13 Inflammatory bowel disease
Epidemiology
The incidence of new cases of ulcerative colitis in Europe and USA is 2–8 per 100,000 per year with a prevalence of 40–80 per 100,000 per year. The incidence has remained fairly static over the last 40 years. In the UK, Crohn’s disease occurs with a similar frequency to ulcerative colitis with around 4 per 100,000 per year and a prevalence of 50 per 100,000. The rates in central and southern Europe are lower. In South America, Asia and Africa, Crohn’s disease is uncommon but appears to be on the rise (Mpofu and Ireland, 2006).
Aetiology
Environmental
Diet
Evidence that dietary intake is involved in the aetiology of IBD is inconclusive, although several dietary factors have been associated with IBD, including fat intake, fast food ingestion, milk and fibre consumption, and total protein and energy intake. A large number of case-control studies have reported a causal link between the intake of refined carbohydrates and Crohn’s disease (Gibson and Shepherd, 2005). The mechanism for diet as a trigger is very poorly understood.
Those that are breastfed as infants have a reduced risk of developing IBD (Mpofu and Ireland, 2006).
Smoking
There is a higher rate of smoking amongst patients with Crohn’s disease than in the general population, with up to 40% of patients with the disease being smokers. Smoking worsens the clinical course of the disease and increases the risk of relapse and the need for surgery. Fewer patients with ulcerative colitis smoke (approximately 10%). Former smokers are at the highest risk of developing ulcerative colitis, while current smokers have the least risk. Stopping smoking can provoke the emergence of ulcerative colitis. This indicates that smoking may help to prevent the onset of the disease. The explanation for this is unclear. However, it is thought that in addition to its effect on the inflammatory response, the chemicals absorbed from cigarette smoke affect the smooth muscle inside the colon, potentially altering gut motility and transit time. In some studies, nicotine has been shown to be an effective treatment for ulcerative colitis (Guslandi, 1999).
Drugs
Non-steroidal anti-inflammatory drugs (NSAIDs) such as diclofenac have been reported to exacerbate IBD (Felder et al., 2000). It is thought this may result from direct inhibition of the synthesis of cytoprotective prostaglandins. Antibiotics may also precipitate a relapse in disease due to a change in the enteric microflora. The risk of developing Crohn’s disease is thought to be increased in women taking the oral contraceptive pill, possibly caused by vascular changes.
Appendicectomy
Appendicectomy has a protective effect in both Crohn’s disease and ulcerative colitis (Radford-Smith et al., 2002). It is unclear whether this protective effect is immunologically based or whether individuals who develop appendicitis and consequently have an appendicectomy are physiologically, genetically or immunologically distinct from the population that is predisposed to IBD.
Genetic
Fifteen percent of first degree relatives have IBD. There is mounting evidence that Crohn’s disease and ulcerative colitis result from an inappropriate response of the immune system in the mucosa of the gastro-intestinal tract to normal enteric flora (Ahmad et al., 2004). Since the mid-1990s there has been considerable progress in understanding the contribution of genetics to IBD susceptibility and phenotype.
Ethnic and familial
Jews are more prone to IBD than non-Jews, with Ashkenazi Jews having a higher risk than Sephardic Jews. In North America, IBD is more common in whites than blacks. First-degree relatives of those with IBD have a 10-fold increase in risk of developing the disease. A familial link is supported from research showing a 15-fold greater concordance for IBD in monozygotic (identical) than dizygotic (non-identical) twins (Jess et al., 2005).
Pathophysiology
Disease location
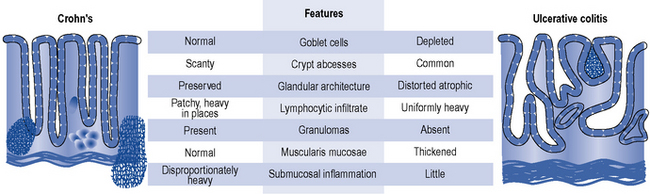
The character and distribution, both macroscopic and microscopic, of chronic inflammation define and distinguish ulcerative colitis and Crohn’s disease. Table 13.1 shows the differences in location and distribution of ulcerative colitis and Crohn’s disease. Figure 13.1 details the histological differences.
Table 13.1 Location and distribution of ulcerative colitis and Crohn’s disease
| Ulcerative colitis | Crohn’s disease | |
|---|---|---|
| Location | Colon and rectum 40% proctitis 20% pancolitis 10–15% backwash ileitis |
Entire gut (mouth to anus, rectal sparing) 45% ileocaecal disease 25% colitis only 20% terminal ileal disease 5% small bowel disease 5% anorectal, gastroduodenal, oral disease |
| Distribution | Continuous, diffuse | Often discontinuous and segmental ‘skip lesions’ |
| Full thickness (transmural) | ||
| No granulomas | Granulomatous inflammation | |
| Inflammation of mucosa | ||
| Ulceration if fine and superficial | Deep ulceration with mucosal extension | |
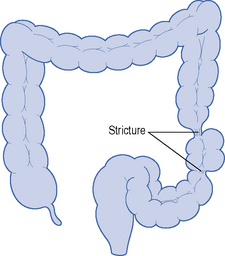
| Fissures, fistulae and stricture | Absent | Common |
| Perianal disease | Absent | Present |
Clinical manifestation
The clinical differences between Crohn’s disease and ulcerative colitis are described in Table 13.2.
