9
Infertility
Introduction
Infertility is a condition that affects approximately one in six couples at some stage in their lives. The cause may be related to a problem with the man, woman or both. In view of the intimate nature of the problem, infertility is often associated with personal distress and embarrassment; effective treatment is available to help an increasing proportion of these couples.
Definitions
Infertility is defined by the World Health Organization (WHO) as the inability of the couple to achieve a clinical pregnancy within 12 months of beginning regular unprotected sexual intercourse. A couple can have primary infertility – no previous pregnancies within the relationship – or secondary infertility, where the couple has had at least one pregnancy.
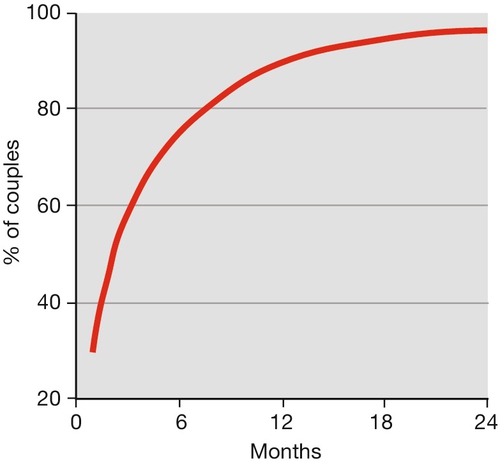
Infertility is rarely absolute, and most couples have a degree of subfertility. Around 84% of the normal fertile population will conceive within 1 year, and 92% by the end of 2 years. Cumulative pregnancy rates and live birth rates are the terms used to express the chance of conception within a given time interval. Figure 9.1 illustrates the cumulative pregnancy rate for the normal fertile population.
Fecundability is the percentage of women exposed to the risk of a pregnancy for one menstrual cycle, who will subsequently produce a live-born infant (normal range – 15–28%). Fecundability usually diminishes slightly with each passing month of not conceiving.
Age and fertility
Normal fertility declines as the woman’s age increases. A woman is born with a finite number of oocytes; around 1 million. This falls to approximately 250 000 at puberty, and by the time the menopause is reached, the number of oocytes has fallen to below 1000. During her reproductive life, a woman will release only 500 mature oocytes – a form of pre-conceptual natural selection – while the remaining oocytes undergo atresia or apoptosis. The rate of oocyte loss is a dynamic process with the rate of follicular recruitment increasing from birth to age 14 and then progressively decreasing until the menopause. At the menopause, which occurs at an average age of 51, there are no functioning oocytes.
The decline in fertility is directly related to the declining oocyte population and the eggs’ inherent quality. There is a small, but noticeable, fall in monthly fecundity rates from the age of 31 years, a more pronounced decrease from the age of 36 years, and a very steep decline over the age of 40 years. In assisted conception procedures, this decline is also observed with a gradual decline in success rates from age 32. In addition, in both natural and assisted conception pregnancies, there is a substantial increase in spontaneous miscarriage rates with advancing maternal age. Although older men are less fertile, the effect of age on men’s fertility is less pronounced than in women.
Causes of infertility
The causes of infertility can be categorized in a simple manner but in reality, more than one problem can be identified in a couple. Causes include: ovulation disorders (25%); male factor (25%); unexplained (25%) and tubal factors (15%). Remaining causes include endometriosis related infertility.
Diagnosis
The diagnosis of infertility is a process of exclusion, identifying couples where the cause is clear, those in whom there is a possible cause and those in whom the cause is unexplained. The aim of investigation should be to reach a diagnosis as soon as possible, using only tests that are of proven value.
History and examination
Factors that provide clues to the aetiology are outlined in Tables 9.1 and 9.2. Other important factors to be noted are the woman’s age and the duration of infertility – generally, the older the woman is and the longer the period of infertility, the poorer the prognosis. The order in which the investigations are performed varies, depending on whether the couple has primary or secondary infertility, with an earlier assessment of tubal patency in the latter. Early assessment is also indicated if a specific abnormality is suspected from the history, and for an older patient.
Table 9.1
Examination of a woman
| Examination | Reason |
| Height and weight for body mass index (BMI) | High or low BMI associated with lower fertility |
| Body hair distribution | Hyperandrogenism |
| Galactorrhoea | Hyperprolactinaemia |
| Uterine structural abnormalities (most usefully determined by transvaginal ultrasound) | May be associated with infertility |
| Immobile and/or tender uterus | Endometriosis or pelvic inflammatory disease, associated with tubal damage |
Table 9.2
Examination of a man
| Examination | Reason |
| Scrotum | Varicocele |
| Size (volume) of the testes | Small testes associated with oligospermia |
| Position of the testes | Undescended testes |
| Prostate | Chronic infection |
Examination of the woman
Height and weight should be recorded and used to calculate the body mass index (BMI), using the formula: weight (kg)/height (m)2. The normal range is between 19 and 25. A change of weight of > 10% in the preceding year may cause a disturbance of the menstrual pattern and anovulation. A BMI at either extreme is detrimental to fertility (see later).
Increased body hair is associated with hyperandrogenism, most commonly because of polycystic ovary syndrome. Breast examination may demonstrate galactorrhoea, which is associated with hyperprolactinaemia. Pelvic examination is important, to look for signs of structural abnormalities, infection and pathological processes, such as endometriosis or pelvic inflammatory disease.
Examination of the man
Examination of the man is not essential in the absence of any relevant history. If, however, the semen analysis is abnormal, examination of the genitalia may be helpful, looking specifically at size (volume); consistency and position of the testes; the outline of the epididymis (for the presence of the vas deferens) and finally, the scrotum, for any evidence of swellings.
Investigations and their interpretation
The investigations should be arranged in a logical manner with reference to the history, along with appropriate general health screening (Box 9.1). Additional tests may be necessary, depending on the clinical circumstances (Box 9.2).
Male factors
Classification
Male factor infertility can be a problem of sperm production, sperm function or sperm delivery. Sperm production may be completely absent (azoospermia) in, for example, testicular failure. More commonly, a patient may present with a reduced count of sperm of normal appearance (oligospermia). Additionally, a high proportion of the sperm may be poorly motile, lacking the normal forward progressive movement (asthenospermia) or may appear morphologically defective (teratospermia) with abnormalities of the head, midpiece or tail.
Normal sperm function – the ability of the sperm to reach, bind and fertilize the oocyte – is more difficult to demonstrate. At present, there are no reliable methods of measuring sperm function, other than monitoring the proportion of sperm moving and assessing the speed of their progress. Antisperm antibodies can affect sperm motility.
Problems with sperm delivery may be caused by absence or blockage of the vas deferens or epididymis. It may also be related to impotence, premature ejaculation or a physical inability to have normal sexual intercourse.
