Inflammatory and Neoplastic Disorders of the Anal Canal
Thomas P. Plesec
Scott R. Owens
Embryology and Anatomy of the Anal Canal
The anal canal forms during the fourth to seventh weeks of gestation after partitioning of the cloacal membrane into ventral (urogenital membrane) and dorsal (anal membrane) portions.1 The epithelium of the superior two thirds of the primitive anal canal is derived from the endodermal hindgut; the inferior one third develops from the ectodermal proctodeum. The dentate line (i.e., pectinate line) is located at the inferior limit of the anal valves and delineates the fusion of these two epithelial derivatives. The dentate line also indicates the approximate former site of the anal membrane that ruptures in the eighth week of gestation. The outer layers of the wall of the anal canal are derived from the surrounding splanchnic mesenchyme.
The anal canal, which is 3 to 4 cm long, is defined surgically by the borders of the internal anal sphincter (Fig. 32.1).2,3 The surgical anal canal begins at the apex of the anal sphincter complex. This palpable landmark (i.e., anal rectal ring) is located approximately 1 to 2 cm proximal to the dentate line. It ends where the nonkeratinizing squamous mucosa terminates at the perianal skin.4 The internal sphincter is the most distal portion of the internal circular layer of the muscularis propria and is continuous with the muscularis propria of the colorectum. The surface of the anal canal is lined by vertical mucosal folds called anal columns (i.e., columns of Morgagni), and it is separated by anal sinuses (i.e., sinuses of Morgagni). The columns connect at the most distal end by a horizontal row of mucosal folds known as the anal valves. Anal valves are typically most evident in children, but they may become more prominent with advancing age.
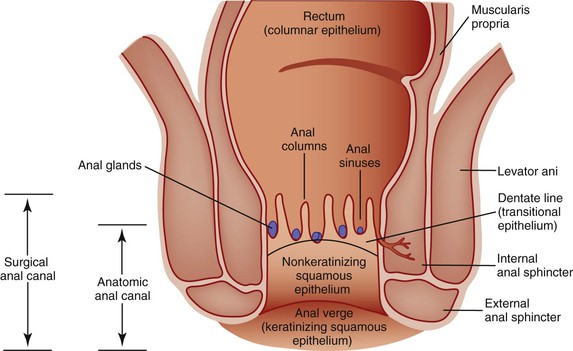
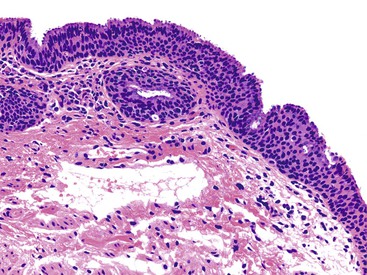
The location of the anal valves corresponds to the dentate line, which is located approximately at the midpoint of the surgically defined anal canal. The dentate line corresponds to the squamocolumnar junction. This is not an abrupt transition, but a transition zone that extends for several millimeters to slightly more than 1 cm. Microscopically, the epithelium lining the anal transition zone varies from a type that resembles the lower genitourinary tract to stratified squamous, columnar, or cuboidal tissue, often with islands of colorectal-type epithelium (Fig. 32.2).1 Despite its resemblance to bladder epithelium, the anal transition zone expresses cytokeratin (CK) 7 and CK19 but not CK20.5,6 Immunohistochemical studies with human melanoma black 45 (HMB-45), an anti-melanoma monoclonal antibody, and S100 protein have demonstrated melanocytes in the anal transitional epithelium, although they are usually more prominent in the anal squamous zone.7
Microscopically, the mucosal lining superior to the transition zone is columnar, whereas the mucosa inferior to the transition zone is stratified squamous. The squamous mucous membrane is devoid of hair and other cutaneous appendages and does not keratinize. The anal canal ends at the anal verge, where the anal squamous mucosa merges with the true anal skin. At this point, hair follicles, sweat glands, and apocrine glands are detected.
The anal ducts are long, tubular structures that closely approach or penetrate the internal sphincter muscle and may undermine the rectal mucosa. These ducts are lined by transitional epithelium and mucus-producing cells, which are most common at the terminal portion of the ducts before their opening into the anal crypts. Nodules of lymphoid tissue are often seen surrounding these ducts. The epithelium lining the ducts shows a similar immunohistochemical profile to that of the overlying transitional mucosa (i.e., CK7 positive and CK20 negative).5,8
The dual embryologic origin of the anal canal results in a dual blood supply, venous and lymphatic drainage, and nerve supply.9–11 The superior two thirds of the anal canal is supplied by the superior rectal artery, a continuation of the inferior mesenteric artery. The venous drainage of the superior anal canal flows into the superior rectal veins, which are tributaries of the inferior mesenteric vein. The lymphatic drainage of the superior two thirds of the anal canal flows to the inferior mesenteric lymph nodes. The inferior one third of the anal canal is supplied primarily by the inferior rectal arteries, which are branches of the internal pudendal arteries. The venous drainage of this portion of the anal canal flows to the inferior rectal veins, which are tributaries of the internal pudendal veins, and ultimately to the internal iliac veins. Lymph drains into the superficial inguinal lymph nodes. The nerve supply of the superior two thirds of the anal canal is part of the autonomic nervous system; the inferior third is supplied by the inferior rectal nerve through the sacral plexus.
These differences in embryology, blood supply, drainage, and innervation are clinically relevant, particularly when evaluating congenital malformations of the anal canal and predicting patterns of spread of neoplasms. In surgical pathology practice, it may be difficult to determine whether a tumor has arisen within the distal rectum, the anal canal, or the anal margin or perianal skin, because these anatomic zones overlap. Bulky tumors often obliterate the normal anatomy. The American Joint Committee on Cancer (AJCC) suggests that a tumor is rectal in origin if its epicenter is located at least 2 cm proximal to the dentate line, whereas a tumor is considered to be an anal canal tumor if it is less than 2 cm from the dentate line.4 Identification of skin appendages helps to differentiate perianal skin carcinomas from true anal canal cancers. A perianal cancer is also defined by a tumor that is located within a 5 cm radius of the anus and that is completely visualized after gentle traction is placed on the buttocks. An anal canal tumor should not be completely visualized after gentle traction.
Embryologic Abnormalities of the Anus and Anal Canal
Clinical Features
Anorectal malformations occur in approximately 1 of 1500 to 5000 live births12,13 and affect both sexes equally. They range from minor anal anomalies to complex cloacal malformations. The most common congenital anomaly of the anus is anal atresia, which accounts for as many as 75% of all anorectal malformations.14 Anorectal agenesis accounts for approximately 10% of all anal atresias. As much as two thirds of congenital anal anomalies are associated with anomalies in other organ systems, most often the genitourinary system but also the central nervous system, skeleton, cardiovascular system, and gastrointestinal (GI) tract.14–16 Soon after birth, patients fail to pass meconium or have meconium that extrudes from a fistulous opening.17
Pathogenesis
Anorectal malformations have many causes, and genetic factors are considered an important component of their pathogenesis. Approximately 5% to 10% of malformations arise in children with a chromosomal abnormality, most commonly trisomy 21, and several monogenetic syndromes include anorectal malformations among their features.16 Anorectal malformations are more common in siblings of similarly affected patients.18 Ninety-five percent of patients with trisomy 21 and anorectal malformations have imperforate anus without a fistula, compared with only 5% of all patients with anorectal malformations. Congenital abnormalities, including anorectal malformations, have been associated with conception by using assisted reproduction methods.19
Pathology
Anorectal malformations are recognized by their gross anatomic features. The Wingspread classification of anorectal malformations is subdivided into high, intermediate, and low atresia based on the level of termination of the anorectum in relation to the levator ani muscle.20,21 This classification has proved quite useful because it shows good correlation with the type of surgical approach needed for repair.22 Fistula formation is a common finding in anorectal malformations, particularly in high and intermediate forms of anal atresia, and it may be rectovesical, rectoprostatic, rectourethral, or anocutaneous in boys and rectovaginal or anoperineal in girls.
An updated classification of anorectal malformations, the Krickenbeck scheme (Table 32.1), emphasizes the type of fistula associated with the malformation. The type of fistula helps to determine the location of the blind pouch and to guide the surgeon’s expectations regarding the length of the atretic segment to be resected at the surgical pull-through procedure.22 In patients with a cloacal disorder, a single perineal orifice is found where the urinary tract, vagina, and rectum converge into a common channel.
Table 32.1
Krickenbeck Classification of Anorectal Malformations
| Major Clinical Groups | Rare Variants |
| Perineal (cutaneous) fistula | Pouch colon |
| Rectourethral fistula | Rectal atresia or stenosis |
| Prostatic | Rectovaginal fistula |
| Bulbar | H-fistula |
| Rectovesical fistula | Other |
| Vestibular fistula | |
| Cloaca | |
| No fistula | |
| Anal stenosis |
Adapted from Holschneider A, Hutson J, Pena A, et al. Preliminary report on the international conference for the development of standards for the treatment of anorectal malformations. J Pediatr Surg. 2005;40:1521-1526.
Prognosis and Treatment
A posterior sagittal approach is considered the best method for defining and repairing anorectal anomalies.18 This approach has greatly improved outcomes in anorectal malformation repair over the past several decades.22,23
The level of anal atresia (i.e., high, intermediate, or low) in relation to the levator ani muscle is directly related to functional prognosis and rates of fecal continence or constipation after a surgical pull-through procedure.20 Patients with anorectal agenesis have a poor prognosis for fecal continence because they lack a functional puborectalis sling mechanism and internal and external anal sphincters. The prognosis for anal atresia located inferior to the levator ani muscle depends on the presence of functional sphincters and intact sensation. Atresias isolated to the anus, such as imperforate anal membrane, carry the best surgical prognosis.
For patients with cloacal disorders, prognostic factors include the quality of the sacrum, the quality of the muscles, and the length of the common channel. Surgical repair of a common channel less than 3 cm long is feasible for most pediatric surgeons, but for a common channel longer than 3 cm, the repair should be performed at a specialized center by an experienced surgeon.18
In the first 24 to 48 hours after birth of an infant with an anorectal malformation, the major issues are identification of life-threatening anomalies and deciding whether to repair the defect immediately or to perform a protective colostomy with repair at a later date. These decisions are based on the infant’s physical examination, the extent of the malformation, and any significant changes that occur in the first 24 hours of life. After this critical period passes and the malformation features are delineated, the surgeon can repair the defect.
Benign Tumors and Tumor-like Lesions of the Anus and Anal Canal
Hemorrhoids
Clinical Features
Epidemiologic studies indicate that 4.4% of the population have hemorrhoids, although this figure is likely a gross underestimate because many patients avoid seeking medical attention. Men and women are equally affected, and the peak age at diagnosis is between 45 and 65 years of age. In the United States, whites are affected more often than blacks.24
Painless bleeding is the most common sign of hemorrhoids. Pain may occur if the hemorrhoids become thrombosed or strangulated. Hemorrhoids rarely lead to anemia, and if a patient has anemia, other potential causes should be investigated. With age, the hemorrhoidal tissue may gradually engorge and extend farther up into the anal canal, where it becomes susceptible to the effects of straining at defecation. Internal hemorrhoids usually become symptomatic only when they prolapse, become ulcerated, bleed, or thrombose. External hemorrhoids may be asymptomatic or associated with discomfort, acute pain, or bleeding from thrombosis or ulceration.
Pathogenesis
In the anal submucosa, hemorrhoids are cushions of fibrovascular and connective tissue that consist of direct arteriovenous communications, mainly between the terminal branches of the superior rectal and superior hemorrhoidal arteries. These cushions serve a protective role during defecation.11,25 Hemorrhoidal tissues may arise from abnormal dilation of the internal hemorrhoid venous plexus, distention of the arteriovenous anastomoses, prolapse of these cushions, or elevations of anal sphincter pressure with resultant vascular congestion. Any cause of elevated intraabdominal pressure, such as straining at defection, inadequate fiber intake, prolonged lavatory sitting, constipation, diarrhea, and conditions such as pregnancy, ascites, and pelvic space-occupying lesions, congest the vascular cushions.24 Portal hypertension alone is not associated with hemorrhoid formation, but hemorrhoidal bleeding in the setting of portal hypertension may be related to a coagulopathic disorder rather than venous engorgement.
Pathology
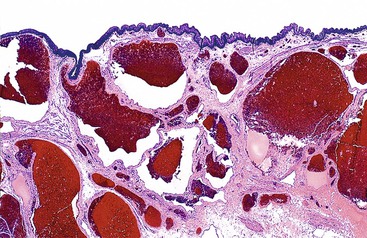
Hemorrhoidal tissue is located on the left lateral, right anterior, and right posterior aspects of the anal canal. Hemorrhoids are classified according to their site of origin; the dentate line serves as an anatomic and histologic border. External hemorrhoids originate distal to the dentate line, arising from the inferior hemorrhoidal plexus, and are lined by modified squamous epithelium. Internal hemorrhoids originate proximal to the dentate line, arising from the superior hemorrhoidal plexus, and are covered with rectal or transitional mucosa. Microscopically, excised specimens show evidence of dilated, thick-walled, submucosal vessels and sinusoidal spaces, often with thrombosis and hemorrhage into the surrounding connective tissues (Fig. 32.3).
All tissues excised as clinical hemorrhoids should be examined histologically because the differential diagnosis of an anal mass and anal bleeding includes colorectal or anal carcinoma, anal melanoma, inflammatory bowel disease, and infection.26 Anal fibroepithelial polyps (discussed later) are often confused clinically with hemorrhoids because of their similar gross appearance.
Prognosis and Treatment
Nonoperative measures, such as sitz baths, analgesics, topical anesthetics, increased dietary fiber, and stool softeners, can be offered to patients with mild symptoms or minimally symptomatic hemorrhoids. If these methods fail, sclerotherapy, rubber band ligation, cryotherapy, or surgical therapy should be considered. Surgical treatment should be tailored to each patient according to the extent of symptoms, coexisting anorectal diseases, and the degree of external anorectal component.
Complications of hemorrhoidal disease are mostly related to treatment. Mild complications include pain, urinary retention, and constipation. Severe complications include fistula formation, rectal prolapse, and incontinence.
Hypertrophied Anal Papillae or Fibroepithelial Polyps
Clinical Features and Pathogenesis
Hypertrophied anal papillae, also known as anal fibroepithelial polyps, are benign polypoid projections of anal squamous epithelium and subepithelial connective tissue. They result from enlargement of anal papillae, which are barely perceptible triangular protrusions located at the base of the anal columns (i.e., columns of Morgagni). They are found in 45% of patients who undergo proctoscopic examination and are thought to be acquired structures.27 They are twice as common in men as in women. They range in size from 0.3 to 1.9 cm, with a mean diameter of approximately 1 cm.