Table 13.2 Clinical differences between ulcerative colitis and Crohn’s disease
| Symptom | Ulcerative colitis | Crohn’s disease |
|---|---|---|
| Prominent symptom | Bloody diarrhoea | Diarrhoea, abdominal pain, weight loss 30%, no gross bleeding |
| Fever | ++ | ++ |
| Abdominal pain | Variable | ++ |
| Diarrhoea | +++ | +++ |
| Rectal bleeding | +++ | ++ |
| Weight loss | + | ++ |
| Sign of malnutrition | + | ++ |
| Abdominal mass | − | ++ |
| Dehydration | +++ | ++ |
| Iron-deficiency anaemia, raised CPR/ESR, hypoalbuminaemia | ++ | ++ |
CPR, C-reactive protein; ESR, erythrocyte sedimentation rate; +, the likelihood this symptom will be present; −, symptom absent in patient.
Crohn’s disease
Extra-intestinal complications of IBD
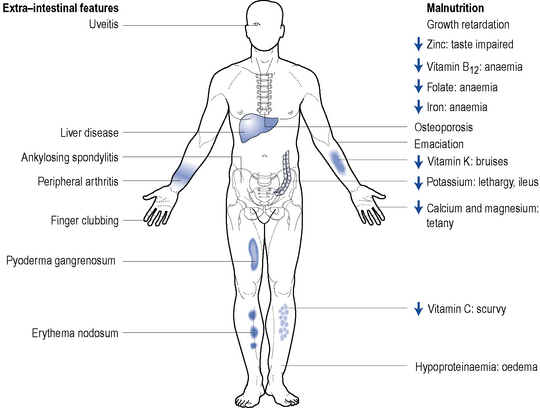
Around 20–30% of patients with IBD will present with extra-intestinal manifestations. They are more commonly seen in patients where IBD affects the colon. Complications affect the joints, skin, bone, eyes, liver and biliary tree and are more common in active disease. Figure 13.3 highlights some of the extra-intestinal features of IBD.
Thromboembolic
Thromboembolic complications occur in around 1–2% of IBD patients. The most common cause of death in hospitalised IBD patients is pulmonary embolism (Solem et al., 2004).
Investigations
Clinical assessment tools
The Crohn’s Disease Activity Index (CDAI) or the Harvey–Bradshaw Index (HBI) is used in most clinical trials to define remission in Crohn’s disease. However, in clinical practice the CDAI is rarely used as it needs to be measured prospectively and is complex. The HBI is a simple measure of stool frequency, pain and other clinical features that is increasingly used to document selection for and response to biologic therapy. A CDAI of <150 or an HBI ≤3 suggests patient is in remission (NICE, 2010).
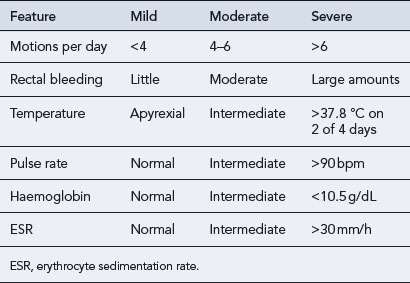
The Truelove and Witts criteria is a useful tool in defining the severity of ulcerative colitis (see Table 13.3). A severe attack is defined as more than six bloody stools a day plus one or more of the following: pulse > 90 beats/min, temperature >37.8 °C, haemoglobin <10.5 g/dL or ESR >30 mm/h. In this case, the patient should be admitted to hospital.
Treatment of inflammatory bowel disease
Nutritional therapy
Nutritional therapy can be considered as an adjunctive or primary treatment. Although a potential problem for all patients with IBD, patients with Crohn’s disease are at particular risk of becoming malnourished and developing a variety of nutritional deficiencies (Fig. 13.3). It is, therefore, important for patients to receive optimal nutrition and dietary manipulation as needed. Low-fibre diets help to reduce clinical symptoms of intestinal obstruction in Crohn’s disease (Fernandes-Banares et al., 1999). Functional and structural damage to the small bowel can cause malabsorption problems and occasionally patients may require a low-lactose diet. Patients with poor oral intake and loss of appetite often respond to supplemental enteral feeds. Enteral nutrition in the form of an elemental or polymeric diet can be used as primary therapy and is widely employed in paediatrics (Heuschkel and Walker-Smith, 1999).
Drug treatment
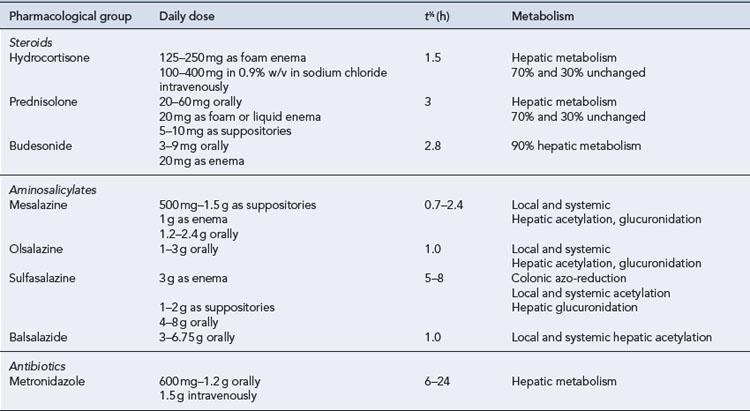
Disease confined to the anus, rectum or left side of the colon is more appropriately treated with rectally administered topical preparations where the drug is applied directly to the site of inflammation (Table 13.4).