Semen analysis
This provides information about spermatogenesis and an aspect of sperm delivery, but gives little information about sperm function. The WHO has produced a normal range of values for semen, based upon semen analyses performed upon samples obtained from men with a time to pregnancy interval of up to 12 months (Table 9.3). The values, however, are empirical and do not reflect a cut-off point below which pregnancy will not occur. Rather, there is an increase in probability of conception with increasing numbers of sperm and motility up to 40 million/mL and 40%, respectively with a relative plateau thereafter.
Table 9.3
WHO criteria for semen analysis (2010) (2.5–97.5 centiles)
| Volume | 1.2–7.6 mL |
| Concentration | 9–259 × 106/mL |
| Total motility | 34–81% |
| Progressive motility | 28–75% |
| Normal forms | 3–48% |
| Vitality | 53–92% |
Reproduced with permission from WHO.
A man’s sperm count varies considerably, and in the presence of one abnormal result, a second count should be arranged. As spermatogenesis takes approximately 3 months to complete, the samples ought to be produced at least 3 months apart. Samples should be produced by masturbation or after intercourse into a non-lubricated condom after a period of abstinence of between 3 and 5 days. The sample should be analysed in an accredited laboratory and according to WHO guidelines.
Tests of sperm function
Sperm function tests are no longer used in routine clinical practice, with more emphasis now placed on the identification of the number of abnormal/normal sperm as part of the routine analysis. Some sperm function tests, such as the ability of sperm to swim through culture medium, are employed in specialized reproductive medicine units, where more complicated treatment may be contemplated.
The post-coital test involves asking the couple to have sexual intercourse timed to the woman’s mid-cycle. Then, 6–12 h later, a sample of endocervical mucus is taken, looking for the presence or absence of sperm. Some studies have shown a positive correlation between the finding of motile sperm in the mucus and the chance of subsequent pregnancy. The use of this test, however, is controversial, as other studies have shown that the finding of a positive or negative result does not alter the chance or timing of a pregnancy. As a result, most centres have abandoned this procedure.
Antibodies can develop against sperm in response to injury or infection of the testis and epididymis. Men who have had a vasectomy and attempted reversal are the commonest group in whom antisperm antibodies are identified. The antibodies can be serum (IgG) or bound (IgA), and attach principally to the tail, midpiece or head of the sperm. Tests used to detect antisperm antibodies include the mixed agglutination reaction (MAR) test, and the immunobead test. Levels between 17% and 49% are likely to be associated with a fall in fertility, and levels greater than 50% are thought to significantly affect fertility.
Female factors
Ovulation
Ovulation is an ‘all or nothing’ phenomenon, with usually one oocyte released per ovulatory cycle.
Causes of anovulation
Ovarian failure is found in about 50% of women with primary amenorrhoea, and 15% of those presenting with secondary amenorrhoea. Most women with primary amenorrhoea will have an established diagnosis before presenting to an infertility clinic. The cause may be genetic, e.g. Turner syndrome (45,XO) or autoimmune. In those presenting with secondary amenorrhoea and ovarian failure, there may be an obvious cause, such as previous ovarian surgery, abdominal radiotherapy or chemotherapy. There will also be a proportion of women in whom no reason can be identified – idiopathic premature menopause.
Weight-related anovulation
Weight plays an important part in the control of ovulation. A minimum degree of body fat (considered to be around 22% of body weight) is needed to maintain ovulatory cycles. Substantial weight loss leads to the disappearance of the normal 24-h secretory pattern of gonadotrophin-releasing hormone (GnRH), which reverts to the nocturnal pattern seen in pubescent girls. As a result, the ovaries develop a multifollicular appearance on ultrasound. Prolonged exercise can, by increasing the muscle bulk and decreasing the body fat, have the same effect, and it is not uncommon for women athletes or ballerinas to be amenorrhoeic. Excessive weight can also have an adverse effect on ovulation. This probably results from excess oestrone, generated in the adipose tissue by conversion from androgens, interfering with the normal feedback mechanism to the pituitary gland.
Excess weight has a profound effect on female fertility, with a significant reduction in the chance of a successful pregnancy: it reduces the chance of conception, increases the risk of miscarriage, as well as substantially increasing the risk of obstetric complications during the pregnancy and at delivery. The distribution of the fat is important, with central (visceral) fat having a bigger impact than peripheral fat distribution. The waist–hip ratio, which more reliably picks up visceral fat distribution, seems a more reliable guide to the impact of fat on fertility than the body mass index (BMI).
Polycystic ovary syndrome
Of the women presenting with anovulatory infertility, 50% will have polycystic ovary syndrome (PCOS) (see Chapter 8).
Luteinized unruptured follicle syndrome
In certain patients, the oocyte may be retained following the luteinizing hormone (LH) surge, the so-called ‘luteinized unruptured follicle syndrome’ (LUF). Repeated pelvic ultrasound scans fail to show the expected collapse of the follicle at ovulation, and the follicle persists into the luteal phase. As no longitudinal studies have shown this to be a persistent finding in the same woman, there is uncertainty regarding its relevance to fertility.
Hyperprolactinaemia
Hyperprolactinaemia is diagnosed in 10–15% of cases of secondary amenorrhoea. About one-third of these women will have galactorrhoea, and occasionally, there may be some evidence of visual impairment (bitemporal hemianopia) due to pressure on the optic chiasma from a pituitary adenoma.
Tests of ovulation
Only a pregnancy categorically confirms ovulation. However, there are a number of investigations that imply that ovulation has taken place:
![]() history – over 90% of women with regular menstrual cycles will ovulate spontaneously
history – over 90% of women with regular menstrual cycles will ovulate spontaneously
![]() urinary LH kit – this picks up the mid-cycle surge of LH that starts the cascade reaction leading to ovulation. Biochemical measurement of the LH surge requires repeated blood tests and is restricted to specialist reproductive medicine units
urinary LH kit – this picks up the mid-cycle surge of LH that starts the cascade reaction leading to ovulation. Biochemical measurement of the LH surge requires repeated blood tests and is restricted to specialist reproductive medicine units
![]() mid-luteal phase progesterone – this is the most commonly used test of ovulation. A luteal phase progesterone value of > 28 nmol/L is found in conception cycles, and as a result, this value is generally regarded as evidence of satisfactory ovulation. However, it is important to time the blood sample carefully – between 7 and 10 days before the next menstrual period. This can only be determined with some knowledge of the length of the patient’s normal menstrual cycle.
mid-luteal phase progesterone – this is the most commonly used test of ovulation. A luteal phase progesterone value of > 28 nmol/L is found in conception cycles, and as a result, this value is generally regarded as evidence of satisfactory ovulation. However, it is important to time the blood sample carefully – between 7 and 10 days before the next menstrual period. This can only be determined with some knowledge of the length of the patient’s normal menstrual cycle.
