CHAPTER 110 Inferior Vena Cava and Its Main Tributaries
INTRODUCTION
Inferior Vena Cava
Normal Anatomy and Congenital Anomalies
The IVC extends from the confluence of the common iliac veins at the level of L5 vertebral body, to the right atrium of the heart in right prevertebral location, next to the abdominal aorta and is surrounded by a rich network of lymphatic vessels (Fig. 110-1). It is partially covered anteriorly by the peritoneal membrane. The retroperitoneal space where the IVC is located can communicate with the perirenal spaces and the anterior and posterior interfascial spaces.1
Duplicated IVC: There are two IVCs below the level of the renal veins—each connected to the ipsilateral common iliac vein. The left IVC joins the left renal vein, which then crosses anterior to the abdominal aorta and drains into the right IVC (Fig. 110-2). There may be variants in this anatomy and there may be significant discrepancy in the size of the two IVCs.
Azygos continuation of IVC: The infrarenal portion of IVC receives blood from the renal veins. It passes posterior to the diaphragmatic crura, enters the thorax as the azygos vein, and then joins the superior vena cava at the azygos arch. The hepatic and right adrenal veins drain directly into the right atrium. The gonadal veins drain into the ipsilateral renal veins. The right renal artery crosses abnormally anterior to the IVC (Fig. 110-3).
Circumaortic left renal vein: There are two left renal veins. The superior-anterior renal vein receives the adrenal vein and crosses the aorta anteriorly to join the IVC. The second renal vein is approximately 1 to 2 cm more inferior and posterior. It receives the left gonadal vein and crosses posterior to the aorta to join the IVC (Fig. 110-4).
Retroaortic left renal vein: The renal vein crosses posterior to the aorta to join the IVC.
Etiology and Pathophysiology (Including any Special Anatomic Considerations)
Embryogenesis
Knowledge of the IVC embryogenesis is necessary for a better understanding of the IVC anatomic aberrations. The IVC is composed of four segments which form during the 6 to 8 weeks postconception.2,3 This is due to continuous appearance and regression of three paired embryonic veins, which include the posterior cardinal veins, the subcardinal veins, and the supracardinal veins. The first step in this complex process is the formation of the posterior supracardinal and more anterior subcardinal veins. Then, the most caudal segment of the right supracardinal vein becomes the infrarenal vena cava. The hepatic segment of IVC is derived from the vitelline vein, which conveys blood from the viscera. The suprarenal segment is formed via a subcardinal-hepatic anastomosis. The renal segment forms via anastomosis of the right supra-subcardinal and post-subcardinal veins. The infrarenal segment arises from the right supracardinal vein.
Manifestations of Disease
Clinical Presentation
With retroaortic left renal vein, an increased incidence of testicular varicoceles has been reported, presumably due to compression of the left renal vein by the abdominal aorta. With the circumcaval ureter type, there could be partial right ureteral obstruction or recurrent urinary tract infection. With the absence of infrarenal IVC or entire IVC, patients may present with venous insufficiency of the lower extremities or idiopathic deep venous thrombosis. Approximately 5% of patients younger than 30 years with idiopathic deep venous thrombosis show IVC absence on CT. Almost 10% of these patients with a coexisting thrombophilia have congenital absence of the IVC.4
Imaging Indications and Algorithm
Imaging Techniques and Findings
Reporting: Information for the Referring Physician
TUMORS OF THE IVC AND MAIN TRIBUTARIES
Prevalence and Epidemiology
Primary IVC tumors (leiomyosarcomas) are very rare with only one large series published in the literature.5 It is more common to see tumors invading the IVC through its tributary veins arising from separate abdominal organs. One of the most common causes of neoplastic invasion of the IVC lumen is the renal cell carcinoma (RCC) that can be seen invading the IVC through the renal vein in 4% to 10% of the cases. Hepatocellular carcinoma (HCC) frequently invades the portal vein but also on rare occasions can invade the IVC through the hepatic veins. Primary adrenocortical carcinoma is a rare adrenal tumor that invades the IVC. Multiple other retroperitoneal tumors can compress and invade the IVC, including lymphomas, metastasis of gonadal or uterine tumors, pheochromocytomas, and other retroperitoneal sarcomas.
Imaging Indications and Algorithm
Best Imaging Modality
Contrasted MRI or CT of the abdomen and pelvis is the modality of choice for the assessment of these complex tumors.6–8 The scanning protocol should include the right atrium of the heart as well as the entire pelvis and common femoral veins. Early postcontrast phases (arterial and portal-venous) may demonstrate heterogeneous luminal enhancement due to admixing artifacts at the level of the renal veins that can obscure tumor or produce a false positive diagnosis of IVC thrombus; therefore additional 2- to 4-minute delayed images are recommended, which will provide more homogeneous luminal enhancement for improved ability to detect the presence or absence of intraluminal tumor or thrombus.