Table 13.4 Comparison of commercially available preparations for rectal administration in inflammatory bowel disease
| Generic name (proprietary name) | Formulation | Site of release |
|---|---|---|
| Sulfasalazine (Salazopyrin®) | Suppositories Retention enema |
Rectum Transverse, descending colon and rectum |
| Mesalazine (Pentasa®, Salofalk®) | Retention enema | Transverse, descending colon and rectum |
| Mesalazine (Asacol®, Salofalk®) | Foam enema | Rectum and rectosigmoid colon |
| Mesalazine (Asacol©®, Pentasa®, Salofalk®) | Suppositories | Rectum |
| Prednisolone sodium phosphate (Predsol®) | Retention enema Suppositories |
Transverse, descending colon and rectum Rectum |
| Prednisolone sodium metasulphobenzoate (Predenema®) | Retention enema | Transverse and descending colon |
| Prednisolone sodium metasulphobenzoate (Predfoam®) | Foam enema | Rectum and rectosigmoid colon |
| Prednisolone sodium phosphate (Predsol®) | Retention enema | Transverse and descending colon |
| Hydrocortisone acetate (Colifoam®) | Foam enema | Rectum and rectosigmoid colon |
| Budesonide (Entocort©®) | Retention enema | Rectum and rectosigmoid colon |
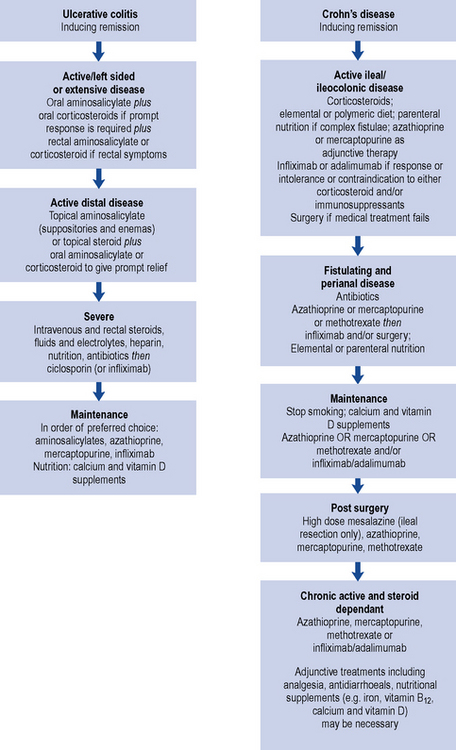
An algorithm for drug treatment in IBD is shown in Fig. 13.4.
Corticosteroids
Formulations
Oral prednisolone will control mild and moderate IBD and 70% of patients improve after 2–4 weeks of 40 mg/day. This is gradually reduced over the next 4–6 weeks to prevent acute adrenal insufficiency and early relapse. An example of a reducing oral prednisolone regime is detailed in Box 13.1.
Box 13.1 An example of a reducing regimen for oral steroids
Local regimen used at the Oxford Radcliffe Hospitals NHS Trust
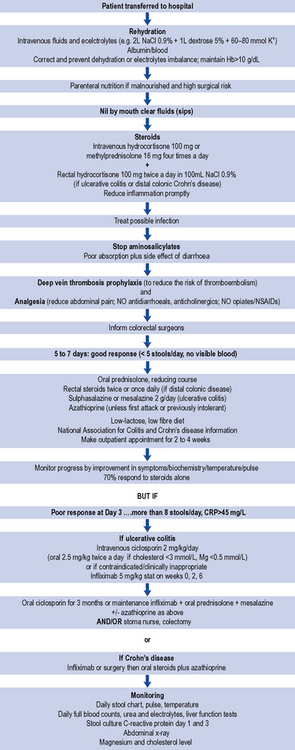
Prednisolone at doses higher than 40 mg/day increases the incidence of adverse effects and has little therapeutic advantage. Short-term side effects include moon face, acne, sleep and mood disturbance, dyspepsia, hypokalamia, hypernatraemia and glucose intolerance. Prolonged use can cause cataracts, osteoporosis and increased risk of infection. Doses below 20 mg are not generally effective in active disease (St Clair Jones, 2006). A typical treatment algorithm for the management of an acute attack of IBD is presented in Fig. 13.5.
Other steroids
Budesonide, available orally and rectally, is currently licensed for Crohn’s disease affecting the ileum and descending colon. It is less effective than conventional corticosteroids in inducing remission in active Crohn’s disease, but has fewer side effects than prednisolone because of its rapid and extensive first-pass metabolism. However, the absorbed drug has a higher affinity for glucocorticoid receptors 50–100 times that of prednisolone and so long-term treatment is not advocated. Budesonide has shown to be of benefit in microscopic colitis (Travis et al., 2005). Budesonide enemas are effective in inducing remission in distal ulcerative colitis and are comparable to conventional steroids but probably less effective than mesalazine enemas (Marshall and Irvine, 1997).