They may be asymptomatic, in which case they are usually found in isolation as a solitary, firm, palpable mass on digital examination or develop in association with an irritation, infection, or chronic fistula or fissure in the anal canal.27–29 They may coexist with hemorrhoids and, not surprisingly, are confused with hemorrhoids clinically.30
Pathology
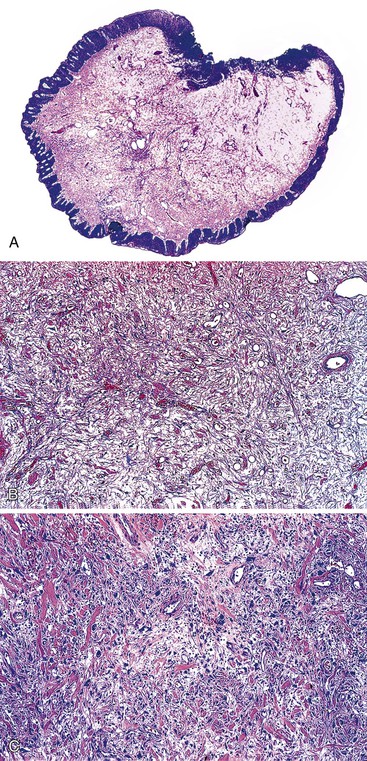
Hypertrophied anal papillae or fibroepithelial polyps may have the clinical appearance of hemorrhoids and are often submitted to the pathologist with this designation.29 Unlike hemorrhoids, fibroepithelial polyps usually do not contain microscopic evidence of dilated or thick-walled vessels, recent or remote hemorrhage, or organizing thrombi. The mucosa covering the polyp is typically squamous, and the submucosal tissue is composed of the loose fibrovascular connective tissue characteristic of this region (Fig. 32.4). Some polyps may contain large, multinucleated, or stellate CD34-positive stromal cells or hyalinization of stromal vessels, likely caused by a reactive process of the stroma.31,32
Some pathologists prefer to reserve the diagnosis of hypertrophied anal papillae for cases in which the stroma is composed of loose fibrovascular tissue, and they use the term anal fibroepithelial polyp when the stroma shows predominantly fibrous changes.28 In essence, hypertrophied anal papillae and anal fibroepithelial polyps are identical to fibroepithelial polyps (i.e., acrochordons) of the cutaneous skin.
The differential diagnosis of a polypoid mass in the anal canal includes hemorrhoids, infection, abscess, and more serious disorders such as anal carcinoma or anal melanoma. Routine histologic examination is usually adequate to determine the underlying pathologic process. Occasionally, the squamous epithelium of a fibroepithelial polyp contains unsuspected intraepithelial neoplasia.
Prognosis and Therapy
Hypertrophic anal papillae tend to enlarge with time and may convert from an asymptomatic to a symptomatic mass associated with pruritus, anal discharge, and discomfort. Surgical resection in symptomatic cases often results in relief of symptoms. When anal papillae accompany an underlying chronic process, treatment often attempts to correct the primary cause and remove the hypertrophied papillae.28
Inflammatory Cloacogenic Polyps and Mucosal Prolapse
Clinical Features
Inflammatory cloacogenic polyps (ICPs) occur predominantly in middle-aged patients, although ICPs in children have been described.33 Men and women are equally affected. The most common presentation is rectal bleeding or mucous discharge. Patients who report straining at defecation are at increased risk for ICPs.
ICPs represent one aspect of mucosal prolapse disorders of the GI tract, which include inflammatory cap polyps, inflammatory myoglandular polyps, polypoid prolapsing folds of diverticular disease, colitis cystic profunda, and polyps associated with the solitary rectal ulcer syndrome. Any type of mucosal polyp may undergo secondary prolapse changes, with resultant misplacement of mucosal elements into the submucosa or deeper layers.
The pathogenesis of ICP is thought to be similar to that of other mucosal prolapse disorders.34 It is likely related to chronic mucosal prolapse that occurs in long-term disorders with constipation and defecation and with associated ischemic, inflammatory, and reactive changes of the overlying mucosa.35–37
Pathology
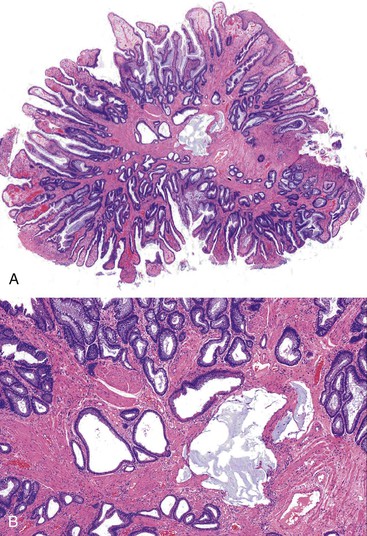
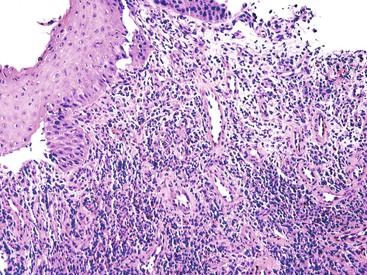
ICPs are located in the anterior anal canal, may be single or multiple, and are typically sessile (see Chapter 22). The gross size varies, but most are between 1 and 2 cm in the greatest dimension. The histologic features include fibrosis of the lamina propria, thickening of the muscularis mucosae, hyperplasia of mucosal glands (often with a villous-like configuration) leading to a serrated contour of the epithelium, and telangiectasia of surface vasculature with or without fibrin thrombi (Fig. 32.5). The muscularis mucosae is typically thickened and irregular, with frequent extension of fibromuscular strands into the lamina propria that results in the formation of diamond-shaped crypts and deposition of mucosal elastin. The finding of elastin is distinctive because it is otherwise not seen in the normal rectum. The surface epithelium is characteristically composed of a mixture of colorectal, transitional, and squamous mucosa. Ischemic-type erosion of the surface epithelium is a common finding, and it may contribute to regenerative or hyperplastic (serrated) epithelial changes.
Differential Diagnosis
A variety of entities enter into the differential diagnosis of an ICP.38 Because of their location in the anal canal and low-power appearance, ICPs may resemble tubulovillous adenomas or traditional serrated adenomas of the distal rectum. Recognition of the regenerative (non-neoplastic) epithelial cytology and eroded surface in ICPs and of the absence of cytologic dysplasia helps to distinguish an ICP from an adenoma. ICPs typically contain transitional and squamous epithelium. The fact that adenomas are somewhat uncommon in the age group in which ICPs are often diagnosed is also useful.
In some ICPs, prominent misplacement of the reactive epithelium into the underlying fibrous stroma combined with hyperplastic musculature, also known as proctitis cystica profunda, may simulate an invasive mucinous carcinoma (see Chapter 22). Although prolapse changes may also be found in invasive carcinomas, they are more prominent in ICPs, and the misplaced epithelium is usually accompanied by lamina propria tissue. Non-neoplastic epithelium surrounding mucin pools may be found in cases of benign proctitis cystica profunda, whereas neoplastic epithelium is often found floating within mucin pools in cases of invasive mucinous adenocarcinoma. In some cases, features of prolapse may be the only finding in the mucosa overlying a malignancy, and an underlying malignancy should always be considered when biopsy findings of a mass lesion reveal prolapse changes but no neoplasm.39
The finding of inflammation and a granulation tissue cap may cause diagnostic confusion with a juvenile or hamartomatous polyp or an inflammatory polyp (i.e., inflammatory bowel disease-related or sporadic).38 This distinction may be particularly difficult because any type of colorectal polyp can undergo secondary prolapse. A low-power view of the polyp often helps the pathologist to differentiate prolapse from an injury resulting from a preexisting (often neoplastic) lesion. ICPs show muscularization in the base of the polyp, whereas juvenile or inflammatory polyps never have this feature. Inflammatory polyps in inflammatory bowel disease usually occur in the context of chronic colitis in adjacent mucosa.
Simple excision of ICPs is usually curative.36 Recurrences are uncommon.
Inflammatory Disorders of the Anal Canal
Anal Tears, Fissures, and Ulcers
Clinical Features
Acute anal fissures are common, although the exact incidence is unknown because most patients do not undergo clinical evaluation.40 Chronic anal fissures affect men and women equally, and they account for as many as 10% of patients who are seen in colorectal clinics. Although there are no uniform criteria, chronicity of an anal fissure usually is defined as persistence for at least 6 weeks with transverse internal anal sphincter fibers visible on anoscopy. Other features of chronicity include an indurated edge and a hypertrophic anal papilla. When fissures become large, deep, and chronic, they are often referred to as an anal ulcer.
Pathogenesis
The term anal tear refers to an acute linear tear in the mucosa of the anal canal. Although most anal tears heal spontaneously, the development of an anal fissure is associated with spasm of the internal anal sphincter and a reduction in mucosal blood flow that leads to delayed or failed healing of the initial mucosal injury.41 Chronic anal fissures are further propagated by increased resting internal anal sphincter tone. In patients with a normal sphincter tone, other factors have been linked to the development of an anal fissure, such as human immunodeficiency virus (HIV) infection, anal-receptive intercourse, sexual abuse, Crohn’s disease, and previous obstetric operations or anorectal malformation repair.40
Pathology
Anal fissures typically extend from the dentate line to the anal verge in the posterior midline of the anal canal overlying the lower portion of the internal sphincter. Histologically, anal fissures or ulcers are characterized by nonspecific acute and chronic inflammation, granulation tissue, and reactive changes of the squamous epithelium at the edges of the fissure (Fig. 32.6). Chronic fissures are frequently associated with hypertrophy of the anal papillae at the proximal end of the lesion.41 Histologically, hypertrophic anal papillae are similar to other types of benign fibroepithelial polyps. When a fissure is located in an unusual location or fails to heal after treatment, inflammatory bowel disease (usually Crohn’s disease), neoplasm, or infectious processes should be considered.42–44
Prognosis and Treatment
Most acute fissures are superficial and heal quickly. Healing may be facilitated by increased dietary fiber and warm sitz baths. For chronic anal fissures, medical management is recommended for first-line treatment with the aim of reducing internal anal sphincter resting pressure.40,45,46 Medical treatments include the use of nitric oxide donors such as topical glyceryl trinitrate (GTN) to increase anodermal blood flow and botulinum toxin to reduce resting internal anal sphincter tone.
If these methods fail or the fissures recur, surgical management may be indicated by using approaches such as anal dilation, internal sphincterotomy, or fissurectomy.41 Surgical management permits healing of chronic fissures in as many as 100% of patients, and rates of recurrence are low.40 Patients with recurrent anal fissure after surgical management should be evaluated for alternative causes, such as Crohn’s disease, sexually transmitted disease, sexual abuse, and HIV infection.
Anal Abscesses and Fistulas
Most anal fistulas or abscesses are idiopathic, although they may occur in patients with Crohn’s disease or carcinoma in this region.47 Anorectal abscesses may also be found in patients with hidradenitis suppurativa.48–50 In hidradenitis patients, wound healing is often complicated by coexistent obesity and diabetes.
Anal abscesses and fistulas represent different stages of anorectal suppurative disease. Most suppurative processes in this location arise after infection of an anal duct, which provides a pathway from the anal canal to perineal soft tissues.51,52 In the acute phase, an abscess may form, whereas a fistula represents the chronic phase of infection, occurring in 30% to 50% of patients with an anal abscess.53,54 There are no reliable ways to predict which patients will have disease progression to a fistula.
Histologic specimens obtained from fistulas show nonspecific acute and chronic inflammation, fibrosis, and granulation tissue. A giant cell foreign body reaction to fecal matter may be seen in specimens from fistula tracts of the anus and should not be confused with the well-formed, sarcoid-like granulomatous reaction typical of Crohn’s disease. In patients with hidradenitis suppurativa, the histologic findings in the anal canal are identical to those in other affected locations.
Any cause of infection or fistula formation should be considered in the differential diagnosis of an anal abscess or fistula. A thorough histologic examination, including multiple sections, should be conducted to rule out an underlying neoplasm, and special stains for acid-fast bacilli, fungi, or other infectious organisms should be performed, particularly if granulomatous inflammation is found. The patient’s clinical history, including inflammatory bowel disease, should be carefully reviewed.
Antibiotics are often ineffective because of poor penetration into inflamed areas. Incision and drainage remain the primary treatment modality for anal abscesses, but recurrences are relatively common54 Wide surgical excision is curative in most cases of idiopathic anal abscess or fistula.
Common Infections of the Anal Canal
Clinical Features and Pathogenesis
Individuals who engage in unprotected anal intercourse are at greatest risk for acquiring anorectal infections. Anorectal infections are most common among men who have sex with men (MSM). The symptoms of an anorectal infection depend on the specific infection or pathologic process but are often indistinguishable from those of inflammatory bowel disease. The most common symptom is a frequent or continuous urge to have a bowel movement. Other symptoms include anorectal pain or discomfort, anal discharge (i.e., purulent, mucoid, or blood stained), tenesmus, urgency of defecation, rectal bleeding, and constipation. Systemic symptoms such as fever may also occur, but many patients are asymptomatic.55
Infections of the anal canal are most often caused by sexually transmitted pathogens. The most common agent identified is Neisseria gonorrhoeae (gonorrhea), found in 30% of patients, followed by Chlamydia trachomatis (19%), herpes simplex virus (HSV) type 2 (16%), and Treponema pallidum (syphilis; 2%). HSV type 1 accounts for 13% of anorectal herpes infections and likely represents anal-oral transmission.55 The incidence of anorectal disease caused by C. trachomatis, the causative agent of lymphogranuloma venereum (LGV), is increasing.56,57 More than one infection may exist in a single individual.
Pathology
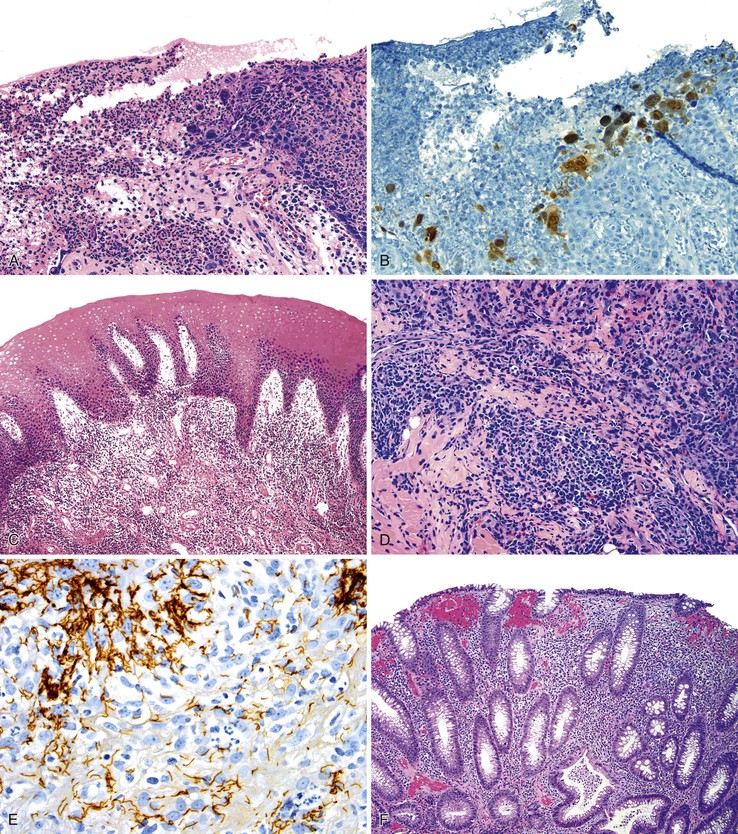
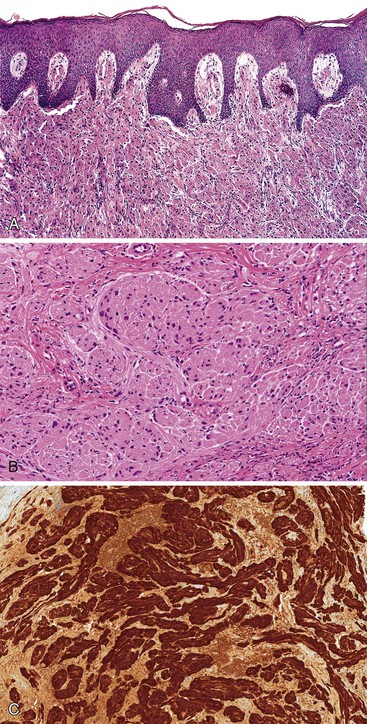
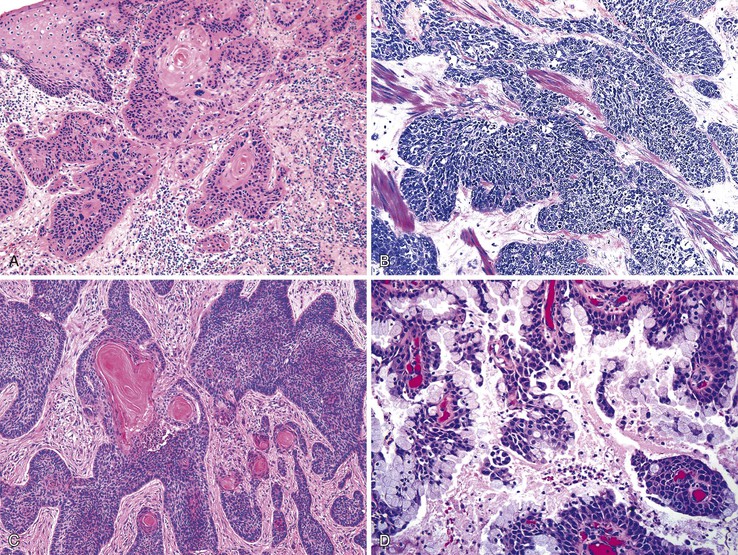
Different pathogens infect different types of mucosa. HSV and T. pallidum infect the stratified squamous epithelium of the perianal area and anal verge. As in other sites, HSV infections are associated with small vesicles that ulcerate and are seen histologically as mucosal ulcerations with associated acute and chronic inflammation and granulation tissue. Virally infected multinucleated cells with smudged chromatin may be seen in routine sections stained with hematoxylin and eosin, although immunohistochemical stains are of great value in confirming the presence of virally infected cells (Fig. 32.7, A and B). In contrast, syphilis is commonly associated with a chronic inflammatory infiltrate rich in plasma cells and may show associated granulomatous inflammation (see Fig. 32.7, C to E).
