Chapter 431 Infective Endocarditis
Etiology
Viridans-type streptococci (α-hemolytic streptococci) and Staphylococcus aureus remain the leading causative agents for endocarditis in pediatric patients. Other organisms cause endocarditis less frequently and, in ≈6% of cases, blood cultures are negative for any organisms (Table 431-1). No relationship exists between the infecting organism and the type of congenital defect, the duration of illness, or the age of the child. Staphylococcal endocarditis is more common in patients with no underlying heart disease; viridans group streptococcal infection is more common after dental procedures; group D enterococci are seen more often after lower bowel or genitourinary manipulation; Pseudomonas aeruginosa or Serratia marcescens is seen more frequently in intravenous drug users; and fungal organisms are encountered after open heart surgery. Coagulase-negative staphylococci are common in the presence of an indwelling central venous catheter.
Table 431-1 BACTERIAL AGENTS IN PEDIATRIC INFECTIVE ENDOCARDITIS
COMMON: NATIVE VALVE OR OTHER CARDIAC LESIONS
UNCOMMON: NATIVE VALVE OR OTHER CARDIAC LESIONS
HACEK group†
PROSTHETIC VALVE
* These fastidious bacteria plus some fungi may produce culture-negative endocarditis. Detection may require special media, incubation for more than 7 days, or serologic tests.
† The HACEK group includes Haemophilus species (H. paraphrophilus, H. parainfluenzae, H. aphrophilus), Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species.
‡ Candida species, Aspergillus species, Pseudallescheria boydii, Histoplasma capsulatum.
Clinical Manifestations (Table 431-2)
Table 431-2 MANIFESTATIONS OF INFECTIVE ENDOCARDITIS
HISTORY
SYMPTOMS
SIGNS
LABORATORY
CNS, central nervous system.
Diagnosis
The critical information for appropriate treatment of infective endocarditis is obtained from blood cultures. All other laboratory data are secondary in importance (see Table 431-2). Blood specimens for culture should be obtained as promptly as possible, even if the child feels well and has no other physical findings. Three to five separate blood collections should be obtained after careful preparation of the phlebotomy site. Contamination presents a special problem inasmuch as bacteria found on the skin may themselves cause infective endocarditis. The timing of collections is not important because bacteremia can be expected to be relatively constant. In 90% of cases of endocarditis, the causative agent is recovered from the 1st 2 blood cultures. The laboratory should be notified that endocarditis is suspected so that, if necessary, the blood can be cultured on enriched media for longer than usual (>7 days) to detect nutritionally deficient and fastidious bacteria or fungi. Antimicrobial pretreatment of the patient reduces the yield of blood cultures to 50-60%. The microbiology laboratory should be notified if the patient has received antibiotics so that more sophisticated methods can be used to recover the offending agent. Other specimens that may be cultured include scrapings from cutaneous lesions, urine, synovial fluid, abscesses, and, in the presence of manifestations of meningitis, cerebrospinal fluid. Serologic diagnosis or PCR of resected valve tissues is necessary in patients with unusual or fastidious microorganisms (Table 431-3).
Table 431-3 DIAGNOSTIC APPROACH TO UNCOMMON PATHOGENS CAUSING ENDOCARDITIS
| PATHOGEN | DIAGNOSTIC PROCEDURE |
|---|---|
| Brucella spp. | Blood cultures; serology; culture, immunohistology, and PCR of surgical material |
| Coxiella burnetti | Serology (IgG phase I >1 in 800); tissue culture, immunohistology, and PCR of surgical material |
| Bartonella spp. | Blood cultures; serology; culture, immunohistology, and PCR of surgical material |
| Chlamydia spp. | Serology; culture, immunohistology, and PCR of surgical material |
| Mycoplasma spp. | Serology; culture, immunohistology, and PCR of surgical material |
| Legionella spp. | Blood cultures; serology; culture, immunohistology, and PCR of surgical material |
| Tropheryma whipplei | Histology and PCR of surgical material |
PCR, polymerase chain reaction.
