Inflammatory Disorders of the Small Intestine
Marie E. Robert
Joanna A. Gibson
Disorders of Malabsorption
Box 16.1 lists intestinal malabsorptive disorders by disease category.
Gluten-Sensitive Enteropathy (Celiac Disease)
Epidemiology and Clinical Features
Gluten-sensitive enteropathy (GSE), or celiac disease, is an autoimmune-mediated disorder that results in damage to the small-intestinal mucosa and leads to malabsorption of nutrients. Although this disease was described more than a century ago,1,2 only in the past 60 years has the role of dietary gluten in its pathogenesis been recognized.3 GSE has a worldwide distribution but is most common in countries with populations of European descent (Europe, North and South America, and Australia), where the prevalence is approximately 1%.4,5 Although it is less common in Asia and Africa, GSE is expected to become more prevalent in these countries as wheat consumption increases.4,6 A recent change in the gluten reaction landscape is the emergence of “gluten sensitivity” as a separate, poorly understood, and possibly immune- (not autoimmune-) mediated condition, which along with wheat allergy and GSE, forms a spectrum of gluten-related disorders.5 The discussion here is limited to GSE.
GSE may appear at any time in life, from early childhood to late adulthood. It is presumed that patients who are diagnosed in adulthood have had a mild form of disease for a long period. Classic symptoms include abdominal discomfort, diarrhea, and steatorrhea.7,8 Some patients do not have diarrhea but instead exhibit other signs and symptoms, including short stature, infertility, neurologic disorders, recurrent aphthous stomatitis, or dermatitis herpetiformis.5,7–11 In adults with occult GSE, complaints of fatigue often uncover the presence of iron deficiency anemia, which ultimately leads to the correct diagnosis.12 Some patients are entirely asymptomatic and initially have only histologic changes on biopsy analysis. The term latent celiac disease has been used to describe patients with this presentation (see later discussion).13,14 Factors governing the type of clinical presentation and the expression of symptoms are poorly understood. It was once thought that the development and severity of symptoms were related to the length of intestine involved rather than the severity of mucosal pathology in a single biopsy specimen;14 however, recent studies appear to refute that theory.15,16 Attention is now being focused on the action of gluten-sensitized lymphocytes to increase mucosal cytokine levels as a determiner of clinical symptoms, regardless of severity or length of mucosa showing villous blunting.16–18
Pathogenesis
GSE is an immunologic disorder that occurs in genetically susceptible hosts. Environmental influences are important as well. The familial nature of the disease was originally established in a study of 17 probands and their families, who underwent biopsies of the small bowel.7 An increased incidence of GSE was found in first-degree relatives of symptomatic patients. Studies have documented that 10% to 20% of asymptomatic first-degree relatives have mild histologic changes, such as increased intraepithelial lymphocytes (IELs) without villous blunting, or have mild mucosal lesions.7,19,20 In such a setting, the decision to institute a gluten-free diet depends on a variety of factors.
Genetic studies have established the strong association with major histocompatibility complex class II, with as many as 95% of patients carrying either the human leukocyte antigen HLA-DQ2 allele (the majority) or the HLA-DQ8 allele.21–25 However, these genetic phenotypes do not explain the pathogenesis entirely, because 30% of the normal population carry the DQ2 haplotype without disease, and not all patients with GSE carry this specific phenotype.21,23 Studies have identified at least seven non-HLA genes associated with an increased risk of GSE, including CCR3, and the interleukin locus IL2/IL21. Some of the identified genes are also common in type 1 diabetes and rheumatoid arthritis, suggesting that they impart a generalized increased risk of autoimmunity.26–29 The reader is referred to several reviews on the genetics of GSE.26,27,30–32
In the proper genetic environment, exposure to the prolamin fractions of gluten found in wheat, rye, and barley (gliadin, secalin, and hordein, respectively), results in a local immune response.8,33 Additional environmental components may be related to previous infections.34–36 For example, the E1b protein of adenovirus 12 has been shown to contain amino acid sequence homology with α-gliadin antibodies, and T cells that recognize these viral proteins cross-react with gliadin. The crucial role of intestinal tissue transglutaminase has also been elucidated. Tissue transglutaminase (tTG), a ubiquitous enzyme in human tissues, catalyzes the formation of cross-links between glutamine and lysine residues in substrate proteins.37 tTG is believed to stabilize extracellular matrix molecules in the setting of mucosal injury and granulation tissue. In 1997, Dieterich and colleagues demonstrated that dietary gliadin, which is rich in the amino acid glutamine, serves as a substrate for tTG.37 It is thought that the resulting cross-linked molecules (gliadin-gliadin or gliadin-tTG) become antigenic epitopes for a subsequent immune response. Of the more than 50 T cell–stimulating epitopes in gluten proteins, a 33-mer peptide that may be the primary initiator of the inflammatory response in GSE has been identified.38 Investigations into the role of tTG in GSE have led to improved diagnostic tests (described later).39
The immune response to dietary gluten involves both antibody- and cell-mediated injury. Antibody-induced injury may occur via antigen-antibody complex deposition with complement activation and also by the induction of cell-mediated cytotoxicity. Activated mucosal T cells have been shown to cause epithelial injury, probably through the release of cytokines, including IL15.33,40,41
The clinical diagnosis of GSE depends on detection of the appropriate combination of symptoms, laboratory evidence of malabsorption, and the presence of serum autoantibodies. New and increasingly accurate serologic tests continue to be added to the “diagnostic tool kit” for GSE.42 These include the enzyme-linked immunosorbent assay for immunoglobulin A (IgA) anti-tTG antibodies, which has a high degree of sensitivity (77% to 100%) and specificity (91% to 100%). tTG antibody determination has become the serologic test of choice for GSE, largely replacing antigliadin and antiendomysial antibody tests.43–45 The antiendomysial antibody (EMA) test is as sensitive and specific as the anti-tTG test with human recombinant protein, but the immunofluorescence EMA test is labor intensive and relies on a more subjective interpretation than the anti-tTG determination does.6 Today, the EMA is often used as a confirmatory test when tTG test results or clinical or biopsy findings are ambiguous.
Traditional antigliadin antibody testing no longer has a place in GSE diagnosis because of its lack of specificity. However, a new generation of antigliadin antibodies, known as deamidated gliadin peptides (DGP), are being used in a promising IgG-based test that has shown high concordance with both tTG and EMA tests, with possibly greater sensitivity in children and in early disease.5,42,46–48 This test also has the advantage of detecting GSE in IgA-deficient patients.
HLA testing to detect susceptible HLA phenotypes (DQ2 and DQ8) has an increasing role in the diagnostic workup of GSE because the absence of DQ2 or DQ8 virtually excludes the diagnosis.49,50 Finally, transglutaminase-specific IgA deposits can be found by immunofluorescence in frozen sections of biopsy specimens obtained from small-intestinal mucosa in patients with GSE, even those with normal to near-normal histology.51–53 Although this technique is not currently in widespread use, it could become more prevalent in the future, especially in confirming histologically mild disease.53 For a more detailed discussion of the current approach to clinical diagnosis and management of GSE, several excellent reviews are available.5,42,45,48–50,54,55
Pathologic Features
Background
In Western countries before 1990, GSE was a diagnosis that was always made with ease and confidence on the basis of small bowel biopsies. The pathologic diagnosis required flat or nearly flat small bowel mucosa, usually accompanied by increased IELs and lamina propria inflammation in biopsy specimens obtained distal to the duodenal bulb. A diagnosis of untreated GSE was not normally considered if the biopsy specimen did not show significant loss of villous architecture.
Since the early 1990s, several changes in the understanding of GSE have altered the diagnostic algorithm for this disease and eliminated the gold standard status of small bowel biopsies.5,42,56 These changes include (1) recognition that latent or occult (minimally symptomatic) GSE in adults represents a large, previously unappreciated disease population13,14,56; (2) revision of histologic criteria to include minimal inflammatory changes with intact or only mildly abnormal architecture as a part of the histologic spectrum of GSE14,56–58; and (3) the discovery that tTG is the target autoantigen of antibodies in patients with GSE and the subsequent development of a highly accurate diagnostic test for anti-tTG antibodies, which can now be used in combination with the tests described previously.37,43,44,59
These developments have had a great impact on pathologists, who can now correlate minimal histologic findings with objective clinical and serologic evidence of GSE. They have also decreased the need for reliance on small bowel biopsy findings, which are often subject to interpretive challenges because of orientation and nonspecificity of changes. Today, the sophisticated pathologist and clinician must understand that evaluating patients for GSE may or may not include the need for mucosal biopsy.42 Small-intestinal mucosal biopsies can always be used to help fulfil diagnostic criteria, or they may be reserved for patients in whom results are inconclusive, the caveat being that histologic findings in the latter category of patients may also be inconclusive (Box 16.2).