Other tests
Less commonly employed tests include serial ultrasound scans to monitor the growth, and subsequent disappearance, of a Graafian follicle. A luteal phase endometrial biopsy looking for appropriately timed secretory changes is no longer considered of value. Basal body temperature was previously considered to be of value as there is a rise of 0.5°C if ovulation has occurred (due to the thermogenic effect of a rise in serum progesterone) but in practice, this is now rarely used.
Testing ovarian reserve
Ovarian reserve is defined as the number of viable oocytes in the ovary. This is particularly important in women contemplating more complex fertility treatment, and may provide a guide to their response to treatment. Determining the level of follicle-stimulating hormone (FSH) at the beginning of the menstrual cycle, is probably the most commonly employed test. A raised FSH taken between days 2 and 5 of the menstrual cycle indicates impaired ovarian reserve, and a likely poor response to ovarian stimulation. Other methods that appear to be more reliable include:
![]() measuring the antral follicle count – the number of small developing follicles seen in the ovary on ultrasound
measuring the antral follicle count – the number of small developing follicles seen in the ovary on ultrasound
![]() measuring the ovarian volume – this is an indication of ovarian activity, as ovaries decrease in size with advancing age and decline in oocyte numbers
measuring the ovarian volume – this is an indication of ovarian activity, as ovaries decrease in size with advancing age and decline in oocyte numbers
![]() measuring the concentration of anti-Müllerian hormone (AMH). AMH is produced in small developing follicles and, unlike FSH, can be measured reliably throughout the menstrual cycle.
measuring the concentration of anti-Müllerian hormone (AMH). AMH is produced in small developing follicles and, unlike FSH, can be measured reliably throughout the menstrual cycle.
Although tests of ovarian reserve may be important in identifying women who may not respond well to fertility treatment or will have a shorter reproductive lifespan, there is limited information on their value in predicting overall natural fertility if the woman has a regular menstrual cycle and is ovulating.
Further investigations
Pelvic ultrasound is useful in defining ovarian morphology, and is more reliable than pelvic examination in identifying other potentially relevant pelvic pathology, such as fibroids, ovarian cysts and endometrial polyps.
Chlamydia serology has been used as a screening test for tubal pathology. Those with a positive antibody titre are more likely to have tubal pathology, because of the association between chlamydial infection and salpingitis.
Serum testosterone measurement is indicated if there is evidence of hirsutism, to exclude more sinister disorders, such as androgen-secreting tumours of the ovary or adrenal gland. In women with an elevated testosterone, measurement of 17-hydroxyprogesterone is relevant in order to exclude late onset congenital adrenal hyperplasia. Thyroid function tests are commonly performed, as approximately 7% of women in this age group will have a thyroid disorder, which may have an impact on a pregnancy if not appropriately treated.
A progestogen challenge test may be useful in women with a history of amenorrhoea and normal levels of FSH and prolactin. It is used to determine whether the woman is clinically oestrogenized. This acts as a guide to what would be the most appropriate medication to use to induce ovulation. If the bleeding is normal following 5 days of an oral progestogen, the patient is well oestrogenized, whereas if it is absent or scanty, the woman is relatively poorly oestrogenized. The presence of a withdrawal bleed also demonstrates the presence of endometrium and the patency of the genital tract.
Tubal patency
Classification
The fallopian tube can be blocked distally – at the fimbrial end – or, less commonly, at the proximal end – the cornu. In addition, the tubo–ovarian relationship may be disrupted by peritubal adhesions. Fimbrial disease has varying degrees of severity:
![]() agglutination of the fimbria to produce a narrowed opening – known as a phimosis
agglutination of the fimbria to produce a narrowed opening – known as a phimosis
![]() complete agglutination to form a hydrosalpinx (fluid-filled tube).
complete agglutination to form a hydrosalpinx (fluid-filled tube).
In addition, tubal damage can also involve the endosalpinx, with intraluminal adhesions and flattening of the mucosal folds. Microsurgery to relieve tubal blockage, therefore, may not restore tubal function.
The important features for fertility prognosis appear to be:
![]() degree of dilatation of the fallopian tube
degree of dilatation of the fallopian tube
![]() extent of the fibrosis of the wall of the tube
extent of the fibrosis of the wall of the tube
![]() damage to the endosalpinx
damage to the endosalpinx
![]() if one or both tubes are affected.
if one or both tubes are affected.
Tests of tubal patency
In the absence of a positive history suggestive of pelvic pathology, a negative physical examination, and a negative Chlamydia antibody titre, the least invasive method for assessing tubal patency should be employed.
Hysterosalpingography (HSG)
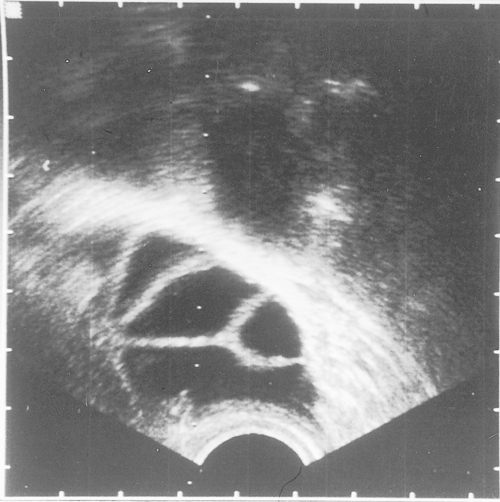
HSG is widely employed in the assessment of tubal patency. It involves inserting a cannula into the cervix and passing radio-opaque fluid into the uterine cavity and fallopian tubes, demonstrating their outline (Fig. 9.2). The test is performed under X-ray screening on an outpatient basis. If the HSG is normal, the diagnosis can be relied upon in 97% of cases. However, if the HSG is abnormal, the diagnosis can only be relied upon in 34% of cases (false positive rate 66%), and a laparoscopy is required to confirm the nature of the abnormality.
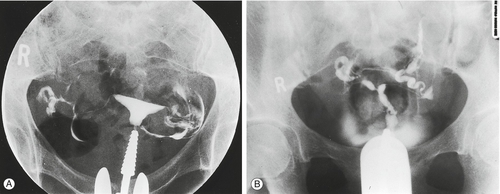
Fig. 9.2Hysterosalpingography (HSG) of (A) normal uterus and (B) abnormal uterus.
In (B) the radio-opaque dye does not flow through the fallopian tubes, thus indicating blockage.