Dark-field microscopy of exudates from anorectal ulcers may be inaccurate because of contamination from commensal spirochetes found in the normal flora of the colorectum; however, T. pallidum immunohistochemical stains have become a useful tool for surgical pathologists.58 Demonstration of antibodies in the serum confirms the syphilis diagnosis. Serologic tests for syphilis include the use of nonspecific cardiolipin antigens (i.e., Venereal Disease Research Laboratory [VDRL] slide test and rapid plasma reagin [RPR] test). These tests can be used to diagnose primary and secondary syphilis and to monitor treatment response. The specific treponemal antigen tests (i.e., enzyme immunoassay, T. pallidum hemagglutination assay, T. pallidum particle agglutination assay, and fluorescent treponemal antibody absorption test) remain positive even after successful treatment and can detect latent syphilis in untreated adults.
Infections occurring between the anal verge and the dentate line tend to be extremely painful because of the abundance of sensory nerve endings in this area. Chlamydial infections and gonorrhea target the columnar epithelium of the rectum. The pathologist may see evidence of cryptitis, crypt abscesses, and reactive epithelial changes in affected individuals (see Fig. 32.7, F). Granulomas may occur. Polymerase chain reaction amplification for C. trachomatis DNA is the standard diagnostic test for anorectal chlamydial infection. Because the rectum has few sensory nerve endings, infections that spare the anus may be painless.55
Differential Diagnosis
A solitary anal ulcer (e.g., in HSV infection) may be misdiagnosed clinically as a chronic anal fissure. Granulomatous inflammation can lead to the formation of a rectal mass in primary and secondary syphilis, and it must be differentiated from other causes of a rectal mass, such as a neoplasm. Condylomata lata occurring in the perianal region in syphilis patients appear as moist, wartlike lesions, and they may be confused with human papillomavirus (HPV) infection. The pain, tenesmus, bloody discharge, and constipation of untreated LGV may mimic Crohn’s disease. In the tertiary stage, anorectal fistulas and strictures may occur in LGV.
Inflammatory Bowel Disease
The histopathologic features of inflammatory bowel disease are detailed in Chapters 16 and 17. However, involvement of the anal canal is sufficiently common to warrant additional comments, particularly because neoplastic disorders may manifest with similar findings in this region.
Clinical Features and Pathogenesis
In ulcerative colitis, involvement of the anus is typically nonspecific and indistinguishable from the appearance in patients without colitis. In contrast to ulcerative colitis, at least 50% of patients with Crohn’s disease have perianal or anal disease.59 The anal canal is involved in approximately 25% of patients with small intestinal Crohn’s disease, more than 40% of patients with large bowel disease and rectal sparing, and more than 90% of patients with large bowel disease that includes the rectum.60 In some patients, anal involvement is the first or only clinical manifestation of Crohn’s disease.61
The clinical features of Crohn’s disease involving the anus have been well characterized.61,62 Symptoms include recurrent sepsis, local pain, discharge, ulceration, fecal incontinence, sleep disruption, psychological disturbance, and sexual dysfunction.63 Signs most characteristic of Crohn’s disease affecting the anus are chronic inflammatory changes, induration of the anal skin, multiplicity of lesions, and skin discoloration. Anal canal lesions include fissures, ulcers, strictures, perianal fistulas that open into or near the anal canal, abscesses, rectovaginal fistulas in women, and cancer.59 Anal skin lesions are usually perianal skin tags or hemorrhoids. Anal skin tags are the most common finding and tend to be larger than those from patients without Crohn’s disease. Fissures and fistulas are the next most common finding.59 Fissures in Crohn’s disease tend to be large, deep, and located more atypically compared with traumatic anal fissures. Most fistulas in anal Crohn’s disease are classified as simple (see Prognosis and Treatment), although in some patients, they may contain multiple openings, sometimes occurring at a considerable distance from the anal canal (i.e., watering-can perineum).61 The cause of Crohn’s perianal fistulas is uncertain, but in some patients, it is thought to be a fistula-in-ano arising from inflamed or infected anal glands or penetration of fissures or ulcers in the rectum or anal canal.61
Pathology
In ulcerative colitis, chronic active inflammation tends to involve rectal mucosa in the proximal anal canal.64 If histologic findings are encountered more distally in the anal canal, they are limited to nonspecific chronic inflammation of the submucosal tissues with associated mild reactive changes of the squamous or transitional epithelium. Anal fissures, abscesses, or fistulas are occasionally found, and these more severe changes should prompt clinical consideration of Crohn’s disease.
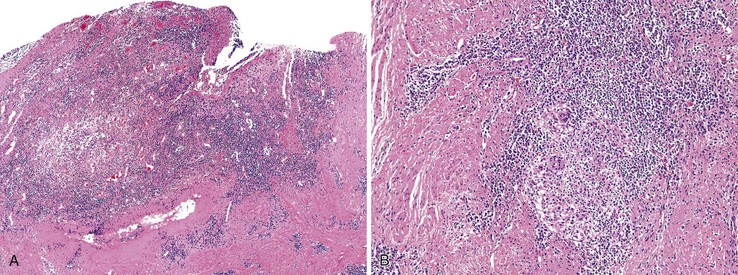
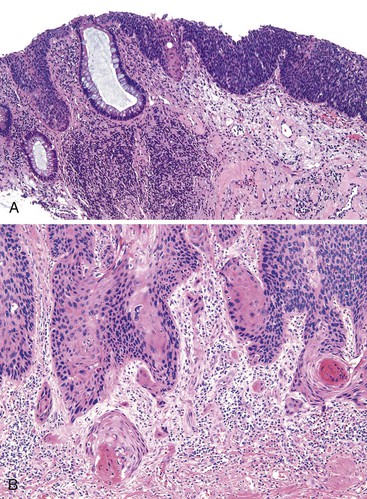
The anal findings in Crohn’s disease vary. They include anal fissures, fistulas, ulcers, abscesses, and tags.61 Unfortunately, the histologic features seen in Crohn’s-related fissures or fistulas are not different from those in patients without Crohn’s disease. A feature supporting the diagnosis of anal Crohn’s disease is sarcoid-like (noncaseating) granulomatous inflammation close to the anal mucosa, particularly in a young person with no other obvious reason for an inflammatory disorder of the anal canal (Fig. 32.8). As in other GI sites, a diagnosis of Crohn’s disease requires correlation of the histologic changes with all available clinical, endoscopic, and radiologic information.
Differential Diagnosis
The differential diagnosis of granulomatous inflammation in the anal canal includes foreign body reaction to nonspecific fistulas, sarcoidosis, tuberculosis, granuloma inguinale caused by Calymmatobacterium granulomatis, and LGV caused by sexual transmission of C. trachomatis.55,65–67 Tuberculous granulomas are typically caseating. Stains for acid-fast bacilli and other microbiologic studies are helpful. Patients with tuberculosis of the anal canal invariably have pulmonary disease. The finding of Donovan bodies with a Warthin-Starry stain supports a diagnosis of granuloma inguinale, whereas the finding of follicular lymphohistiocytic and plasma cell infiltrates in association with neural hyperplasia may suggest chlamydial infection.68 Hidradenitis suppurativa may be confused with complex fistulizing perianal Crohn’s disease. A correct diagnosis can often be made by examination under anesthesia and by probing the cutaneous tracts and demonstrating that they traverse only to the subdermal space and do not communicate with the rectum or other parts of the digestive tract.69
Prognosis
The prognosis for patients with anal Crohn’s disease is related to the extent of involvement, and for most patients, it is excellent.70 Various classification schemes for perianal Crohn’s disease have been proposed, although most are not widely used because they have not shown a reproducible relation to meaningful clinical outcomes, such as fistula formation.61 Most clinicians classify perianal disease as simple or complex.
Proper classification involves thorough evaluation of the perianal area for involvement by skin tags, fistulas, fissures, abscesses, and stricturing disease and an endoscopic examination of the rectum to assess for inflammation. Fistulas may be classified as simple, indicating that the fistula tract arises below the external sphincter, has a single external opening, has no pain or fluctuation to suggest perianal abscess, is not a rectovaginal fistula, and has no evidence of anorectal stricture. Complex fistulas arise above the external anal sphincter and may contain one or more of the features described. Rectal Crohn’s disease may make a simple fistula more complicated to manage, but overall, patients with Crohn’s disease and simple fistulas have higher rates of healing than non-Crohn’s patients with complex fistulas.71
Treatment
Management of perianal Crohn’s disease includes medical and surgical treatment. It most often begins with conservative medical treatment that includes antibiotics, steroids, antimetabolites such as azathioprine or 6-mercaptopurine, anti–tumor necrosis factor-α agents, tacrolimus, and cyclosporine. These agents are used to reduce the number of draining fistulas and are most effective in simple perianal disease.61,71
Combined with small bowel disease, perianal Crohn’s disease is an absolute contraindication to restorative proctocolectomy (i.e., ileal pouch anal anastomosis) in the otherwise well-motivated subset of Crohn’s patients seeking a continence-preserving procedure.72 Surgical management is reserved for patients with complex fistulizing disease. As many as 50% of patients require surgical management of their perianal disease, ranging from local procedures such as sepsis drainage and seton placement to segmental resections such as proctectomy with ostomy creation.61,71
Benign Tumors of the Anal Canal
Adnexal Tumors
Clinical Features
Many adnexal tumors have been described in the perianal skin. The most common lesion is hidradenoma papilliferum.73,74 Hidradenoma papilliferum occurs almost exclusively in women and within a wide age range. In most patients, it manifests as a solitary, painless mass of the perianal skin or perineum, although ulceration of the overlying skin has been reported in some cases.74 Rarely, trichoepitheliomas or apocrine gland adenomas may arise in the perianal skin.75,76 Trichoepitheliomas occur in both sexes and may reach several centimeters in diameter when they occur in the perianal skin, unlike trichoepitheliomas of other skin regions.75
Most adnexal tumors of the perianal skin have an apocrine or sweat gland origin, although trichoepitheliomas are derived from hair follicles. Immunolabeling for estrogen and progesterone receptors provides a reliable marker for anogenital sweat glands in women but not conventional sweat glands, in keeping with the fact that benign apocrine gland tumors occur almost exclusively in the female anogenital region.74
Pathology
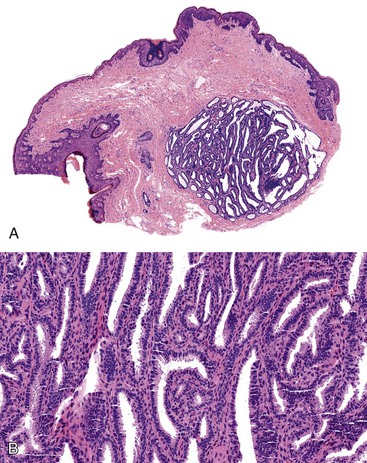
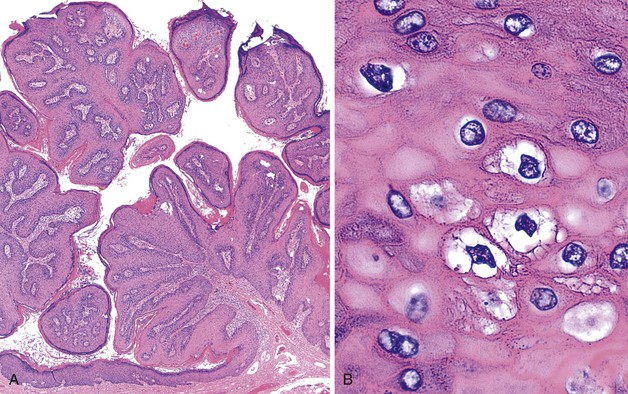
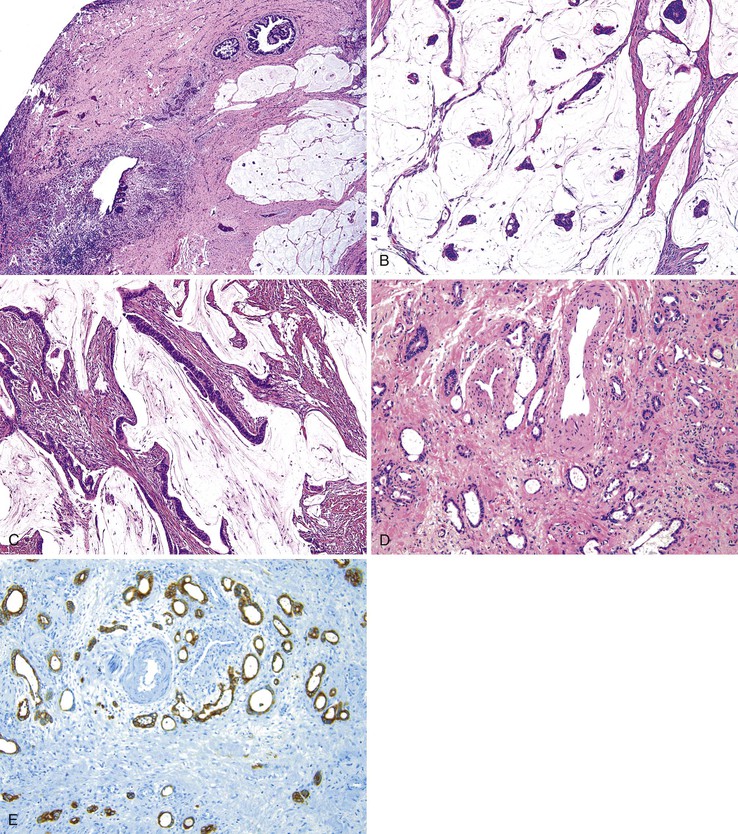
Grossly, perianal adnexal tumors appear as solitary subcutaneous nodules with a smooth red to tan surface, although cases of ulceration mimicking a more aggressive lesion have been reported.77 In the case of hidradenoma papilliferum, pathologic examination reveals a complex papillary glandular pattern characterized by a prominent myoepithelial layer. Some stratification of the epithelial cells and mild pleomorphism may be seen (Fig. 32.9). Trichoepitheliomas often appear as a solid mass without ulceration of the overlying skin. Histologic examination reveals uniform eosinophilic cells with centrally located, minimally atypical nuclei. The epithelial cells form tracts throughout the supportive stroma that are two or more cells thick, and they form pseudopapillae in regions of loose matrix, reminiscent of abortive pilar differentiation.78 Local excision is usually curative.
