Chapter 122 Infectious Endophthalmitis
Organisms that cause endophthalmitis
Bacteria, fungi, protozoa, and parasites are all capable of producing endophthalmitis (Box 122.1).
Bacteria
Gram-positive cocci
Staphylococci
S. epidermidis has been increasingly identified as a cause of human infection often associated with foreign bodies, such as implanted catheters, and has become the most common cause of postoperative endophthalmitis.1–7 Hospitals may not subspeciate coagulase-negative staphylococci, reporting them all as S. epidermidis.
Gram-positive bacilli
Bacillus
The genus Bacillus has more than 13 members, the most widely known of which is B. anthrax. The most common intraocular pathogen is B. cereus, with B. subtilis also identified as a cause of endophthalmitis.9 Bacillus is an aerobic spore-forming rod that is Gram-positive or Gram-variable in stain. The size varies from 3 × 0.4 µm to 9 × 2 µm. These organisms grow singly, in chains, or in diplobacillary form. In nature they are usually found in decaying organic matter, dust, soil, vegetables, water, and human flora. B. cereus is an important cause of food poisoning and may cause bacteremia as a result of wound or burn infections. It produces multiple extracellular products, including antimicrobial substances, enzymes, and toxins. The enterotoxins are diarrheal and emetic in action, and there are two additional toxins that may be correlated with virulence. Some toxins have produced severe inflammation when injected into the eye.10 Identification by the laboratory is usually as a cultural contaminant.
Risk factors for Bacillus infection include intravenous (IV) drug use, sickle-cell disease, foreign bodies including IV catheters, immunosuppression from malignancy, neutropenia, corticosteroid use, and acquired immunodeficiency syndrome (AIDS). Bacillus is now the most commonly identified organism in traumatic endophthalmitis.11–14 The infection is particularly virulent and may destroy the eye in 12–24 hours. It is unique in inducing fever and leukocytosis in endophthalmitis.
Propionibacterium
Propionibacterium organisms are Gram-positive or Gram-variable rods that are often pleomorphic, anaerobic, and nonsporulating. Propionibacteria are a predominant component of skin flora and are also found on mucosal surfaces of the mouth, intestines, urethra, and vagina. They are the most common clinical isolate of Gram-positive, nonsporulating bacteria. They are found in acne, prosthetic joints, cerebrospinal fluid shunts, endocarditis, and osteomyelitis. They have been identified as a cause of postoperative endophthalmitis of a chronic granulomatous nature, almost exclusively found in patients with intraocular lenses (IOLs).15–20 P. acnes is almost always the clinical isolate.
Gram-negative bacilli
Pseudomonas
Pseudomonas produces a wide variety of clinical syndromes, including endocarditis on prosthetic valves and in IV drug users, lower respiratory infections in persons with compromised defense mechanisms, bacteremia in immunocompromised patients, meningitis and brain abscesses, corneal ulcers, keratitis, ophthalmia neonatorum, scleral abscess, and conjunctivitis. Pseudomonas is the most common cause of Gram-negative endophthalmitis. Most cases of endophthalmitis are produced by P. aeruginosa, but other species have also been isolated in clinical cases.11,21 Sensitivities include aminoglycosides and ceftazidime.21,22
Enterobacteriaceae
Klebsiella
The genus Klebsiella contains a group of three species of bacteria, including K. pneumoniae. They are a relatively common isolate in Gram-negative endophthalmitis23 and are characteristically resistant to multiple antibiotics. Enterobacter organisms are opportunistic pathogens that rarely produce human disease. When they function as opportunistic pathogens, however, they may be resistant to first-generation cephalosporins. Serratia spp. are opportunistic pathogens that have only been recognized as capable of producing human disease since the 1960s. They are more likely to colonize the respiratory and urinary tracts of hospitalized patients than other Enterobacteriaceae. Most hospital infections are caused by catheterization and instrumentation of the urinary and respiratory tracts. These organisms have multiple drug resistances but are most often sensitive to amikacin.