Hysterosalpingo-contrast sonography (HyCoSy)
This technique involves a standard pelvic ultrasound scan at which galactose-containing ultrasound contrast medium is inserted into the uterine cavity, outlining any abnormalities such as submucosal fibroids and endometrial polyps, before passing down the fallopian tubes to confirm tubal patency. The technique offers a similar level of diagnostic accuracy to HSG.
Diagnostic laparoscopy with dye hydrotubation
Diagnostic laparoscopy (‘lap and dye’) remains the ‘gold standard’ investigation. It provides a direct view of the pelvic organs, and also offers the possibility to treat minor pathology discovered during the investigation. Methylthioninium chloride (methylene blue) dye is inserted through a cannula in the cervix to demonstrate tubal patency. Hysteroscopy is often carried out at the same time in order to examine the uterine cavity. The diagnostic laparoscopy requires admission to hospital, usually as a day case, and a general anaesthetic. As such, it is the most invasive and expensive investigation of tubal patency so some specialists undertake a HSG as the first-line assessment.
Selective salpingography
If HSG or laparoscopy detects a proximal blockage, further investigation may be considered. The blockage could simply be due to spasm of the muscle of the uterine cornua, or to genuine pathology. A fine guidewire is inserted into the internal tubal ostium under direct fluoroscopic control or by direct vision using a hysteroscope. This may dislodge a small plug of amorphous debris, restoring patency. This technique appears to increase the likelihood of pregnancy in women with proximal tubal damage.
Salpingoscopy
More detailed investigation of the interior of the fallopian tube is possible but is only really necessary if the question of pursuing tubal surgery is raised. A fine telescope, called a salpingoscope, can be passed down an operating laparoscope and inserted into the ampullary portion of the fallopian tube. Approximately 50% of patients with macroscopically damaged fallopian tubes will have fine intratubal adhesions. If present, these adhesions adversely affect the outcome of subsequent tubal surgery.
Falloposcopy
Advances in fibreoptics have allowed the development of a very fine instrument, a falloposcope, with a diameter of < 1 mm, which can be inserted into the fallopian tube via the uterine cavity. Falloposcopy appears to be effective in detecting tubal pathology, and can be performed on an outpatient basis. The equipment is fragile and expensive, however, and its use is limited to a small number of centres, mainly as a research tool.
It is important to note that all of these techniques are useful in demonstrating patency, but none is capable of directly assessing tubal function.
Treatment
Approximately one-fifth of couples with infertility conceives spontaneously during investigation or while awaiting treatment. However, the probability of this is related to the age of the woman; how long the couple has been trying to conceive for; whether they have been pregnant before; tubal patency and sperm motility. Consequently, these factors must be taken into account when considering conservative treatment with early referral to assisted conception services generally appropriate for most couples after the initial investigations have been completed. The couple should be counselled about general health matters such as smoking, alcohol intake and diet. It is appropriate to recommend folic acid to the woman as routine prevention of neural tube defects. Where the diagnosis is established, specific treatments can be employed.
Anovulation
There are various ways of inducing ovulation, depending on the underlying cause. Successful ovulation induction should be continued for long enough to give the optimum chance for conception – generally 12 months.
Hyperprolactinaemia is treated with a dopamine agonist, either bromocriptine or cabergoline.
Excess or decreased body weight should be managed by dietary adjustments. Although ovulation can generally be induced by exogenous gonadotrophins, this should be avoided, particularly if the woman is underweight or obese, as there is an increased risk of pregnancy complications. Women with moderate obesity often show resistance to treatment with clomiphene citrate and gonadotrophins, requiring much higher doses to induce ovulation.
Anovulation in oestrogenized patients
Most of these women have polycystic ovary syndrome (85% of patients presenting with oligomenorrhoea and 25% of those with amenorrhoea). First-line treatment in this condition is oral anti-oestrogen therapy, usually with clomiphene citrate.
Clomiphene citrate’s mode of action is to increase the plasma FSH concentration, mainly by competitively blocking the negative feedback effects of endogenous oestradiol on the hypothalamus. FSH is the principal hormone responsible for follicular recruitment and development.
Initially, a dose of 50 mg daily for 5 days is given at the beginning of a cycle (days 2–6 inclusive). If there is no response (judged by luteal phase progesterone estimations), the dose is increased to a usual maximum of 100 mg daily for 5 days. Rarely, in obese patients, the dose may be increased to 150 mg daily for 5 days. Clomifene may induce multiple follicles and consequently, there is a 10% chance of a multiple pregnancy.
Ovulation can be achieved successfully in approximately 80% of cycles, with cumulative pregnancy rates of up to 81% after 12 months’ treatment.
Side-effects while using clomiphene citrate include:
![]() vasomotor symptoms (hot flushes) (11%)
vasomotor symptoms (hot flushes) (11%)
![]() pelvic discomfort (7%)
pelvic discomfort (7%)
![]() nausea (2%)
nausea (2%)
![]() breast discomfort (2%).
breast discomfort (2%).
These are seldom severe, and simple explanation and reassurance are usually all that is required. More significant problems include visual disturbances (1.5%) and cholestatic jaundice (rare). In both these situations, the drug should be discontinued and not used again. Some women do not respond to oral ovulation–induction agents, such as clomiphene; these patients will require treatment with exogenous gonadotrophins.
Exogenous gonadotrophins are derived from two sources: extracted from postmenopausal women’s urine or, more recently, created in vitro by genetically engineered mammalian cells. Although the original urinary-derived gonadotrophins contained an equal mixture of FSH and LH (75 IU per ampoule), refinement of the extraction techniques has resulted in highly purified FSH compounds (> 99% of protein content is FSH). The recombinant genetically engineered FSH preparations attain similar levels of purity.
All these medications require parenteral administration, by either subcutaneous or intramuscular injection. Around 4% of women undergoing gonadotrophin treatment will develop ovarian hyperstimulation syndrome and this will be severe in 0.5%. The incidence of multiple pregnancy with gonadotrophin treatment is around 20%, although with careful monitoring and ‘step up’ of the dose, this can be reduced to < 5%. The aim is to achieve development of a single follicle using a low-dose regimen, starting at one ampoule (75 IU) daily for 10 days, and then increasing the dose, if necessary, by small increments until satisfactory follicular growth occurs. Response is monitored by ovarian ultrasound, sometimes combined with serum oestradiol measurement.
When the follicle reaches maturity, ovulation is induced by administering human chorionic gonadotrophin (hCG) (5000 IU). This replaces the physiological LH surge. Gonadotrophin therapy is highly successful in restoring normal fertility to women with hypothalamic amenorrhoea (see below), or to women with PCOS. For women with unexplained infertility clomiphene and exogenous gonadotrophins have not been shown to improve the chance of conception and referral for in vitro fertilization (IVF) is appropriate.
