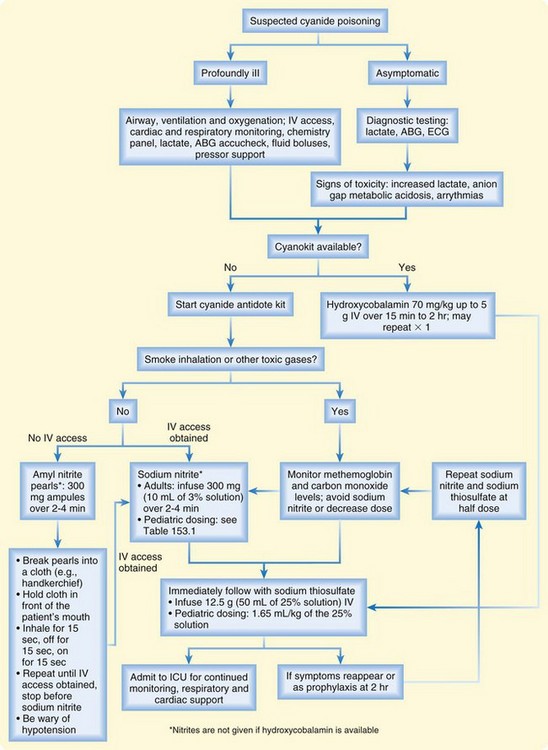
Aggressive supportive care and early administration of antidote therapy are the mainstays of treatment (Fig. 153.1 and Box 153.4). Patients with cyanide exposure are often critically ill at presentation and may rapidly progress to respiratory and cardiovascular collapse. Antidote therapy options include the cyanide antidote kit (CAK) and hydroxycobalamin (Cyanokit).
Box 153.4 Treatment Algorithm for Cyanide Poisoning
The CAK contains three products: amyl nitrite, sodium nitrite, and sodium thiosulfate. The nitrates induce methemoglobinemia and thus allow cyanide to dissociate from cytochrome oxidase and preferentially bind to methemoglobin. This process sequesters cyanide in the serum and allows cellular metabolism to resume. The sodium thiosulfate component enhances the innate pathway of cyanide metabolism. The amyl nitrite component of the CAK is contained in pearls that are crushed to produce a vapor that is inhaled. This component is generally reserved for situations in which intravenous access is delayed or cannot be achieved. The sodium nitrite comes in 300-mg ampules, which are infused over 2 to 4 minutes in adult patients. The sodium thiosulfate dose is 12.5 g administered intravenously. The patient’s blood pressure must be monitored carefully because the nitrites can induce hypotension. Methemoglobin levels must be monitored regularly during nitrite therapy because methemoglobin does not carry oxygen. The methemoglobin levels should be kept at less than 30%. Nitrites should not be administered to fire victims, who may have carbon monoxide poisoning, because carboxyhemoglobin also does not carry oxygen. Inducing methemoglobinemia in these patients may worsen oxygen delivery to tissues. In such cases, the physician should use the sodium thiosulfate alone, the hydroxycobalamin alone, or both the thiosulfate and hydroxycobalamin together. In the pediatric patient, the nitrite dose must be adjusted for the hemoglobin level. Table 153.2 provides dosing guidelines for such a situation. Both the sodium nitrite and sodium thiosulfate components of the CAK may be readministered as clinically indicated.
Table 153.2 Pediatric Nitrite Dosing According to Hemoglobin Value*
| HEMOGLOBIN VALUE (g/dL) | 3% SODIUM NITRITE SOLUTION (mL/kg) |
|---|---|
| 7.0 | 0.19 |
| 8.0 | 0.22 |
| 9.0 | 0.25 |
| 10.0 | 0.27 |
| 11.0 | 0.30 |
| 12.0 | 0.33 |
| 13.0 | 0.36 |
| 14.0 | 0.39 |
* If giving sodium nitrite empirically to a patient with no history of anemia, do not wait for hemoglobin results; assume that the hemoglobin level is 12 g/dL, and dose accordingly.
Adapted from Berlin CMJ. The treatment of cyanide poisoning in children. Pediatrics 1970;46:793–6.
Hydroxycobalamin (also hydroxocobalamin, vitamin B12a) is the other antidote for cyanide poisoning and is much simpler and safer to use. Marketed as the Cyanokit, it has been approved in the United States for the treatment of cyanide poisoning. Hydroxycobalamin has a high affinity for cyanide and binds with cyanide to form cyanocobalamin (vitamin B12), which is excreted in the urine. Transient hypertension and a self-limited reddish discoloration of the skin, mucous membranes, and urine are the only adverse effects of hydroxycobalamin. The adult dose is 5 g administered intravenously over 15 to 30 minutes; the pediatric dose is 70 mg/kg. The dose may be repeated as clinically indicated up to a maximum of 10 g.2 Hydroxycobalamin can be used alone or in conjunction with the sodium thiosulfate component of the CAK. Hydroxycobalamin can be safely administered simultaneously with sodium thiosulfate, although the two agents cannot be administered through the same intravenous access site. Hydroxycobalamin and sodium thiosulfate may be synergistic in cyanide elimination.3,4 Hydroxycobalamin does not induce methemoglobinemia.
Hydrogen Sulfide
Epidemiology
Hydrogen sulfide is a known knockdown agent. It causes a patient to become rapidly comatose, with profound metabolic acidosis. Because of its rotten egg odor, it is commonly referred to as “sewer gas” or “stink damp.”5 In addition to being a well-known sewer hazard, hydrogen sulfide exposure can be seen in plumbing, mining, tanning, fisheries, drilling, and miscellaneous chemical manufacturing occupations. This agent can be produced by the decomposition of animal or organic material. It can also be produced by the addition of an acid to a metal sulfide. A clue to the presence of hydrogen sulfide is the blackening or tarnishing of silver coins, which results from the conversion of silver to silver sulfide on exposure to hydrogen sulfide.