Recommendations
Imaging Techniques and Findings
CT Findings
Intraluminal leiomyosarcoma: presents as a tumor thrombus in the lumen of the vessel. This solid neoplasm shows contrast enhancement in postinjection images. The tumor thrombus characteristically produces expansion of the IVC and can also occlude the lumen, causing venous congestion in the proximal organs such as the liver or kidneys (Fig. 110-5). Lack of a known primary tumor suggests the diagnosis of primary leiomyosarcoma of the IVC.
Adrenocortical carcinoma: Presents as a large heterogeneous adrenal mass typically with venous invasion.10 It usually compresses and displaces adjacent organs such as the kidney or liver and it can be confused with renal cell carcinoma or hepatocellular carcinoma. For this particular point, the use of sagittal or coronal reconstructions can be useful to determine the origin of the mass. As mentioned previously, the right adrenal veins drain directly into the IVC but the left adrenal carcinoma invades the IVC through the left renal vein, potentially being confused with venous invasion from renal cell carcinoma arising from the upper pole of the left kidney.
Renal cell carcinoma: typically presents as a solid, heterogeneous renal mass that can extend into the renal veins and IVC (Fig. 110-6).8,9 There may be gonadal vein invasion. This tumor also can display direct retroperitoneal extension and lymph node metastasis.
Hepatocellular carcinoma: is a solid hepatic tumor that is most commonly seen in patients with cirrhosis. After contrast injection, it typically enhances in the arterial phase and washes out in the delay phase. It invades the IVC through the hepatic veins.11 As other tumoral thrombus, it typically expands the veins (Fig. 110-7).
DIFFERENTIAL DIAGNOSIS
SYNOPSIS OF TREATMENT OPTIONS
IVC COMPLICATIONS IN ORTHOTOPIC LIVER TRANSPLANTATION ANATOMY
Definition
The liver is transplanted in an orthotopic position, that is, the native liver is removed and the donor liver is transplanted in the normal anatomical site.12 Because of the firm attachment of the retrohepatic inferior vena cava (IVC) to the caudate lobe, the most direct method of removal is to take the IVC with it. An end-to-end anastomosis is then performed between the recipient and donor IVC. In order to minimize complications arising from the clamping of the portal vein and IVC during the operation, a veno-venous bypass must be performed. Another type of IVC anastomosis performed is the “piggy-back” anastomosis. In this technique, the liver is lifted off the IVC, by dividing the short caudate veins. The suprahepatic IVC of the implanted liver is then anastomosed to the hepatic venous confluence of the recipient by an end-to-side anastomosis and the donor infrahepatic IVC is tied off. The main advantage of this technique is that it does not need the veno-venous bypass because the recipient IVC is not clamped. Although the procedure is both technically difficult and time-consuming, there is one less anastomosis to perform.
In living or pediatric transplants, a split-liver transplantation is performed, where the donor liver may be provided or the donor liver may be reduced to a right lobe (segments 5 through 8), left lobe (segments 1 through 4) or left lateral segment (segments 2 and 3). With the first two, the cava is preserved, allowing either the caval replacement or piggy-back technique to be employed. The left lateral segment reduction is performed in spilt-liver transplants only and involves removal of the IVC; thus the piggy-back method is necessary.13
Prevalence and Epidemiology
Liver transplantation has become the method of choice for treatment of patients with irreversible severe liver dysfunction.13 Today, liver transplantation is a standardized treatment modality for well-defined indications.14 According to the United Network of Organ Sharing (UNOS), to-date 97,166 liver transplants have been performed. It is increasingly successful with 1-year patient survival rate of approximately 84% and 3-year survival rate of 76%.14 IVC complications in orthotopic liver transplantation, that is, stenosis and thrombosis, are diagnosed in fewer than 1% of transplant cases.15
Manifestations of Disease
Imaging Techniques and Findings
Ultrasound Imaging
On ultrasound findings, the normal hepatic vein and IVC have a triphasic waveform. Ultrasound findings in IVC thrombosis are absent flow within the vein on color Doppler with no spectral Doppler flow. An intraluminal echogenic thrombus can also be seen in complete or partial thrombosis of the IVC. In IVC stenosis, there is a three- to fourfold increase in velocity as compared to the prestenotic segment. The velocity in the IVC has to be measured at the sites of anastomosis, suprahepatic and infrahepatic, in end-to-end anastomosis and at the end-to-side anastomosis in piggy-back anastomosis (Figs. 110-9 and 110-10). A significant caval stenosis may result in reversed flow or absence of phasicity in the hepatic veins.16
Angiography
Angiography is definitive in diagnosing IVC stenosis and can provide treatment at the same time as well. Pressure gradients can be measured in the IVC and the atrium during angiography. Percutaneous treatment for IVC stenosis and thrombosis includes angioplasty and/or stenting (Figs. 110-11 and 110-12).