Gross Pathology
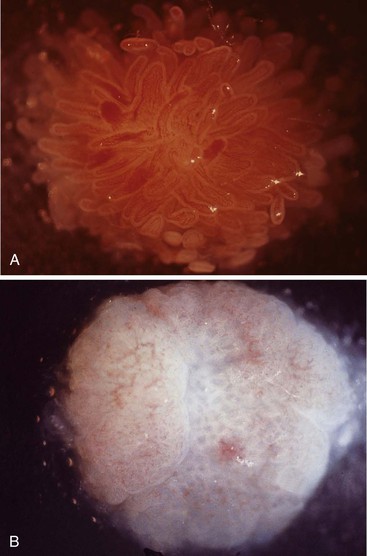
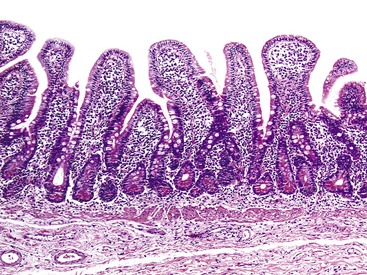
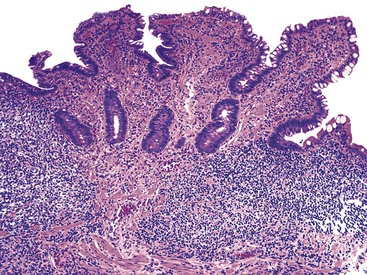
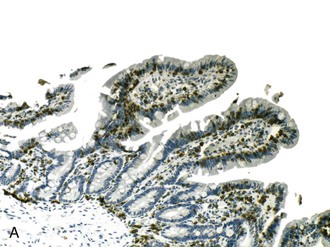
In a landmark study, Rubin and associates described the appearance of normal duodenal mucosa on biopsy samples with the use of a hand lens and showed that the mucosa is characterized by numerous slender villi with a delicate capillary network that floats in formalin like “the tentacles of a sea anemone.”60 In contrast, mucosa specimens from patients with GSE have a barren, stubby surface, without normal villi, containing widely spaced, irregular capillaries (Fig. 16.1). However, endoscopic findings in patients with GSE are subtle and often unreliable. A flat, scalloped appearance of the duodenal mucosa, most likely reflecting loss of villi, may be seen.61 One endoscopic study found that a reduction of folds, scalloping, mosaic pattern, and nodular mucosa were sensitive but not specific endoscopic findings in GSE, because they were also seen in some dyspeptic patients without GSE.62 A meta-analysis of studies testing the diagnostic sensitivity and specificity of video capsule endoscopy in GSE reported that the images seen were sufficient to correctly diagnose the condition.63 However, there are insufficient data on the performance of this tool in the mild histologic forms that are commonly at issue today. Confocal laser microscopy shows promise as an endoscopic technique that can recognize villous irregularity and blunting, with the caveats that interobserver variability is high and the technique is not yet widely available.64
Microscopic Pathology: Biopsy Strategy
GSE is a disease of the proximal small intestine in most patients, with severity usually greatest in the duodenum and proximal jejunum. The ileum may be involved in severe cases, but it is not a reliable site for biopsy diagnosis.61,65–67 It is now widely recognized that many patients with GSE do not have diffusely abnormal duodenal histology, but instead show mild and patchy involvement in duodenal mucosal biopsies. The duodenal bulb, once strictly avoided in the evaluation of GSE because of its susceptibility to peptic injury and prominent Brunner glands, is now a recommended biopsy site because it is reliably involved in patients with patchy disease.68,69 In a pediatric study, 16% of children had patchy villous atrophy, but all of them had involvement of the duodenal bulb, and in four patients the bulb was the only site of abnormality.68 Duodenal bulb biopsies may also be more sensitive in patients following a low-gluten diet.70 Further evidence supporting the validity of the duodenal bulb as a diagnostic site is found in the study by Walker and co-workers (2010), in which paired IEL counts were identical in the duodenal bulb and in the descending (second) duodenum in GSE patients.71 Based on studies in both adults and children, it appears that the most reliable biopsy protocol to detect all cases of GSE is a three- (or preferably five-) biopsy regimen that includes the duodenal bulb, the proximal duodenum, and the distal duodenum.68,69,72,73
Once the small bowel biopsy specimen is received in the laboratory, accurate evaluation in cases of suspected GSE requires proper orientation in tissue blocks to assess mucosal architecture and to avoid overinterpretation of short villi (which are invariably seen in poorly oriented sections) as abnormal. Although specimens are rarely perfectly oriented, an attempt should be made to find three or four well-oriented villi in a row to assess architecture. Similarly, now that duodenal bulb biopsies are requested in this setting, care must be taken to distinguish peptic injury (neutrophils, gastric surface metaplasia, and surface injury) and villous blunting resulting from mucosal Brunner glands from GSE. IEL counts should be helpful in making this distinction.
Histology of Untreated Disease
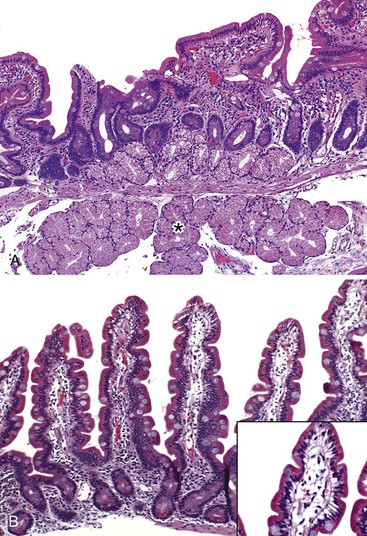
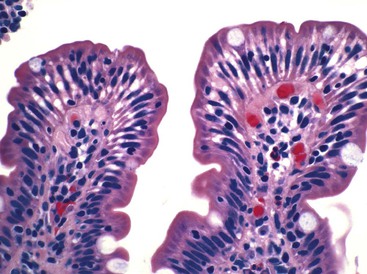
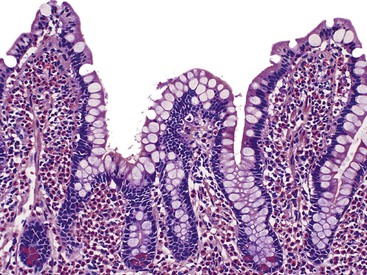
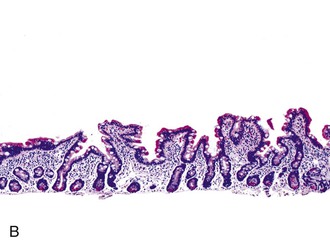
In normal small bowel mucosa, the ratio of villus height to crypt depth is between 3 : 1 and 5 : 1, depending on the anatomic location (Fig. 16.2). Epithelial cells lining the villi contain basally located nuclei with abundant mature cytoplasm and a preponderance of absorptive cells admixed with goblet cells. A faint density, representing the microvillus brush border, can be appreciated on hematoxylin and eosin (H&E)-stained tissue sections. Studies have confirmed that there are approximately 20 lymphocytes per 100 epithelial cells in healthy people, with minor variations.74–78 The lamina propria normally contains a mixture of plasma cells, lymphocytes, and occasional eosinophils. Each of the three mucosal components (i.e., architecture, epithelium, and lamina propria) should be carefully examined in cases of suspected GSE.
The pathology of GSE is described here in conjunction with a grading scheme that can be used in pathology reports (Table 16.1). At the time of this writing, the Marsh-Oberhuber classification is being used regularly in Europe but has not gained wide acceptance in the United States for routine use in pathology reports. Inclusion of the Marsh-Oberhuber subtype requires prior knowledge (e.g., by serology) that the patient has GSE, and biopsy requisition forms often do not provide that information.56,58 In addition, differences between types 0, 1, and 2 in the Marsh scheme are subtle, so there is a high degree of inter-observer variability among pathologists. To avoid these issues, it is reasonable to use the term mucosal lesion or villous blunting,79 as detailed in the following discussion, with a modifier (i.e., mild, moderate, or severe) to reflect the degree of villous blunting.
Table 16.1
Diagnostic Terminology for Reports
| Histology | Pathology Report* |
 |
Note: Increased intraepithelial lymphocytes may be seen in a variety of conditions, including mild forms of GSE. If suspicion for GSE exists, correlation with serologic tests is necessary. Helicobacter pylori gastritis, peptic injury, and NSAID injury should also be considered.
Note: The findings are nonspecific but may represent GSE in the proper clinical setting. Correlation with serologic studies is indicated. (Include differential diagnosis as appropriate.)
Note: The findings are characteristic of GSE in the proper clinical setting. Correlation with serologic studies is indicated. (Include differential diagnosis as appropriate.)
Note: The findings are characteristic of GSE in the proper clinical setting. Correlation with serologic studies is indicated. (Include differential diagnosis as appropriate.)
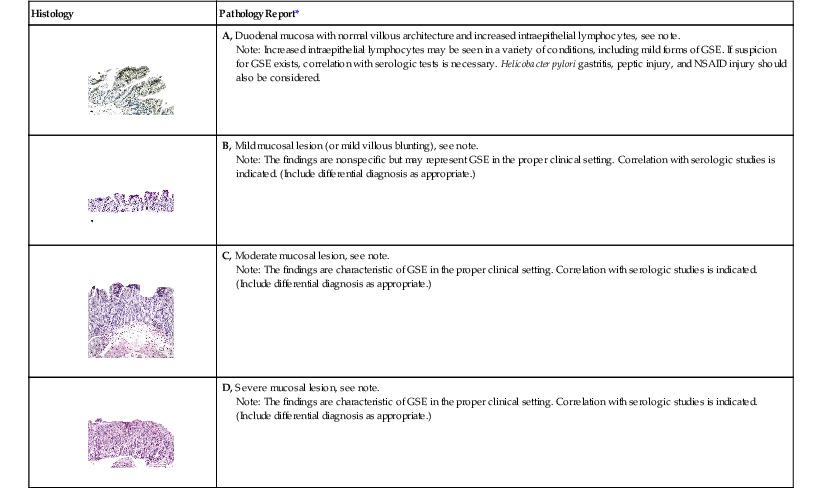
* The content of the note will depend on the clinical information provided and findings in gastric and other biopsies. For ease of reading and clarity, do not include an exhaustive list of all causes of small bowel inflammation. Rather, tailor the report to fit the clinical situation.
GSE, Gluten-sensitive enteropathy; NSAID, nonsteroidal anti-inflammatory drug.
Nonetheless, it is important from both a research and a clinical perspective to be aware of the Marsh-Oberhuber classification scheme (Table 16.2).56,58 This system describes five histologic lesions associated with GSE, termed preinfiltrative (type 0), infiltrative (type 1), infiltrative-hyperplastic (type 2), flat-destructive (type 3), and atrophic-hypoplastic (type 4). Two modifications to this scheme have been proposed in an attempt to simplify the diagnosis for pathologists and clinicians.80,81 Neither has been universally accepted at the time of this writing, but they are included for the sake of completeness in Table 16.3.
Table 16.2
Marsh-Oberhuber Classification of Celiac Disease
| Type* | IELs per 100 Epithelial Cells | Crypts | Appearance of Villi |
| Preinfiltrative type 0 | Normal (<40) | Normal | Normal |
| Infiltrative type 1 | >40 | Normal | Normal |
| Hyperplastic type 2 | >40 | Hypertrophic | Normal |
| Destructive type 3a | >40 | Hypertrophic | Mild blunting |
| Destructive type 3b | >40 | Hypertrophic | Moderate blunting |
| Destructive type 3c | >40 | Hypertrophic | Severe blunting (flat) |
| Hypoplastic type 4 | >40 | Atrophic | Severe blunting (flat) |

* Types 0 to 2 are rarely seen and occur most frequently in patients with dermatitis herpetiformis, asymptomatic patients, and first-degree relatives of patients with gluten-sensitive enteropathy (GSE). Types 3a to 3c are usually found in patients with GSE symptoms. Type 4 is rare and is seen in refractory cases (see text).
IELs, Intraepithelial lymphocytes.
Modified from Oberhuber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194.