Aminosalicylates
Diagnosis, disease location, activity, side effect profile, efficacy and cost all affect the choice of aminosalicylate. Available as oral or rectal preparations, aminosalicylates can be used in combination with steroids to induce and maintain remission, in mild to moderate ulcerative colitis. Sulfasalazine is considerably cheaper but the newer aminosalicylates are generally used (Sutherland and MacDonald, 2006). Aminosalicylate maintenance therapy with doses of 1.2 g and above appears to reduce the risk of colorectal cancer by up to 75% (Van Staa et al., 2005).
The use of aminosalicylates in Crohn’s disease is less well established (Dignass et al., 2010). There is evidence of patient benefit with high-dose mesalazine (over 2 g/day) in reducing relapse post-small bowel resection.
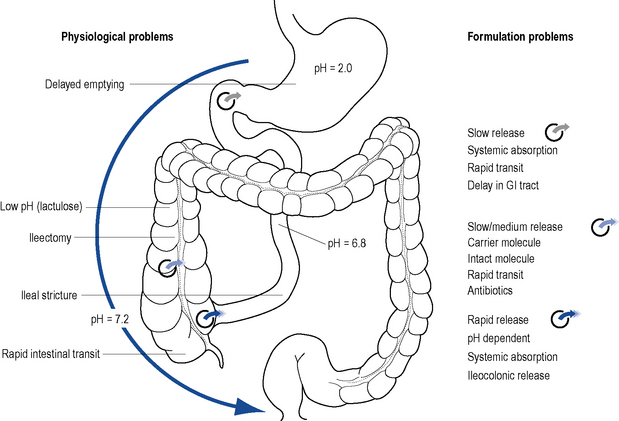
Elimination of sulfapyridine depends on the patient’s acetylator phenotype. Those who inherit the ‘slow’ acetylator phenotype experience more side effects. The dissolution profile of the drug and the site of ulceration determine effectiveness (Fig. 13.6). The optimal dose of sulfasalazine to achieve and maintain remission is usually in the range of 2–4 g per day in 2–4 divided doses. Acute attacks require 4–8 g per day in divided doses until remission occurs, but at these doses associated side effects are often observed.
Formulations
Mesalazine is unstable in acid medium and rapidly absorbed from the gastro-intestinal tract. To increase stability and/or alter the site of release, 5-ASA is modified by different delivery systems (see Fig. 13.6). Mesalazine dose is more important than the delivery system and the lowest systemic absorption preparation should be used. The different delivery systems developed are:
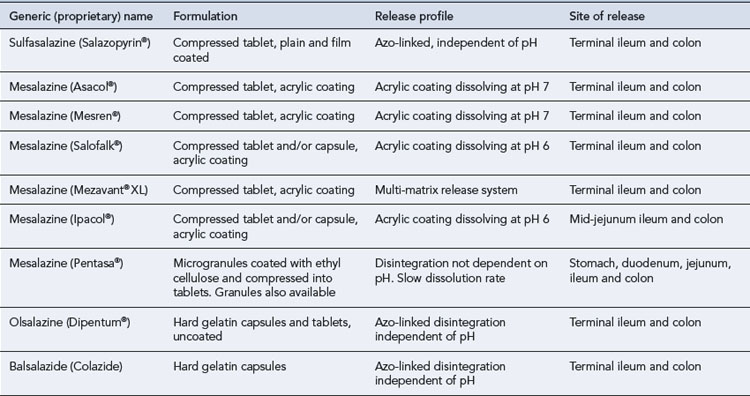
Table 13.5 compares the oral aminosalicylate preparations currently available.
Mesalazine enemas (1 g in 100 mL), foam enemas (1 g per application) or suppositories (250 mg, 500 mg and 1 g) are effective alternatives for treating distal ulcerative colitis and proctitis. The optimum rectal dose is 1 g. Rectal administrations of 5-ASA formulations are significantly better than rectal corticosteroids in inducing remission in ulcerative colitis but steroids are considerably cheaper (Travis et al., 2008). In severe ulcerative colitis, oral and topical formulations should be combined to give prompt symptom relief. Topical and oral 5-ASA is better than either alone (Marteau et al., 2005).