IVC THROMBOSIS
Etiology and Pathophysiology (Including any Special Anatomic Considerations)
The variety of underlying etiologies for IVC thrombosis is extensive and listed in Table 110-1. IVC thrombosis can result from extension of a DVT, but also can be secondary to other factors such as tumor invasion/extension, external compression, hematoma/trauma, coagulation dysfunction, iatrogenic causes, and other miscellaneous etiologies.
IVC, inferior vena cava.
Manifestations of Disease
Imaging Indications and Algorithm
Presence of bilateral leg edema and pulmonary embolism are common indications for imaging the IVC.
Imaging Techniques and Findings
Ultrasound Imaging
A filling defect is seen in the IVC which appears echogenic in an acute thrombus with a distended IVC. A chronic thrombus appears hypoechoic with decreased size of the IVC lumen.17 Doppler ultrasound examinations show monophasic flow without respiratory variation distal to the compression and a significant increase in flow velocity at the point of arterial compression in the common iliac vein in patients with May-Thurner syndrome. DVT may be seen in the left lower extremity on ultrasonography in patients with May-Thurner syndrome.
CT
Definitive diagnosis of venous thrombosis by CT depends on demonstration of an intraluminal thrombus. Whereas a fresh thrombus has a density similar to or higher than that of circulating blood, an old thrombus is of lower density than the surrounding blood on noncontrast CT scans. When the occlusion is complete, the involved segment remains unenhanced on postcontrast CT scans. In case of chronic occlusion, the IVC may become atrophic and calcified. In partial IVC occlusion, the thrombus is seen as a filling defect surrounded by enhanced blood. A “pseudothrombus” artifact is seen due to nonopacified blood flow mixing with enhanced blood giving an artifactual filling defect appearance (Fig. 110-13). CT has 100% sensitivity and 96% specificity in detecting DVT when compared to conventional venography (Fig. 110-14).18 In patients with May-Thurner syndrome, pelvic CT images in the transverse plane show iliac vein compression by the overlying right common iliac artery in patients with left-sided deep vein thrombosis.19 In case of complete caval obstruction, extensive venous collaterals may also be identified.
MR
On spin-echo (SE) images, venous thrombus appears as a region of persistent intraluminal signal which can be confused with slow flowing blood which can also appear as high signal intensity. On SSFP or gradient recalled echo (GRE) images, venous thrombus appears as an area of lower signal intensity. SSFP and GRE pulse sequences are faster than SE imaging and hence are preferred if gadolinium-chelate contrast agents cannot be administered because of a contraindication.20 Acquisition of SSFP (or GRE) in cine mode using ECG gating will help differentiate flow artifacts from true thrombus. On cine MR, thrombus will appear as fixed intraluminal filling defects that persist throughout the cardiac cycle. Alternatively, flow artifacts will not persist and will be seen only on a few phases of the cardiac cycle. On gadolinium-enhanced MRI, in addition to a filling defect in complete thrombosis, lack of enhancement is seen in a bland thrombus; however, enhancement is seen in a tumor thrombus (Fig. 110-15). In addition, there will be focal dilation of the vena cava in an acute thrombosis and presence of venous collateral vessels in chronic thrombosis.
Differential Diagnosis
Synopsis of Treatment Options
Thrombolytic Agents
Most thrombolytic agents have been reported in the treatment of IVC thrombosis. The relative merits of thrombolytic therapy must be weighed against the risks of hemorrhagic complications. Urokinase, tissue-type plasminogen activator (tPA), and streptokinase have all been used. Typically, delivery is catheter-directed with or without a pulse spray. Patients require concurrent heparin therapy; however, tPA protocols do not use concurrent heparin because of the risk of bleeding complications.21
IVC INTERVENTIONS
Definitions
IVC intervention includes angioplasty, placement of stents and filters, biopsy, and shunt creation. IVC angioplasty or stenting is performed to bypass areas of occlusion or stenosis. Inferior vena cava filters are used to prevent pulmonary embolism (PE) in patients with contraindications to, complications of, or failure of anticoagulation therapy and patients with extensive free-floating thrombi or residual thrombi following massive PE. IVC biopsies are performed for tissue diagnosis in cases with suspected tumor thrombi or primary IVC malignancies. IVC biopsy can be performed by transcatheter aspiration,22 brush biopsy, and scoop biopsy.23 IVC shunt is the surgical anastomosis between portal and systemic veins and the surgical anastomosis between the portal vein and the vena cava.