Table 16.3
Classification Schemes for Pathologic Evaluation of Gluten-Sensitive Enteropathy
| Marsh, 1992 | Oberhuber et al., 1999 | Corazza and Vilanaci, 2005 | “New Proposal,” Ensari, 2010 |
| Type 1 | Type 1 | Grade A | Type 1 |
| Type 2 | Type 2 | Grade A | Type 1 |
| Type 3 | Type 3A | Grade B1 | Type 2 |
| Type 3B | Grade B1 | Type 2 | |
| Type 3C | Grade B2 | Type 3 | |
| Type 4 | Type 4 | Obsolete | Obsolete |
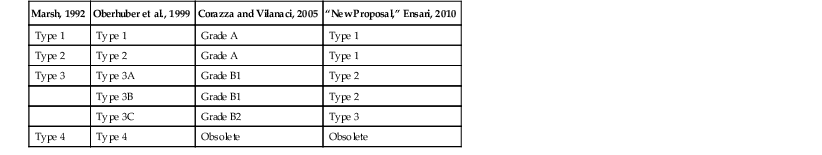
Modified from Ensari A. Gluten-sensitive enteropathy (celiac disease): controversies in diagnosis and classification. Arch Pathol Lab Med. 2010;134:826-836.
Biopsies without Villous Blunting
Preinfiltrative lesions (type 0) exhibit normal mucosa and are seen predominantly in patients who have dermatitis herpetiformis without evidence of malabsorption.
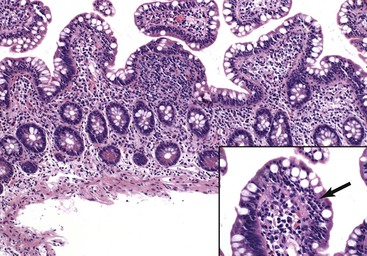
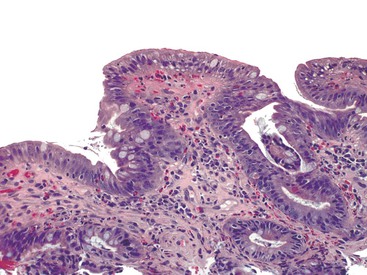
Infiltrative lesions (type 1) have an increased number of IELs with preserved villous architecture, whereas infiltrative-hyperplastic lesions (type 2) show the additional feature of elongated crypts. Both of these patterns of injury are seen in patients with GSE (with or without symptoms), in first-degree relatives of patients with GSE, and in patients with dermatitis herpetiformis. Phenotypic analyses have shown that the increase in IELs is the result of increased numbers of γ/δ T cells.82–84 Biopsy specimens with these subtle changes are challenging to interpret; clinical correlation with serology is required to confirm a diagnosis of GSE, because similar histology is seen in other conditions. By definition, the villus height and the villus-to-crypt ratio is normal or near-normal (Fig. 16.3). The diagnosis relies on the detection of increased IELs, assessment of which is now a routine part of small bowel biopsy interpretation (see later discussion). The proportion of patients with these minimal histologic changes in whom severe histologic lesions and symptoms eventually develop is unknown.13,14,85 One small study found that 4 (33%) of 12 patients with mild changes on duodenal biopsies progressed to GSE with flat mucosa at follow-up.85 However, in a larger study with 8 to 25 years of follow-up, GSE eventually developed in only 5 (2.1%) of 236 patients with increased IELs or a slight reduction in villus-to-crypt ratio.86 These data are complicated by the newer knowledge that patchy histologic findings are more common in GSE than once appreciated. Therefore, sampling error in these studies cannot be excluded.
Counting Intraepithelial Lymphocytes
Numerous studies have shed light on appropriate methods to count IELs (Table 16.4). Reassessment of the normal range of IELs in the second portion of the duodenum has been performed by several investigators by using H&E-stained sections and sections stained for CD3 antigen, establishing an upper limit of 20 lymphocytes per 100 epithelial cells in normal mucosa in the duodenum.74–78 Careful analysis by using CD3 and γ or δ T-cell stains have shown that IEL counts of greater than 25 per 100 epithelial cells (or a ratio of greater than 1 : 4) merit suspicion for potential celiac disease, whereas counts greater than 29 per 100 epithelial cells are found almost exclusively in patients with GSE.75,78,87 However, this kind of analysis is impractical for routine use.
Table 16.4
Methods of Counting IELs in Architecturally Normal Duodenal Biopsies*
| Interpretation | Counts | Stain |
| Diagnosis by Counting IELs per 100 Enterocytes (300-500 Cells Counted) | ||
| Upper limit of normal | 20/100 | H&E |
| 25/100 | CD3 | |
| Borderline increased | 25-29/100 | H&E or CD3 |
| Definitely increased | >29/100 | H&E or CD3 |
| Diagnosis by Villous Tip Method (5 Villi, 20 Enterocytes per Villous Tip, Mean Count) | ||
| Upper limit of normal | 5/20 | H&E or CD3 |
| Definitely increased | ≥6/20 | H&E or CD3 |
* Avoid all lymphoglandular complexes. Average the sum of at least five villi.
H&E, Hematoxylin and eosin; IELs, intraepithelial lymphocytes.
A more practical approach, the villous tip counting method, has been validated in at least four studies.71,75,85,87 In H&E- or CD3-stained sections, averaging the number of IELs per 20 epithelial cells in at least five villous tips is done to obtain a rapid and comparable assessment of IEL counts. With this approach, lymphocyte counts of 6 to 12 IELs per 20 epithelial cells in the tips of villi in architecturally normal mucosa are found in patients with serologic or other evidence of GSE. The IEL counts are lower than in untreated flat GSE and higher than in normal controls. Although CD3 stains were used for cell counting in one study,87 equivalent results were obtained by using H&E stains in two others.75,85 The counts obtained by using the villus tip method correlate well with previously cited studies reporting that greater than 29 IELs per 100 epithelial cells is abnormal.78 A slightly different counting approach was taken in a landmark prospective study that compared IEL counts with serology in a random adult population.71 IELs were counted in a total of 50 enterocytes (in groups of 10) along the sides and tops of villi. In this study, counts of greater than 25 IELs per 100 epithelial cells detected GSE, whereas higher cutoffs missed 50% of cases. Importantly, this study found equally elevated IEL numbers in 3.8% of non-GSE controls, most of whom had Helicobacter pylori gastritis. The term lymphocytic enteropathy (or duodenal lymphocytosis) has emerged to describe the spectrum of patients with increased IELs and normal villous architecture. The term lymphocytic duodenosis has been proposed to refer to the subset of this group that are proved serologically to not have GSE.71
Numerous conditions other than GSE are associated with the presence of increased IELs in architecturally normal duodenal biopsies. These include H. pylori gastritis (Fig. 16.4), injury caused by use of nonsteroidal antiinflammatory drugs (NSAIDs), viral gastroenteritis (Fig. 16.5), tropical sprue, cow’s milk protein sensitivity, bacterial overgrowth, and some immune conditions.71,88–96
In summary, the following approach is recommended for biopsies in patients who have normal or near-normal villous architecture and a perceived increase in IELs or who have the clinical potential for a diagnosis of GSE (i.e., positive tTG serology). First, perform a low-power scan of villi and villus tips, looking for either a diffuse or a patchy increase in IELs. Avoid areas of lymphoglandular complexes, because they lead to falsely elevated IEL counts. If a striking increase in IELs is detected, there is no need to perform an IEL cell count. The number of IELs can be evaluated by the villus tip method for subtle cases, if desired, without the need for a CD3 stain. Because correlation with serology is required in all cases, use of excessive time or expense counting IELs in the diagnostic setting is not recommended. Pathology reports should contain a descriptive diagnosis, such as “duodenal mucosa with preserved villous architecture and increased intraepithelial lymphocytes,” with a note recommending correlation with clinical or serologic evidence of GSE (see Table 16.1), because many disorders can lead to an increase in IELs in the small intestine. Some of these disorders are listed in Table 16.5.54,88–90
Table 16.5
Disorders Showing Histologic Overlap with Gluten-Sensitive Enteropathy
| Conditions Associated with Increased Intraepithelial Lymphocytes* | Conditions Associated with Villous Blunting |
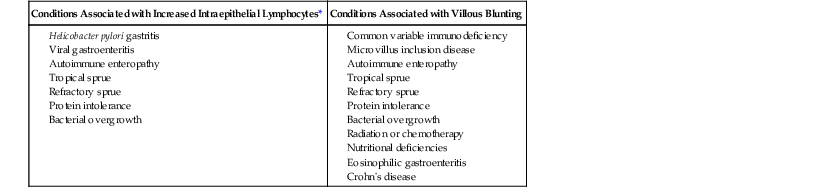
* Not all conditions associated with increased intraepithelial lymphocytes also show villous blunting.
Biopsies with Blunted Villi
The flat-destructive pattern (Marsh type 3) is the classic mucosal lesion associated with symptomatic GSE. Duodenal biopsy specimens reveal loss of normal small-intestinal architecture resulting from a decrease in the height of the villi (villous blunting) accompanied by crypt hyperplasia. Type 3 was further subdivided by Oberhuber into types 3a (mild blunting), 3b (moderate blunting), and 3c (severe blunting).58 These types correspond to mild, moderate, and severe mucosal lesions (or villous blunting).97
It is suggested that pathology reports be accompanied by a note, the content of which depends on the degree of clinical information available at the time of sign-out (see Table 16.1). If clinical information is not provided, the note might read, “These histologic findings are consistent with GSE in the appropriate clinical setting.” In contrast, if a prior history of GSE is known or positive tTG antibodies have been identified, the note could read, “The histologic findings support a clinical diagnosis of GSE.” The terms mucosal lesion and villous blunting are nonspecific and useful to help convey that an architectural abnormality exists but the cause is unknown at the time of sign-out. It also reminds pathologists that villous blunting is a nonspecific finding that may be seen in a variety of conditions.