Differential Diagnosis
In the case of hidradenoma papilliferum, the finding of a solitary perianal mass with ulceration may indicate a more aggressive lesion, such as an infiltrating squamous carcinoma. The main differential diagnosis of a trichoepithelioma is basal cell carcinoma, because both lesions consist of a proliferation of basaloid cells. The formation of pseudopapillae is characteristic of trichoepitheliomas, whereas peripheral nuclear palisading and retraction artifact are important features of basal cell carcinomas.78,79
Granular Cell Tumor
Clinical Features and Pathogenesis
Granular cell tumors are common benign tumors that originate from Schwann cells. They occur in many locations, including the GI tract. When the GI tract is involved, the perianal region is a common site (21% in a large series).80,81 They are most commonly diagnosed in adults in the fifth decade of life and are more common in women. The most common presentation is an incidental, asymptomatic, solitary mass.80
Pathology
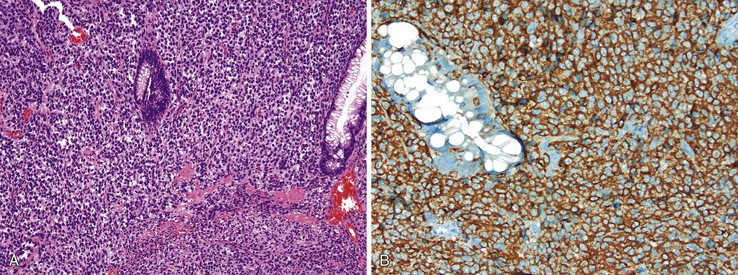
Grossly, granular cell tumors appear as firm, solitary subcutaneous nodules that are white or yellow on cut section. Histologically, these lesions are composed of sheets of cells with small, uniform, hyperchromatic nuclei and with characteristic eosinophilic granular cytoplasm that is diastase resistant on periodic acid–Schiff (PAS) staining. The granularity of the cytoplasm corresponds to vacuoles that contain cellular debris, which is evident by electron microscopy (Fig. 32.10).
The distinctive histologic features of a granular cell tumor can distinguish it from other subcutaneous perianal masses. A positive immunostain for S100 protein may be used to support the diagnosis of a granular cell tumor. These tumors are also strongly positive for CD68 (KP1). Of particular significance is the occurrence of pseudoepitheliomatous hyperplasia of the epithelium overlying granular cell tumors in as many as 50% of cases, which may be mistaken for an infiltrating squamous carcinoma.80 S100 protein–positive granular cells located immediately beneath the atypical epithelium should help to indicate its benign nature. Local excision is usually curative.
Squamous Neoplasms of the Anal Canal
Risk Factors and Pathogenesis
Risk factors for squamous tumors and neoplasms of the anal canal include anal-receptive intercourse, heavy smoking, a history of sexually transmitted diseases, HIV-positive status, and immunosuppression.82–86 In one study, 92% of HIV-positive MSM demonstrated evidence of anal HPV infection, compared with 66% of MSM who were HIV negative. In women, lower genital tract squamous neoplasia is a risk factor, and numerous similarities to the incidence and epidemiology of cervical and vulvar neoplasia have been observed.87 Women with multifocal genital intraepithelial neoplasia have a 16-fold increase in the rate of anal canal intraepithelial neoplasia.86 Solid organ transplant recipients have a 10- to 100-fold increased risk of anogenital neoplasia.
There are more than 100 types of HPV. Of the approximately 30 types that infect the anogenital tract, the most common are 6, 11, 16, and 18.88 The finding of HPV in an anal carcinoma is related to the sensitivity of the technique used,89 but some evidence suggests geographic or population differences in HPV genotypes associated with anal cancers. The prevalence of HPV-16–associated anal squamous cell carcinoma (SCC) was found to be significantly lower in India and South Africa compared with more developed countries.90
Although HPV infection is identified in more than 80% of anal SCC,91 it is not sufficient to cause malignancy by itself. Evidence for this claim is largely related to two phenomena. First, HPV infection is highly prevalent among sexually active individuals, but cancer does not develop in most patients infected with high-risk genotypes (discussed later).92,93 In most patients, cell-mediated immunity is responsible for clearance of the infection, and neutralizing antibodies prevent subsequent infections.94 Second, in experimental studies, infection with high-risk (oncogenic) HPV genotypes is insufficient to induce transformation and tumor progression95,96 This may reflect the inability of HPV to integrate into the host genome. For example, in benign lesions, the viral genome replicates as an extrachromosomal episome, whereas in malignant tumors, the viral genome becomes integrated into the host cell chromosomes.97–100 Viral integration does not appear to occur randomly. In a systematic review of known HPV integration sites, integration at 8q24, the location of the MYC oncogene, and 3p14, the location of the FHIT tumor suppressor gene, were the most common sites reported.101
High-risk HPVs, most commonly genotypes 16 and 18, but also 31, 33, 35, 39, 45, 50, 51, 53, 56, 58, 59, 68, and others, encode for at least three oncoproteins, known as E5, E6, and E7, which are growth stimulating and have transforming properties. With integration of HPV DNA into the host genome, E6 and E7 expression is increased.102 The E6 protein binds to the TP53 tumor suppressor protein, which is encoded by the TP53 gene and is a critical regulator of cell growth and response to stress103,104 E6 binding to TP53 leads to its rapid degradation.104 Similarly, E7 protein binds to and inactivates the retinoblastoma-associated protein (RB1) tumor suppressor protein, which normally restricts cell proliferation to the basal layer.105–107 In most human cancers with TP53 or RB1 mutations, the proteins are inactivated. However, wild-type TP53 and RB1 genes are common in anogenital cancers.108–111 By inactivating TP53 and RB1 and deregulating cellular growth, the action of E6 and E7 on these tumor suppressor proteins appears functionally equivalent to genetic mutations and increases the risk of anogenital cancer. Consistent with this notion, HPV-negative cervical and anal cancers have been found to harbor inactivating mutations in TP53 and RB1.111,112
HPV integration promotes general changes of the host genome, such as chromosomal instability. The degree of chromosomal instability is highly correlated with levels of E7 overexpression, although whether the instability precedes or follows viral integration is unclear.113–115 Recurrent chromosomal alterations associated with chromosomal instability have been described in anal squamous carcinoma that include deletion of chromosome arms 11q, 3p, 4p, 13q, and 18q and nonrandom copy number increases of chromosomes 17 and 19.116,117 In contrast, chromosomal instability is relatively uncommon in anal carcinomas of HIV-positive patients, suggesting that immunosuppression may promote carcinogenesis through an alternate pathway.118,119
There is no known association between any HPV subtype and specific tumor morphology. However, HPV-6 and HPV-11 are most likely to be associated with condyloma acuminatum, and HPV-16 and HPV-18 are found in a significant minority of conventional condylomata and in lesions with high-grade dysplasia89,120–122 Verrucous carcinoma is most commonly associated with infection by HPV subtypes 6 and 11, although occasionally HPV-16 and HPV-18 have been found in these lesions.123–125 Similar to the documented progression of HPV-associated premalignant conditions of the uterine cervix, epidemiologic studies indicate that the development of anal canal intraepithelial neoplasia is associated with infection by HPV-16 and HPV-18 and less commonly by HPV-6 and HPV-11.126,127
Evidence in support of the precursor potential of anal squamous intraepithelial neoplasia (ASIN) lies in its close similarity to dysplasia of the uterine cervix, including histologic similarities between the preinvasive and invasive lesions, its occurrence at a younger average patient age compared with its invasive counterpart, and its common occurrence adjacent to invasive cancers, particularly those associated with HPV infection in MSM.84,86 Perianal intraepithelial neoplasia, including bowenoid papulosis and Bowen disease, also have an association with HPV infection; in particular, HPV-16 has been demonstrated in bowenoid papulosis and Bowen’s disease.128,129
HPV vaccines have been developed to protect recipients against HPV types 16 and 18 (HPV2/Cervarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) and HPV types 6, 11, 16, and 18 (HPV4/Gardasil, Merck, West Point, PA). These vaccines were first approved and recommended for administration to girls in 2009 and 2006, respectively; in late 2011, HPV4/Gardasil was recommended for administration to boys.130 One analysis found that vaccinating boys and girls, rather than girls alone, could reduce the HPV-related cancer burden in boys by 65% compared with girls-only vaccination. The investigators further estimated that inclusion of boys in a HPV vaccination program would lead to an 86% reduction in the incidence of anal cancer compared with a 63% reduction with girls-only vaccination.131
Nomenclature of Preinvasive Anal Squamous Neoplasia
During the past several decades, various terms have been used to describe the same anal squamous pathology. Consensus groups such as the AJCC, the Bethesda System, and the World Health Organization (WHO) have used different nomenclature. This chapter uses the WHO nomenclature, but a brief discussion of the latest attempt at unifying squamous neoplasia nomenclature is warranted because it may become standard in the future (Table 32.2).
Table 32.2
Nomenclature for Preinvasive Anal Squamous Lesions
| Lesion | AIN | ASIN (WHO) | SIL (CAP/LAST) |
| Mild dysplasia | I | ASIN-L | LSIL |
| Moderate dysplasia | II | ASIN-H | HSIL |
| Severe dysplasia | III | ASIN-H | HSIL |
| Carcinoma in situ | III | ASIN-H | HSIL |
| Condyloma acuminatum | Condyloma acuminatum | Condyloma acuminatum | LSIL (condyloma acuminatum) |
| Bowen disease | NA | PSIN-H | HSIL |
| Bowenoid papulosis | NA | PSIN-H | HSIL (bowenoid papulosis) |
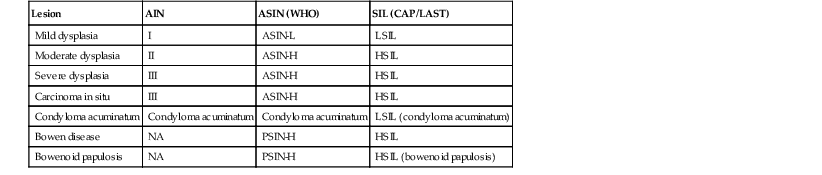
AIN, Anal intraepithelial neoplasia; ASIN-H, high-grade anal squamous intraepithelial neoplasia; ASIN-L, low-grade anal squamous intraepithelial neoplasia; CAP/LAST, College of American Pathologists/Lower Anogenital Squamous Terminology Project; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NA, not applicable; PSIN, perianal squamous intraepithelial neoplasia; SIL, squamous intraepithelial lesion; WHO, World Health Organization.
In 2012, the College of American Pathologists (CAP) and the American Society for Colposcopy and Cervical Pathology released the product of a joint Lower Anogenital Squamous Terminology (LAST) Project, in which the groups reported their recommendations for a unified terminology for all HPV-related squamous lesions in the lower anogenital tract.132 They contended that the clinical, epidemiologic, and biologic similarities between the various anogenital sites justified the use of a single nomenclature for HPV-associated lesions. When presented with a lesion, a pathologist cannot reliably predict from which site it was derived (e.g., cervix, vagina, anus) or whether it came from a male or female patient. There are no differences in the molecular or biomarker phenotypes in the various HPV-related lower anogenital sites.
The CAP/LAST investigators contend that HPV interacts with all the anogenital squamous epithelium by two basic mechanisms. Squamous cells transiently support the virus, which manifests as a low-grade squamous intraepithelial lesion (LSIL). Alternatively, precancerous lesions resulting from viral oncogene expression cause a clonal proliferation of relatively undifferentiated cells in a high-grade squamous intraepithelial lesion (HSIL). With time, persistent HPV infection results in a substantial risk of malignancy. In the LAST nomenclature, additional pathologic descriptors such as ASIN, condyloma acuminatum, or bowenoid papulosis may be added in parentheses as needed.
Some investigators and clinicians are skeptical of applying the cervical HPV screening and treatment paradigm to anal pathology. They cite reasons such as unclear natural history data for anal HPV infection. Many of the studies of anal squamous neoplasia focus on MSM, which may be a significantly different population from women with cervical neoplasia. For example, HPV infection and anal intraepithelial neoplasia tend to persist in MSM as the population ages, whereas cervical HPV infection and intraepithelial neoplasia dramatically decrease with patient age.91 Some researchers report high-grade anal squamous intraepithelial neoplasia (ASIN-H) with a relatively high risk of progression to invasive carcinoma; Scholefield and colleagues estimate 10% at 5 years.133 However, one meta-analysis estimates the risk to be much lower: 0.15% per year for HIV-positive MSM and 0.02% per year for HIV-negative MSM.91 No randomized trials have investigated the benefits and risks of managing ASIN.
Condyloma Acuminatum
Clinical Features
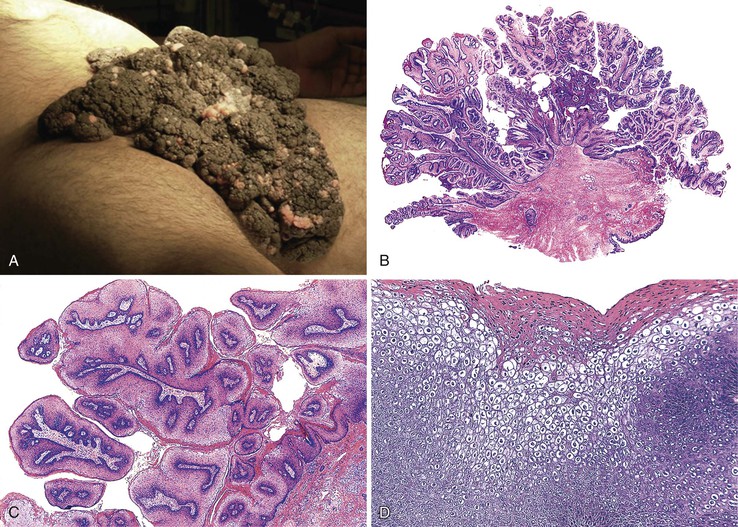
Condyloma acuminatum (i.e., common anogenital wart) is the most common tumor of the anal and perianal region.120 These lesions typically grow on warm, moist mucosal regions, characteristic of the anogenital skin. As many as 1% of sexually active people have anal condylomata, and the lesions often occur in association with other sexually transmitted diseases.89 Anal condylomata may be associated with penile warts in men or vulvar warts in women, but they may also occur as the sole area of infection, particularly in the MSM population. Nonsexual transmission may occur, particularly in children.134
Pathology
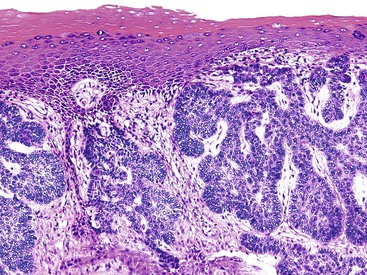
The perianal skin is the most commonly affected area, although condylomata located solely within the anal canal may occur. Grossly, condylomata are soft, fleshy, and tan, gray, or pink papillomatous growths that often occur in groups or clusters.