Fungi
Candida
Candida is predominantly a unicellular organism that is small, thin-walled, ovoid, and reproduces by budding. Yeast forms, hyphae, and pseudohyphae may all be identified in clinical specimens; their identification is facilitated by staining with KCl 10%. On culture, it grows as a smooth, creamy-white colony that usually must be identified by physiologic rather than morphologic means. Candida is widespread in soil, hospital environments, inanimate objects, food, and as a commensal of humans, being isolated from diseased skin, the gastrointestinal tract, female genitalia, and the urine of patients with Foley catheters. The incidence of Candida infections has increased with the use of immunosuppression, indwelling catheters, and with increased IV drug use.24,25 Candida typically causes endophthalmitis as a complication of candidemia26 or as a result of epidemics of contaminated irrigating solutions used in intraocular surgery.27 It typically responds to amphotericin, triazoles,28 and 5-fluorocytosine.
Histoplasma capsulatum
Histoplasmosis is the most common human fungus infection in the USA. Virtually all persons in the Ohio river valley and along the lower Mississippi river have been infected. Two eye syndromes are produced by H. capsulatum. The presumed ocular histoplasmosis syndrome consists of a morphologic triad of fundus scarring consisting of peripheral punched-out spots, macular disciform scars, and peripapillary scarring. This is thought to be a late effect of H. capsulatum after hematogenous spread has created earlier choroidal infections. Organisms have not been identified in this form of the disease. Endophthalmitis associated with disseminated histoplasmosis has been described in an immunocompromised host.29 Amphotericin is the drug of choice in active disease but is not indicated in presumed ocular histoplasmosis syndrome.
Helminths, protozoa, and ectoparasites
Helminths
Taenia solium, a trematode, is the pork tapeworm for which humans are the only definitive host. Ingestion of the organism allows the development of the intermediate-stage Cysticercus cellulosae. This organism may invade almost any area of the body, including the vitreous cavity. Other helminths of ocular importance are Toxocara canis and T. cati. The predominant hosts for these organisms are dogs and cats, respectively. There are a large number of viable eggs, particularly from T. canis, in the environment. Eggs are spread by direct ingestion or, in the case of dogs, by eating infected meat. T. canis in children produces a chronic inflammatory granulomatous disease involving the vitreous and retina.30
Protozoa
Toxoplasmosis is the most common protozoon causing eye disease.31 Toxoplasma gondii is an obligate intracellular protozoon that is ubiquitous in nature, infecting all herbivorous, carnivorous, and omnivorous animals. The definitive host is the cat. The ingestion of raw or uncooked meat allows tissue cysts to enter the gastrointestinal tract where they are broken down. They then invade the walls of the gastrointestinal tract and spread throughout the body to many tissues. The organisms remain viable for the life of the host. Although most humans are asymptomatic for the infection, a recurrent panuveitis may be an ocular manifestation of infestation.
Experimental endophthalmitis
Meyers-Elliott and Dethlefs32 injected Klebsiella oxytoca organisms into the vitreous cavity of the phakic rabbit. Pathologic evaluation demonstrated widespread polymorphonuclear leukocyte invasion throughout ocular tissues within 24 hours and significant photoreceptor degeneration within 48 hours. Peak numbers of organisms could be cultured from the eye at 24 hours, but they declined spontaneously, with no organisms being recovered after 72 hours. Pathologic signs continued to increase once the cavity was sterile, however, implicating endotoxins as important to ongoing tissue damage. Davey and colleagues33 injected K. pneumoniae and Pseudomonas aeruginosa into the vitreous of the phakic rabbit and noted that bacterial growth peaked at 48 hours, with the number of organisms falling spontaneously after this. Measurable changes in biochemical parameters of the vitreous did not seem to account for this phenomenon; the authors postulated that it might be a characteristic of Gram-negative infections. Meredith and coworkers34 created an experimental model of Staphylococcus epidermidis endophthalmitis by injecting various numbers of organisms into the vitreous cavity of the aphakic rabbit. Low numbers of organisms produced mild disease with slow progression; some infections appeared to be self-limited. Larger numbers of organisms produced infections of greater intensity, which were almost uniformly steadily progressive. Organisms could not be recovered from the vitreous cavity after 96 hours, however, regardless of the size of the initial inoculum. This fact suggests that progressive inflammatory signs were related to factors other than continuing active infection. Other models of S. epidermidis have yielded organisms from the phakic eye as long as 7 days after their injection into the vitreous. Peyman35 produced endophthalmitis with S. aureus in phakic rabbits to compare various treatment regimens, reporting uniformly poor results with loss of the eye in untreated animals.