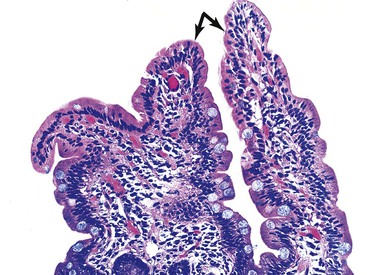
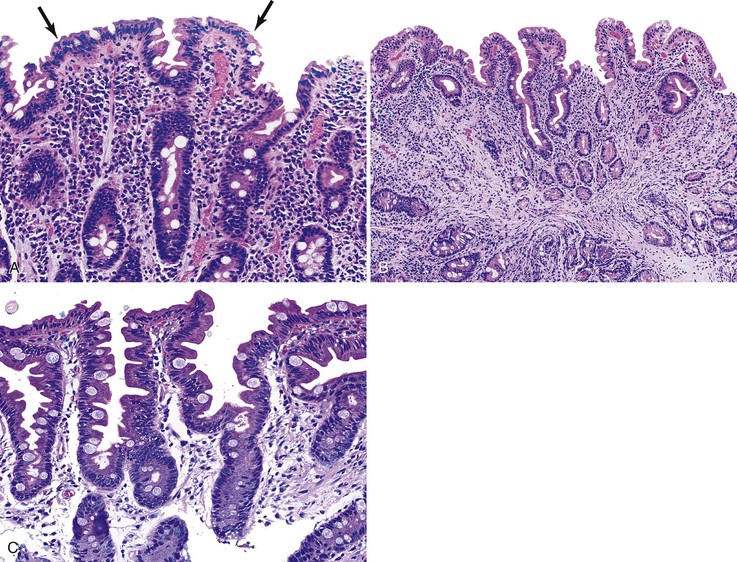
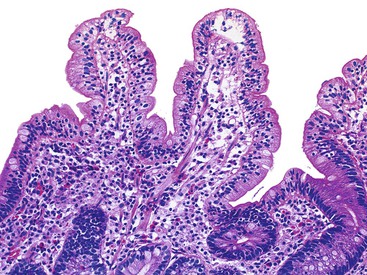
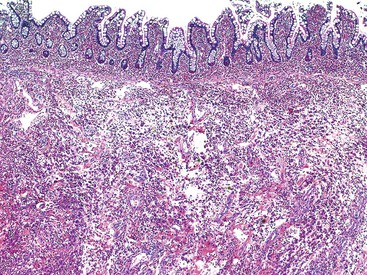
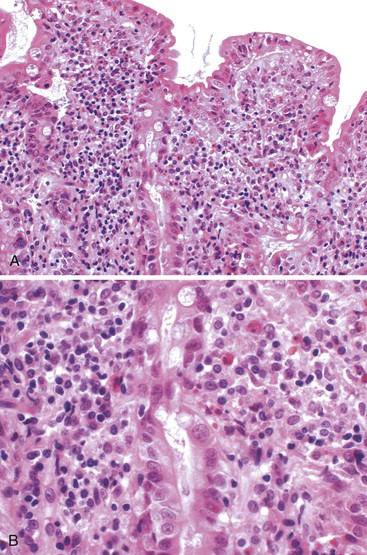
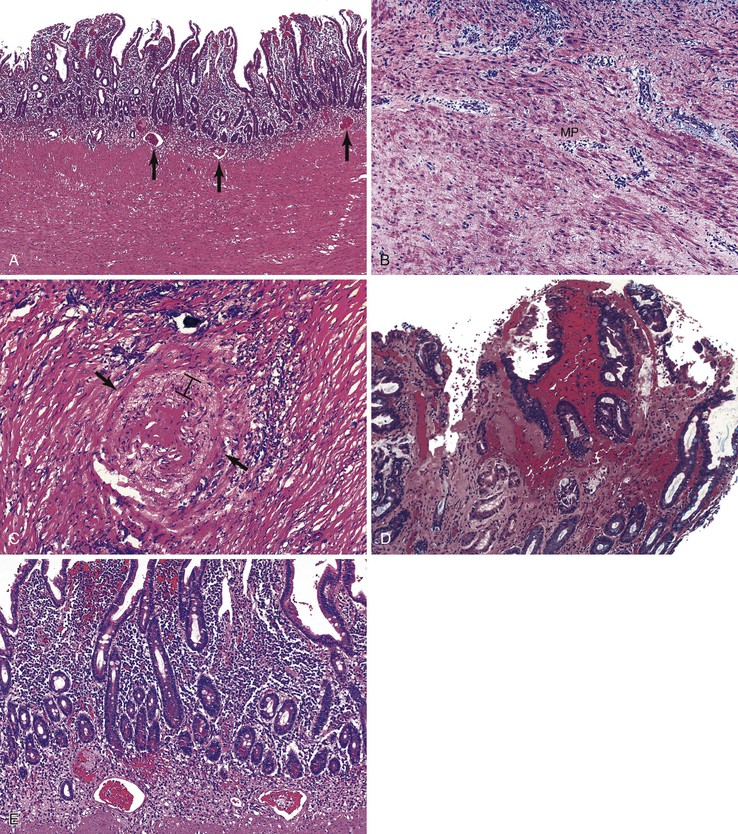
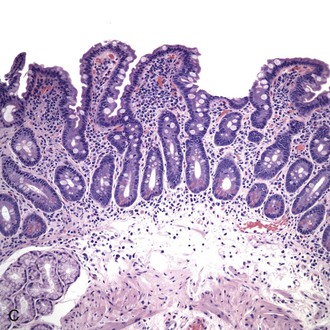
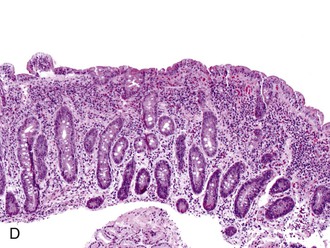
The histology of GSE with villous blunting is characteristic but variable. Biopsy specimens from the duodenum reveal loss of normal architecture caused by a decrease in the height of villi. In many cases, the mucosa appears completely flat. In others, the villi may be broad and only mildly to moderately shortened, or they may be irregular. In clinical practice, histologic overlap between mild, moderate, and severe blunting is often observed in single biopsy specimens (Fig. 16.6). Loss of villus height is usually matched by crypt elongation or hyperplasia, so the overall width of the mucosa usually remains unchanged.61 The degree of injury observed in surface and crypt epithelia can vary widely and does not always reflect the degree of villous blunting or the severity of symptoms.61 When the condition is severe, the surface epithelium shows loss of its columnar shape, mucin depletion, vacuolization, and enlargement of cell nuclei (see Fig. 16.6, A). Cells may show loss of polarity with stratification, pyknosis, and fragmentation of nuclei. IELs are increased, especially in the tips of villi.98–100 The epithelium situated along the length of blunted villi may appear entirely normal, despite its proximity to severely affected cells at the surface. Hyperplastic crypts often show a variable degree of nuclear enlargement and increased mitoses, most likely reflecting an increase in proliferative activity.
The lamina propria in GSE typically shows a variable increase in plasma cells. Neutrophils and crypt abscesses are uncommon but may be present focally. However, the finding of diffuse, or marked, acute inflammation should prompt consideration of other diagnoses, such as peptic duodenitis, Crohn’s disease, and refractory sprue.
Atrophic-hypoplastic lesions (type 4) are rare and are similar to the lesions described in biopsy samples from patients with refractory, or unclassified, sprue (discussed later).10 The finding of atrophic mucosa may correlate with lack of response to a gluten-free diet.
Histology of Treated Disease (Response to a Gluten-Free Diet)
Small bowel biopsy specimens obtained as soon as 1 week to even several months after complete exclusion of dietary gluten may show only a modest improvement in villous architecture and persistently elevated numbers of IELs despite an excellent clinical response.15,66 However, in most cases, these features return to normal or near-normal states (i.e., a mild mucosal lesion) within 2 to 3 months after institution of a gluten-free diet (see Fig. 16.6, C). Some patients show persistence of moderate or even severe lesions despite marked clinical improvement. Repeat biopsies are not required if the clinical response to a gluten-free diet is positive; they are indicated only if the clinical response is poor and other disorders (e.g., refractory sprue, lymphoma, infection) need to be excluded.
Refractory (Unclassified) Sprue
Refractory celiac disease (RCD) is defined as an absent or incomplete clinical response to a gluten-free diet, usually in a patient with prior serologic or genetic evidence of GSE.97,101,102 If no evidence of gluten sensitivity ever existed, the term unclassified sprue is appropriate. Inadvertent gluten ingestion should be ruled out, as should other potential causes of diarrhea or malabsorption, including pancreatic insufficiency, concomitant collagenous colitis, lymphoma, and rare entities such as adult-onset autoimmune enteropathy.98–100 Therefore, idiopathic refractory sprue remains a diagnosis of exclusion. In their review of the English-language literature through 2009, Rubio-Tapia and Murray highlight a number of points regarding RCD. The prevalence is rare but unknown; definitions vary from center to center; the pathogenesis is poorly understood; and therapies are based on anecdotal practices and opinion.101 However, the following information appears to be supported by numerous studies.
Many patients with refractory sprue show abnormalities in small bowel intraepithelial and lamina propria T lymphocytes, such as abnormal phenotype and monoclonality.103–108 Intraepithelial T lymphocytes in some patients with refractory sprue show loss of the surface markers CD3, CD4, and CD8 with maintenance of cytoplasmic CD3ε and have oligoclonal or monoclonal rearrangements of the T-cell receptor γ gene.103–105,107 These findings have led to the theory that a significant proportion of refractory sprue cases represent a form of in situ, or cryptic, T-cell lymphoma. RCD is categorized into two types based on the immunophenotype and clonality of IELs. Patients with RCD I or normal T-cell phenotype (CD3+ CD8+) often respond to generic immunosuppression regimens and have a low risk of progression to enteropathy-associated T-cell lymphoma106,109; patients with RCD II or abnormal T cells (CD3 cytoplasmic +/surface − CD8−) are usually refractory to immunosuppression and are at increased risk of progression.97,103,104,109,110 There is no agreement on appropriate therapy, but numerous chemotherapeutic and immunomodulatory regimens have been used.103,105,111,112 Specific regimens that have been attempted include cladribine,111 recombinant IL10,113 and alemtuzumab (an anti-CD52 monoclonal antibody).114
In clinical practice, immunostains for CD3 and CD8 theoretically may help distinguish responsive celiac disease (CD3+ CD8+) from RCD II. However, it is well documented that some RCD II patients have a normal IEL phenotype by immunohistochemistry.115,116 Polymerase chain reaction (PCR) may be used to detect T-cell receptor gene rearrangements with the use of paraffin-embedded tissue.117
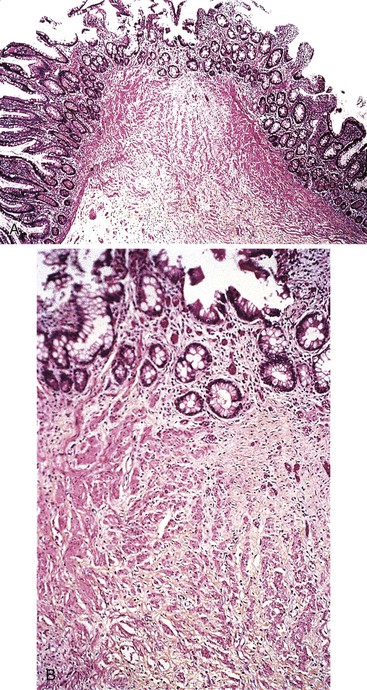
From a histologic (not causative) point of view, small bowel biopsies in refractory sprue usually reveal moderate to severe villous flattening despite adherence to a gluten-free diet. The lesions are typically patchy and variable in distribution, although diffuse flattening of the entire small intestine may occur. In a longitudinal study of 10 patients with refractory sprue, several histologic features were strongly associated with a refractory course.116 In that study, collagenous sprue developed in five patients. In addition, basal plasmacytosis and mucosal thinning (corresponding to Marsh type 4) were found almost exclusively in specimens from patients with refractory disease.