From Moreillon P, Que YA: Infective endocarditis, Lancet 363:139–148, 2004.
Treatment
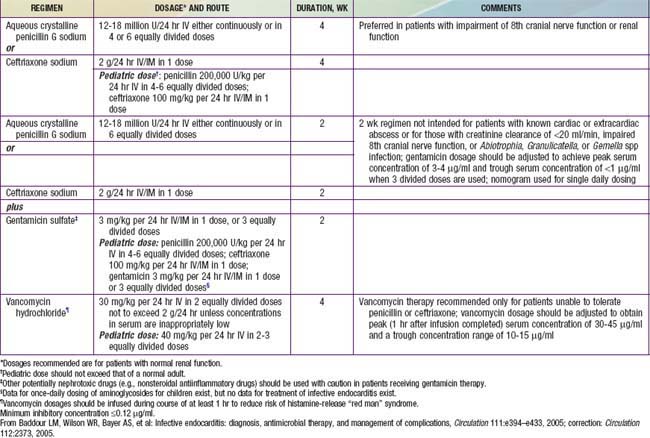
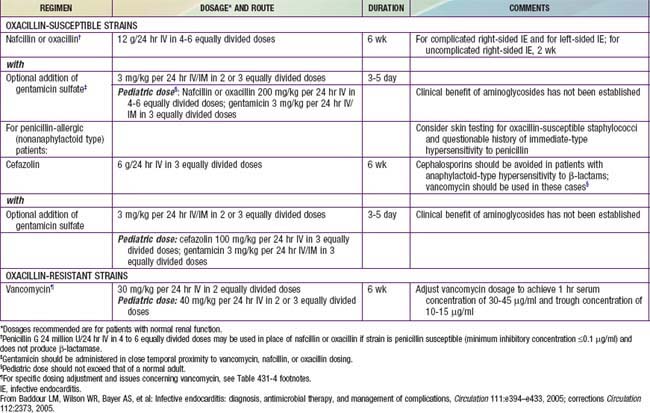
Antibiotic therapy should be instituted immediately once a definitive diagnosis is made. When virulent organisms are responsible, small delays may result in progressive endocardial damage and are associated with a greater likelihood of severe complications. The choice of antibiotics, method of administration, and length of treatment should be coordinated with consultants from both cardiology and infectious diseases (Tables 431-4 and 431-5). Empirical therapy before the identifiable agent is recovered may be initiated with vancomycin plus gentamicin in patients without a prosthetic valve and when there is a high risk of S. aureus enterococcus or viridans streptococci (the 3 most common organisms). High serum bactericidal levels must be maintained long enough to eradicate organisms that are growing in relatively inaccessible avascular vegetations. Between 5 and 20 times the minimal in vitro inhibiting concentration must be produced at the site of infection to destroy bacteria growing at the core of these lesions. Several weeks are required for a vegetation to organize completely; therapy must be continued through this period so that recrudescence can be avoided. A total of 4-6 wk of treatment is usually recommended. Depending on the clinical and laboratory responses, antibiotic therapy may require modification and, in some instances, more prolonged treatment is required. With highly sensitive viridans group streptococcal infections, shortened regimens that include oral penicillin for some portion have been recommended. In nonstaphylococcal disease, bacteremia usually resolves in 24-48 hr, whereas fever resolves in 5-6 days with appropriate antibiotic therapy. Resolution with staphylococcal disease takes longer.