Beyer et al.36 studied the role of the posterior capsule in the development of S. aureus endophthalmitis in the primate. Nine monkeys had bilateral lens extraction; in one eye a large capsulotomy was performed, while in the other the capsule was intact. Inoculation of 105 S. epidermidis organisms was made into the anterior chamber, and the vitreous was cultured after 72 hours. Only one culture was positive with the capsule intact, but all nine cultures were positive when the capsule was opened. The experiment was repeated with a posterior-chamber lens implanted. None of ten eyes with an intact capsule and IOL was culture-positive, whereas 40% of the eyes with the capsule open and a lens in place were culture-positive and an additional 20% showed histopathologic signs of vitreous inflammation. An intact posterior capsule thus appeared to inhibit the spread of infection from the anterior chamber into the vitreous cavity, an effect that was not compromised by the addition of a posterior-chamber IOL.
Anaerobic organisms have also been used to produce clinical disease in the rabbit. The injection of 1000 organisms of Fusobacterium necrophorum into the vitreous cavity of the phakic rabbit produced clinical infection in 100% of eyes. Propionibacterium acnes was studied in the aphakic rabbit with and without a posterior-chamber IOL.37 Injection of 108 organisms into the anterior chamber produced a severe infection, while inoculation of 2.5 × 106 produced clinical inflammation that peaked at 3 days but persisted for up to 24 days. The presence of an IOL appeared to favor the development of chronic, low-grade inflammation.
Clinical findings
Postoperative infection
Postoperative infection is the cause of roughly two-thirds of all cases of endophthalmitis in most clinical series. Although infectious endophthalmitis may follow any operative procedure performed on the eye, most cases follow cataract extraction, and almost all are bacterial in origin. Studies from a single institution suggest that the incidence of endophthalmitis over the past several decades has been declining. At the Bascom Palmer Eye Institute, the incidence from 1984 to 1994 was 0.09%, dropping to 0.05% from 1995 to 2001.38 Recent studies indicate that causative organisms in infection after cataract surgery are usually genetically identical to the patient’s own flora.39,40 In 75–95% of the reported cases, the causative organisms are Gram-positive. A significant percentage of cases of apparent infectious endophthalmitis proved to be culture-negative.2,3,41
Cataract extraction
Allen42 reviewed 30 000 intracapsular cataract procedures performed at the Massachusetts Eye and Ear Infirmary from 1964 to 1977, and found an incidence of endophthalmitis of 0.057%. A review of 23 625 cases of extracapsular cataract extraction from Bascom Palmer Eye Institute revealed an incidence of 0.072%.5 More recent figures from two studies in the phacoemulsification era suggest an incidence of 0.03%43 to 0.04%38,44 National registries in Sweden45 and Norway46 identified rates of 0.1% and 0.11–0.16%, respectively.
Heavy cellular debris is present in the vitreous, and there may be focal accumulations of whitish material or sheets of opacification within the vitreous. The intraocular pressure may be low, normal, or high. The pupil often dilates poorly, making examination with an indirect ophthalmoscope difficult. Retinal periphlebitis47 has been reported as an early sign, but in most cases the retinal vessels are seen poorly, if at all. With more severe disease, large areas of opacity are seen within the vitreous; there may be a red reflex, or only a dark appearance to the posterior cavity.