Etiology and Pathophysiology (Including any Special Anatomic Considerations)
Inferior vena cava syndrome causes accumulated ascites and edema in the lower limbs, scrotum, and abdominal wall. Placement of a stent in the narrowed area of the vena cava with prompt dilation of the vascular lumen and correction of venous flow decreases venous pressure distal to the stenosis and results in improvement of congestive symptoms.24
IVC Filters
Choice of access site depends on a patient’s anatomy, the site of venous thromboemboli, and the type of filters available. Generally, the right internal jugular vein or the right femoral vein is the preferred route, but left-sided venous approaches or approaches from arm veins can be used in some circumstances (Fig. 110-16). Ultrasound scanning can be used to confirm entry site and to guide puncture in difficult cases. After local anesthetic injection, the subcutaneous tissues are infiltrated and the vein is punctured under strict antiseptic conditions. A cavogram is performed to confirm anatomy and the presence or absence of intraluminal filling defects and to identify the renal veins and anatomical variants. Conscious sedation with midazolam and fentanyl can be used. Following the cavogram, the diameter of the IVC is calculated; most filters cannot be placed if the cava is larger than 28 mm, the exception being the bird’s nest filter. The filter is usually placed below the renal veins but can be placed in a suprarenal position when there is renal vein thrombosis or thrombus extending proximal to the renal veins, during pregnancy, and when there is thrombus proximal to an indwelling filter. The procedure usually takes less than 60 minutes.25
IVC Shunts
Different types of IVC shunts include the side-to-side portocaval shunt and the mesocaval shunt.
Side-to-Side Portacaval Shunt (SSPCS). This shunt may be performed for hepatic venous obstruction due to isolated hepatic vein thrombosis (Fig. 110-17). The data on SSPCS for hepatic vein obstruction is somewhat limited—an earlier review reported only two studies with more than 10 patients each.26 The reservation against performing SSPCS in patients with hepatic vein thrombosis is the likely technical difficulty in approximating the portal vein to the infrahepatic vena cava in the presence of a hypertrophied caudate lobe. Another argument is that because a portacaval shunt involves hepatic hilar dissection, it may increase the technical difficulties if a subsequent liver transplantation is needed because of progression of liver disease. Despite these reservations, excellent long-term graft patency and symptom-free survival of 81% to 94% has been reported in series from dedicated centers.
Mesocaval Shunt (MCS). MCS is the preferred shunt in the setting of isolated hepatic vein thrombosis in many studies.27 Advantages of MCS are its technical simplicity and avoidance of hilar dissection. The prerequisite for this shunt is a patent IVC and it provides an effective hepatic decongestion even in those patients in whom an enlarged caudate lobe presses on the IVC.28 The shunt can be an autologous internal jugular vein or prosthetic (Dacron or PTFE). The main disadvantage of the Dacron grafts is high incidence (up to 50% or more in some series) of postoperative thrombosis, whereas the reported long-term patency of the internal jugular vein grafts exceeds 80%.29 Reported 5-year survival after MCSs for hepatic venous outflow obstruction ranges between 57% and 95%. Because the mesocaval shunt is a partial shunt (portal vein flow is maintained), there is a low rate of encephalopathy, rebleeding, and improved quality of life.
IVC Stents
IVC stents are placed routinely to bypass areas of occlusion or stenosis (Fig. 110-18). Placement of long-standing indwelling venous catheters or creation of surgical anastomoses in patients undergoing liver transplantation increases the risk of IVC stenosis. Percutaneous stent insertion can produce rapid and sustained relief of symptoms, either as a primary treatment or in patients in whom other methods have failed or symptoms have recurred. Since this technique was first reported in 1986, it has become widely used in the palliation of IVC obstruction.
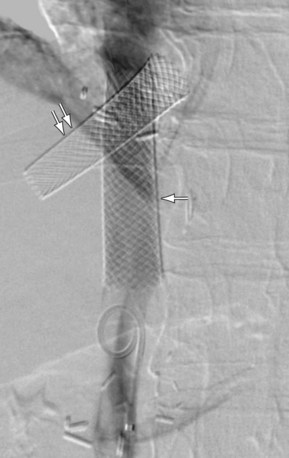
 FIGURE 110-18 Stents placed in the IVC (arrow) and hepatic vein (double arrows) for stenosis in a post-liver transplantation patient.
FIGURE 110-18 Stents placed in the IVC (arrow) and hepatic vein (double arrows) for stenosis in a post-liver transplantation patient.
KEY POINTS
 Inferior Vena Cava
Inferior Vena Cava
 Tumors of the IVC and main tributaries
Tumors of the IVC and main tributaries
Bass JE, Redwine MD, Kramer LA, et al. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics.. May-Jun 2000;20(3):639-652.
Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician.. Jan 2008;54(1):49-55.
Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics.. Sep-Oct 2003;23(5):1093-1104.