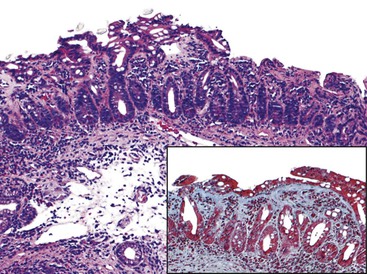
More recently, collagenous sprue has been reported in several series as a pattern of injury seen in the setting of RCD or unclassified sprue.101,117,118 Similar to its counterpart in the colon, collagenous sprue is a female-predominant injury pattern characterized by increased deposition of collagen beneath the surface epithelium basement membrane.119 Rigorous diagnostic criteria, including entrapment of small capillaries and fibroblasts in the collagen layer, should be applied to establish this diagnosis (Fig. 16.7). A trichrome stain to highlight collagen is often helpful. Collagen deposition may be patchy and often appears late in the course of illness. Associated collagenous gastritis and collagenous colitis may occur. Clonal T-cell populations are sometimes found by PCR analysis of fixed tissue.117 Whereas early reports suggested dismal clinical outcomes,116,119 recent literature provides evidence that collagenous sprue represents a histologic pattern with a heterogenous clinical outcome. Some patients respond to a gluten-free diet and steroids, others require total parenteral nutrition and chemotherapy, and some die of intractable disease.101,117,118
Malignancy in Gluten-Sensitive Enteropathy
An association between GSE and an increased incidence of lymphoma and carcinoma has been reported.120–122 Although some studies have suggested a relative risk of approximately 30-fold,120 recent population-based studies suggest that the increased risk may be lower.123–127 In two large population-based studies of patients with latent celiac disease, defined as positive serology with normal biopsy, there was no increase in mortality or lymphoma risk on follow-up.123,126 The role of adherence to a gluten-free diet in decreasing the risk of malignancy in patients with GSE is controversial.120,128,129 A number of studies have confirmed that strict dietary compliance, especially beginning in childhood, probably confers protection from malignant complications.130–133 Intestinal lymphoma in GSE, first documented in 1937,134 has occurred in 5% to 10% of patients in some series.135–137 Lymphomas that arise in this setting have been variably categorized and were originally classified as reticulum cell sarcomas138 or malignant histiocytosis.139 In some reports, the term ulcerative jejunitis (now understood to be synonymous with lymphoma) has been used to describe tumors that develop in the setting of malabsorption.140 The term enteropathy-associated T-cell lymphoma has also been applied.141 In 1985, Isaacson and colleagues characterized these tumors as T-cell lymphomas.142 However, B-cell lymphomas may rarely develop in association with refractory sprue as well.116
Lymphoma may manifest as solitary or multiple tumors and may involve any portion of the small bowel, with or without involvement of the mesenteric lymph nodes.135–137 Clinically, the onset of lymphoma is frequently associated with relapse of malabsorption in previously gluten-responsive patients.
Whereas older studies cite an increased frequency of carcinomas, especially those arising in the GI tract, in patients with GSE,135–137,143 recent population studies in Sweden and the United Kingdom suggest no increase in the incidence of solid tumors in GSE. They do, however, confirm an increase in lymphoproliferative disorders.123–126
Other Complications in Gluten-Sensitive Enteropathy
A variety of intestinal and extraintestinal pathologic conditions occur in patients with GSE. These include lymphocytic gastritis,144 collagenous gastritis,145 lymphocytic colitis,146 collagenous colitis,98,147,148 dermatitis herpetiformis,11,149 nonspecific intestinal ulceration,150,151 and other associations.152,153
Differential Diagnosis
As with mild infiltrative lesions, the flat appearance of type 3 lesions is not specific for GSE (see Box 16.1).154,155 Flattening of small-intestinal villi can be seen in several conditions, such as tropical sprue,156 bacterial overgrowth,157,158 unclassified sprue,116 specific food allergies,159 and common variable hypogammaglobulinemia (see Table 16.5).93 It is also observed in infants and young children with viral gastroenteritis or cow’s milk intolerance. Small bowel Crohn’s disease and eosinophilic gastroenteritis may rarely result in villous blunting.160,161 Distinction of these entities from GSE rests primarily on clinical, not pathologic, data. However, in the rare instance of a flat mucosal biopsy in common variable hypogammaglobulinemia, lack of lamina propria plasma cells and concurrent infection with Giardia are clues to the correct diagnosis. Biopsy specimens in tropical sprue only rarely show completely flat mucosa, and a history of residence in tropical climates allows distinction from GSE. Above all, serum antibody tests and response to a gluten-free diet are of paramount importance in distinguishing GSE from these other conditions.
Other Disorders of Protein (Cow’s Milk and Soy Protein) Intolerance
The syndromes of cow’s milk or breast milk and soy protein intolerance in infancy and childhood are well documented.94,159,162–167 Hypersensitivities to other foods have been reported in both children and adults but are not as well understood. Here, the discussion is limited to milk and soy allergies, because these disorders are associated with reproducible histologic patterns of injury in the GI tract. Clinically, a variety of symptoms develops in affected infants introduced to either cow’s milk or soy-based formula, including acute (frequently bloody) diarrhea, vomiting, abdominal pain, and weight loss. Laboratory abnormalities may be seen, including hypoalbuminemia, anemia, and metabolic acidosis.167 Peripheral eosinophilia may or may not be present. Symptoms usually improve rapidly after removal of the inciting agent. However, in some individuals, the symptoms may be prolonged, and steroid therapy may be required.
The pathogenesis of GI protein allergies is diverse, with approximately half related to IgE-mediated hypersensitivity and the other half related to non-IgE, T cell–mediated reactions.168 Early prospective studies documented that histologic injury in soy protein intolerance develops within 12 hours after ingestion and may resolve within 4 days after dietary exclusion.159 Studies have revealed a prominent helper type 2 (Th2) T-cell phenotype in cow’s milk–specific lymphocytes and a lack of immunosuppressive cytokines in children with milk-induced GI disease.169 In some patients, overlap is seen between milk protein intolerance and the more generalized condition of eosinophilic gastroenteritis. Both disorders may be associated with peripheral eosinophilia. In patients with eosinophilic gastroenteritis, the initial symptoms may apparently be related to milk protein intolerance. However, their clinical course is more refractory, and they are also more likely to have an atopic phenotype.162
Several clinicopathologic entities with overlapping features have been described that result from soy or milk protein intolerance, including proctocolitis, food protein–induced enteropathy, and food protein–induced enterocolitis syndrome.167,168,170 In proctocolitis, histologic abnormalities consist of mild to severe mucosal eosinophilic infiltrates, intraepithelial eosinophils associated with epithelial injury, and edema.162 The small bowel may show manifestations in food protein–-induced enteropathy or enterocolitis, particularly in the ileum and to a lesser extent in the duodenum. Varying degrees of villous blunting may be seen, with mucosal edema, mucin depletion, and increased IELs and eosinophils. In severe cases, the histologic features may resemble untreated GSE. However, the finding of increased eosinophils helps distinguish these two entities. Eosinophils are not always significantly increased in protein intolerance, and in such cases, clinical correlation with the patient’s age and dietary history is required to establish the diagnosis. Colorectal changes in allergic disease are discussed in more detail in Chapter 17.
Tropical Sprue
Epidemiology and Clinical Features
Tropical sprue is a chronic malabsorptive syndrome, often associated with folate and vitamin B12 deficiency, that occurs in residents of or visitors to the Indian subcontinent and parts of Southeast Asia, Central America, and the Caribbean.171–176 Notable exceptions are Jamaica and sub-Saharan Africa, where the disease is rare. In tropical populations, adults are more severely affected than children. The disease occurs in both endemic and epidemic forms. Patients experience chronic nonbloody diarrhea, weight loss, bloating, and abdominal cramps. In severe cases, secondary vitamin deficiencies can lead to complications of nutritional deficiencies (such as subacute combined degeneration of the spinal cord caused by B12 deficiency).
The cause of tropical sprue remains unknown, although the preponderance of evidence supports an infectious origin.175,177–182 The illness probably begins with an acute infection of the small or large intestine that may be bacterial, viral, or parasitic in origin.177 Subsequently, a chronic infection, with colonization of the small bowel by aerobic enterotoxigenic bacteria, is believed to occur, and this probably results in ongoing injury to enterocytes. Bacteria isolated from patients with tropical sprue include Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae.177 However, a consistent and specific causal agent has not been identified. An environment conducive to bacterial overgrowth may result from the release of enteroglucagon, a potent inhibitor of peristalsis, during the acute infectious phase of the disease.177 A study from India documented contamination of the small bowel with aerobic bacteria and prolonged orocecal transit time in patients with tropical sprue; both abnormalities reversed after prolonged antibiotic treatment.178 The theory of bacterial overgrowth is strongly supported by the response of some patients to broad-spectrum antibiotics, such as tetracycline, along with folic acid. In addition, studies have shown an increased frequency of HLA antigens Aw19 and Aw13 in some patients with tropical sprue. However, the role of HLA antigens in the pathogenesis of this disease remains poorly understood.183
The entire small bowel is usually affected, including the ileum,172,184 and this leads to vitamin B12 and folate deficiency in most symptomatic patients. Vitamin deficiencies may lead to proliferative injury (e.g., megaloblastic change) to the intestinal crypts and other epithelia.185 Megaloblastic change, or macrocytosis, refers to the nuclear and cytoplasmic enlargement of epithelial cells that occurs in the setting of interrupted DNA synthesis and cell division (see Chapter 6).
Pathologic Features
Asymptomatic native populations susceptible to tropical sprue often harbor pathologic changes that include villous shortening and increased inflammation. The degree of abnormality in asymptomatic people is usually minor but is occasionally pronounced.172,174 Evidence of malabsorption is also frequently found in asymptomatic native residents.