Infection caused by Staphylococcus epidermidis and other species of coagulase-negative staphylococci may have the clinical onset delayed by 5 days or more after surgery. Even then, the clinical signs and symptoms may be mild and may be difficult to distinguish from a noninfectious inflammatory process.1,4,48,49 There was no hypopyon or pain in 25% of confirmed cases in the Endophthalmitis Vitrectomy Study (EVS).50 In this study a number of clinical features at initial presentation were associated with microbiologic factors. More severe initial findings suggest infection with Gram-negative bacteria, Streptococcus or Staphylococcus aureus. Factors noted at initial diagnosis correlating with Gram-negative and Gram-positive organisms other than Gram-positive coagulase micrococci included corneal infiltrate, cataract wound abnormalities, afferent pupillary defect, loss of red reflex, initial light perception-only vision, and symptom onset within 2 days of surgery. Gram-negative organisms were not identified in those eyes in which retinal vessels were visualized preoperatively; 61.9% of those eyes had equivocal or no growth. Diabetes mellitus was associated with a higher yield of Gram-positive, coagulase-negative micrococci, while there was a shift toward other Gram-positive organisms in eyes undergoing secondary IOL implantation compared with those that had initial cataract surgery.51,52
Late-onset disease may occur with predisposing anatomic problems such as a persistent conjunctival filtering bleb or the presence of a vitreous wick.53 Chronic, low-grade inflammation that ultimately proves to be of an infectious origin may occur in rare instances and has been termed chronic postoperative endophthalmitis or delayed-onset endophthalmitis.16,54 This may occur secondary to coagulase-negative Gram-positive organisms (such as S. epidermidis)16,54 and also results from infections with the anaerobic species Propionibacterium acnes.15–20 In reviews of cases of endophthalmitis after cataract extraction, a significant incidence of intraoperative complications has been found.16–18,55–57 Postoperative filtering blebs, wound leaks,55 and vitreous wick are also found more frequently in infected eyes.2 Infection can also result after the cutting of sutures holding the cataract wound or after an invasive procedure to incise the posterior capsule.2,58
The type of cataract incision has recently attracted attention as a possible contributor to the incidence of postoperative infection. A case–control study demonstrated a threefold greater risk of endophthalmitis with clear corneal incisions than with scleral tunnel incision.59 However, this has not been confirmed by newer studies.60,61 Temporal incisions were noted to have a higher incidence of infection than superior incisions in another study.62 A case–control study of secondary IOL implantation showed endophthalmitis to be associated with diabetes mellitus, transscleral suture fixation of posterior-chamber IOLs, polypropylene haptics, preoperative eyelid abnormalities, re-entry of the eye through a previous wound, and postoperative wound defects.63
Gram-positive organisms are found in 75–90% of culture-positive cases.3,51 Most common is S. epidermidis, followed by S. aureus and Streptococcus spp. Gram-negative organisms accounted for only 6% of the culture-positive cases in the Endophthalmitis Vitrectomy Study.3,51 Fungi are rare, with the exception of epidemics such as those of Candida parapsilosis64 and Paecilomyces lilacinus,65 which were traced to infected irrigating solutions. Bacterial epidemics have also been traced to an infected phacoemulsifier (Pseudomonas),66 and to infected viscoelastic material (Bacillus spp.).67 Culture-negative cases account for 25–35% of cases of pseudophakic endophthalmitis.2,3,51,68
Corneal transplantation
Since endophthalmitis after corneal transplantation is rarely seen, its characteristics are less well defined. In two large series of corneal transplants, an incidence of 0.11% and 0.08% of postoperative endophthalmitis was reported.38,69 In a review of over 90 000 cases between 1972 and 2002, the incidence after PKP was 0.38%.70 Guss et al.69 studied 445 corneal transplant cases and demonstrated that, in addition to three acute cases, there were eight other cases, six of which occurred after an ulcerative process in the graft. Endophthalmitis of delayed or late onset can also result from suture abscess formation or from bacterial access to the anterior chamber associated with a loose suture.71 In an ulcerative process, entry may occur because of disruption of continuity of the graft, or the bacteria may invade through an intact but thinned cornea. Endophthalmitis following corneal transplantation has also been associated with a vitreous wick. Unlike endophthalmitis following cataract surgery, the onset of the disease may be relatively painfree and is heralded by an increased anterior-chamber reaction, hypopyon, and loss of red reflex. The bacteria usually involved in these cases are Gram-positive, with Staphylococcus spp. and Streptococcus spp. being equally represented; fungal and Gram-negative cases are least common. In the series of Leveille et al.,72 three of four acute cases were associated with a contaminated donor rim; this was not noted in any of the cases reported by Guss et al.69 The prognosis in post-corneal transplantation cases is poor; nine of the 11 cases reported by Guss et al.69 had final vision of light perception or no light perception.