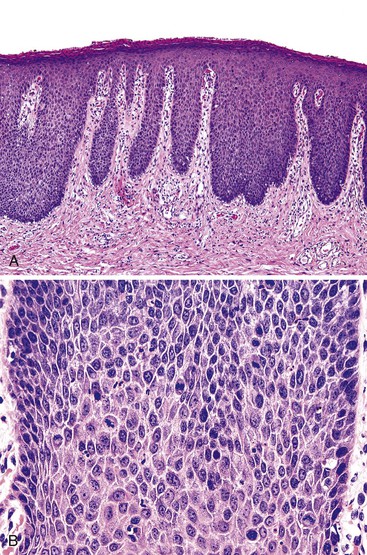
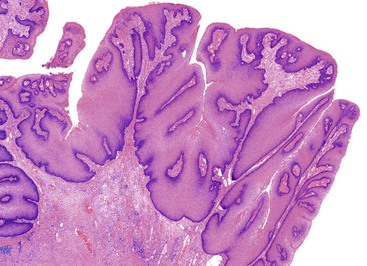
Histologically, the papillomatous appearance is best appreciated at low magnification. The squamous epithelium shows marked acanthosis and a variable expansion of the stratum corneum layer. Surface parakeratosis is common. The rete pegs may appear elongated with broad bases. On higher magnification, the surface epithelium contains squamous cells with an enlarged, irregular, and hyperchromatic nucleus and an accompanying perinuclear halo. These cells, known as koilocytes, are the histologic hallmark of HPV infection (Fig. 32.11). Dyskeratotic cells are usually easily found, as well as multinucleate cells, which are also characteristic of HPV infection. Basal cell hyperplasia is typically associated with orderly and progressive maturation of the epithelium. The squamous epithelium is well delineated from the underlying stroma, which often contains chronic inflammation, edema, and vascular dilation.
Differential Diagnosis
The differential diagnosis of condyloma includes verrucous carcinoma on one end of the spectrum and benign anal or perianal lesions, such as seborrheic keratosis, fibroepithelial polyp, and prolapse-type polyp, on the other end. Condyloma acuminatum may be impossible to distinguish from verrucous carcinoma, particularly in biopsy samples or when the clinical impression is not provided. Verrucous carcinomas are usually larger than condylomata acuminata, and verrucous carcinomas display exophytic and endophytic growth patterns, whereas condylomata usually are only exophytic. Separating condyloma from another type of benign lesion rests on identifying an HPV cytopathic effect in the condyloma or the characteristic histologic features in the other benign lesions. Immunohistochemistry or in situ hybridization studies for HPV can be helpful in equivocal cases.
Prognosis
There is controversy regarding the malignant potential of condylomata. Although they usually harbor low-risk HPV genotypes, as many as 35% demonstrate high-risk HPV, most commonly genotypes 16 and 18.122 High-grade intraepithelial neoplasia has been described in as many as 20% of HIV-infected patients with condylomata, and cases of invasive squamous carcinoma arising in an anal condyloma have been reported.121
Treatment
The most common treatments for anal condylomata are ablative or cytodestructive.135 Methods of ablative treatment include cryotherapy, local excision, electrocautery, and laser therapy. Cytodestructive therapies include podophyllotoxin and trichloroacetic acid. Both forms of treatment are associated with a high recurrence rate, which is attributed to latent HPV in clinically unremarkable adjacent epithelium. Immunosuppression is also associated with an increased rate of recurrence after surgical removal.136 The immunomodulatory agent imiquimod has potent antiviral and antitumor activity without the common side effects of the other forms of treatment.137
Anal Squamous Intraepithelial Neoplasia
Clinical Features
Anal squamous intraepithelial neoplasia (ASIN), as listed in the WHO nomenclature, may be found in tissues removed for a variety of disorders.138 Similar to its cervical counterpart, ASIN has been identified in anal mucosa adjacent to invasive carcinomas of the anal canal and has been an incidental finding in resection specimens from this region.139
Its prevalence in the general population is estimated at 2 to 3 cases per 1000 individuals, but the prevalence is much higher in at-risk populations, such as MSM, HIV-positive individuals, women with cervical or vaginal squamous carcinoma, and immunocompromised individuals.138,140 ASIN has been documented at much greater frequencies in HIV-positive MSM compared with MSM who are HIV negative. For example, one study found ASIN in 36% of HIV-positive men and 7% of HIV-negative men.141 The difference also seems to hold true for ASIN-H; 49% of HIV-positive MSM had ASIN-H and 17% of HIV-negative MSM had ASIN-H in one study.142
Clinically, ASIN may appear as raised, scaly, white, erythematous, pigmented, fissured, or ulcerated areas in the anal canal, although it is often subclinical and may be an incidental finding.143 ASIN may also be identified as acetowhite epithelium, which contrasts with iodine-positive non-neoplastic mucosa by anal colposcopy.
Pathology
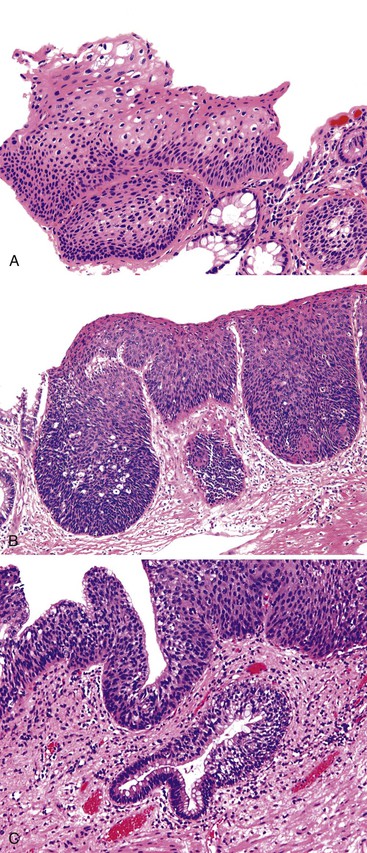
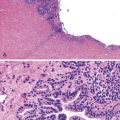
ASIN is characterized by cells with enlarged nuclei, irregular nuclear membranes, and increased nucleus-to-cytoplasm ratios, with a lack of cytoplasmic maturation toward the luminal surface.132 Mitotic figures may be encountered beyond the normal regenerative basal zone of the squamous epithelium. Individual cell keratinization and dyskeratosis are common and may represent markers of HPV infection. These changes typically occur in the absence of inflammation.
ASIN is divided into two grades, primarily based on the level at which orderly cytoplasmic maturation begins and the level at which mitotic activity stops. In low-grade anal squamous intraepithelial neoplasia (ASIN-L), mitotic figures and cellular pleomorphism are predominantly located in the basal third of the epithelium, with evidence of surface maturation in the upper two thirds or evidence of an HPV cytopathic effect (i.e., koilocytic atypia) is present. In contrast, ASIN-H is characterized by cellular pleomorphism, mitotic figures, and lack of cytoplasmic maturation extending into the middle and superficial thirds of the epithelium (Fig. 32.12). These preinvasive changes may colonize the underlying anal ducts and glands, which may not be detected or excised during treatment. Intraepithelial neoplasia that extends into the ducts can be graded using the same principles as surface dysplasia; however, the pathologist must be cautious with the use of tangential sectioning (see Fig. 32.12, C).
Differential Diagnosis
The differential diagnoses of ASIN include other neoplasms within the squamous epithelium such as melanoma and primary or secondary Paget disease. Some ASIN-H lesions may demonstrate a pattern of pagetoid spread that can only be differentiated from Paget disease by immunohistochemistry (see Paget Disease of the Anus).144
Differentiating Reactive Changes or Immature Squamous Metaplasia from High-Grade Squamous Intraepithelial Lesions
Distinguishing reactive changes from ASIN-L in biopsy material is fraught with high observer variability, and usually rests on deciding whether or not unequivocal HPV cytopathic effect is present (Table 32.3).145 Unfortunately, there are no reliable adjunctive pathologic methods to separate these two processes.
Table 32.3
Differential Diagnosis of Anal Intraepithelial Neoplasia and Separation from Reactive Lesions
| Feature | Reactive Lesions | ASIN-L | ASIN-H |
| N : C ratio | Low | Low | High |
| Koilocytes | No | Yes | Often |
| Mitotic activity | Basal layers only | Lower one third of mucosa | Upper two thirds of mucosa (may have abnormal mitotic figures) |
| Maturation | Orderly | Dysmaturation in lower one third of mucosa only | Dysmaturation in upper layers to full thickness |
| Cell polarity | Normal polarity | Relatively well-maintained polarity | Loss of polarity |
| Proliferation | Basal layers | Lower one third of mucosa | Throughout mucosa |
| Staining for p16 | Negative or patchy positivity | Varies | Blocklike positivity (nuclear and cytoplasmic) |
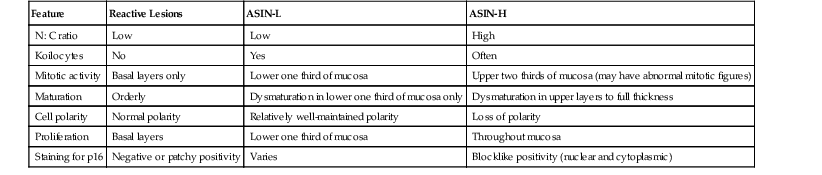
ASIN-H, High-grade anal squamous intraepithelial neoplasia; ASIN-L, low-grade anal squamous intraepithelial neoplasia; N : C, nucleus-to-cytoplasm ratio; p16, p16 protein.
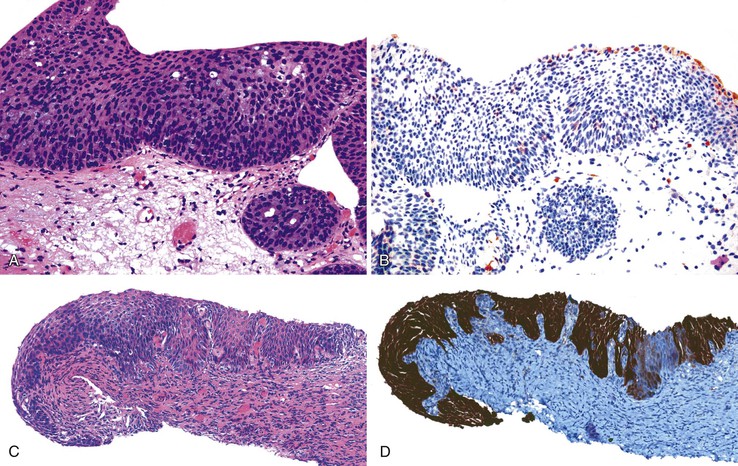
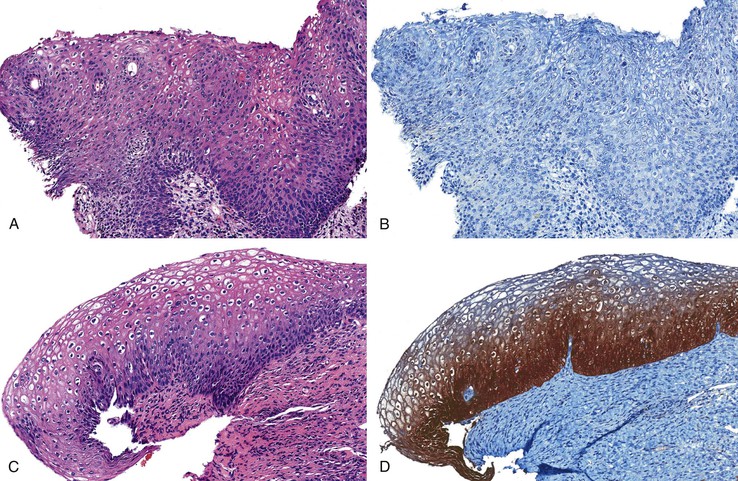
Separating reactive changes from ASIN-H, a precancerous lesion, is clinically important and more feasible (Fig. 32.13). Although reactive changes or immature squamous metaplasia and ASIN-H may show immature cells located beyond the basal layer, ASIN-H demonstrates a more disorganized or jumbled arrangement of the epithelium, and reactive processes tend to retain an orderly configuration of the squamous epithelial cells. Identification of inflammation or regular nuclei with open chromatin and prominent nucleoli favor a reactive process, whereas ASIN-H usually shows nuclear hyperchromasia and irregular nuclear contours.
When this differential is encountered, p16 immunohistochemistry often becomes necessary to separate reactive processes from ASIN-H. Weak and patchy immunoreactivity favors a non-neoplastic process, whereas diffuse, strong, and block positivity argues in favor of ASIN-H (Table 32.4).132 The latest recommendations argue against the use of multiple adjunctive immunohistochemical tests (e.g., p16 and Ki67) in the workup of a possible ASIN-H case.132 Evidence supports p16 as the best marker for ASIN-H, but Ki67146–148 and ProEx C (Becton Dickinson, Franklin Lakes, NJ)149–151 may also be useful when difficulties with p16 arise.
Table 32.4
Use of P16 Immunohistochemistry in the Diagnosis of Anal Squamous Intraepithelial Neoplasia
| Diagnostic Problem | P16 Recommended? | Interpretation or Reasoning |
| Reactive versus ASIN-H | Yes | Block positive staining supports ASIN-H diagnosis |
| Diagnosis of ASIN-L versus ASIN-H (equivocal for ASIN-H) | Yes | Block positive staining supports ASIN-H diagnosis |
| Professional disagreement about histologic diagnosis (provided ASIN-H is in differential diagnosis) | Yes | Block positive staining supports ASIN-H diagnosis |
| Diagnosis of negative or reactive on histology | No | Staining may be confusing |
| Diagnosis of ASIN-L on histology | No | Staining may be confusing |
| Diagnosis of ASIN-H on histology | No | Staining may be confusing |
ASIN-H, High-grade anal squamous intraepithelial neoplasia; ASIN-L, low-grade anal squamous intraepithelial neoplasia; P16, p16 protein.
From Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136:1266-1297.
Distinguishing Low-Grade and High-Grade Squamous Intraepithelial Lesions
In immunocompetent hosts, low-grade squamous lesions usually are transient and not precancerous, although this is often not the case in immunocompromised patients. Aside from the traditional morphologic features that separate ASIN-L from ASIN-H, the CAP and Lower Anogenital Squamous Terminology Project investigators identify at least two special circumstances for “bumping up” a lesion with the cytoarchitectural features of ASIN-L to ASIN-H: atypical mitotic figures or significant nuclear atypia beyond that expected in typical ASIN-L and thin ASINs, which are immature intraepithelial lesions less than 10 cells thick. As long as there are mitotic figures or cytologic features of ASIN above the basal layer, thin ASIN lesions should be considered ASIN-H. In both circumstances, a diffuse, strong, and blocklike positivity for p16 immunohistochemistry helps to confirm ASIN-H (Fig. 32.14).132
Differentiating High-Grade Squamous Intraepithelial Lesions from Superficially Invasive Squamous Cell Carcinoma
Early invasion by SCC can be difficult to diagnose with certainty, particularly in the setting of a condyloma, because condylomata often have an undulating interface with the underlying stroma (Fig. 32.15). ASIN may colonize the anal ducts and glands, mimicking stromal invasion. It is best to maintain strict criteria for stromal invasion by a squamous carcinoma. Invasive carcinomas tend to arise in mucosa extensively involved by high-grade intraepithelial neoplasia. Several features support the diagnosis of invasion:
• Blurring of the epithelial-stromal interface with loss of the regular arrangement of the squamous basal cells152
The pathologist must be careful not to misinterpret artifacts or reactive changes such as those accompanying prior biopsy, intense stromal inflammation, or pseudoepitheliomatous hyperplasia as evidence of invasive carcinoma.
Natural History and Treatment
Progression of ASIN largely depends on the immune status of the patient and extent of disease.153 Small series of immunosuppressed patients have found that as many as two thirds of cases progress from ASIN-L to ASIN-H within 2 years.141 After ASIN has developed, the rate of progression to carcinoma is uncertain, but some estimate that as many as 5% to 10% of patients will ultimately develop invasive disease.143 In one long-term surveillance study of 35 patients with ASIN-H, invasive carcinoma developed in 3 of 6 immunosuppressed patients (all with multifocal disease) in the follow-up period, but cancer did not develop in any of the other 3 patients.153 However, compared with some small observational studies, a meta-analysis found much lower progression rates; as few as 0.02% of cases progressed to invasive cancer per year.91
There are no well-established guidelines regarding appropriate screening for ASIN, even in at-risk populations, and groups such as the American Society of Colon and Rectal Surgeons154 and the Association of Coloproctology of Great Britain and Ireland133 have opposite opinions regarding the benefit of screening for ASIN. Management guidelines range from watchful waiting to topical immunomodulatory or ablative therapies to surgical excision. Management of ASIN should probably be individually tailored, taking into account the patient’s symptoms and immune status and the size and focality of disease.133
When treatment is determined, the goal is to eradicate ASIN, prevent the development of anal carcinoma, and maintain anal function. The success of surgical resection is largely related to the extent of involvement and the ability to achieve a disease-free margin, although HIV status also seems to be an important factor.143 Unfortunately, many of these treatment modalities have been associated with high rates of recurrence, particularly in HIV-positive patients and those with extensive or multifocal disease.154
Perianal Squamous Intraepithelial Neoplasia
Many of the previously discussed clinical and pathologic principles for ASIN apply to perianal lesions. However, two perianal-specific terms are worthy of additional review: bowenoid papulosis and Bowen disease. The use of these terms has been discouraged in anal pathology by some because they have engendered confusion with regard to their cutaneous counterparts. In practice, both conditions are considered to be HPV related in most cases and should be approached as such.