Prevention
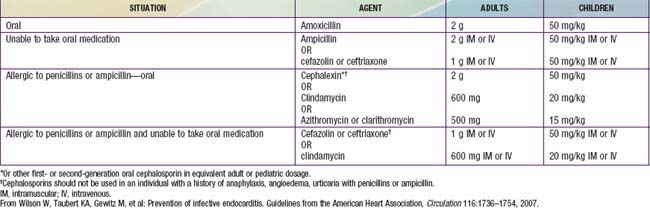
Recommendations by the American Heart Association for antimicrobial prophylaxis before dental and other surgical procedures received a major revision in 2007. A substantial reduction in the number of patients who require prophylactic treatment and the procedures requiring coverage was recommended. The primary reasons for these revised recommendations were that (1) infective endocarditis is much more likely to result from exposure to the more frequent random bacteremias associated with daily activities than from a dental or surgical procedure; (2) routine prophylaxis may prevent “an exceedingly small” number of cases; and (3) the risk of antibiotic-related adverse events exceeds the benefits of prophylactic therapy. Improving general dental hygiene was felt to be a more important factor in reducing the risk of infective endocarditis resulting from routine daily bacteremias. The current recommendations limit the use of prophylaxis to those patients with cardiac conditions associated with the greatest risk of an adverse outcome from infective endocarditis (Table 431-6). Prophylaxis for these patients is recommended for “all dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa.” Furthermore, “placement of removable prosthodontic or endodontic appliances, adjustment of orthodontic appliances, placement of orthodontic brackets, shedding of deciduous teeth and bleeding from trauma to the lips or oral mucosa” are not indications for prophylaxis. Given that many invasive respiratory tract procedures do cause bacteremia, prophylaxis for many of these procedures in considered reasonable. In contrast to prior recommendations, prophylaxis for gastrointestinal or genitourinary procedures is no longer recommended in the majority of cases. Prophylaxis for patients undergoing cardiac surgery with placement of prosthetic material is still recommended. Given the highly individual nature of these recommendations and the continued concern amongst some cardiologists over their adoption, direct consultation with the child’s cardiologist is still the best method for determining a specific patient’s ongoing need for prophylaxis (Table 431-7).
Table 431-6 2007 STATEMENT OF THE AMERICAN HEART ASSOCIATION (AHA): CARDIAC CONDITIONS ASSOCIATED WITH THE HIGHEST RISK OF AN ADVERSE OUTCOME FROM INFECTIVE ENDOCARDITIS FOR WHICH PROPHYLAXIS WITH DENTAL PROCEDURES IS REASONABLE
CONGENITAL HEART DISEASE (CHD)*
Cardiac transplantation recipients who develop cardiac valulopathy
* Except for the conditions listed here, antibiotic prophylaxis is no longer recommended by the AHA for any other form of CHD.
† Prophylaxis is reasonable because endothelization of prosthetic material occurs within 6 mo after the procedure.
From Wilson W, Taubert KA, Gewitz M, et al: Prevention of infective endocarditis. Guidelines from the American Heart Association, Circulation 116:1736–1754, 2007.
American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 Guideline update on valvular heart disease: focused update on infective endocarditis. J Am Coll Cardiol. 2008;52:676-685.
Ashrafian H, Bogle RG. Antimicrobial prophylaxis for endocarditis: emotion or science? Heart. 2007;93:5-6.
Ayres NA, Miller-Hance W, Fyfe DA, et al. Indications and guidelines for performance of transesophageal echocardiography in the patient with pediatric acquired or congenital heart disease: report from the task force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2005;18:91-98.
Bach DS. Perspectives on the American College of Cardiology/American Heart Association guidelines for the prevention of infective endocarditis. J Am Coll Cardiol. 2009;53:1852-1854.
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications. Circulation. 2005;111:e394-e433.
Bendig EA, Singh J, Butler TJ, et al. The impact of the central venous catheter on the diagnosis of infectious endocarditis using duke criteria in children with Staphylococcus aureus bacteremia. Pediatr Infect Dis J. 2008;27:636-639.
Bolger AF. The rationale for the new infective endocarditis guidelines. Curr Cardiol Rep. 2009;11:101-106.
Connaughton M, Rivett JG. Infective endocarditis. BMJ. 2010;341:1267-1268.
Cooper HA, Thompson EC, Laureno R, et al. Subclinical brain embolization in left-sided infective endocarditis. Circulation. 2009;120:585-591.