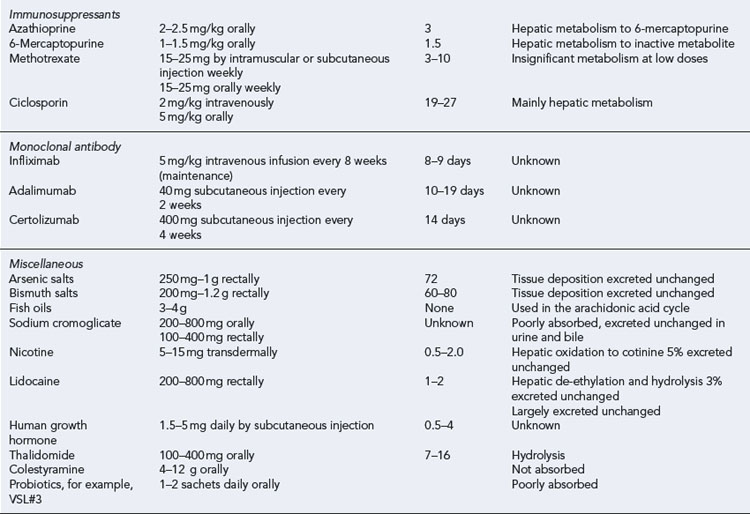
Immunosuppressants
Thioguanines (azathioprine, mercaptopurine)
The main indications for thioguanines are when patients
Seventy percent of patients will tolerate azathioprine. Of the remaining 30%, most will tolerate mercaptopurine. The most common side effects occur within 2–3 weeks of starting treatment and rapidly stop on withdrawal. These include flu-like symptoms (myalgia, headache), nausea and diarrhoea. Nausea is reduced by taking the medicine with food. Although rare (<3%), leucopenia can develop suddenly and unpredictably. Hepatotoxicity and pancreatitis have also been reported in less than 5% of patients. Thioguanine has been used but is associated with a greater risk of hepatotoxicity. There is no evidence that the incidence of lymphoma increases with the use of these agents (St Clair Jones, 2006).
The value of assessing thiopurine methyl transferase (TPMT) activity and genotype, especially prior to initiating treatment, is debatable. Patients who develop leucopenia and are TPMT deficient have a greater risk of myelotoxicity. However, this may not apply in IBD. Some gastroenterologists measure TPMT if a patient has relapsed on doses greater than 2 mg/kg/day to identify fast metabolisers who may respond to higher doses. Measuring TPMT activity to identify the 0.3% of non-metabolisers who are at high risk of rapid leucopenia has also been recommended (Carter et al., 2004).
Methotrexate
Methotrexate is teratogenic and all male and female patients should be counselled about using contraception while taking the medication and also for 3 months after therapy is withdrawn. Guidance to improve the safety of methotrexate use and minimise the potential risk of overdose has been issued (NPSA, 2006). Measures include the issue of patient hand-held monitoring cards detailing dose and blood test results, comprehensive written and verbal medicines information, dispensing one strength of tablet (2.5 mg) and ensuring inpatient medication charts clearly state once-weekly dosing and the number and strength of tablets routinely used. An aide memoire commonly recommended to patients to remember dose frequency is to take methotrexate on Mondays and folic acid on Fridays.
Ciclosporin
Ciclosporin is a calcineurin inhibitor that acts at an early stage on precursors of helper T-cells by interfering with the release of interleukin-2. This inhibits the formation of the cytotoxic lymphocytes which cause tissue damage. Both controlled and uncontrolled studies suggest that ciclosporin is effective rescue therapy for severe ulcerative colitis failing to respond to intravenous steroids (Campbell et al., 2005). Its use in Crohn’s disease is unproven.
The effectiveness of ciclosporin at doses of 2–5 mg/kg/day in treating IBD has been studied in patients refractory to conventional drug therapy (Van Assche et al., 2003). A dose of 2 mg/kg has been shown to be as effective as 4 mg/kg. Patient response to this treatment has varied, with adverse effects causing withdrawal of treatment in some cases. However, some patients have stopped concurrent steroid therapy and have remained in remission for some time.
When patients with severe colitis fail to show a response to treatment with parenteral steroids, then ciclosporin at an intravenous dose of 2 mg/kg/day should be considered. If patients respond to parenteral ciclosporin they can subsequently be maintained on an oral dose for 3–6 months. If the patient has a low plasma magnesium (< 0.5 mmol/L) or low cholesterol (<3 mmol/L), they are at an increased risk of ciclosporin-induced seizures when given intravenously. In these circumstances, treatment with an oral ciclosporin preparation, for example, Neoral®, at a dose of 5 mg/kg/day is preferred. Ciclosporin therapy is used for many patients, but normally as a bridge to colectomy or starting maintenance treatment with azathioprine or mercaptopurine. Infliximab is an alternative ‘rescue therapy’ for acute severe colitis (NICE, 2008a) if ciclosporin is contraindicated or clinically inappropriate. A multi-centre randomised controlled trial (CONSTRUCT) is underway comparing the clinical and cost-effectiveness of infliximab and ciclosporin in the treatment of steroid resistant acute severe colitis.
Monitoring of immunosuppressants
The incidence of lymphomas in patients receiving immunosuppressants when compared with other treatments is of concern. Patients should be advised to avoid live vaccines while taking immunosuppressants, including corticosteroids. Guidance on appropriate action following abnormal blood results is provided in Box 13.2.