In the WHO classification, perianal squamous intraepithelial neoplasia (PSIN) is synonymous with Bowen disease, which consists only of high-grade intraepithelial neoplasia or carcinoma in situ. Although the WHO has seemingly eliminated low-grade PSIN, low-grade lesions (PSIN-L), especially in the form of condylomata acuminata, do occur, although the “flat” PSIN-L lesions may be exceedingly difficult to reproducibly recognize, akin to the vulvar lesions.155 The clinicopathologically defined bowenoid papulosis (also PSIN-H) may continue to have diagnostic relevance because of its more indolent course.
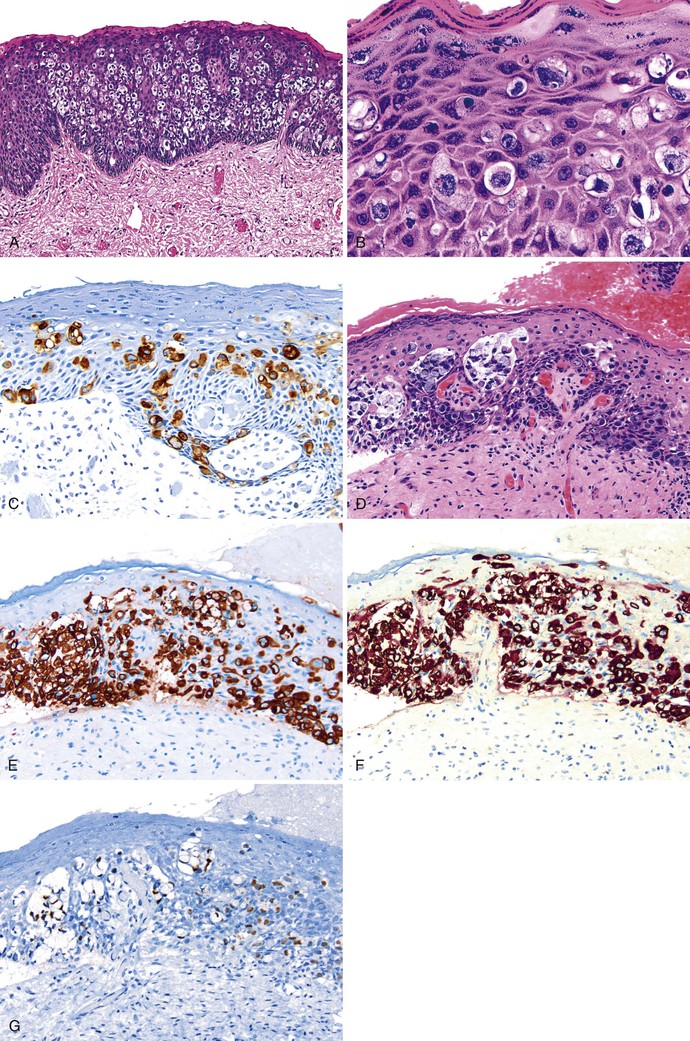
Bowen disease (PSIN-H) is rare in the perineal region.156 Bowen disease tends to involve the anal margin and adjacent perianal skin only as an extension of perineal Bowen disease. PSIN-H appears as erythematous, scaly plaques that may itch and burn; although in 25% of patients, it may be an incidental finding.157 Bowen disease mainly affects middle-aged to elderly individuals and has a known association with other primary internal and extracutaneous malignancies. In the skin, Bowen disease is associated with sun exposure, whereas in the anal region, high-risk HPV subtypes have been detected.
Bowen disease appears as areas of firm, irregular-bordered, reddish-brown plaques with a scaly surface, and some patients may have ulceration. Histologic examination of these plaques reveals a striking full-thickness disorganization of the squamous epithelium, characterized by abundant, large, atypical cells and loss of orderly surface maturation on a background acanthosis, hyperkeratosis or parakeratosis, and particularly dyskeratosis (Fig. 32.16). Mitotic figures occur frequently and can be seen at all levels of the epithelium along with multinucleated cells and koilocytes. Accessory skin structures are often incorporated into the lesion as it spreads laterally throughout the epidermis. The superficial dermis underlying the neoplastic epithelium often contains a chronic inflammatory infiltrate.
Bowen disease and bowenoid papulosis are two forms of PSIN-H and cannot be reliably separated based on histology alone. Bowenoid papulosis consists of small papules, whereas Bowen disease is composed of large and often multifocal plaques. Bowenoid papulosis also shows clearly demarcated borders from the surrounding normal skin and usually does not involve the skin appendages. Bowen disease often requires formal histologic mapping to discern the extent of disease.156,158 Bowen disease is also included in the differential diagnosis of Paget disease and melanoma. Differentiation of Bowen disease from these entities is discussed later.
Bowen disease of the anus is a chronic and slowly progressive condition that tends to spread intradermally. It is prone to local recurrence after wide excision if disease-free (negative) margins are not achieved.156,157 Even when negative margins are attained, up to 25% of patients have recurrent disease within 3 to 4 years. In a few patients with Bowen disease who are not treated adequately by wide local excision, transformation to invasive disease may occur.157
In the past, the standard form of treatment of Bowen disease was histologic mapping followed by wide local excision to achieve negative margins.156,158 However, despite preoperative mapping, as many as 63% of patients had recurrences within 1 year after wide local excision.159 Topical 5-fluorouracil or imiquimod has been effective for patients with extensive disease.160,161
Bowenoid papulosis was initially described as a lesion of the genitalia of young adults, most often diagnosed in the third decade of life. In addition to its common occurrence on the penis and vulva, anal lesions may occur.30 Clinically, bowenoid papulosis is characterized by one or more small, reddish-brown papules in the anogenital region that persist for a few weeks to several years.162 Most patients are asymptomatic, although some complain of pruritus associated with areas of involved perianal skin.
Bowenoid papulosis appears as slightly raised, firm, red to brown papules that may be scaly and are usually located on the perianal skin. Classically, these lesions are sharply circumscribed and more easily treated by excision. The papules rarely exceed a few millimeters in diameter. Despite their rather benign gross appearance, the histology of bowenoid papulosis is similar to conventional PSIN-H or Bowen disease.162 The squamous epithelium is typically acanthotic, and the surface of the epithelium is hyperkeratotic. At higher magnification, the epithelium shows cytologic atypia and disordered maturation, with scattered dyskeratotic and mitotic cells seen throughout the thickness of the epithelium (see Fig. 32.16). Parakeratosis and hypergranulosis may also be identified.
Based purely on histology, small or partial biopsies of bowenoid papulosis cannot be reliably separated from Bowen disease. When a pathologist encounters PSIN-H histology in the setting of a young patient with small anogenital papules, a note documenting the possibility of bowenoid papulosis may be indicated. If the entire lesion is excised and its small size is confirmed, the pathologist can more confidently consider the lesion as a bowenoid papulosis variant of PSIN-H. The distinction is important because clinical follow-up of bowenoid papulosis suggests it is usually a benign condition. Most cases resolve spontaneously without treatment, although in some patients, it is chronic and slowly progressive. Squamous lesions that may cause diagnostic confusion with or progress to invasive SCC are summarized in Table 32.5.
Table 32.5
Clinical and Pathologic Features of Noninvasive Human Papilloma Virus–Associated Anal Squamous Lesions
| Lesion | Clinical Presentation | Gross Features | Microscopic Features | Molecular Features | Treatment | Outcome |
| PSIN-H (bowenoid papulosis) | Young, sexually active patients | Small, smooth papules < 5 mm | Disordered organization Basaloid proliferation of atypical cells with mitotic figures beyond the lower one half of the epithelium |
HPV subtypes 16 and 18 most common | Generally conservative; spontaneous resolution reported in some patients | Generally excellent |
| PSIN-H (Bowen disease) | M : F = 2 to 1 More common in whites and increasing in MSM |
Scaly plaques, often multiple May be associated with condyloma |
Disordered organization Basaloid proliferation of atypical cells with mitotic figures beyond the lower one half of the epithelium |
HPV subtypes 16 and 18 most common | Excision of discrete lesions with mapping biopsies; topical therapies (5-FU, imiquimod) | Up to 5% progress to invasive carcinoma, especially in immunosuppressed, patients infected with HPV subtypes 16 or 18, and those with extensive disease |
| Condyloma acuminatum | Sexually active patients Increasing in MSM and HIV+ patients |
Exophytic papillomatous lesions 1-20 mm in diameter Often multiple |
Papillary proliferation of acanthotic squamous epithelium with orderly maturation Prominent granular cell layer and koilocytes |
HPV subtypes 6 and 11 most common Significant minority with HPV subtypes 16 and 18 |
Optional: topical podophyllin or imiquimod (patient applied); cryotherapy, podophyllin resin, TCA, curettage intralesional interferon (provider applied) Surgical excision for large, persistent, or primarily intraanal lesions |
Generally benign course Immunosuppressed patients have high risk of recurrence, including high-grade intraepithelial neoplasia or carcinoma |
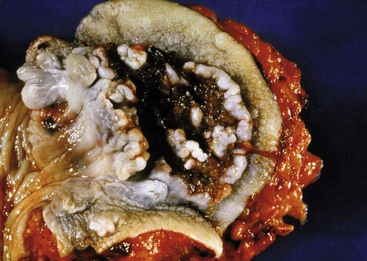
| Verrucous carcinoma | M > F Mean age: fifth decade No association with sexual orientation Rare in children |
Large, bulky cauliflower-like mass Prone to ulceration and bleeding |
Very well-differentiated squamoproliferative lesion May have koilocytes Broad, pushing invasive front |
HPV subtypes 6 and 11 most common | Wide local excision Radiation and chemotherapy if complete resection not possible |
Generally good Morbidity and mortality related to extensive disease with locally destructive growth |
| ASIN | Young, sexually active MSM and women with anogenital HPV-associated lesions Often incidental |
Usually flat High-resolution anoscopy with application of 3% acetic acid and Lugol iodine solution helps identify lesions |
Low-grade lesions are usually recognized by koilocytes (HPV effect) High-grade lesions indistinguishable from PSIN-H (see above) |
HPV subtypes 16 and 18 most common | Watchful waiting (especially for low grade) Topical therapy (5-FU, TCA, imiquimod) Anoscopy-directed ablation Surgical excision |
Rate of progression to invasive carcinoma varies (up to 10% at 5 years for ASIN-H); high-risk patients include immunosuppressed (HIV+), infected with HPV subtypes 16 or 18, and those with extensive disease |
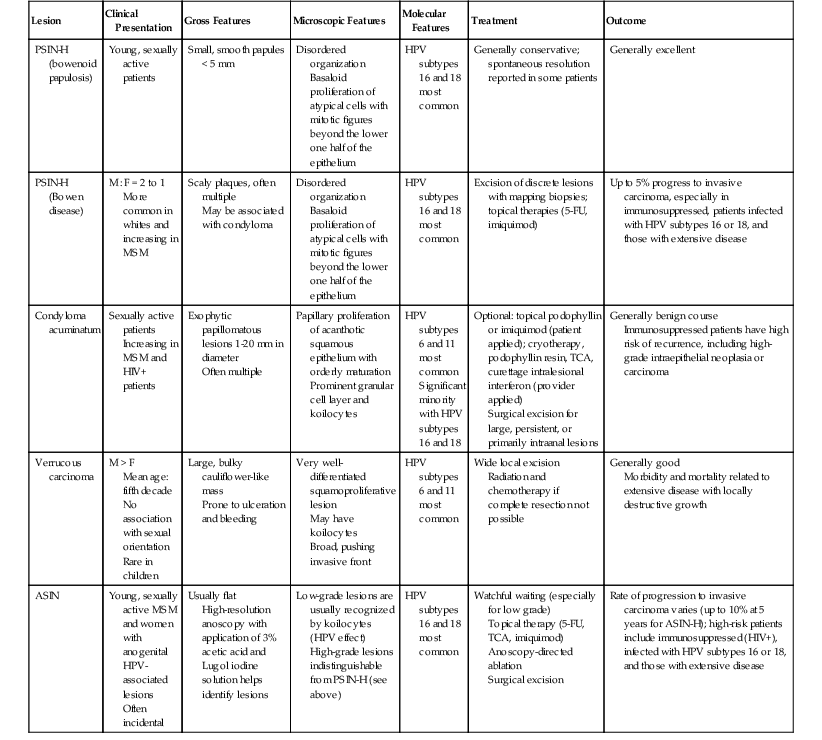
ASIN, Anal squamous intraepithelial neoplasia; 5–FU, 5-fluorouracil; HPV, human papillomavirus; M : F, male to female ratio; MSM, men who have sex with men; PSIN-H, high-grade perianal squamous intraepithelial neoplasia; TCA, trichloroacetic acid, +, positive.
Anal Canal Squamous Cell Carcinoma
Clinical Features
Carcinomas of the anal canal are rare but increasing in frequency. For 2013, 7060 new cases of anal cancer were expected to be reported in the United States, corresponding to approximately 5% of all cancers involving the colon, rectum, and anus.163 The incidence of anal cancer is slightly greater among women than men (1.5 to 1), and the rate is also slightly higher for African Americans.164 Anal squamous neoplasia is increasing in incidence, particularly among MSM with or without HIV infection and among women with multifocal anogenital neoplasia. As many as 80% to 90% of anal SCCs are associated with HPV infection.140,165
Carcinomas that develop above (proximal to) the dentate line are two to three times more common in women than men and usually are diagnosed in the sixth decade of life. The clinical characteristics of neoplasms in this location include bleeding, pain, change in bowel habits, and pruritus ani. Carcinomas in this location often arise in the absence of any known preexisting condition. In contrast, carcinomas that develop below (distal to) the dentate line are four times more common in men than women. Coexisting conditions are more common for carcinomas in this region and include anal condyloma, Bowen disease, chronic fistula (as in patients with Crohn’s disease), chronic pruritus, and a history of radiation treatment. The clinical manifestation of carcinomas at this location ranges from small, firm nodules for early-stage lesions to large, ulcerated tumors in advanced-stage lesions (Fig. 32.17). The clinicopathologic features of anal cancers in relation to their anatomic locations are summarized in Table 32.6.