If standard ovulation–induction treatment fails in patients with PCOS, laparoscopic ovarian diathermy can be offered. The ovarian capsule is pierced four times for 5 s with a needle point diathermy. Encouraging results have been reported, with spontaneous ovulation returning in up to 71% of cycles, without the risk of hyperstimulation or multiple pregnancy. In women where spontaneous ovulation does not occur, most become more responsive to clomiphene or gonadotrophin therapy. Unfortunately, the effect is time-limited, with chronic anovulation returning in 50% of women within 2 years. There is also the risk of iatrogenic adhesion formation and reduction in the ovarian reserve leading to premature menopause if diathermy is used excessively.
Metformin, an oral antidiabetic drug, is of value in helping to induce ovulation in obese women with PCOS; metformin has a significant impact when used either alone or in combination with clomiphene.
Anovulation in oestrogen-deficient women
Women who have a low FSH, normal prolactin, and either a low serum oestradiol or a negative progestogen withdrawal test (hypogonadotrophic hypogonadism), require exogenous gonadotrophins in order to ovulate (see above).
Tubal disease
There are two treatment options in the presence of tubal disease, namely surgery and IVF.
Tubal surgery
Surgery used to be the principal treatment for occlusive tubal disease but, as the results of IVF have improved, tubal surgery is performed less frequently.
Selection of women
Women considered for tubal surgery need to be carefully selected, taking into consideration:
![]() the woman’s age
the woman’s age
![]() site and extent of the tubal damage
site and extent of the tubal damage
![]() other factors that might influence fertility.
other factors that might influence fertility.
As with other treatments, age has an important effect on the outcome, and IVF may be a better option for women in their late 30s or 40s. Distal tubal occlusion carries a poorer prognosis than proximal disease, and surgery should be reserved only for cases in which damage is relatively minor. In women with minor damage, the live birth rate after surgery is around 40% over a period of 18 months. Women with a moderate to severe distal abnormality, and those with damage at more than one site on the same tube, have a poor prognosis and IVF is more appropriate. With limited proximal damage, pregnancy rates of nearly 50% can be achieved after tubal surgery.
Techniques
Conventionally, tubal surgery has been performed by laparotomy, through a low transverse incision, with microsurgical techniques used to restore tubal patency. However, tubal surgery is now largely performed laparoscopically. Even completely occluded tubes have been opened successfully laparoscopically, using either a CO2 laser or electrodiathermy. In skilled hands, the results compare very favourably with conventional tubal surgery using an operating microscope.
More recently, selective salpingography (passing a fine catheter through the uterus and along the fallopian tube under X-ray screening) has been used successfully to treat some women with proximal obstruction caused by a plug of amorphous debris.
Risks of tubal surgery
Women who have undergone tubal surgery have a 10-fold increased risk of having an ectopic pregnancy and should be advised to undergo an ultrasound scan at around 6 weeks’ gestation to confirm the site of a subsequent pregnancy.
Endometriosis
Endometriosis is discussed in Chapter 13. There is no evidence that treating minimal peritoneal endometriosis with drugs improves natural fertility. Indeed, medical treatment of endometriosis involves creating anovulation for up to 6 months and this effectively delays the couple trying for a pregnancy. However, there is some evidence to suggest that surgery to ablate the endometriotic lesions and to divide adhesions that may have formed from endometriosis increases the chance of natural conception. Similarly, prior to IVF, treatment of endometriosis with GnRH analogues for 3 months may significantly improve the likelihood of pregnancy.
If the endometriosis involves the ovary or the fallopian tube, surgical treatment appears to be beneficial by correcting the anatomical defect. The results of surgery, even where the endometriosis is quite severe, appear to be quite encouraging. However, if pregnancy does not occur within 9–10 months, IVF should be considered.
Male factor problems
Azoospermia and a raised serum FSH
Azoospermia and a raised serum FSH signify spermatogenic failure (non-obstructive azoospermia). This can be confirmed by testicular biopsy. Occasionally, islands of spermatogenesis can be identified and sperm can be extracted from a testicular biopsy and used for intracytoplasmic sperm injection (ICSI) as part of IVF treatment (see later). If no sperm is identified at surgical sperm retrieval, the only remaining option is the use of donor sperm.
Donor insemination (DI)
Men who donate sperm do so for altruistic reasons and within the UK, sperm donors are no longer anonymous. All potential donors are carefully screened for a family history of medical and genetic conditions, and for infection, particularly HIV, hepatitis B and C. For the latter reason, semen is frozen in straws and quarantined for a minimum of 6 months. The donor is screened again for infection at the end of this period, and only if this second screen is negative, is the sperm used for treatment.
Semen is inserted into the woman’s uterus at the time of ovulation (see below). Ovulation is usually predicted by serial pelvic ultrasound scans or by the detection of the LH surge using a urinary assay. The chance of conception in the first cycle of treatment is 18.8%, falling rapidly to around 6% per cycle for the next 12 months.
Azoospermia and a normal FSH
Azoospermia in the presence of a normal FSH signifies a block of the vas deferens or epididymis. The most common group of men in this category are those who have had a vasectomy. Using microsurgical techniques, the vas can be re-anastomosed (vasovasostomy) or attached to the epididymis (vasoepididymostomy), depending on the site of the obstruction. Although good anatomical results can be achieved, pregnancy rates are often disappointing, partly because the build-up of pressure distal to the obstruction may have damaged the delicate epididymis, and partly because antisperm antibodies may have formed. The time from the original vasectomy to the reversal procedure provides a useful guide to prognosis. With good surgical technique, pregnancy rates are approximately 60%, compared to 30% if the interval from vasectomy to surgery is >10 years.
Men in whom spermatogenesis is normal but surgery is not possible, may be suitable for epididymal sperm aspiration in combination with IVF and ICSI. The sperm can be obtained under local anaesthesia by placing a needle percutaneously into the epididymis, or using more conventional microsurgical techniques under a general anaesthetic. As the sperm sample is usually of poor quality, direct ICSI into the oocytes is necessary (see below).
A significant proportion of men who have congenital absence of the vas (CAV) have been found to carry a variant of cystic fibrosis. They have compound heterogenicity, where each chromosome 7 carries a different mutation at the site of the transmembrane conductance regulator gene, which is responsible for cystic fibrosis. These couples therefore need careful screening for the common cystic fibrosis mutations. If his partner is found to be a carrier of one of the same mutations, there would be a 1:4 chance of their children being affected with cystic fibrosis.
Hypogonadotrophic hypogonadism
Hypogonadotrophic hypogonadism is rare but can be treated successfully with exogenous gonadotrophins, FSH and hCG or by using a gonadotrophin releasing hormone (GnRH) infusion pump.