Day MD, Gauvreau K, Shulman S, et al. Characteristics of children hospitalized with infective endocarditis. Circulation. 2009;119:865-870.
Falcone M, Barzaghi N, Carosi G, et al. Candida infective endocarditis. Medicine. 2009;88:160-168.
Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis. J Am Coll Cardiol. 2009;53:436-444.
Fournier PE, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture–negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis. 2010;51(2):131-140.
Fowler VGJr, Boucher HW, Corey R, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653-664.
Gavaldá J, Len O, Miró JM, et al. Brief communication: treatment of Enterococcus faecalis endocarditis with ampicillin plus ceftriaxone. Ann Intern Med. 2007;146:574-579.
Gerber MA. New AHA guidelines for prevention of infective endocarditis. Pediatr Infect Dis J. 2008;27:647-648.
Guerrero MLF, López JJG, Goyenechea A, et al. Endocarditis caused by Staphylococcus aureus. Medicine. 2009;88:1-22.
Harrison JL, Hoen B, Prendergast BD. Antibiotic prophylaxis for infective endocarditis. Lancet. 2008;372:1317-1319.
Hartzell JD, Torres D, Kim P, et al. Incidence of bacteremia after routine tooth brushing. Am J Med Sci. 2005;329:178-180.
Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84:162-173.
Humpl T, McCrindle BW, Smallhorn JF. The relative roles of transthoracic compared with transesophageal echocardiography in children with suspected infective endocarditis. J Am Coll Cardiol. 2003;41:2068-2071.
Levitas A, Zucker N, Zalzstein E, et al. Successful treatment of infective endocarditis with recombinant tissue plasminogen activator. J Pediatr. 2003;143:649-652.
Marin M, Muňoz P, Sánchez M, et al. Molecular diagnosis of infective endocarditis by real-time broad-range polymerase chain reaction (PCR) and sequencing directly from heart valve tissue. Medicine. 2007;86:195-202.
Medical The. Letter: Major changes in endocarditis prophylaxis for dental, GI and GU procedures. Med Lett. 2007;49:99-100.
Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363:139-148.
Morpeth S, Murdoch D, Cabell CH, et al. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147:829-835.
Murdoch DR, Corey R, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med. 2009;169:463-473.
Rai K, Supriya S, Hedge AM. Oral health status of children with congenital heart disease and the awareness, attitude and knowledge of their parents. J Clin Pediatr Dent. 2009;33:315-318.
Reyes MP, Ali A, Mendes RE, Biedenbach DJ. Resurgence of Pseudomonas endocarditis in Detroit, 2006–2008. Medicine. 2009;88:294-301.
Richey R, Wray D, Stokes T, et al. Prophylaxis against infective endocarditis: summary of NICE guidance. BMJ. 2008;336:770-771.
Seña AC, Seed P, Nicholson B, et al. Kingella kingae endocarditis and a cluster investigation among daycare attendees. Pediatr Infect Dis J. 2010;29:86-88.
Stokes T, Richey R, Wrayob D, et al. Prophylaxis against infective endocarditis: summary of NICE guidance. Heart. 2008;94:930-934.
Tissieres P, Gervaix A, Beghetti M, et al. Value and limitations of the von Reyn, Duke, and modified Duke criteria for the diagnosis of infective endocarditis in children. Pediatrics. 2003;112:e467.
Valente AM, Jain R, Scheurer M, et al. Frequency of infective endocarditis among infants and children with Staphylococcus aureus bacteremia. Pediatrics. 2005;115:e15-e19.
Vollmer T, Piper C, Horstkotte D, et al. 23S rDNA real-time polymerase chain reaction of heart valves: a decisive tool in the diagnosis of infective endocarditis. Eur Heart J. 2010;31:1105-1113.
Weber R, Berger C, Balmer C, et al. Interventions using foreign material to treat congenital heart disease in children increase the risk for infective endocarditis. Pediatr Infect Dis J. 2008;27:544-550.
Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis. Guidelines from the American Heart Association. Circulation. 2007;116:1736-1754.