Box 13.2 Guidance on dealing with abnormal blood results for patients on immunosuppressant therapy
| WBC | <4 × 109 L−1 |
| Neutrophils | <2 × 109 L−1 |
| Platelets | <150 × 109 L−1 |
| AST/ALT | >3 × normal range |
| WBC | <4 × 109 L−1 |
| Neutrophils | <2 × 109 L−1 |
| Platelets | <150 × 109 L−1 |
| AST/ALT | >3 × normal range |
Ciclosporin should be stopped and the relevant expert advice obtained if any of the following occur:
Biologic agents
Biologic agents that are licensed for use in IBD are infliximab (Remicade®) and adalimumab (Humira®). At present there are no direct comparative studies of the two agents. Certolizumab pegol (Cimzia®) has also shown benefit and is an alternative in some cases; however, it is currently not licensed for use in IBD. All these monoclonal antibodies inhibit the functional activity of the pro-inflammatory cytokine TNF-α which damages cells lining the gut, causing pain, cramping and diarrhoea. Colonic biopsies post-treatment with these agents show a substantial reduction in TNF-α and a reduction in the commonly elevated plasma inflammatory marker C-reactive protein. Table 13.6 summarises the anti-TNF agents used. All appear to have similar efficacy, although there are more data on infliximab than adalimumab or certolizumab. Natalizumab, another monoclonal antibody, has also shown promise in treating active Crohn’s disease. It works by inhibiting the migration of leucocytes into the CNS thereby reducing inflammation. It is unlicensed in IBD.
| Agent | Licensed/(unlicensed) indication | Dose |
|---|---|---|
| Infliximab | Crohn’s disease | |
| Induction of remission in severe active disease | 5 mg/kg intravenously at weeks 0 and 2 | |
| Maintenance treatment | 5 mg/kg intravenously 6 weeks after initial dose then every 8 weeks or further dose of 5 mg/kg if signs and symptoms recur | |
| Fistulising | 5 mg/kg intravenously at weeks 0, 2 and 6 (consult product literature) | |
| Infliximab | Ulcerative colitis | |
| Induction of remission in moderate to severe active disease | 5 mg/kg intravenously at weeks 0, 2 and 6 | |
| Maintenance of remission in severe active disease | 5 mg/kg intravenously every 8 weeks; discontinue if no response 14 weeks after initial dose | |
| Extra-intestinal manifestations (unlicensed) | 5 mg/kg intravenously | |
| Adalimumab | Crohn’s disease | |
| Induction of remission in severe active disease | 80 mg subcutaneously at week 0, 40 mg at week 2 and or accelerated regime of 160 mg at week 0, 80 mg at week 2 | |
| Maintenance treatment | 40 mg subcutaneously on alternate weeks, increasing to weekly if loss of response | |
| Certolizumab pegol | Crohn’s disease (unlicensed) | |
| Used in moderate to severe disease when patients who have lost response to or are intolerant of infliximab or adalimumab | ||
| Induction | 400 mg subcutaneously at weeks 0, 2 and 4 | |
| Maintenance treatment | 400 mg subcutaneously every 4 weeks |
National guidance has been issued on the use of infliximab in ulcerative colitis (NICE, 2008a,b) and infliximab and adalimumab in the treatment of Crohn’s disease (NICE, 2010). The latter guidance indicates treatment should be started with the less expensive drug, taking into account drug administration costs, required dose and product price per dose. This may need to be varied for individual patients due to differences in the method of administration and treatment schedules.
A combination of infliximab with azathioprine has been shown to be superior to monotherapy in inducing remission and mucosal healing in Crohn’s patients naïve to both agents. The rate of infliximab-related infusion reactions was also less with combination treatment and there was no significant difference seen with the rate of serious infection (Colombel et al., 2010). For scheduled treatment, the concomitant immunomodulator should be reviewed and stopped after 6–12 months. It remains unclear whether the same applies to other anti-TNF agents. In practice, the same principles apply with other immunomodulators such as methotrextate. Natalizumab should not be combined with an immunosuppressant or prolonged steroids as this may increase the risks of progressive multifocal leucoencephalopathy (D’Haens et al., 2010).
There is currently no specific guidance on how long anti-TNF agents should be continued for. Preliminary evidence suggests that many patients in clinical remission for greater than 1 year, with a normal C-reactive protein and complete mucosal healing on endoscopy will remain in remission during the following year after stopping treatment. Whether remission is then sustained or whether the behaviour of disease is altered in the long term is currently unknown (D’Haens et al., 2010). NICE guidance on the treatment of Crohn’s disease recommends that infliximab or adalimumab treatment should be given until treatment failure (including the need for surgery) or until 12 months after initiation of treatment, whichever is shorter. Patients should then have their disease reassessed and continue treatment if there is clear evidence of ongoing active disease and treatment is still clinically appropriate. Treatment can be restarted if patients subsequently relapse.
Infliximab
Infliximab is a chimeric human murine monoclonal antibody, licensed for treating severe active Crohn’s disease (with or without fistulae) which is refractory or intolerant to corticosteroids or conventional immunosuppressants alone or if surgery is inappropriate. Treatment may be repeated if the condition responded to the initial course but subsequently relapsed. Infliximab is also licensed for moderate to severe active ulcerative colitis (acute exacerbation) requiring hospitilisation and/or possible surgical intervention and if unresponsive to conventional treatments. Infliximab may half the need for colectomy in steroid refractory patients with acute ulcerative colitis. It remains to be determined whether infliximab is superior to ciclosporin (D’Haens et al., 2010).
In severe active Crohn’s disease, infliximab is administered by intravenous infusion, at a dose of 5 mg/kg over a 2-h period, repeated after 2 weeks. If a clinical response is seen, then a maintenance dose of 5 mg/kg every 8 weeks should be given. Alternatively, a dose could be given when signs and symptoms recur. Fixed interval dosing may be superior to intermittent dosing because of the reduced risk of immunogenicity (NICE, 2010).