Glaucoma filtration surgery
The risk of developing endophthalmitis after filtering surgery is similar to the risk following cataract extraction,5,38,73–76 but most of these cases occur months to years after the original procedure. A prodrome of browache, headache, or eye pain is not uncommon.77 There may be an antecedent conjunctivitis, but often the abrupt onset of pain and redness constitutes the presenting signs and symptoms. An inferior location to the bleb and use of antifibrotic agents increase the likelihood of subsequent infection.78–80 The blebs may appear intact in these cases, although some may be Seidel-positive.53,80 Thin, avascular, and leaking blebs appear to be at increased risk of infection.77 The material within the bleb is white or yellow, giving a “white-on-red” appearance against the conjunctival erythema. Eyes with glaucoma drainage devices are also at risk for infection.75 The spectrum of bacteria isolated from culture-positive bleb infection is quite different from that of endophthalmitis following cataract surgery, with 31–57% demonstrating Streptococcus spp. as the causative organism53,77,79,81–83 More recent series have found more cases caused by Staphylococcus spp. and Enterococcus spp. than older reports. Gram-negative species are also more common than after acute post-cataract infections.83 Visual outcomes remain generally poor in these cases, even with modern therapy. In two large series, 50% of eyes had final visual acuity of 20/400 or better,82,83 in part because of the influence of Streptococcus spp. infections on the outcome.
Pars plana vitrectomy
The incidence of endophthalmitis after pars plana vitrectomy appears to be about the same as that after other intraocular procedures.38,84 The diagnosis is most difficult to make because the normal postoperative pain and intraocular inflammation after vitrectomy may mask the symptoms. The diagnosis rests on findings that are more severe than usual; appearance of hypopyon is often rapid and should cause particular concern.84,85 In one case with intraocular silicone, findings were limited to a whitish material collecting between the silicone and the retina.86 The spectrum of bacteria in these cases is similar to other acute postoperative infections. In spite of this, the prognosis is uniformly poor, and retention of vision is rare.
The first large, multicenter studies in the era of small gauge vitrectomy suggested a significant higher rate of endophthalmitis with 25-gauge sutureless vitrectomy (0.23 and 0.84)87,88 Since then, several large series have not confirmed these findings.89–92 Optimized wound construction, modifications in case selection and a lower threshold for suturing appear to reduce the incidence of endophthalmitis in small gauge cases to the level of standard 20-gauge cases.
Intraocular injection
Introduction of organisms into the eye may occur during a pars plana injection of intraocular gas for pneumatic retinopexy.93 In recent years intraocular injection of medications to treat age-related macular degeneration, macular edema, retinal vein occlusions, cytomegalovirus retinitis and uveitis have increased in frequency dramatically, resulting in increased numbers of cases of endophthalmitis. The reported incidence of endophthalmitis following intravitreal injections varies significantly. A recent review paper described a rate of endophthalmitis from 0.014% up to 0.87% per injection. The overall incidence was 0.051% (50/98 962).94 Injection technique used varied from study to study. To this day there is no clear consensus on a “standard” injection protocol as for the use of a sterile drape, gloves, surgical mask or topical antibiotics. So far, only the use of povidone iodine and a lid speculum is routinely recommended.95 Cultures most frequently showed coagulase-negative staphylococci as causative organism, with atypical organisms being more common after the use of triamcinolone.94 With triamcinolone, the typical clinical signs of endophthalmitis may be masked by antiinflammatory effects and the presentation may be difficult to differentiate from a pseudohypopyon without infection which can occur after triamcinolone injection caused by deposition of the injected material in the anterior chamber.96
Strabismus surgery
Endophthalmitis is a rare but devastating complication of strabismus surgery. True endophthalmitis probably always occurs after an inadvertent suture perforation, although a scleral abscess at the site of a suture could possibly lead to an intraocular infection. Lethargy, asymmetric eye redness, eyelid swelling, and fever have been reported as presenting signs, but diagnosis may be delayed.97 Prognosis is poor in these eyes, possibly because delays in diagnosis are common.97,98
Post-traumatic endophthalmitis