As stated earlier, the entire small bowel, including the ileum, is usually affected in tropical sprue.172,184 This is in contrast to GSE, in which pathologic lesions are present in the duodenum and jejunum and only rarely extend into the ileum. The patterns of distribution of these two diseases are consistent with their pathogenesis. Tropical sprue is caused by colonization of the bowel by bacteria, whereas GSE is a response to a dietary antigen that is present in highest concentration in the proximal gut.
Gross Pathology
In early studies of small bowel mucosa (with a dissecting microscope) biopsy specimens from symptomatic patients and from some asymptomatic patients in endemic areas revealed an abnormal villous pattern characterized by fusing and broadening of the villi.172,184,185 The jejunum is usually more severely affected than the ileum, which may appear normal under the dissecting microscope despite being abnormal histologically.184
Microscopic Pathology
There is significant overlap between the histologic features of tropical sprue and those of GSE. Small bowel biopsies in tropical sprue reveal varying degrees of villous blunting, crypt hyperplasia, and increased mitotic activity (Fig. 16.8).178,185,186 Increased lamina propria lymphocytes, plasma cells, and eosinophils, as well as IELs, are also seen. In tropical sprue, IELs are more numerous in the crypts than in the surface cells—unlike GSE, in which the reverse situation occurs.187 In severe cases, decreased mitotic activity is combined with a lack of surface epithelial cell maturation. The cells acquire a cuboidal shape, become mucin depleted, and accumulate cytoplasmic fat droplets.172,174,184,185 As previously mentioned, concomitant folate and B12 deficiency may lead to mucosal atrophy, manifested by crypts lined by cells with markedly enlarged (megaloblastic) nuclei. Completely flat mucosa, common in GSE, is almost never seen in tropical sprue.
Electron microscopic studies of small bowel biopsy specimens in tropical sprue have revealed irregularities of microvilli, increased lysosomes, accumulation of fat in surface epithelium, and the presence of dense material beneath the basal lamina.174
Differential Diagnosis
The histologic changes in tropical sprue are nonspecific and can be seen in a variety of conditions (see Fig. 16.8). A clinical history of residence in, or prolonged visits to, endemic areas is critical in establishing a correct diagnosis. Parasitic and other specific infections must be excluded before a diagnosis of tropical sprue can be made confidently. Biopsy specimens that show completely flat mucosa should arouse suspicion for other diseases such as GSE. However, mild to moderate mucosal lesions may be seen in both GSE and tropical sprue. Clinical improvement after treatment with broad-spectrum antibiotics further supports a diagnosis of tropical sprue.
Bacterial Overgrowth Syndrome (Blind-Loop Syndrome, Stasis Syndrome)
Pathogenesis
Bacterial overgrowth by coliform bacteria in the small intestine occurs in a variety of conditions, all of which predispose the individual to decreased intestinal motility and stasis.157,188,189 These include motor and neural disorders such as scleroderma, diabetes mellitus, pseudo-obstruction, and amyloidosis; structural defects such as diverticulosis and strictures; genetic conditions such as pancreatic deficiency resulting from cystic fibrosis; and surgical manipulations that lead to isolated bowel segments, such as Billroth II gastrojejunal anastomosis or short bowel syndrome.190–199 Immune deficiency states, such as AIDS and common variable immune deficiency, may also result in bacterial overgrowth and may contribute to the diarrheal illness that frequently occurs in these conditions.157,189 Clinically significant bacterial overgrowth also occurs in older adult patients without any other predisposing features, especially those with disabilities.200,201 Several studies have documented bacterial overgrowth in patients with irritable bowel syndrome, although this remains controversial.202,203
Clinical Features
Regardless of the underlying cause, this syndrome is characterized by overgrowth of anaerobic bacteria (normally confined to the colon) within the small intestine.157,188,189 Patients with this condition typically have diarrhea and steatorrhea, although malabsorption of vitamin B12, carbohydrates, and other nutrients can occur as well. Proposed theories of pathogenesis include deconjugation of bile salts by anaerobic bacteria, which leads to fat malabsorption.106 In addition, certain nutrients, such as vitamin B12, may be depleted by luminal bacteria. Ultrastructural studies of this condition also have revealed evidence of damage to surface epithelium and the brush border, with some evidence of defective fat transport and absorption.204
Several methods of diagnosis have been used to detect bacterial overgrowth. The traditional gold standard is small-intestinal aspirate culture showing growth of at least 105 colony-forming units of bacteria per milliliter of small bowel fluid (normal small bowel has <104 colonies per milliliter).205 Typically, multiple organisms are identified and include species of bacteroides, enterococcus, and lactobacillus. Other diagnostic tests include the 14C-labeled d-xylose and hydrogen breath tests.206 When bacterial overgrowth is suspected or proven, antibiotic therapy results in marked improvement.157,207–209
Pathologic Features
Small bowel biopsy specimens from patients with bacterial overgrowth may be histologically normal despite clinical evidence of malabsorption. When abnormal, they typically reveal mild to moderate villous blunting with increased chronic inflammation in the lamina propria and epithelium, as well as crypt hyperplasia (Fig. 16.9).157,204,210 Vacuolated epithelial cells, probably containing lipid, may also be seen. One study found that villous blunting, defined as a villus to crypt ratio less than 3 : 1, was the most common abnormality in small bowel biopsy specimens from patients with bacterial overgrowth versus controls. However, in the same study, more than half of the specimens from patients with bacterial overgrowth were scored as histologically unremarkable.211 When present, histologic changes tend to be patchy and vary from segment to segment, so that biopsies taken during the same procedure from different sites may show different degrees of abnormality. This pattern of involvement helps to distinguish bacterial overgrowth from GSE.
As in other conditions characterized by villous blunting, the histologic features are entirely nonspecific and do not correlate with severity of clinical symptoms.204
Eosinophilic Gastroenteritis
Eosinophilic gastroenteritis includes a spectrum of diseases characterized by eosinophilic infiltration of one or more segments of the GI tract, peripheral eosinophilia in some patients, and the frequent coexistence of allergies or asthma.160,212–216 This rare condition typically appears in children or young adults with symptoms related to the particular segment of GI tract that is involved with disease. The stomach is the most common site of involvement, followed by the duodenum, jejunum, and ileum, which are involved approximately equally. Small bowel disease and gastric disease often occur together.160 Eosinophilic esophagitis, a related but distinct clinical entity that can occur in isolation or in association with eosinophilic gastroenteritis, is discussed in Chapter 14. Because of the rarity of this disease, many epidemiologic features are not well understood. Following earlier descriptions, Klein and co-workers categorized eosinophilic gastroenteritis into three anatomic patterns (mucosal, mural, and serosal), based on the layer of the bowel wall that is primarily infiltrated by eosinophils213 (Table 16.6). Approximately 50% of patients have mucosal disease, followed by mural disease, and, least commonly, serosal disease.213,217 There appears to be a slight male predominance.
Table 16.6
Eosinophilic Gastroenteritis
| Type | Clinical Characteristics | Pathology |
| Mucosal | Diarrhea, hemorrhage, protein-losing enteropathy | Mucosal eosinophils with degranulation, crypt abscesses, variable villous blunting |
| Mural | Abdominal pain, obstruction, nausea, and vomiting | Thickened wall, mural and subserosal eosinophilic infiltrates, edema; mucosa may be normal |
| Serosal | Abdominal pain, obstruction, ascites, nausea, and vomiting | Eosinophils and edema limited to serosa and subserosa, ascitic fluid with abundant eosinophils |
Adapted from Klein NC, Hargrove RL, Sleisenger MH, et al. Eosinophilic gastroenteritis. Medicine (Baltimore). 1970;49:299-319.
Clinical Features
Symptoms of eosinophilic gastroenteritis vary depending on the layer of the bowel wall that is involved. The mucosal form most frequently manifests with GI hemorrhage, nausea, vomiting, and diarrhea. Protein-losing enteropathy and other forms of malabsorption may also occur in this type. Patients with primarily mural disease usually have abdominal pain and symptoms of small bowel obstruction, such as nausea and vomiting. The serosal form often manifests with ascites, as well as abdominal pain, nausea, and vomiting.
Once the condition has been accurately diagnosed, treatment consists of steroids. Surgical resection of obstructed segments of small bowel is frequently performed before diagnosis, because it is difficult to establish a diagnosis by biopsy if the mucosa is not involved or cannot be sampled. Although some diagnostic importance is given to the finding of peripheral eosinophilia, it may be absent in as many as 25% of patients.204 The natural history of eosinophilic gastroenteritis remains largely unknown. In a recent study, three different courses of disease were identified: single flare, recurring disease, and continuous course. In the same study, approximately 40% of patients demonstrated spontaneous remission, while the remainder were treated and showed greater than 95% response to steroids.218
Pathogenesis
The pathogenesis of eosinophilic gastroenteritis remains unknown but may be different for the three types described (mucosal, mural, and serosal). Familial cases do occur,219 but no specific genetic abnormalities or inheritance patterns have been identified. In some patients with mucosal disease, eosinophilic gastroenteritis appears to be related to food allergy.212,214,216,220 IgE is elevated in some patients. On the other hand, several studies have shown that the degree of tissue injury and the severity of symptoms are related to the degree of eosinophilic infiltration and to eosinophilic activation and degranulation.119,219,221,222 Extracellular major basic protein and eosinophil cationic protein are present at increased levels in affected mucosa.223 Experimental models with electron microscopy have demonstrated neuronal damage secondary to eosinophil infiltration and degranulation.224,225 Eotaxin 1, a highly selective eosinophil chemokine, is important for the migration of eosinophils into the GI tract in health and disease.226,227 IL5 may work synergistically with eotaxin 1 in eosinophil migration.228 The triggers for chemokine activation in eosinophilic gastroenteritis are unknown but are presumably related to altered immune responses to environmental antigens in susceptible hosts.
Pathologic Features
Eosinophils are a normal constituent of the gastrointestinal lamina propria, to varying degrees depending on the site; they serve a protective role against infections, particularly parasitic infestations. However, when eosinophil numbers are increased, the diagnosis of eosinophilic gastroenteritis should be considered. The pathologic changes in eosinophilic gastroenteritis depend on the anatomic location of disease.213,222 Because mucosal biopsies are frequently normal in the mural and serosal forms of disease, the finding of normal mucosa in biopsy specimens from patients with suspected eosinophilic gastroenteritis does not exclude the diagnosis. In the mucosal type, an increase in both intact and degranulated eosinophils is seen in the lamina propria and infiltrating the epithelium (Fig. 16.10). Some specimens may show eosinophilic crypt abscesses, surface erosion, or ulceration. The infiltrate may be severe and diffuse or mild and patchy. Biopsies from multiple sites are often needed to detect the presence of mucosal disease.160,214,219,222,229 In the small intestine, the mucosal architecture is usually normal, or it may show mild villous blunting. However, severe mucosal lesions (flat mucosa) have also been reported in rare instances.214 In addition to the lamina propria, eosinophils may accumulate in the muscularis mucosae and the superficial submucosa. The density of eosinophils is often greatest in the deepest portion of the mucosa.