Idiopathic oligospermia
This is the most common diagnosis in male factor infertility. A wide range of oral treatments have been employed to improve fertility but there is no firm evidence that the use of any oral medication can improve conception rates. Multivitamins have been associated with an improvement in sperm parameters and are recommended for all men whose partners are trying to conceive. The mainstay of treatment is ICSI using sperm prepared in culture medium.
Varicocele
There is ultrasound evidence of a varicocele in 15% of the general male population. Surgery to correct the defect is not justified in the absence of symptoms and in the presence of a normal semen analysis. Even in the presence of oligospermia, there is no evidence that the sperm count (or the conception rate) can be improved with surgery and therefore surgery is no longer recommended in otherwise asymptomatic men.
Unexplained infertility
If no cause can be found, then the main treatment would be to proceed to IVF. Although conservative management is an option, the chance of a natural pregnancy can be calculated using a mathematical model to guide whether waiting or referral to assisted conception would be appropriate.
Assisted conception
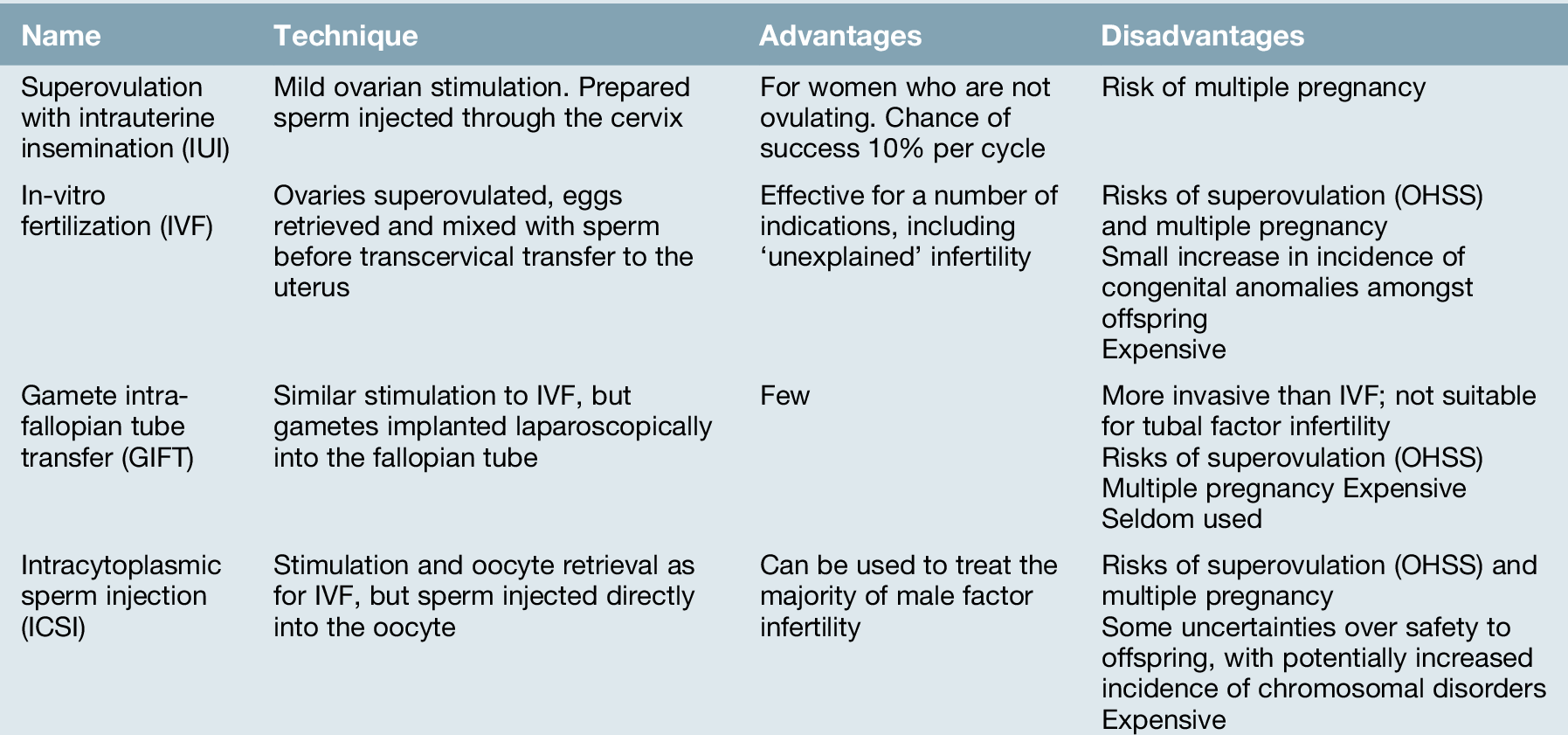
‘Assisted conception’ techniques are those in which gametes, either sperm or eggs, are manipulated to improve the chance of conception (Table 9.4).
Intrauterine insemination (IUI)
IUI was historically used to treat couples with unexplained infertility, a ‘mild’ male factor infertility and mild endometriosis, but is now mainly restricted to those with coital difficulties, and couples requiring donor sperm (donor insemination, DI). Sperm is prepared in culture medium, separating the seminal fluid, poorly motile sperm and other cellular debris in the ejaculate, and producing a clean sample of highly motile sperm. This is then placed directly into the uterine cavity via a fine plastic catheter. IUI is used alone, with the insemination timed to natural ovulation, or in conjunction with controlled ovarian stimulation. The latter involves ovulation–induction agents, such as clomiphene or gonadotrophins, which are used to recruit up to two mature follicles. When the follicles reach an appropriate size, around 17 mm, ovulation is induced with hCG, and the prepared sperm is inserted into the uterine cavity.
In the UK, in 2011, 2087 women had a total of 4091 cycles of DI. Overall, the live birth rates following IUI are around 8–9% per cycle. One complication of controlled ovarian stimulation is the high rate of multiple pregnancy, which in some older studies has been as high as 29%. For this reason, careful dosing, monitoring and a willingness to cancel the cycle due to an excessive response of the ovaries to ovulation induction are required.
In-vitro fertilization (IVF)
The term ‘in-vitro fertilization’ refers to the mixing of sperm and egg outside the body.
Indications
IVF was originally developed for women with tubal disease. However, the indications for IVF have expanded considerably and now include:
![]() male factor infertility
male factor infertility
![]() severe endometriosis
severe endometriosis
![]() failed ovulation induction
failed ovulation induction
![]() unexplained infertility
unexplained infertility
![]() preimplantation diagnosis for genetic disease
preimplantation diagnosis for genetic disease
![]() surrogacy
surrogacy
![]() egg donation.
egg donation.