Pathophysiology
Hydrogen sulfide is as potent as cyanide in binding to cytochrome aa3, inhibiting oxidative phosphorylation, and leading to metabolic acidosis and impaired oxygen utilization.5
Presenting Signs and Symptoms
Hydrogen sulfide is a rapid knockdown agent. An individual exposed to a large concentration of hydrogen sulfide may quickly lose consciousness. Exposure to low concentrations may cause headache, dizziness, and nausea; at higher concentrations, exposure can lead to dyspnea, chest pain, and a decreased level of consciousness. At the highest concentrations, exposures may quickly cause collapse, coma, seizures, respiratory arrest, and asphyxiation. Table 153.3 illustrates the correlation of hydrogen sulfide air concentrations with clinical effect.6
Table 153.3 Hydrogen Sulfide Air Concentrations and Clinical Effects
| HYDROGEN SULFIDE CONCENTRATION (ppm) | CLINICAL EFFECTS |
|---|---|
| 30 |
Adapted from the Agency for Toxic Substances and Disease Registry. Toxicological profile for hydrogen sulfide. Atlanta: Agency for Toxic Substances and Disease Registry, U.S Department of Health and Human Services, 2006.
Differential Diagnosis and Medical Decision Making
The differential diagnosis of hydrogen sulfide exposure includes the many medical and toxicologic causes of altered mental status, metabolic acidosis, and hypoxia. See Box 153.3 for an illustration of the differential diagnosis of hydrogen sulfide exposure.
Treatment
The most important concept in hydrogen sulfide treatment is removal of the patient from the source of exposure (Box 153.5). Patients with significant signs and symptoms or a history of significant exposure should be given 100% oxygen. Comatose patients or those in severe respiratory distress should be intubated and given 100% oxygen. Principles of decontamination should be followed as indicated. The patient’s clothes should be removed, and the skin should be decontaminated using water. Ocular examination with fluorescein and irrigation with saline solution are appropriate to address ocular exposures.
Box 153.5 Treatment of Hydrogen Sulfide Poisoning
1. Remove the patient from the source of exposure by using a self-contained breathing apparatus.
2. Decontaminate the patient with water.
4. Intubate if the patient is comatose or has severe respiratory distress, and provide 100% oxygen.
5. If the patient is hypotensive, provide intravenous fluids and inotropes or pressors.
6. In moderate to severe cases in patients who present early, administer sodium nitrite: 300 mg (10 mL of 3% solution) over 2 to 4 minutes in adults. Measure methemoglobin levels 30 minutes after infusion.
7. Hyperbaric oxygen therapy may be tried in patients with moderate to severe cases if it is readily available at the treating institution.
The sodium nitrite component of the CAK can be administered to patients presenting early after moderate to severe exposure to hydrogen sulfide. Animal evidence supports the early administration of nitrites as antidote therapy for hydrogen sulfide toxicity.7,8 Nitrates can be given intravenously in the same manner as described for cyanide poisoning.
Hyperbaric oxygen therapy (HBOT) has been used to treat hydrogen sulfide poisoning with anecdotal success.9,10 Its use is based on a theoretical benefit, and the indications are controversial. Transfer of an unstable patient for HBOT is not justified by the current literature. HBOT may be considered if it is readily available at the treating institution.
Follow-Up, Next Steps of Care, and Patient Education
Tips and Tricks
• Without a history of exposure, the diagnosis of cyanide poisoning is challenging.
• Hydrogen sulfide poisoning should be suspected whenever a person is found unconscious in an enclosed space, especially if an odor of rotten eggs is present.
• Hydroxycobalamin (Cyanokit) has fewer adverse effects compared with nitrites in treating cyanide poisoning and is preferred in the setting of smoke inhalation.
• Many patients with gas exposure respond rapidly to oxygen therapy; lack of response should raise suspicion of cyanide or hydrogen sulfide poisoning.
• Hyperbaric oxygen therapy for hydrogen sulfide toxicity is indicated only when it is readily available at the treating facility.
• Patients who do not survive cyanide poisoning are candidates for organ donation.
1 Morocco AP. Cyanides. Crit Care Clin. 2005;21:691–705.
2 Sauer SW, Keim ME. Hydroxocobalamin: Improved public health readiness for cyanide disasters. Ann Emerg Med. 2001;37:635–641.
3 Hall AH, Rumack BH. Hydroxycobalamin/sodium thiosulfate as a cyanide antidote. J Emerg Med. 1987;5:115–121.
4 Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol. 2009;39:541–552.
5 Knight LD, Presnell SE. Death by sewer gas: case report of a double fatality and review of the literature. Am J Forensic Med Pathol. 2005;26:181–185.
6 Agency for Toxic Substances and Disease Registry. Toxicological profile for hydrogen sulfide. Atlanta: Agency for Toxic Substance and Disease Registry. U.S. Department of Health and Human Services; 2006.
7 Smith RP, Gosselin RE. Hydrogen sulfide poisoning. J Occup Med. 1979;21:93–97.
8 Smith RP, Kruszyna R, Kruszyna H. Management of acute sulfide poisoning: effects of oxygen, thiosulfate, and nitrite. Arch Environ Health. 1976;31:166–169.
9 Smilkstein MJ, Bronstein AC, Pickett HM, Rumack BH. Hyperbaric oxygen therapy for severe hydrogen sulfide poisoning. J Emerg Med. 1985;3:27–30.
10 Belley R, Bernard N, Cote M, et al. Hyperbaric oxygen therapy in the management of two cases of hydrogen sulfide toxicity from liquid manure. CJEM. 2005;7:257–261.