The pathology of the mural form of eosinophilic gastroenteritis is characteristic (Fig. 16.11). Resection specimens show thickening and induration of the intestinal wall on gross examination. Ulceration may be present.160,230 Microscopically, prominent edema and a marked eosinophilic infiltrate are the hallmarks of mural eosinophilic gastroenteritis. Submucosal edema is frequently prominent and may also extend into the muscle layer. As in mucosal biopsies, eosinophilic infiltration is usually of a severe degree, but it can also be patchy. Dense eosinophilic infiltrates may extend into the subserosa.160,222,230,231 In serosal eosinophilic gastroenteritis, edema and eosinophils are limited to the subserosal layers. In this setting, the diagnosis can usually be made by ascitic fluid cytology.
Differential Diagnosis
The diagnosis of eosinophilic gastroenteritis in resection specimens is usually straightforward. The differential diagnosis includes many other conditions that can lead to elevated tissue eosinophil levels, such as parasitic infections, vasculitis, and Crohn’s disease. Parasites must be excluded by careful examination of tissue sections and by stool examination. In vasculitis, the inflammation tends to be centered in and around blood vessels, and ischemic changes may be present. Although clinical and radiologic confusion with Crohn’s disease often occurs, the distinction from Crohn’s disease is relatively simple in histologic sections. The differential diagnosis in mucosal biopsies is more difficult, especially if the stomach is not involved and the degree of eosinophilic infiltration is patchy. However, certain features of Crohn’s disease, such as focally enhanced neutrophilic exudates and granulomas, are never seen in eosinophilic gastroenteritis. Occasionally, cases of GSE can be associated with increased mucosal eosinophils.222 However, clinical correlation with serum autoantibodies and attention to the other characteristic features of GSE usually make distinguishing it from eosinophilic gastroenteritis straightforward. The concurrent or subsequent development of GSE and dermatitis herpetiformis has been reported in patients with eosinophilic gastroenteritis.232
Nutritional Deficiencies
A variety of nutritional deficiencies may lead to characteristic morphologic abnormalities in the small intestine. Changes related to vitamin B12, protein, zinc, and iron deficiencies are described here.
Vitamin B12 Deficiency
Vitamin B12 deficiency, such as that resulting from pernicious anemia, is characterized by macrocytosis, a decrease in the number of mitoses, and villous blunting (Fig. 16.12).233,234 These changes, which are similar to those seen in folate deficiency, chemotherapy, and radiation injury, are reversed by B12 administration. Several patient populations are at risk for B12 deficiency, including the elderly, gastric bypass patients, premature infants, and cancer patients with long-term decreased nutritional intake.235,236 Tropical sprue may also result in B12 deficiency and may show identical small-intestinal pathology. After treatment, the mitotic rate has been shown to return to normal within several days, whereas the macrocytic and architectural changes take longer (approximately 2 months) to normalize. Vitamin B12 is necessary for normal DNA synthesis. The mechanism by which B12 deficiency leads to macrocytosis is thought to be impaired DNA synthesis and inhibition of cell division.233 Of note, B12 deficiency secondary to pernicious anemia rarely, if ever, is associated with evidence of malabsorption.
Protein Deficiency
Protein malnutrition, although rare in Western societies, remains an important cause of death in infants and children in African and Asian countries. Kwashiorkor is a syndrome associated with low protein intake but normal or high caloric intake, and marasmus is a condition of both low protein and low caloric intake. Most patients also show concurrent parasitic infection or tropical sprue, which complicates the histologic picture.237–240
Pathologic features in untreated kwashiorkor or marasmus typically include mild to moderate villous blunting, increased mononuclear lymphocytes in the lamina propria, normal or mildly decreased mucosal width, cuboidalization of epithelial cells, and a decrease in the number of mitotic figures.237–240 In some studies, Paneth cells are also decreased in number, and cytoplasmic lipid droplets are present in epithelial cells.241 Macrocytosis may be present and may reflect dietary folate deficiency. The overall appearance is similar to that of GSE. Changes vary from minimal to severe and correlate with the degree of protein malnutrition, but severe changes are uncommon.
Electron microscopic studies in malnutrition reveal abnormalities in the microvillus brush border, decreased endoplasmic reticulum, dilated cytoplasmic vesicles, and phagocytic vacuoles.242 Functional studies have revealed a decrease in the activity of mucosal disaccharidase, as well as other enzymes, that may contribute to nutrient malabsorption.237,238 Duodenal biopsy specimens from patients with kwashiorkor had lower levels of heparan sulfate proteoglycan when compared with those from patients with marasmus.240 The biopsy appearance in marasmus is less pronounced than in kwashiorkor, despite a higher degree of caloric depletion. The mucosa is typically slightly thin, with a marked decrease in mitotic rate. However, a near-normal crypt-to-villus ratio is usually seen.239
One long-term study found a return to normal histology only after 4 to 10 years of protein supplementation.238
Acrodermatitis Enteropathica (Zinc Deficiency)
Acrodermatitis enteropathica is a rare autosomal recessive syndrome characterized by the presence of dermatitis, alopecia, diarrhea, growth retardation, and depressed mental function in early childhood.243,244 It occurs in all races and affects males and females equally. The discovery, in 1973, that acrodermatitis enteropathica is caused by zinc deficiency has allowed the successful treatment of this once-fatal condition by dietary supplementation with zinc sulphate.245 The disorder is caused by mutations in the SLC39A4 gene on chromosome 8q24.3, which encodes a protein that appears to be involved in zinc transportation.246 In patients with acrodermatitis enteropathica, zinc remains bound to this peptide and is unavailable for absorption.247
Small bowel mucosal biopsies in patients with acrodermatitis enteropathica may be entirely normal. However, a number of studies have reported varying degrees of villous blunting, lamina propria inflammation, and edema.243,248,249 In rare cases, flat lesions similar to those seen in GSE have been documented.249 The degree of abnormality may reflect the severity of zinc deficiency at the time of biopsy. In any case, the histologic features are nonspecific. Several electron microscopic studies have revealed peculiar rhomboid and ovoid lysosomes within Paneth cells in patients with acrodermatitis enteropathica.248,250 These changes may be related to zinc deficiency; at least one study reported their disappearance after zinc replacement.250
Iron Deficiency
Iron deficiency is a well-known result of malabsorption such as that seen in GSE. However, iron deficiency itself may lead to mucosal injury and subsequent steatorrhea and malabsorption. In a study of 14 infants and children with iron deficiency anemia, in whom GSE had been excluded, duodenal biopsies revealed mild to moderate villous blunting and increased lamina propria inflammation in approximately 50% of the patients.251 The mechanism of injury is unknown but may be related to the role of iron in the normal metabolism of epithelial cells.252
Peptic Duodenitis and Duodenal Ulcer
Peptic duodenitis and duodenal ulcer, once considered separate disease entities, are now recognized to represent two ends of the spectrum of peptic injury.253,254 The proximal duodenum may be viewed as an extension of the gastric antrum, but without the protective coating of mucus and bicarbonate-secreting surface cells. The duodenal bulb, in particular, is physiologically exposed to a higher acid content than the remainder of the small intestine. In the setting of increased gastric acid secretion, such as in antral-predominant H. pylori gastritis, duodenal inflammation and ulceration may occur. An extreme version of peptic injury is also seen in Zöllinger-Ellison syndrome, in which ulceration and diffuse Brunner gland hyperplasia may also involve the distal duodenum and jejunum.255
Current understanding of the pathophysiology of peptic duodenitis is that chronic exposure to acid induces both gastric surface metaplasia and hyperplasia of Brunner glands in the duodenal bulb.254,256–261 In patients with H. pylori gastritis, foci of gastric surface metaplasia may become colonized with bacteria and incite an inflammatory response that can lead to ulceration.254,260–262 This hypothesis is strongly supported by the finding that 95% to 100% of duodenal ulcers occur in patients with H. pylori gastritis and that duodenal ulcers heal faster and recur less frequently after eradication of H. pylori.260,263,264
Kreuning and coworkers were among the first to document the presence of duodenitis, which they studied in asymptomatic volunteers.253,256 Patients with dyspepsia are more likely to have moderate to severe duodenitis or duodenal ulcer.253 A high proportion of asymptomatic and symptomatic patients (64% and 94%, respectively) have foci of gastric surface metaplasia and prominent mucosal Brunner glands in biopsy specimens from the duodenal bulb.253,256 Fundic gland (gastric) heterotopia, found less frequently than gastric surface metaplasia in duodenal mucosal specimens, is associated with the development of chronic duodenitis and duodenal ulcer disease.254,256,265
Pathologic Features
Peptic duodenitis is characterized by the presence of three main pathologic features, all of which may vary in severity: increased plasma cell infiltration, neutrophils in the lamina propria or epithelium (or both), and reactive epithelial changes, including villous blunting.253,254,256,259 Gastric surface metaplasia is not always used as an absolute criterion for peptic duodenitis, because it may be found in cases without inflammation and therefore may simply represent an adaptive response to chronic acid exposure.254 However, most cases of peptic duodenitis show extensive gastric surface metaplasia, which can be highlighted with a periodic acid–Schiff (PAS) stain (Fig. 16.13). The distribution of changes is often patchy and can be missed as a result of sampling error.
In mild cases, near-normal mucosa can be seen with only a borderline increase in plasma cells and subtle reactive epithelial changes. These changes are not usually associated with symptoms and therefore may be physiologic.253,254,256 In moderately active peptic duodenitis, the epithelium is infiltrated by neutrophils and shows mucin depletion, a syncytial growth pattern, and more marked reactive epithelial changes, such as nuclear hyperchromasia and increased mitoses. Surface cells containing abundant PAS-positive mucin (gastric surface metaplasia) are usually easily identifiable. Brunner glands are hyperplastic and, as a result, may be prominent in the mucosa above the level of the muscularis mucosae. Severe cases show erosion or ulceration. If the ulcer is caused by peptic injury, concomitant H. pylori gastritis is frequently present.260,262
Differential Diagnosis
Pathologic changes in peptic duodenitis are nonspecific. NSAID injury, Crohn’s disease, celiac disease, and infections may have a similar histologic appearance. Clinical correlation and stains for organisms (including H. pylori) should be performed to help differentiate among these conditions. However, the degree of gastric surface metaplasia, combined with prominent Brunner glands, especially in a patient with documented H. pylori gastritis, often helps in establishing a diagnosis of peptic duodenitis.