Cuevas C, Raske M, Bush WH, et al. Imaging primary and secondary tumor thrombus of the inferior vena cava: multi-detector computed tomography and magnetic resonance imaging. Curr Probl Diagn Radiol.. May-Jun 2006;35(3):90-101.
Sheth S, Fishman EK. Imaging of the inferior vena cava with MDCT. AJR Am J Roentgenol.. Nov 2007;189(5):1243-1251.
Vaidya S, Dighe M, Kolokythas O, Dubinsky T. Liver transplantation: vascular complications. Ultrasound Q.. Dec 2007;23(4):239-253.
1 Gore RM, Balfe DM, Aizenstein RI, Silverman PM. The great escape: interfascial decompression planes of the retroperitoneum. AJR Am J Roentgenol. 2000;175(2):363-370.
2 Phillips E. Embryology, normal anatomy, and anomalies. In: Ferris EJ, Hipona FA, Kahn PC, et al, editors. Venography of the Inferior Vena Cava and its Branches. Baltimore: Williams & Wilkins; 1969:1-32.
3 Bass JE, Redwine MD, Kramer LA, et al. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000;20(3):639-652.
4 Gayer G, Luboshitz J, Hertz M, et al. Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. AJR Am J Roentgenol. 2003;180(3):729-732.
5 Mingoli A, Cavallaro A, Sapienza P, et al. International registry of inferior vena cava leiomyosarcoma: analysis of a world series on 218 patients. Anticancer Res. 1996;16(5B):3201-3205.
6 Cuevas C, Raske M, Bush WH, et al. Imaging primary and secondary tumor thrombus of the inferior vena cava: multi-detector computed tomography and magnetic resonance imaging. Curr Probl Diagn Radiol. 2006;35(3):90-101.
7 Sheth S, Fishman EK. Imaging of the inferior vena cava with MDCT. AJR Am J Roentgenol. 2007;189(5):1243-1251.
8 Sheth S, Scatarige JC, Horton KM, et al. Current concepts in the diagnosis and management of renal cell carcinoma: role of multidetector CT and three-dimensional CT. Radiographics. 2001;21(Spec No):S237-S254.
9 Russo P. Renal cell carcinoma: presentation, staging, and surgical treatment. Semin Oncol. 2000;27(2):160-176.
10 Ng L, Libertino JM. Adrenocortical carcinoma: diagnosis, evaluation and treatment. J Urol. 2003;169(1):5-11.
11 Okada Y, Nagino M, Kamiya J, et al. Diagnosis and treatment of inferior vena caval invasion by hepatic cancer. World J Surg. 2003;27(6):689-694.
12 Corbally MT, Rela M, Heaton ND. Standard orthotopic operation, retransplantation and piggybacking. In: Williams R, Portmann B, Tan KC, editors. The Practice of Liver Transplantation. London: Churchill Livingstone; 1995:135-142.
13 Starzl TE. Liver transplantation. Gastroenterology. 1997;112(1):288-291.
14 Keeffe EB. Summary of guidelines on organ allocation and patient listing for liver transplantation. Liver Transpl Surg. 1998;4(5 Suppl 1):S108-S114.
15 Nghiem HV, Tran K, Winter TC3rd, et al. Imaging of complications in liver transplantation. Radiographics. 1996;16(4):825-840.
16 Glanemann M, Settmacher U, Langrehr JM, et al. Portal vein angioplasty using a transjugular, intrahepatic approach for treatment of extrahepatic portal vein stenosis after liver transplantation. Transpl Int. 2001;14(1):48-51.
17 Park JH, Lee JB, Han MC, et al. Sonographic evaluation of inferior vena caval obstruction: correlative study with vena cavography. AJR Am J Roentgenol. 1985;145(4):757-762.
18 Baldt MM, Zontsich T, Stumpflen A, et al. Deep venous thrombosis of the lower extremity: efficacy of spiral CT venography compared with conventional venography in diagnosis. Radiology. 1996;200(2):423-428.
19 Oguzkurt L, Tercan F, Pourbagher MA, et al. Computed tomography findings in 10 cases of iliac vein compression (May-Thurner) syndrome. Eur J Radiol. 2005;55(3):421-425.
20 Erdman WA, Weinreb JC, Cohen JM, et al. Venous thrombosis: clinical and experimental MR imaging. Radiology. 1986;161(1):233-238.
21 Girard P, Hauuy MP, Musset D, et al. Acute inferior vena cava thrombosis. Early results of heparin therapy. Chest. 1989;95(2):284-291.
22 Wendth AJJr, Garlick WB, Pantoja GE, Shamoun J. Transcatheter biopsy of renal carcinoma invading the inferior vena cava. J Urol. 1976;115(3):331-332.
23 Kishi K, Sonomura T, Terada M, Sato M. Scoop biopsy of intracaval tumor thrombi: a preliminary report of a minimally invasive technique to obtain large samples. Eur J Radiol. 1997;24(3):263-268.