In active fistulising Crohn’s disease where disease has not responded to conventional therapy (including antibiotics, drainage and immunosuppressive therapy) or who are intolerant or have contraindications to conventional therapy, an initial dose of 5 mg/kg is given, repeated at 2 and 6 weeks after the first infusion is given. If no response is seen after three doses, no further treatment should be given (NICE, 2010).
In treatment-refractory, moderate to severe acute exacerbation of ulcerative colitis, the licensed dose regimen is 5 mg/kg at weeks 0, 2 and 6. The optimal maintenance strategy after induction therapy is currently unknown. Treatment should be discontinued if no response is seen after 14 weeks. In azathioprine naïve patients responding to infliximab induction, azathioprine is an option instead of infliximab for maintenance (Travis et al., 2008). Although unlicensed, infliximab has shown to be of benefit in extra-intestinal manifestations such as pyoderma gangrenosum and peripheral and axial naturopathies
Other treatments
Miscellaneous treatments
Leukapheresis has been shown to have some benefit in patients with IBD. It involves removal of leucocytes from the blood, either through an adsorptive system or by centrifugation. In each system, venous blood is removed in a continuous flow, anticoagulated, processed to deplete the leukocytes and returned to the circulation (see NICE, 2005). Table 13.7 summarises the pharmacological profile of drugs used in adults with IBD.
Patient care
All patients should be educated about their illness and medication and reminded that even in periods of remission it is important to continue taking prescribed therapy. This should take the form of verbal and written information. Drug use is invariably lifelong and patients are likely to receive several different treatments during the course of their illness due to intolerance or lack of response. Certain patients such as female or those newly diagnosed patients may require more tailored information about the condition or treatment. Patients with poor dexterity may find the use of rectal preparations difficult and these preparations may, therefore, be poorly tolerated. Leaflets about IBD and insurance or employment for patients with IBD have been prepared by the National Association for Colitis and Crohn’s Disease (www.nacc.org.uk). Patients should also be warned about unreliable information sources.
IBD patients often develop microcytic anaemia because of malabsorption and chronic blood loss. Assessment of the blood film and serum ferritin can differentiate between iron deficiency and anaemia of chronic disease. Oral iron supplements are generally poorly tolerated and parenteral iron may be required. Guidance of the treatment of iron-deficiency anaemia in IBD has been published (Gasche et al., 2007). Megaloblastic anaemias are uncommon, although vitamin B12 and folate deficiencies occur and may benefit from appropriate supplementation.
The European Crohn’s and Colitis Organisation have published evidence-based consensus guidelines on the prevention, diagnosis and management of opportunistic infections in IBD (Rahier et al., 2009). These highlight which vaccinations should be offered on diagnosis of IBD. UK practice currently recommends influenza, and pneumococcal vaccine in immunosupressed patients only, along with HPV vaccines for females aged 12–18 years in line with other national guidelines. IBD patients in the UK are also advised to receive the swine flu vaccine if immunocompromised.
Answers
Questions
Answers
Answer
Questions
Answers
Answers
Answers
Methotrexate should be taken once a week, on the same day of the week. Methotrexate is never taken every day. Methotrexate comes in tablet form in two different strengths: 2.5 and 10 mg. The two strengths are different shapes but are a similar colour. It is important that patients keep an up to date record of the dose they are taking and always check the strength of the tablet they have been given each time they get a prescription. To reduce the risk of confusion and possible overdose, many pharmacies only stock 2.5 mg strength tablets. If patients have problems swallowing large numbers of tablets (in this case 10 × 2.5 mg), they can be dispersed in water. As recommended by the NPSA (2006) in their ‘Improving compliance with oral methotrexate’ guidelines, patients should be given a monitoring card which details the dose, strength and quantity of tablets to be taken each week and blood test results.
Questions
Answers
Ahmad T., Tamboli C.P., Jewell D., et al. Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology. 2004;126:1533-1549.
Campbell S., Travis S., Jewell D. Ciclosporin use in acute ulcerative colitis: a long-term experience. Eur. J. Gastroenterol. Hepatol.. 2005;17:79-84.
Carter M.J., Lobo A.J., Travis S.P.L., et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53,(Suppl. V):v1-v16.
Colombel J.F., Sandborn W.J., Reinisch W., et al. Infliximab, azathiorprine, or combination therapy for Crohn’s disease. N. Engl. J. Med., 362. 2010: 1383-1385
D’Haens G.R., Panaccione R., Higgins P.D.R., et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am. J. Gastroenterol. 2010. Advance online publication 2 November 2010. doi: 10.1038/ajg.2010.392. Available at: http://www.nature.com/ajg/journal/vaop/ncurrent/full/ajg2010392a.html
Dignass A., Van Assche G., Lindsay J.O., et al. The second European evidence based consensus on the diagnosis and management of Crohn’s disease: current management 2010. J. Crohns Colitis. 2010;4:28-62.
Felder J.B., Burton I.K., Rajapakse R. Effects of nonsteroidal anti-inflammatory drugs on inflammatory bowel disease: a case–control study. Am. J. Gastroenterol.. 2000;95:1949-1954.