Table 32.6
Clinical and Histologic Features of Squamous Cell Carcinoma and its Variants
| Variant | Demographics | Clinical Features | Location | Histology | Ancillary Tests | Treatment | Survival |
| Conventional SCC | Equal sex distribution or M > F Younger |
Size varies May manifest as large, ulcerated mass with bleeding Pain |
Tend to occur below dentate line | Often keratinizing May be mixed with nonkeratinizing, mucus-producing areas |
Often preceded by HPV-associated lesions High-risk HPV subtypes (16, 18) |
Chemoradiation for anal SCC; abdominoperineal resection for salvage Wide local excision for small perianal SCC (T1) |
70–90% cured with chemoradiation therapy alone; salvage surgery cures up to an additional 70% |
| Nonkeratinizing and basaloid SCC | F > M Older |
Bleeding Pain Pruritus Change in bowel habits |
Tend to occur above dentate line | No or little keratin Basaloid: prominent peripheral palisading May have mucus-producing areas |
Less often associated with HPV Express p63 and cytokeratin 5/6 by IHC |
Chemoradiation for anal SCC; abdominoperineal resection for salvage | 70–90% cured with chemoradiation therapy alone; salvage surgery cures up to an additional 70% |
| Verrucous SCC (including giant condyloma) | M > F Middle age |
Bulky, space-occupying lesion May be strikingly exophytic (giant condyloma) |
Tend to occur below dentate line and in anogenital skin | Deceptively well differentiated Surface maturation and hyperparakeratosis Broad, pushing invasive front |
Associated with low-risk HPV subtypes (6, 11) Sometimes superimposition of high-risk HPV in invasive lesions |
Wide local excision; chemoradiation therapy if excision not possible | Rare tumor-related deaths |
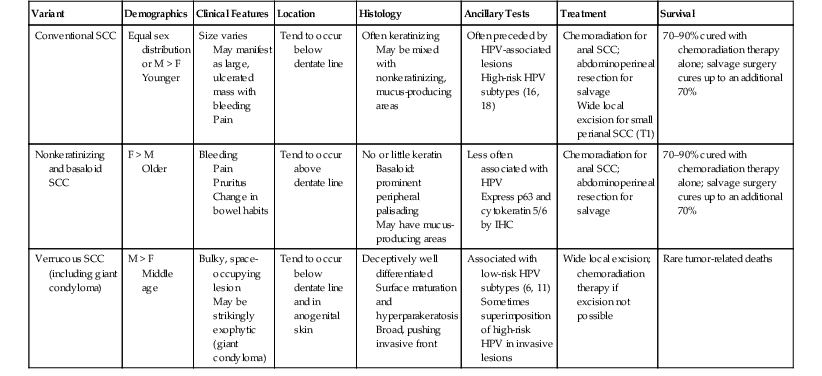
F, Female; HPV, human papillomavirus; IHC, immunohistochemistry; M, male; SCC, squamous cell carcinoma.
Pathology
The WHO recognizes several types of malignancies of the anal canal166 (Table 32.7). Each carcinoma is associated with several histologic patterns, which is probably a reflection of the histologic complexity of the anal canal. The locations of the most common tumors of the anal canal are shown in Figure 32.18.
Table 32.7
World Health Organization Classification of Epithelial Tumors of the Anal Canal
| Category | Types |
| Premalignant lesions | Anal squamous intraepithelial neoplasia, low grade (ASIN-L) |
| Anal squamous intraepithelial neoplasia, high grade (ASIN-H) | |
| Perianal squamous neoplasia (Bowen disease, PSIN-H) | |
| Carcinoma | Paget disease |
| Squamous cell carcinoma | |
| Verrucous carcinoma | |
| Undifferentiated carcinoma | |
| Adenocarcinoma | |
| Mucinous adenocarcinoma | |
| Neuroendocrine tumor (NET) | NET G1 (carcinoid) |
| NET G2 | |
| Neuroendocrine carcinoma (NEC) | Large cell NEC |
| Small cell NEC | |
| Mixed adenoneuroendocrine carcinoma |
Adapted from Bosman FT, World Health Organization, International Agency for Research on Cancer. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press; 2010.
Most malignant neoplasms of the anal canal are SCCs, which are often histologically heterogeneous.167 The second edition of the WHO classification168 subclassified anal canal SCC as large cell keratinizing or nonkeratinizing and basaloid types; however, the prior and current WHO editions eliminated this classification because anal canal invasive SCCs are most often treated with combination chemotherapy and irradiation after diagnostic biopsy rather than primary resection. Because anal canal SCCs are often heterogenous, biopsies cannot sufficiently subclassify them. The WHO 1989 classification system did not yield consistent prognostic groups based on histologic type. The WHO 2010 system166 encourages adding additional descriptors, such as degree of differentiation, predominant cell size, basaloid features, degree of keratinization, or adjacent ASIN, to the generic diagnosis of invasive SCC. Terms such as basaloid, transitional cell, cloacogenic, or mucoepidermoid carcinoma are discouraged.
SCCs located above the dentate line are thought to arise from the anal transitional epithelium. Most tumors are nonkeratinizing SCCs that are similar in appearance to nonkeratinizing squamous cell cancers in other anatomic locations. Basaloid features may predominate (Fig. 32.19). Microscopically, basaloid zones of anal canal SCC frequently show an irregular, angulated, nested, or trabecular pattern composed of relatively small cells without intercellular bridges. Central necrosis is a common histologic feature, and mitotic figures are frequently observed. Some basaloid zones also contain small, cystic foci lined by mucin-producing cells, formerly called mucoepidermoid carcinoma or squamous cell carcinoma with mucinous microcysts. Some pathologists require prominent peripheral palisading within the tumor cell nests, similar to cutaneous basal cell carcinomas, to consider a tumor to have basaloid features. Not uncommonly, a single tumor shows a mixture of histologic patterns, including foci of squamous differentiation.
Tumors in this region may extend proximally or distally, which often obscures their precise site of origin. Examination of the adjacent mucosa may identify ASIN. SCCs with or without basaloid features may occasionally arise from anal duct epithelium, which reflects the common embryologic origin with anal transitional epithelium. In some instances, ASIN may involve the surface mucosa and anal duct epithelium.169 Carcinomas that arise below the dentate line are typically SCCs and tend to be better differentiated and show more keratinizing features than those that arise above the dentate line.170 It is often difficult to grossly or endoscopically ascertain the precise site of origin of any neoplasm, because carcinomas often destroy the normal anatomic landmarks (see Anatomy). Preoperative irradiation and chemotherapy of anal canal carcinomas may also affect the ability to determine the correct site of origin of the neoplasm.126
Special Subtypes
The WHO 2010 classification mentions the importance of the rare small cell anaplastic carcinoma because of its poor prognosis.166 For instance, Shepherd and colleagues171 found that both patients with this histologic subtype, which is characterized by basaloid features, diffuse infiltration, nuclear molding, abundant single cell necrosis, and frequent mitoses, died of their disease within 14 months after surgical resection. Although the WHO and CAP suggest that these tumors should be differentiated from small cell neuroendocrine carcinoma, no guidelines have been offered. Practically, small cell anaplastic carcinoma and small cell neuroendocrine carcinoma of the anal canal have a dismal prognosis and may represent the same neoplasm.
SCCs may show microcyst formation, including Alcian blue/PAS-positive mucin production in some cases. These tumors are rare; Shepherd and colleagues171 found that 10 of 235 cases showed microcyst production in addition to conventional SCC features. Of these 10 cases, 4 had histochemically confirmed mucin production. The prognosis for these tumors appears to be poor, because 6 of the 10 patients died of their disease by the time of the study’s publication.
Differential Diagnosis
SCCs of the anal canal should be differentiated from other primary and metastatic malignancies, including low rectal adenocarcinoma, anal gland carcinoma, melanoma, and small cell neuroendocrine carcinoma. The diagnosis is greatly aided by judicious use of immunohistochemical stains, particularly for poorly differentiated malignant neoplasms.
Prognosis
Staging of anal carcinomas should be performed in accordance with the AJCC criteria (Tables 32.8 and 32.9).4 The most important prognostic indicators of anal carcinoma are depth of invasion and extent of tumor spread. Carcinomas located above the dentate line commonly spread to the lower rectum and involve perirectal and inguinal lymph nodes. Aside from the rare small cell anaplastic carcinoma and carcinoma with microcyst formation, the specific histologic type of squamous carcinoma from this region is not a significant predictor of survival, although stage for stage, nonsquamous carcinomas tend to have a 10% to 20% worse 5-year survival rate compared with anal canal SCC. The degree of differentiation correlates with the presence of lymph node metastases.171 Worse survival has been observed among patients with node-positive compared with node-negative tumors.172,173
Table 32.8
American Joint Committee on Cancer Tumor-Node-Metastatis Classification for Staging Cancer of the Anal Canal
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ (HSIL; AIN 2-3) |
| T1 | Tumor 2 cm or less in greatest dimension |
| T2 | Tumor more than 2 cm but not more than 5 cm in greatest dimension |
| T3 | Tumor more than 5 cm in greatest dimension |
| T4 | Tumor of any size invading adjacent organs (e.g., vagina, urethra, bladder); does not include direct invasion of rectal wall, perirectal skin or subcutaneous tissue, or sphincter |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in perirectal lymph node(s) |
| N2 | Metastasis in unilateral internal iliac and/or inguinal lymph node(s) |
| N3 | Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
AIN, Anal intraepithelial neoplasia, grades 2 to 3; HSIL, high-grade squamous intraepithelial lesion.
From Edge SB, for the American Joint Committee on Cancer. AJCC Cancer Staging Manual. New York: Springer; 2010.
Table 32.9
Stage Groupings Based on the American Joint Committee on Cancer Tumor-Node-Metastasis Classification System for Cancer of the Anal Canal
| Stage | Primary Tumor (T) | Regional Lymph Nodes (N) | Distant Metastasis (M) |
| 0 | Tis (ASIN-H) | N0 | M0 |
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| T3 | N0 | M0 | |
| IIIA | T1 | N1 | M0 |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | |
| T4 | N0 | M0 | |
| IIIB | T4 | N1 | M0 |
| Any T | N2 | M0 | |
| Any T | N3 | M0 | |
| IV | Any T | Any N | M1 |
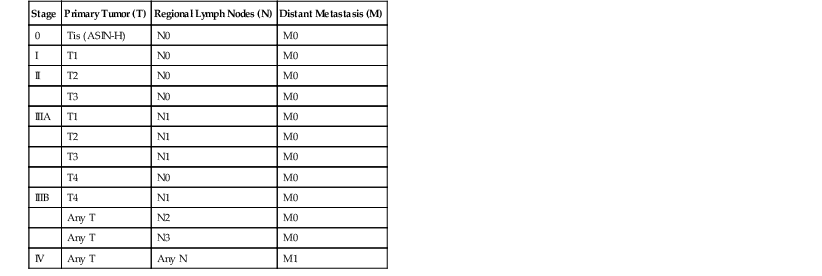
ASIN, Anal squamous intraepithelial neoplasia.
From Edge SB, for the American Joint Committee on Cancer. AJCC Cancer Staging Manual. New York: Springer; 2010.
Carcinomas arising in the perianal skin beyond the junction of anal mucosa and hair-bearing skin are staged by using skin cancer criteria.4,174,175 Carcinomas of the perianal region often grow more slowly than proximally located tumors, and because they are more frequently diagnosed at an early stage, the prognosis tends to be considerably better. Small perianal carcinomas are usually treated with surgical excision and wide margins (≥1 cm) rather than chemoradiotherapy, but larger tumors may require chemoradiation therapy. The outcome of perianal skin SCC also depends on the tumor size and locoregional spread. Metastases to inguinal lymph nodes may occur, but visceral metastases are uncommon.
As reviewed by Eng,176 features that have an impact on local control of anal canal carcinoma include advanced tumor stage, older age at diagnosis, lower radiation dose, and reduced overall treatment time. In HIV-positive patients, CD4+ T-cell counts of less than 200/mm3 can be a negative prognostic indicator of local control, although possible differences in the molecular biology of HIV-positive anal cancers may affect outcome.118,177,178 Highly active antiretroviral therapy (HAART) has revolutionized HIV therapy, but its benefit in anal HPV infection and intraepithelial neoplasia is still debated. Although some larger studies179–181 have found a beneficial effect for HAART on the prevalence of HPV infection or ASIN, or both, others have not.182,183 Studies suggest that HAART does not impact the high incidence of anal carcinoma among HIV-positive patients.184,185 Five-year survival rates are 70% for stage I disease (71.4% for SCC only), 62% for stage II (63.5% for SCC only), 46% for stage IIIA (48% for SCC only), 40% for stage IIIB (43% for SCC only), and 15% for stage IV (21% for SCC only).4
Treatment
Before the 1970s, patients with anal cancer were treated primarily by radical surgery, resulting in an abdominoperineal resection and a permanent colostomy and producing a 5-year survival rate of 40% to 70%. Current protocols for patients with anal cancer include combined chemoradiation therapy. The National Comprehensive Cancer Network (NCCN) guidelines for anal canal cancer recommend combination mitomycin C and 5-flourouracil chemotherapy and multifield radiotherapy as primary therapy for invasive anal canal SCC.174 With this approach, 70% to 90% of patients have a complete clinical response, with no tumor found on subsequent biopsies after 6 weeks.186 One trial found that 29% of patients who did not demonstrate an early complete response (by 11 weeks) had a complete response by week 26, arguing for conservative management within 6 months of completing chemoradiation.187 This remarkable improvement in local control with primary chemoradiotherapy allows preservation of the anal sphincter and an improved quality of life.
The risk of local failure after treatment is greatest within the first 3 years after treatment.172,188–190 Residual disease is defined as persistent disease within 6 months of completion of chemoradiation therapy, and recurrent disease is defined as carcinoma diagnosed 6 or more months after a complete clinical response. Because additional medical therapy is unlikely to provide a cure in this setting, the preferred method of treatment for locally residual or recurrent disease is an abdominoperineal resection.188–190 As many as 70% of patients are cured after salvage surgery. Positive surgical margins are the best predictor of a bad outcome.190
Verrucous Carcinoma and Giant Condyloma Acuminatum of Buschke-Löwenstein
Once thought of as separate entities, verrucous carcinoma and giant condyloma acuminatum (Buschke-Löwenstein tumor) are now considered synonymous. These lesions are characterized by bulky exophytic growth and tumor size that often exceeds 10 cm in the greatest dimension (Fig. 32.20). Despite their inability to metastasize, giant condylomata are locally invasive and therefore considered to be malignant tumors.191,192 They are more common in men, and the incidence is even greater for men younger than 50 years. Rarely, they may occur in children.193 The clinical presentation includes a perianal mass and pain, abscess, or fistula. Because of their large size, these tumors have a propensity to ulcerate, and bleeding is a common complaint.
Their histologic appearance is paradoxically benign. These tumors appear as very-well-differentiated squamoproliferative lesions on microscopic examination (Fig. 32.21; see Fig. 32.20). They contain minimal cytologic atypia but show a broad invasive front. They are typically locally invasive into adjacent chronically inflamed stromal tissue, and examination reveals a pushing rather than an infiltrating tumor margin. It is this downward, destructive growth pattern that best separates verrucous carcinoma from a typical condyloma. The epithelium often shows evidence of surface maturation and extensive crater-like keratinization.
The various treatment strategies include topical chemotherapy, wide local excision, abdominoperineal resection, and adjuvant and neoadjuvant systemic chemotherapy and radiation therapy.194,195 Recurrences are common after surgical resection. As many as 20% of patients die of their disease.191 Because of their slow growth, local excision is the preferred method of management, although for large and bulky tumors that are locally destructive, chemoradiation therapy, cryotherapy, and more extensive surgical management may be required.196
Anal Adenocarcinoma
Clinical Features and Pathogenesis
Primary adenocarcinomas of the anal canal are uncommon.197 Most adenocarcinomas in this region result from secondary involvement by a distal rectal tumor. Primary adenocarcinomas are commonly diagnosed in the seventh decade, and men may be more frequently affected than women.8,164 The clinical presentation typically is a painful mass of the buttock accompanied by a mucin-like discharge. Anorectal bleeding or obstruction is not common.