24 Furui S, Sawada S, Kuramoto K, et al. Gianturco stent placement in malignant caval obstruction: analysis of factors for predicting the outcome. Radiology.. 1995;195(1):147-152.
25 Chung J, Owen RJ. Using inferior vena cava filters to prevent pulmonary embolism. Can Fam Physician. 2008;54(1):49-55.
26 Pisani-Ceretti A, Intra M, Prestipino F, et al. Surgical and radiologic treatment of primary Budd-Chiari syndrome. World J Surg. 1998;22(1):48-53.
27 Slakey DP, Klein AS, Venbrux AC, Cameron JL. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233(4):522-527.
28 Fisher NC, McCafferty I, Dolapci M, et al. Managing Budd-Chiari syndrome: a retrospective review of percutaneous hepatic vein angioplasty and surgical shunting. Gut. 1999;44(4):568-574.
29 Stipa S, Thau A, Schillaci A, et al. Mesentericocaval shunt with the internal jugular vein. Surg Gynecol Obstet. 1978;146(3):391-399.

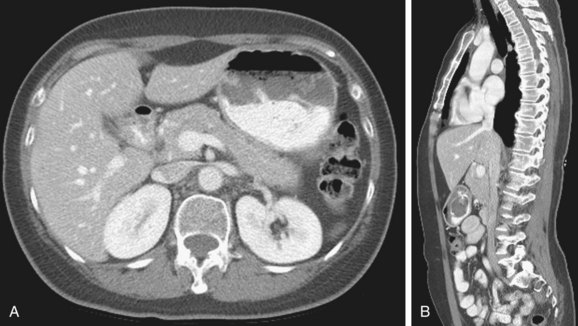
 FIGURE 110-1
FIGURE 110-1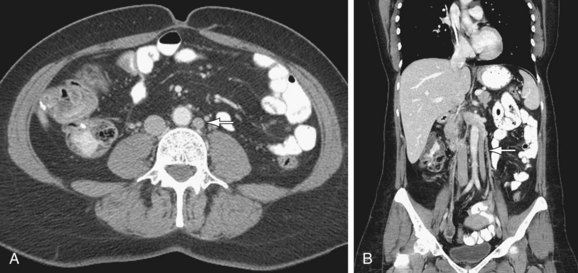
 FIGURE 110-2
FIGURE 110-2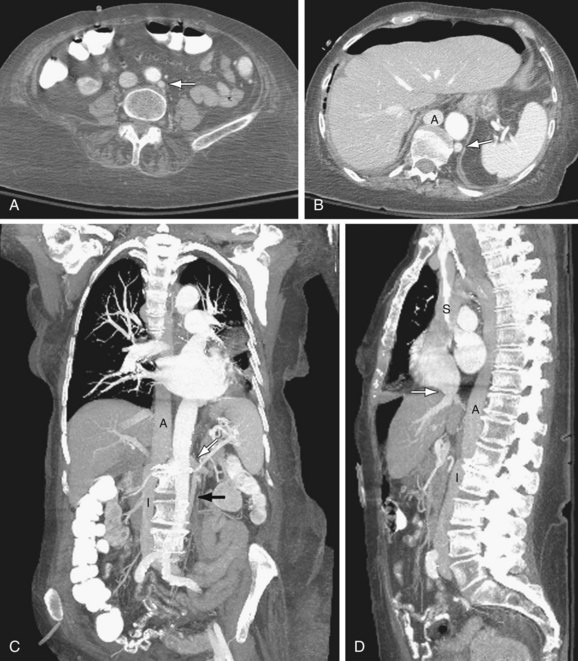
 FIGURE 110-3
FIGURE 110-3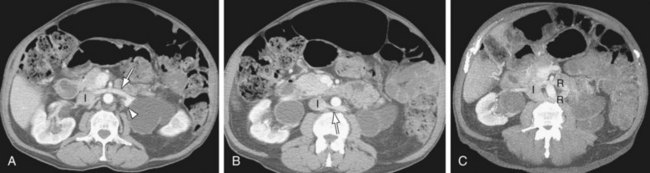
 FIGURE 110-4
FIGURE 110-4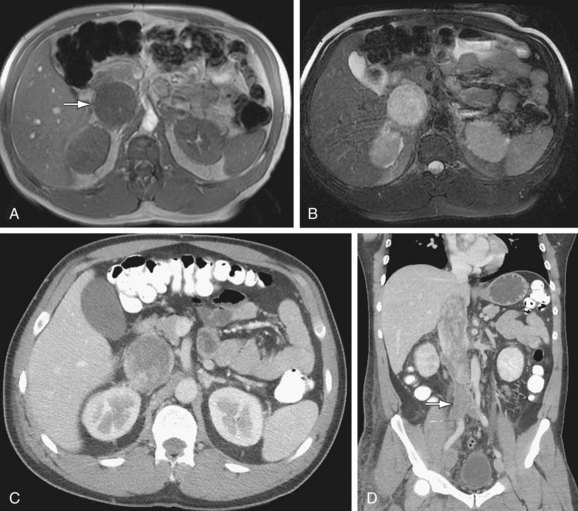
 FIGURE 110-5
FIGURE 110-5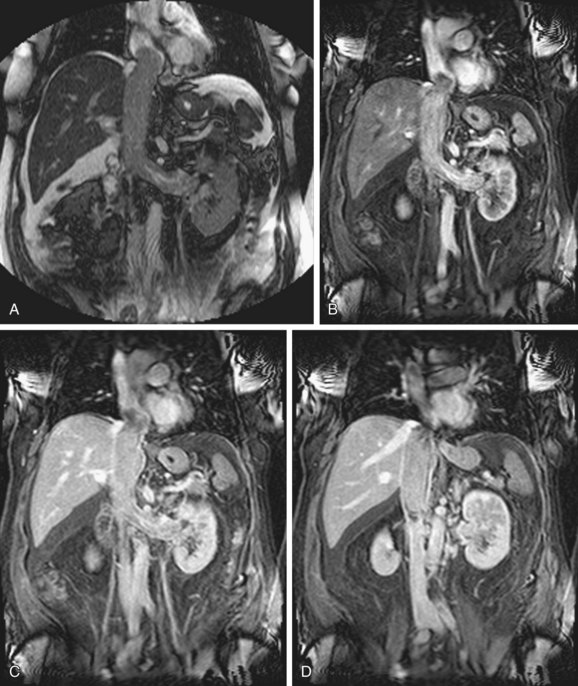
 FIGURE 110-6
FIGURE 110-6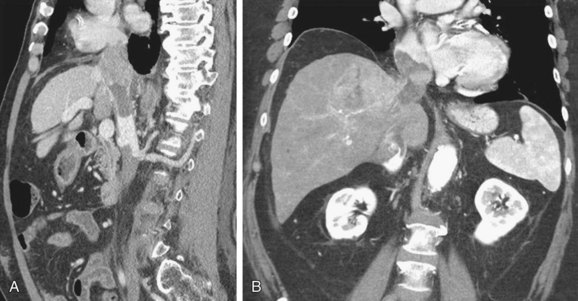
 FIGURE 110-7