Fernandes-Banares F., Honojoso J., Sanchez-Lombrana J.L., et al. Randomised clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalazine in maintaining remission in ulcerative colitis. Am. J. Gastroenterol.. 1999;2:427-433.
Gasche C., Berstad A., Befritis R., et al. Guidelines on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. Inflamm. Bowel Dis.. 2007;13:1545-1553.
Gibson P.R., Shepherd S.J. Personal view: food for thought – western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther.. 2005;21:1399-1409.
Guslandi M. Nicotine treatment for ulcerative colitis. Br. J. Clin. Pharmacol.. 1999;48:481-484.
Heuschkel R.B., Walker-Smith J.A. Enteral nutrition in inflammatory bowel disease of childhood. J. Parenter. Enteral. Nutr.. 1999;23:S29-S32.
Jess T., Riis L., Jespersgaard C., et al. Disease concordance, zygosity, and NOD2/CARD15 status: follow-up of a population-based cohort of Danish twins with inflammatory bowel disease. Am. J. Gastroenterol.. 2005;100:2486-2492.
Marshall J.K., Irvine E.J. Rectal corticosteroids versus alternative treatments in ulcerative colitis: a meta-analysis. Gut. 1997;40:775-781.
Marteau P., Probert C.S., Undgren S., et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54:960-965.
Misiewicz J., Pounder R.E., Venables C.W., editors. Diseases of the Gut and Pancreas, second ed., Oxford: Blackwell Scientific Publications, 1994.
Mpofu C., Ireland A. Inflammatory bowel disease – the disease and its diagnosis. Hosp. Pharm.. 2006;13:153-158.
National Institute for Health and Clinical Excellence. Leukapheresis for Inflammatory Bowel Disease. London: NICE, 2005. Available at: http://www.nice.org.uk/nicemedia/live/11178/31403/31403.pdf
National Institute for Health and Clinical Excellence. Infliximab for acute exacerbations of ulcerative colitis. In Technology Appraisal No. 163. London: NICE; 2008. Available at: http://www.nice.org.uk/nicemedia/pdf/TA163Guidance.pdf
National Institute for Health and Clinical Excellence. Infliximab for subacute manifestations of ulcerative colitis. In Technology Appraisal No. 140. London: NICE; 2008. Available at: http://www.nice.org.uk/nicemedia/live/11959/40412/40412.pdf
National Institute for Health and Clinical Excellence. Infliximab (review) and adalimumab for the treatment of Crohn’s disease (includes a review of technology appraisal guidance 40). In Technology Appraisal No. 187. London: NICE; 2010. Available at: http://www.nice.org.uk/nicemedia/live/12985/48552/48552.pdf
National Patient Safety Agency. Patient safety alert 13. In Improving Compliance with Oral Methotrexate Guidelines. London: NPSA; 2006. Available at: http://www.nrls.npsa.nhs.uk/resources/?entryid45=59800
Radford-Smith G.L., Edwards J.E., Purdie D.M. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut. 2002;51:808-813.
Rahier J.F., Ben-Horin S., Chowers Y., et al. European evidence based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns Colitis. 2009;3:47-91. Available at: https://www.ecco-ibd.eu/
Solem C.A., Loftus E.V., Tremaine W.J., et al. Venous thromboembolism in inflammatory bowel disease. Am. J. Gastroenterol.. 2004;99:97-101.
St Clair Jones A. Inflammatory bowel disease – drug treatment and its implications. Hosp. Pharm.. 2006;13:161-166.
Sutherland L., MacDonald J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev., 2. 2006:CD000543. 10.1002/14651858.CD000543.pub2.
Travis S.P.L., Ahmad T., Collier J., et al. Pocket Consultant Gastroenterology, third ed., Oxford: Blackwell Publishing, 2005.
Travis S.P.L., Stange E.F., Lemann M., et al. European evidence-based consensus on the management of ulcerative colitis: current management. J. Crohns Colitis. 2008;2:24-62.
Van Assche G., D’Haens G., Noman M., et al. Randomised double blind comparison of 4 mg/kg v 2 mg/kg intravenous cyclosporin in severe ulcerative colitis. Gastroenterology. 2003;25:1025-1031.
Van Staa T.P., Card T., Logan R.F., et al. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573-1578.
Grosso A., Bodalia P., Shah M. A review of mesalazine MR formulations in ulcerative colitis. Br. J. Clin. Pharm.. 2009;1:333-336. Available at: http://www.clinicalpharmacy.org.uk/December/review.pdf
Lewis N.R., Scott B.B. Guidelines for osteoporosis in inflammatory bowel disease and coeliac disease. Available at: http://www.bsg.org.uk, 2007.
Quality care: service standards . Quality care: service standards for the healthcare of people who have inflammatory bowel disease (IBD). Available online at: http://www.ibdstandards.org.uk
Van Assche G., Dignass A., Panes J., et al. The second European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis 2010. J. Crohns Colitis. 2010;4:7-27.
Van Assche G., Dignass A., Reinisch W., et al. The second European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations 2010. J. Crohns Colitis. 2010;4:63-101.