After postoperative cases, post-traumatic endophthalmitis is the second largest category, accounting for 20–30% of the cases in large mixed series.41,104–107 The incidence of endophthalmitis after penetrating trauma ranges from as low as 2% to as high as 17%.11,108 In rural injuries, infections have been reported in 30% of the eyes.11 Eyes with intraocular foreign bodies have a risk of infection about twice as high as those without a foreign body.109,110 Other independent risk factors of traumatic endophthalmitis include dirty wound, lens capsule rupture, age greater than 50 years and delayed presentation of more than 24 hours after the injury.111
Of those eyes with open globe injury which are culture-positive at primary trauma repair, not all develop endophthalmitis.110,112 Eyes with more virulent organisms were at greater risk of developing clinical infection in one study.112 The onset of infection after injury varies with the virulence of the organism and is usually accompanied by increasing pain, intraocular inflammation, hypopyon, and vitreous opacities. As with postoperative endophthalmitis, about two-thirds to three-quarters of the cases are due to Gram-positive organisms, with about 10–15% being caused by Gram-negative organisms. An important difference, however, is that in recent series, approximately one-quarter of the infections were due to Bacillus spp., making this the second most common pathogen in most series of post-traumatic endophthalmitis. Most Bacillus infections are associated with intraocular foreign bodies.11–14,41,109,113,114 Unfortunately, infection caused by Bacillus has a particularly poor prognosis, and only two of 25 eyes reported in the literature had a final vision better than being able to count fingers. Fungal infections are also important in series of traumatic endophthalmitis, accounting for 10–15% of the cases; they should be particularly suspected in soil-contaminated injuries. Overall, the reported results of therapy for traumatic endophthalmitis are not as satisfactory as for postoperative endophthalmitis.115 This is most likely due to a higher proportion of cases caused by virulent organisms, the influence of the initial injury on the final visual outcome, as well as potential delay in diagnosis due to post-traumatic inflammation. Although recent series report vision of 20/400 or better for 42–73% of cases of postoperative endophthalmitis, comparable vision following traumatic endophthalmitis is achieved in only 9–50% of cases.41,104,106,109,115,116
Endogenous endophthalmitis
Endogenous endophthalmitis accounts for 5–7% of cases in large mixed series of endophthalmitis. Although cases occur in otherwise healthy patients, most occur in patients with systemic disease, including chronic immune compromising illness such as diabetes mellitus or renal therapy, immunosuppressive disease and therapy, IV drug use, or systemic septicemia.117–120 Patients may present with mild symptoms of decreased vision, redness, pain, and photophobia. The initial diagnosis is not made at the first presentation in 50% of patients.117,119 Bilaterality is common. A search for a systemic focus of infection is indicated when endogenous endophthalmitis is suspected; blood cultures are frequently positive. Assistance of an internist or infectious disease specialist is often sought due to the systemic implications of the condition. Fungal causes are found in 50–62% of cases,117,120 with Candida spp. being the most common isolate in some series and Aspergillus in others.22 In cases of bacterial cause, both Gram-positive and Gram-negative organisms are identified, with the proportions depending on the location of the series.121 Visual outcomes are often poor.119–121 More alarming is the mortality rate, which has been reported to vary from 5%107 to 29%.120
Therapy
For a number of years, antibiotics administered topically, by IV infusion, and by intramuscular injection were the mainstays of therapy for endophthalmitis. Intravenously administered antibiotics alone or in combination with topical medication resulted in few cures. A survey of 103 published cases from 1944 to 1966 revealed that 73% had a final visual result of hand motion or worse.122 The blood–retinal barrier, similar to the blood–brain barrier, effectively blocks significant penetration of antibiotics, particularly hydrophilic ones such as the penicillins, cephalosporins, and aminoglycosides, into the vitreous cavity.123 To provide better antibiotic penetration, frequent subconjunctival injections were subsequently recommended by some authorities.124 This route resulted in somewhat higher, but often insufficient, vitreous levels of certain antibiotics.125–127 To overcome the problem of poor penetration, intravitreal injection of antibiotics was studied by von Sallmann et al.128 and by Leopold,122 with further development by Peyman35 and Forster et al.41 Although there was initial skepticism regarding use of intravitreal antibiotics, the accumulation of experience by various authors has led to their universal acceptance and recommendation in bacterial disease since the 1980s.127