Technique
Hormonal regimen
The aim of the treatment is to recruit, or rescue, a cohort of antral stage follicles, and support their growth through to maturity (superovulation). This is achieved by the administration of exogenous FSH, given by intramuscular or subcutaneous injection. Although there are two pituitary gonadotrophins involved in oocyte development – FSH and LH – only a relatively small background level of LH is needed. Most commercially available FSH-containing compounds are rich in FSH, with little or no LH component. In addition, most modern protocols use some form of pituitary suppression, either a GnRH agonist or an antagonist, principally to block an inappropriate LH surge. As the release of LH is blocked, hCG, which has a similar action, is used as a substitute, given approximately 36 h before oocyte recovery.
Oocyte collection
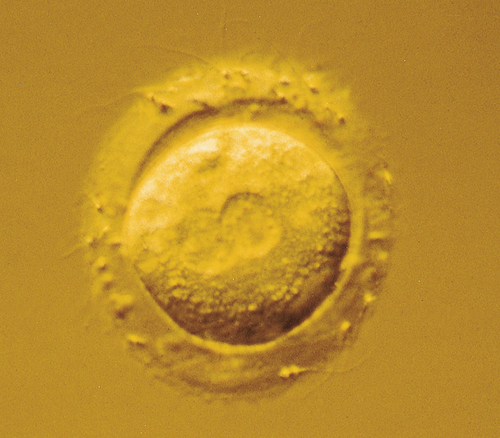
During superovulation treatment, each ovary enlarges to the size of a tennis ball and they generally lie within 1 cm of the posterior vaginal fornix (Fig. 9.3). This allows the oocytes to be collected using a needle passed through the vaginal vault, guided by a vaginal ultrasound probe, whilst the woman is heavily sedated. The oocytes, with their cumulus cell mass, are identified easily (Fig. 9.4) and after removal are placed in an incubator.
Fertilization and incubation
On the morning of oocyte retrieval, the man collects a sperm sample by masturbation. After preparation in culture medium, the sperm are added to the test tubes containing the oocytes. The tubes are inspected 16 h later for the characteristic signs of fertilization, the presence of a male and female pronucleus (see Fig. 9.5). The pronucleate embryos are returned to the incubator for a further 24 or 48 h, with surplus embryos being frozen at this stage.
Embryo transfer
Embryos can be transferred to the uterus at 48 or 72 h after the oocyte collection, at the 4- or 8-cell stage, respectively (Fig. 9.6) or on day 5 at the blastocyst stage. Transferring at the blastocyst stage appears to provide the best chance of pregnancy. Although a maximum of two embryos is transferred to the uterus in women under 40, there is a move in the UK to select out those women most at risk of a twin pregnancy, and electively transfer a single embryo (eSET). Analysis of the Human Fertilisation and Embryology Authority (HFEA) database demonstrates that 87% of all twins occur in those women under the age of 37 in their first cycle of treatment. An eSET policy is widely used in Europe and is associated with a significant reduction in the incidence of multiple pregnancies. In contrast, women over the age of 40, who are more likely to have aneuploid embryos, can have up to three embryos transferred although this has not been associated with a higher live birth rate than when just two embryos are transferred.
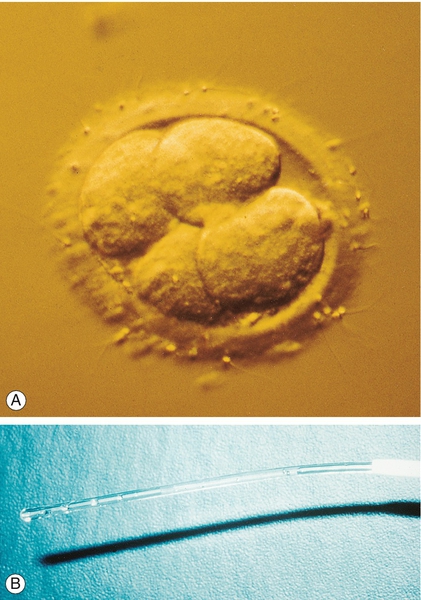
(A) A 4-cell embryo ready for transfer into the uterus. (B) Loaded embryo transfer catheter.
Luteal support
As the pituitary gland has been desensitized (and so will not be producing LH), the luteal phase has to be supported with progesterone suppositories for 14 days, until the result of the pregnancy test is known. Exogenous hCG can also be used for luteal support but significantly increases the risk of ovarian hyperstimulation syndrome (OHSS, see later).
Results
In the UK, in 2011, 48 147 women had a total of 61 726 cycles of IVF or ICSI with an overall live birth rate of 24.5% per cycle started.
Interpreting success rates
There is considerable variation in success rates from clinic to clinic, depending on the clinic’s experience and the mixture of couples treated. In the UK, the Human Fertilisation and Embryology Authority (HFEA, see below), using the data at their disposal, have produced a patient guide providing valuable information on outcomes in each clinic licensed for IVF. The most significant factor in determining an individual couple’s chance of success is the woman’s age, with a dramatic fall in pregnancy rates as age advances (Box 9.3). Success rates gradually decline from 32 years old and so that by the age of 45, the chance of pregnancy with a woman’s own oocytes is approximately 1%.
The live birth rates quoted represent the chance of success in a single cycle. The first cycle of treatment gives the couple the highest chance of success, but the pregnancy rate in subsequent cycles is not dissimilar. The cumulative pregnancy rate in women under the age of 35 is 79% after six attempts.
Embryo freezing
Approximately 25% of couples have ‘spare’ embryos left over after the initial treatment. These can be frozen in liquid nitrogen and replaced during a subsequent natural or artificial cycle, to give a further chance of a pregnancy. With careful selection of embryos for freezing, the live birth rate following frozen embryo transfer can be equivalent to those of ‘fresh’ transfer.
Gamete intrafallopian tube transfer (GIFT)
This also involves the use of superovulation using the same protocols as described for IVF, followed by oocyte collection by vaginal ultrasound guided needle aspiration. Thereafter, the two techniques differ. With GIFT, the ‘best’ three oocytes are selected and a laparoscopy is performed. The fallopian tube is cannulated and the selected oocytes, along with approximately 100 000 sperm, are returned to the tube. Fertilization then occurs in the fallopian tube. However, due to the poor success rates of this strategy compared with IVF, very few centres now offer this technique.
Intracytoplasmic sperm injection (ICSI)
This technique has revolutionized the treatment of male factor infertility. The indications for ICSI are outlined in Box 9.4. If there are no sperm in the ejaculate and none in the epididymis, sperm can still be retrieved from testicular biopsies in approximately half of the men.