Drug Injury of the Small Bowel
Small bowel injury caused by drugs, medications, and other pharmaceuticals occurs in numerous clinical settings and is likely underrecognized and underreported (Box 16.3).266 The patterns of injury are varied and depend on the pharmacologic properties of the specific agent. The most common and consistently observed small bowel drug-related injury is caused by NSAIDs, a widely used class of drugs available both over the counter and by prescription. NSAIDs are used to treat a variety of conditions, ranging from simple headache to cardiovascular disease or arthritis. Other frequently encountered medications that cause small bowel injury include antibiotics, Kayexalate (sodium polystyrene sulfonate in sorbitol), ferrous sulphate, chemotherapeutic agents (e.g., taxanes), and immunosuppressants. With a few exceptions, most of these drugs produce nonspecific histologic patterns of injury, and a high index of suspicion by the clinician and pathologist is required to detect them.
Nonsteroidal Antiinflammatory Drug–Induced Enteropathy
Clinical Features
GI toxicity resulting from NSAID use is increasingly common, causing as many as 100,000 hospital admissions annually in the United States and many more worldwide.267–269 Ulceration of the stomach and proximal duodenum resulting from NSAIDs has long been recognized.270 However, numerous studies have documented ulceration leading to significant hemorrhage, perforation, or obstruction also occurring in the distal small bowel and colon as a result of NSAID use.271–277 The term NSAID enteropathy has been used to describe this condition. Patients may have persistent iron deficiency anemia despite the absence of gastroduodenal involvement.271,272,277 The incidence of small bowel inflammation and ulceration has been reported to be as high as 46% to 70% among long-term NSAID users.273 A more modest increase was found in an autopsy series, wherein 8.4% of patients previously taking NSAIDs had jejunal or ileal ulcers, compared with 0.6% of controls.271
Pathogenesis
The cause of NSAID enteropathy is most likely multifactorial, involving a sequence of events.278 Local topical irritation, enterohepatic recirculation, and increased mucosal permeability secondary to cellular injury and loss of tight junction function, as well as microcirculatory events, all play a role.272,278,279 These events may allow infiltration of luminal antigens, bile, and bacterial products, which in turn incite an inflammatory response. Newer types of NSAIDs, known as selective cyclooxygenase-2 (COX-2) inhibitors, are associated with a significantly lower incidence of intestinal side effects, but they still carry a risk of ulceration and hemorrhage, especially in long-term users.274,277
Pathologic Features
A wide spectrum of histologic changes may occur in the small bowel as a result of NSAID injury. Mild lesions consist of superficial erosions with nonspecific neutrophilic and plasmacytic infiltrates (Fig. 16.14).280–282 Erosions are frequently multiple and can progress to form deep ulcers, which may cause severe hemorrhage.275,283 In one surgical study of 11 patients who underwent emergent small bowel resection for NSAID-induced ulceration, ulcers were observed the ileum in eight and in the jejunum in four, and they were multiple in 50% of cases.283 The ability to obtain jejunal biopsies during push enteroscopy has led to the documentation of more subtle changes, including mild villous blunting in some cases.284 However, severe, diffuse villous blunting has not been reported in NSAID injury.
A rare but more distinctive form of NSAID enteropathy is referred to as diaphragm disease (Fig. 16.15).285–289 This condition involves numerous thin, weblike mucosal septa that project into the lumen and cause significant narrowing and obstruction. The middle small intestine and the ileum are the preferred sites of involvement. The mucosal diaphragms consist of mucosa with reactive epithelial changes, with or without surface erosions, and prominent submucosal fibrosis. The bands of fibrous tissue are characteristically oriented perpendicular to the surface of the mucosa. Mild chronic inflammation is usually present. The fibrosis is thought to result from recurrent ulceration and scarring. These diaphragms are fairly specific for NSAID injury.
Differential Diagnosis
The differential diagnosis of the common ulcerating form of NSAID injury includes peptic and H. pylori–induced duodenitis, Crohn’s disease, and, rarely, infections such as Giardia. The lack of specificity of the histologic changes seen in some of the previously discussed entities can cause diagnostic dilemmas, especially in biopsies of the terminal ileum, where the distinction between NSAID injury and Crohn’s disease has significant clinical implications. Aphthous lesions may occur in both diseases, but significant plasma cell infiltrates, crypt distortion, and pseudopyloric metaplasia are much less common in NSAID injury. In the upper GI tract, gastric biopsies help distinguish H. pylori–related injury from NSAID-induced injury to the proximal duodenum. In addition, peptic duodenitis, which is frequently associated with marked gastric surface metaplasia in the duodenum, is not specifically associated with NSAID injury. Appropriate clinical information is important to differentiate between these disorders. The differential diagnosis for NSAID-induced diaphragm disease includes radiation or chemotherapy injury, chronic ischemia, Crohn’s disease, and the rare entity CMUSE (cryptogenic multifocal ulcerating stenosing enteritis; see later discussion).
Radiation- and Chemotherapy-Induced Enteritis
Radiation Injury
The GI tract is exquisitely sensitive to radiation. The small intestine is the most sensitive portion of the gut, followed by the stomach, colon, and esophagus.290–293 Radiation injury related to therapy is subdivided into acute and chronic forms.294
Acute Radiation Injury
In the acute stage, clinical symptoms related to small-intestinal involvement include diarrhea, abdominal pain, and bloating. The severity of toxicity is related to the radiation dose and to the volume of intestine exposed.295 This syndrome, frequently called radiation enteritis, occurs in 20% to 70% of exposed patients and can appear within hours after exposure.296,297 However, biopsies are not usually obtained at this stage unless the clinical features suggest that other conditions, such as opportunistic infections, may be present. The earliest histologic changes occur in the surface and crypt epithelia, which show mitotic arrest, nuclear enlargement, and hyperchromasia as well as flattening and separation of cells.298 Increased numbers of apoptotic bodies in the crypts are typical.299,300 Ultrastructurally, loss of microvilli, vacuolization of endoplasmic reticulum, and other changes have also been reported.301
After 7 or more days of therapy, the area of the epithelial surface is reduced by approximately 40%,298,302 and the mucosa is notable for villous blunting, crypt hypoplasia, and a marked decrease in the number of lymphocytes in the lamina propria. Ulceration can occur at this stage and may be widespread if exposure is persistent. In addition to epithelial changes, endothelial cells are similarly damaged, resulting in increased vascular permeability and submucosal edema.292,293 The acute syndrome is transient; symptoms and histologic changes usually resolve within several weeks after cessation of radiation.
The most important differential diagnosis in the setting of acute radiation therapy is opportunistic infection. Special stains and cultures should be used to exclude infections in these as in all other immunocompromised patients. Acute radiation injury may also mimic the early phase of ischemic injury. In addition, the degree of epithelial atypia often present in acute radiation injury may occasionally be mistaken for dysplasia, particularly if the clinical history is unknown.
A unique and novel form of radiation brachytherapy is selective internal radiation therapy using biocompatible resin-based yttrium 90 (90Y)-labeled microspheres that are delivered via arterial catheters to selectively target inoperable tumors, such as metastatic lesions of the liver. If delivery of the spheres is imperfect, the spheres can travel to the gastrointestinal tract, leading to mucosal damage, including ulcerations, days to months after exposure.303
Chronic Radiation Injury
Chronic radiation injury develops years after radiation exposure, regardless of the presence or severity of previous acute radiation injury. The reported incidence of chronic radiation injury after radiotherapy has ranged from 1% to 36%.304,305 With the use of current radiotherapy regimens and protective measures, the incidence is now reported to range from 5% to 10%.292,293,306 The incidence and severity of chronic radiation injury has been shown to be related to the overall dosage and to the volume of small bowel irradiated.306,307
Clinical symptoms are variable but usually reflect chronic ulceration (pain, hemorrhage), stricture formation (obstruction), or perforation. The symptoms are related to long-term, irreversible pathologic changes in mesenchymal tissue that ultimately lead to vascular insufficiency. Most likely, these changes occur secondary to sustained overexpression of inflammatory and fibrogenic cytokines.307 Although epithelial and villous architectural changes usually return to normal after acute radiation injury, stromal cells, especially fibroblasts, and endothelial cells undergo a variety of persistent changes. Enlargement of fibroblasts, with expansion and vacuolization of their cytoplasm, an increase in nuclear size, and hyperchromasia are common. Although the cells increase in size, the nucleus-to-cytoplasm ratio typically stays within the normal range.82,308 Submucosal fibrosis and, occasionally, fibrosis of the muscularis propria may be present as well (Fig. 16.16, A and B). Intimal proliferation of small and medium-sized arteries, with partial or total occlusion of the vascular lumen, is a frequent finding in surgical specimens removed because of stricture formation (see Fig. 16.16, C). Ischemic changes in the overlying mucosa are often present (Fig. 16.16, D).82,309 Mucosal biopsies obtained from strictured segments may show ulceration, mucosal fibrosis, crypt branching, and inflammation. Although these findings are nonspecific, they are compatible with chronic radiation injury in the proper clinical setting.
Mucosal telangiectases and vascular proliferations may also develop as a long-term sequela of radiation therapy (see Fig. 16.16, E).308,310 They may be missed on histologic examination because thin-walled vessels frequently collapse after biopsy.
Chemotherapy Injury
Chemotherapy agents frequently cause acute enteritis that may be debilitating.311–314 The pattern of injury reflects four phases of disease: (1) an influx of inflammatory cells into the lamina propria, demonstrated in animal models; (2) loss of epithelial cells and mucosal structure; (3) disruption of large areas of epithelial lining; and (4) recovery with regrowth of epithelium and a return to normal structure and function.313 In fact, the histologic changes seen in small-intestinal biopsies after chemotherapy are similar to those seen in acute radiation injury. Biopsies obtained shortly after chemotherapy show reactive nuclear and cytologic atypia and macrocytosis (Fig. 16.17). These changes reflect an arrest of maturation, which occurs within hours after administration of the drug.315,316 Mitotic figures are usually decreased in number, and an increase in apoptotic bodies is observed in epithelial crypts, along with villous blunting, which may be severe. Cytotoxic chemotherapy is associated with an increase in a variety of pro-apoptotic proteins, including TP53, caspase 3, and Bax and Bak in small-intestinal crypt cells.317 Necrosis of crypt epithelium may follow, with the development of surface erosions. These changes are transient, and the mucosa usually returns to normal within a few weeks after cessation of treatment.