Primary adenocarcinomas of the anal canal (not the rectum) are categorized as those that arise in preexisting anorectal sinuses or fistulas and those that presumably arise from anal glands (without fistulas). They are often slow-growing tumors. Patients with Crohn’s disease are at higher risk for fistula-associated tumors.198 The causal relationship between perianal fistulas and abscesses and the subsequent development of carcinoma is a subject of debate. Some authorities have proposed that the inflammatory process precedes the neoplasm, whereas others think that these rather slow-growing neoplasms undergo secondary fistulization with time. A possible association of anal adenocarcinoma with HPV infection has been described, similar to that observed for endocervical adenocarcinomas.169
Pathology
Primary adenocarcinoma of the anal canal characteristically arises in the deep perianal tissues, without evidence of surface mucosal involvement. On cut section, the tumor appears as an infiltrative mass with an epicenter located deep within the soft tissues and with involvement of the adjacent structures of the anal canal. Most primary anal gland adenocarcinomas are well differentiated, comprising haphazardly dispersed, small glands with scant mucin production (Fig. 32.22). These carcinomas typically show an anal gland immunophenotype; they are CK7 positive and CK20 negative.8
Carcinomas arising within fistulas are usually associated with abundant mucin production (see Fig. 32.22), and they are sometimes referred to as perianal mucinous (colloid) adenocarcinoma.8,199 Perianal mucinous adenocarcinomas are more commonly associated with fistulas and may more often be associated with a rectal phenotype (i.e., CK20 and CDX2 positive).200 Multiple, deep biopsies are often required to establish a diagnosis of colloid carcinoma, because the abundance of mucin may make identification of tumor cells difficult.201,202
Differential Diagnosis
The main differential diagnosis of a primary anal adenocarcinoma is a distal rectal cancer that extends into the anal canal (Table 32.10). Demonstration of a precursor adenomatous component in rectal mucosa helps to demonstrate its origin in rectal columnar epithelium. Immunohistochemical labeling for CK7 and CK20 may also be useful, because primary anal gland adenocarcinomas are universally CK7 positive, whereas most rectal tumors are CK7 negative and CK20 positive. Because 10% to 20% of rectal carcinomas are CK7 positive,5,203,204 CK7 positivity does not exclude the possibility of a rectal primary. CDX2, a marker for colorectal adenocarcinoma, is extremely useful for differentiating an intestinal phenotype from an anal gland phenotype,205 but fistula-associated adenocarcinoma more commonly demonstrates a rectal phenotype than an anal gland phenotype.200 Use of MUC2 or MUC5AC immunohistochemical stains may increase diagnostic specificity.200,206,207
Table 32.10
Distinguishing Features of Primary Rectal and Anal Adenocarcinomas
| Features | Rectal Adenocarcinoma | Anal Gland Carcinoma | Fistula-associated Adenocarcinoma |
| Clinical presentation | Change in bowel habits, rectal bleeding | Painful buttock mass | Mucoid discharge |
| Typical histology | Intestinal-type adenocarcinoma | Simple glands with minimal mucin production | Mucinous (colloid) adenocarcinoma |
| Coexisting conditions | Uncommon | Uncommon | Fistula, Crohn’s disease |
| Immunohistochemistry | CK7−/+, CK20+, CDX2+ | CK7+, CK20−, CDX2− | CK7−/+, CK20+, CDX2+ |

CDX2, Caudal type homeobox 2; CK, cytokeratin, −, negative; +, positive.
Prognosis and Treatment
Primary adenocarcinoma of the anal canal is considered a more aggressive tumor than SCC in this location. Patients are at higher risk for local and distant recurrences.208 Tumor stage, lymph node status, and differentiation status are independently associated with outcome.
Treatment of primary adenocarcinomas of the anal canal is similar to that of rectal cancer. It usually includes surgical resection and aggressive chemoradiation. The overall 5-year survival rate is approximately 30%.208,209
Paget Disease of the Anus
Clinical Features
Primary (extramammary) Paget disease of the anal canal is rare. Among patients with primary anal Paget disease, men and women are equally affected, and most are diagnosed in the fifth to seventh decades of life.210
The clinical appearance of extramammary Paget disease is well described.211,212 Typical lesions consist of erythematous patches or plaques that may be scaly, eroded, or ulcerated, and they may be located anywhere between the dentate line and the perianal skin.210 Pruritus is a common complaint. Lesion size varies greatly, and regions of involvement by neoplastic cells may extend well beyond the impression of involvement.
Secondary anal or perianal Paget disease may occur in association with a distal adenocarcinoma of the rectum, which may be diagnosed before the anal Paget disease, at the same time, or later.211–214 Approximately one half of cases of Paget disease of the anus are associated with a visceral malignancy, such as rectal adenocarcinoma. Perianal Paget disease may also develop as an extension of perineal Paget disease, particularly in postmenopausal white women.215 In this instance, the finding of Paget cells likely represents migration of malignant cells into the anal epidermis from an associated rectal adenocarcinoma or extramammary Paget disease of the perineum.216 These varied patterns of occurrence of anal Paget disease suggest that it may have many causes.213
Pathogenesis
In cases of primary extramammary anal Paget disease, there is general agreement that the neoplastic Paget cell is a secretory (glandular) epithelial cell. Although some authorities favor an eccrine sweat gland origin, most support an apocrine gland origin of Paget cells.217,218 The immunohistochemical demonstration of gross cystic disease fluid protein (GCDFP) expression, which is a marker of apocrine epithelium, in many cases of extramammary Paget disease supports this viewpoint. Paget cells may also be positive for epithelial membrane antigen, carcinoembryonic antigen, androgen receptors, and low-molecular-weight keratins.
Pathology
Histologically, Paget disease is characterized by large, cytologically malignant cells with pale, granular, or vacuolated cytoplasm scattered throughout the epidermis (Fig. 32.23). The cells contain intracytoplasmic mucin, which is usually PAS and Alcian blue positive. The cutaneous appendages may also be involved. Paget cells tend to be more numerous in the basal half of the epidermis. In some cases, intraepithelial glands with intraluminal necrosis may be found.203 The involved epidermis may also show a variety of reactive changes, such as squamous hyperplasia, papillomatous hyperplasia, hyperkeratosis, parakeratosis, or acanthosis.219
Differential Diagnosis
The differential diagnosis of anal Paget disease is pagetoid spread by a primary rectal adenocarcinoma, malignant melanoma, or squamous intraepithelial neoplasia (Table 32.11). Fortunately, a limited panel of immunohistochemical studies can help to establish a correct diagnosis in most cases (Table 32.12). A CK20-positive, CDX2-positive, and GCDFP-negative immunophenotype is typical of Paget disease associated with a coexistent rectal cancer, whereas a CK7-positive, CK20-negative, and GCDFP-positive immunophenotype is typical of primary anogenital Paget disease.203 Paget disease caused by rectal adenocarcinoma usually has a prominent signet ring cell morphology and more abundant cytoplasmic mucin. The cells derived from a rectal adenocarcinoma tend to invade the epidermis more discretely and with less predilection for the basal portions of the epithelium.
Table 32.11
Pathologic Features of Intraepithelial Lesions of the Anus with Possible Pagetoid Cell Spread
| Lesion | Histology | Ancillary Studies |
| Primary Paget disease |
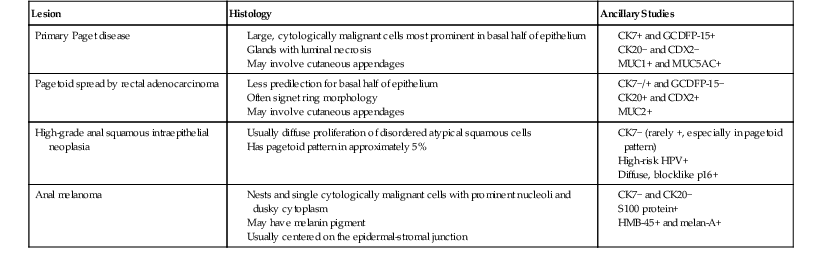
CDX2, Caudal type homeobox 2; CK, cytokeratin; GCDFP-15, 15-kDa gross cystic disease fluid protein; HMB-45, human melanoma black 45; HPV, human papillomavirus; MUC, mucin; −, negative; +, positive.
Table 32.12
Immunohistochemistry of Intraepithelial Lesions of the Anus with Possible Pagetoid Cell Spread
| Lesion | CK7 | CK20 | GCDFP-15 | CDX2 | S100 Protein | HMB-45 and Melan-A | CK5/6 and P63 |
| Primary Paget disease | + | − | + | − | − | − | − |
| Secondary Paget disease or rectal carcinoma | ± | + | − | + | − | − | − |
| Anal gland carcinoma | + | − | − | − | − | − | − |
| Melanoma | − | − | − | − | + | + | − |
| SCC in situ | ± | − | − | − | − | − | + |
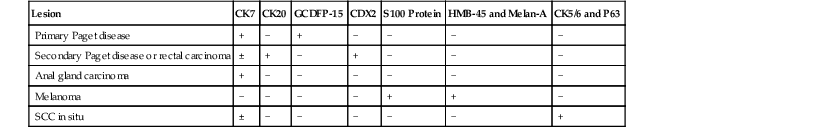
CDX2, Caudal type homeobox 2; CK, cytokeratin; GCDFP-15, 15-kDa gross cystic disease fluid protein; HMB-45, human melanoma black 45; MUC, mucin; P63, P63 protein; SCC, squamous cell carcinoma; −, negative; +, positive; ±, positive or negative.
Stains for markers of melanoma can also be of value. S100 protein, HMB-45, melan-A, and other melanocyte markers are positive in most anorectal melanomas but not in Paget disease or squamous intraepithelial neoplasia.220 The finding of melanin pigment alone does not establish a diagnosis of malignant melanoma because melanin can be detected in Paget cells. In addition, normal melanocytes may be scattered among neoplastic keratinocytes in cases of squamous intraepithelial neoplasia.221
Approximately 5% of high-grade intraepithelial squamous neoplasms demonstrate a nested or pagetoid growth pattern, often called pagetoid SCC. A few cases have been strongly and diffusely positive for CK7, which traditionally has been considered a sensitive and specific marker of primary Paget disease.144,222 Unfortunately, the converse also occurs; florid Paget disease replacement of the squamous epithelium closely mimics high-grade squamous intraepithelial neoplasia, including p16 positivity.219,223 Findings supporting pagetoid SCC in situ include areas of unequivocal full-thickness squamous cell intraepithelial neoplasia and features of the HPV cytopathic effect (i.e., multinucleated giant cells and koilocytes). Pagetoid SCC cells should also coexpress p63 and CK 5/6. Findings that support Paget disease include signet ring cells and intraepithelial mucin.144
Immunostaining for p16 is a useful surrogate marker of HPV infection and is highly associated with anogenital squamous intraepithelial neoplasia but usually not with Paget disease or melanoma.224–226 A small series highlighted the pitfall in relying too heavily on p16 alone, because the study authors documented p16-positive Paget disease cells, which were sometimes diffusely and strongly positive.223
Prognosis and Treatment
Primary anal Paget disease tends to follow an indolent course, although as many as 60% of patients treated by wide local excision develop recurrent disease within 5 years. Recurrences are frequently managed by wide local excision. Despite a tendency for recurrence, the long-term survival of patients with primary anal Paget disease is similar to that of age-matched people without Paget disease.210
Anal Melanoma
Clinical Features and Pathogenesis
Melanomas of the anus account for 0.05% of all colorectal malignancies diagnosed each year.227 Patients usually are diagnosed in the seventh decade of life. Women are affected almost twice as often as men.227,228 There are no known risk factors for anal melanoma.
Anal melanomas are thought to arise from malignant transformation of melanocytes that are normally present in the anal mucosa and transition zone. The clinical presentation is not unlike that seen for a variety of anal lesions, even those as innocuous as hemorrhoids or anal tags, and is characterized by bleeding, pain, or a mass lesion. These often mild and nonspecific features contribute to the typical late-stage diagnosis of anal melanomas.229,230
Molecular diagnostics are playing an increasingly important role in the characterization of all melanomas, including anorectal melanoma. There is a particular focus on treatment-determining somatic mutations. Most clinically significant tests can be performed on formalin-fixed, paraffin-embedded tissue. The two most treatment-specific mutations tested in melanoma are BRAF and KIT, and the results of testing may determine therapy with BRAF inhibitors (e.g., vemurafenib) or tyrosine kinase inhibitors (e.g., imatinib or sunitinib), respectively. Activating codon 600 BRAF (often V600E) mutations are uncommon events in mucosal melanomas, occurring in as many as 11% of lesions.231,232 Other studies have found no BRAF V600E mutations in mucosal melanomas, including some anorectal-specific series 233–236
KIT mutations occur in approximately 26% of mucosal melanomas237 and approximately 15% of anorectal melanomas.236,238 Exons 9, 11, 13, and 17 are the most commonly analyzed in KIT mutation testing, and it seems that mutations in exons 11 and 13 may have the best possibility of treatment response to tyrosine kinase inhibitors.239
Pathology
Anal melanomas usually arise as a polypoid mass adjacent to or at the dentate line.227 Gross pigmentation is uncommon, but microscopic foci of pigmentation may be found in up to 80% of cases. Pigmentation may be obscured by hemorrhage in the lesion. Anal melanomas demonstrate the same immunohistochemical and ultrastructural features as their cutaneous counterparts.220,240 However, similar to melanomas that arise in other mucous membranes, they are typically of the acrolentiginous type. Invasive melanomas in this region are commonly epithelioid, although sarcomatous and desmoplastic melanomas have also been described (Fig. 32.24).220
Differential Diagnosis
Differentiation of anal melanomas from primary anal Paget disease is discussed in the section on Paget disease. Similar to melanomas that occur elsewhere, the histologic features that support a diagnosis of anal melanoma are a nested growth pattern and asymmetric junctional changes in association with the appropriate immunohistochemical labeling pattern (i.e., positive for S100 protein, HMB-45, or melan-A, and other melanocytic markers and negative for cytokeratins).220,240 Junctional changes, although useful when found, may be obscured by ulceration. Because melanomas may also extend proximally into the rectum, they may infiltrate colorectal mucosa in a manner analogous to lymphoma at that site. Negative immunohistochemical labeling for lymphoid markers (i.e., CD20, CD3, and CD45) should be helpful in ruling out this possibility.
Prognosis and Treatment
The prognosis for anal melanoma is poor. Reported 5-year survival rates range from 15% to 35%.227,228,230,241,242 Survival rates are better for younger patients (25 to 44 years old).227 The specific histologic type of anal melanoma or lymph node status does not correlate with survival, but the thickness of the tumor (measured from the top of the overlying intact mucosa or ulcerated tumor) and perineural invasion are related to outcome.240,242,243 Anal melanomas 2 mm thick or smaller have a much better prognosis than those more than 2 mm thick.
Because there is no relationship between the type of surgical management (i.e., abdominoperineal resection versus wide local excision) and local recurrence or outcome, current practice is to perform wide local excision if technically feasible.241,242 Given the lack of benefit of radical surgery in the absence of large or bulky tumors, disease-free survival appears to be a function of distant spread rather than local control.244 Although systemic therapy for melanoma is evolving based on the molecular characteristics of the tumor, the specific effects on anal melanoma are not well established.
Other Rare Neoplasms of the Anal Canal
Rarely, basal cell carcinoma may arise from the perianal skin (Fig. 32.25).245,246 The tumor is usually in the form of an ulcerated nodule with raised, pearly margins, similar to its cutaneous counterpart. Because the prognosis for basal cell carcinomas of this region is distinctly better than that for SCCs of the anal canal, differentiation of this tumor from a well-differentiated basaloid carcinoma of the anal canal is clinically important. Histologically, anal canal carcinomas with basaloid features have more pronounced cytologic atypia and greater numbers of mitoses. Ber-EP4 immunohistochemical stain is typically positive in basal cell carcinoma and negative in SCC.247
Other rare tumors of the anal region include apocrine adenocarcinoma, embryonal rhabdomyosarcoma, and malignant fibrous histocytoma.248–250 Tumors from other sites may involve the anus as a result of metastasis or direct extension. The most common source of metastatic tumor is colorectal carcinoma, although rare cases of renal, lung, or breast carcinoma metastatic to the anus have been reported.26,251,252