FIGURE 110-7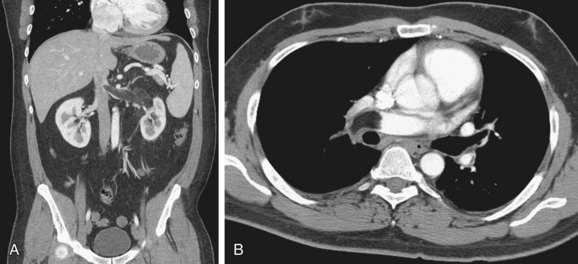
 FIGURE 110-8
FIGURE 110-8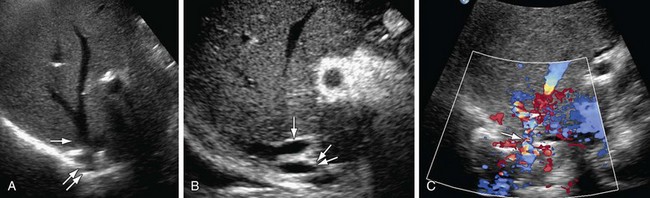
 FIGURE 110-9
FIGURE 110-9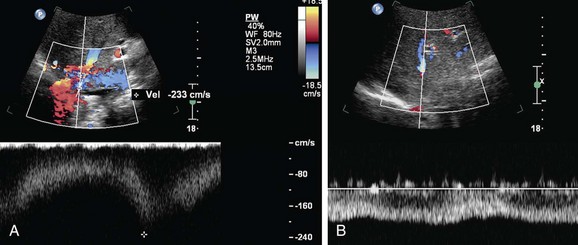
 FIGURE 110-10
FIGURE 110-10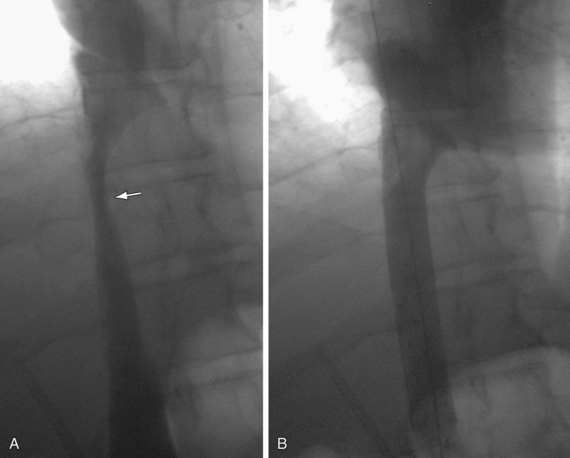
 FIGURE 110-11
FIGURE 110-11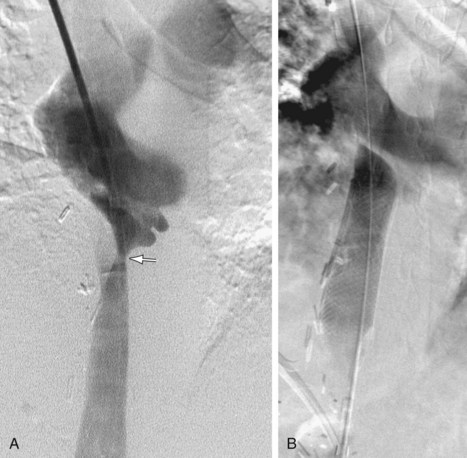
 FIGURE 110-12
FIGURE 110-12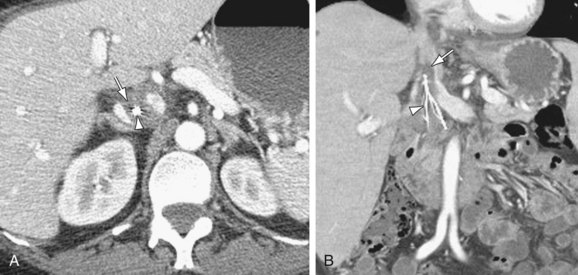
 FIGURE 110-13
FIGURE 110-13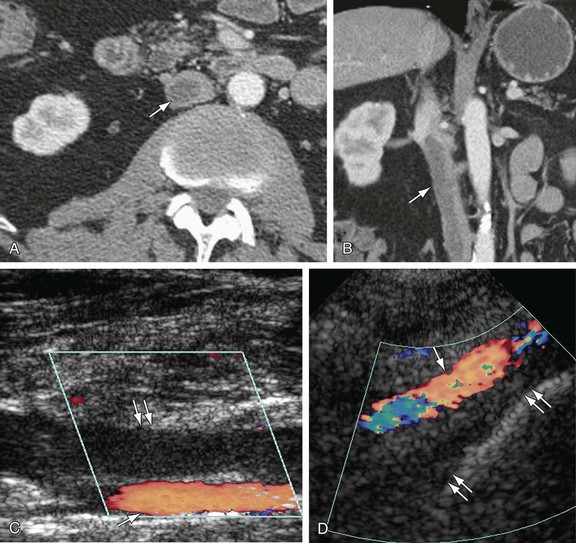
 FIGURE 110-14
FIGURE 110-14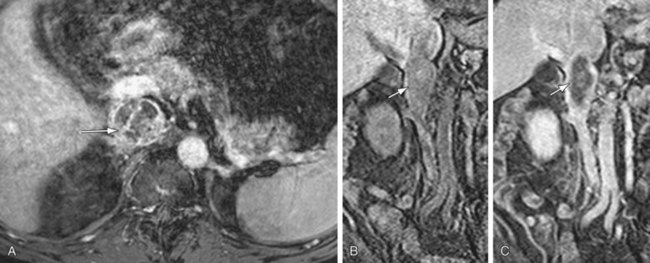
 FIGURE 110-15
FIGURE 110-15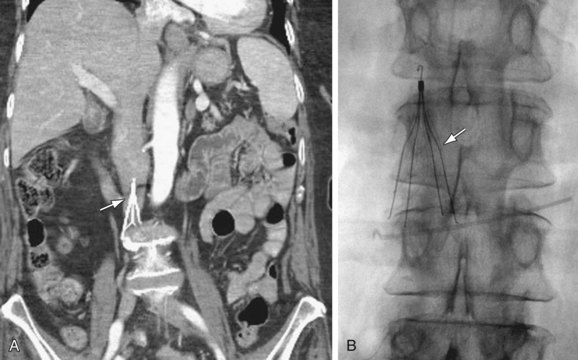
 FIGURE 110-16
FIGURE 110-16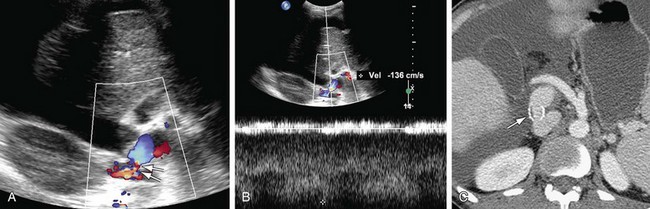
 FIGURE 110-17
FIGURE 110-17