Technique
Oocytes are collected in the standard IVF fashion, and then prepared for ICSI by removing their surrounding cumulus cells. A smooth-ended glass pipette is used to hold the oocyte still, while a sharp ultrafine pipette pierces the egg and deposits one sperm along with a tiny amount of culture medium. Prior to injection the selected sperm needs to be immobilized to avoid damaging the delicate structure of the oocyte. The subsequent embryo is transferred to the uterus or frozen, as per IVF.
Results
The pregnancy outcomes following ICSI are comparable to that of conventional IVF (live birth rate of 24.5% per cycle). An increased risk of genetic and developmental defects in post-ICSI pregnancies has been reported. Furthermore, a proportion of male factor infertility has a genetic basis, and by performing ICSI the genetic abnormality may be passed on to the next generation (whereas otherwise it would not). The significance of these findings is, however, limited with the majority of ICSI offspring being completely normal.
Egg donation
Women with primary ovarian failure will require treatment involving oocyte donation (Box 9.5). This treatment is also increasingly being used for older women. Another indication for egg donation (or for DI where the disorder is in the male partner), is genetic disease. Egg donation is more complicated than sperm donation, as the donors have to undergo IVF treatment to the stage of oocyte collection. Some centres offer an ‘egg sharing’ programme, whereby an infertile couple who cannot afford treatment agrees to go through an IVF cycle but donate half the harvested eggs to a recipient who funds both couples’ treatment. This concept clearly raises ethical and moral concerns, although early research suggests that both parties benefit.
Results
This is a successful treatment, with pregnancy rates generally higher than conventional IVF, and maintained even in women over the age of 40. This is largely due to the young age of the egg donors (on average 25 years old) and illustrates the fact that the quality of the oocyte is the most significant factor in the age-related decline of fertility.
Host surrogacy
Some women have functional ovaries but no uterus, due to either a congenital abnormality or previous hysterectomy. Such a woman could undergo IVF treatment, with the embryos then being transferred to another woman (a ‘host surrogate’) whose uterus has been suitably prepared by hormone treatment. The host will carry the pregnancy and then return the baby to the commissioning couple after delivery. According to English law, the ‘mother’ is the woman who delivers the child. Therefore, the commissioning couple is required to adopt the child, even though it is genetically theirs.
Preimplantation genetic diagnosis (PGD)
Couples who have a history of repeated pregnancy failure due to genetic disease or who have had a child with a specific genetic abnormality may benefit from PGD. The couples undergo a conventional IVF treatment cycle, generally using ICSI as the means of fertilization. The embryos are left until day 3, by which stage they have divided to the 6- to 8-cell stage. Generally one and occasionally two blastomeres (embryonic cells) are removed and analysed for specific chromosomal abnormalities using fluorescent in-situ hybridization (FISH) or a specific gene defect by polymerase chain reaction (PCR). Unaffected embryos are cultured through to the blastocyst stage, and single embryos are replaced by embryo transfer in the usual manner. This allows the couple to start a pregnancy knowing that the child is unaffected by the specific genetic condition generating the concern.
Results
The overall chance of success is slightly lower than for conventional IVF, but greater than 20% per treatment cycle.
Side-effects of assisted conception
Globally, approximately 25% of all IVF pregnancies are twin pregnancies and there remain a few triplet pregnancies as a consequence of IVF. The introduction of elective single embryo transfer (eSET), sanctioned by the HFEA, aims to reduce the multiple pregnancy rate to less than 10% by 2013.
Ovarian hyperstimulation syndrome (OHSS) is a condition where the ovaries over-respond to the gonadotrophin injections:
![]() more than 30 follicles may start to mature, resulting in ovarian enlargement and abdominal discomfort
more than 30 follicles may start to mature, resulting in ovarian enlargement and abdominal discomfort
![]() very high concentrations of oestradiol and progesterone make the woman feel nauseated
very high concentrations of oestradiol and progesterone make the woman feel nauseated
![]() if the condition is severe, protein-rich ascites can accumulate, and more rarely, pleural effusion may result
if the condition is severe, protein-rich ascites can accumulate, and more rarely, pleural effusion may result
![]() the sudden shift in fluid can result in hypovolaemia, with resulting renal and thrombotic problems; the condition can be fatal.
the sudden shift in fluid can result in hypovolaemia, with resulting renal and thrombotic problems; the condition can be fatal.
The incidence of severe OHSS is low (< 1%) and it only occurs if the hCG injection is given during superovulation. Women with polycystic ovaries are the most vulnerable and they have a risk of around 5%. Once a diagnosis is made, treatment is supportive with fluid replacement, generally with protein-rich fluids rather than simple crystalloid solutions. Serum electrolytes are monitored because hyperkalaemia and/or hyponatraemia can develop. Thromboprophylaxis is required because of the risk of venous thromboembolism. Hyperstimulation usually occurs in women who conceive and can last throughout the early first trimester. If the woman does not conceive, the condition is self-limiting and resolves spontaneously.
Fetal abnormality
Approximately 3% of all infants are diagnosed with a congenital anomaly. IVF is associated with a 30–40% increased risk of major congenital anomalies compared with natural conceptions. Notably, this risk is not attributable to the increased risk of congenital anomalies associated with multiple birth, since the excess risk remains among singletons. It appears that the increased risk is partly attributable to the underlying infertility or its determinants as couples who take longer than 12 months to conceive also exhibit an increased risk of anomalies (hazard ratio, HR: 1.20), although this was not as high as that observed in treated infertile couples (HR 1.39). The principal anomalies which occur in IVF pregnancies include a range of gastrointestinal, cardiovascular and musculoskeletal defects and specifically septal heart defects, cleft lip, oesophageal atresia and anorectal atresia. While the relative risk of congenital anomalies amongst IVF pregnancies is increased, the absolute risk remains low.
The UK Human Fertilisation and Embryology Act
This Act, passed by the UK Parliament in 1990, brought about the formation of a regulatory body, known as the Human Fertilisation and Embryology Authority (HFEA). Further powers were given in the revision of the Act in 2008. The HFEA regulates research on human embryos, the storage of gametes and embryos and the use in infertility treatment of donated gametes and of embryos produced outside the body. The HFEA came into operation in August 1991, with the principal aim of ensuring that human embryos and gametes are used responsibly and that infertile couples are not exploited. Assisted conception treatment can only be legally performed in a centre licensed by the HFEA. This licence is renewed on an annual basis.
All women and men who donate gametes (either sperm or eggs) or receive assisted conception treatment have to be registered with the HFEA. The outcome of treatment is also recorded. The HFEA also requires all couples considering assisted conception to be offered counselling by a trained counsellor. Paramount in the HFEA’s philosophy is the welfare of the potential child.