When pars plana vitrectomy became available, a number of potential advantages were recognized.129,130 A significant amount of material may be obtained for culture purposes. Removal of infected vitreous allows the classic principles of incision and drainage to be applied to the eye for the first time. Removing infected material reduces not only the number of living bacteria but also the toxins. Media opacities are cleared more rapidly in those eyes that survive the infection, allowing more rapid restoration of visual function. Maylath and Leopold131 have previously shown that organisms are more effectively cleared from the anterior chamber than from the posterior chamber, and removal of the vitreous allows the vitreous chamber and anterior chamber to become joined in the aphakic eye. Furthermore, it has been suggested that vitreous removal may have a beneficial effect on antibiotic distribution within the eye.129
Antimicrobial therapy
Choice of antimicrobial agent
Microbes causing acute postoperative endophthalmitis are most often the patient’s own bacterial flora. Staphylococcal species account for more than two-thirds of all cases, but Gram-negative organisms are also encountered.3,51 In acute traumatic endophthalmitis, Gram-positive organisms are the most commonly identified, but this includes a high incidence of Bacillus species. In traumatic endophthalmitis, the microbes reflect not only the patient’s flora but also contaminants from the scene of the trauma. Gram-negative infections and mixed infections are encountered more often than in acute postoperative cases.9,11–14,109,115 In delayed postoperative endophthalmitis, Propionibacterium acnes,16–20,134 nonvirulent staphylococci,16,54 and fungi16,65 are most often the causative agents. When the infection is associated with a filtering bleb, Streptococcus species are identified in a high percentage of cases.53,135
1. Bactericidal properties. Because the eye is an immune-privileged site, like the central nervous system, a bactericidal drug rather than bacteriostatic agent is preferred.
2. Broad spectrum of coverage. Coverage must include Gram-positive organisms, especially methicillin-resistant staphylococci and Bacillus species in trauma cases, and Gram-negative organisms.
3. Excellent therapeutic ratio (activity/toxicity) after intravitreal injection. Toxicity has not been well studied for most antibiotics after intravitreal injections. Toxicity is often defined by histologic studies, electron microscopic studies, and electroretinography (ERG) testing. Most antibiotics are tested in rabbits, which are a limited model because of the relative avascularity of rabbit retinas. This relative avascularity may have contributed to the delayed recognition of the vascular occlusive potential of intravitreal aminoglycosides136,137 because of the lack of toxicity studies in primates. Toxicity may be increased by repeat injections of certain antibiotics.
4. Good therapeutic ratio after IV injections. Most antimicrobials penetrate the vitreous cavity poorly after IV injection because of the blood–eye barrier. Intravitreal antimicrobial levels are only rarely reported to reach levels above the minimum inhibitory concentration (MIC) for organisms usually seen in endophthalmitis after IV or oral administration.123,125,138–143 Hydrophilic antibiotics (including aminoglycosides and β-lactam antibiotics) have less potential for penetration into the eye than lipid-soluble compounds. On the other hand, there is significant systemic toxicity to the antimicrobials commonly used in treating endophthalmitis, particularly the aminoglycosides and amphotericin.144 Furthermore, some combinations of antibiotics have a favorable spectrum of coverage (e.g., vancomycin and aminoglycosides), but their toxicities are additive when used simultaneously.
5. Favorable pharmacokinetic properties. Intraocular inflammation enhances penetration of certain antibiotics.123,145–147 Vitrectomy has been shown to enhance the penetration of cefazolin,141 vancomycin,147 and ceftazidime145 into the eye. Repeated IV dosing may contribute to increased penetration into the vitreous cavity after IV administration, particularly in inflamed and previously operated eyes.145–147 After intravitreal administration antibiotics are eliminated through either an anterior or posterior route.132,148 Aminoglycosides are eliminated anteriorly, and the β-lactam antibiotics are removed posteriorly. Vitreous removal shortens the half-life of all antimicrobial agents studied in animal models.132,148,149 Lens removal decreases the half-life of antibiotics eliminated anteriorly.148 Inflammation may increase the half-life of antimicrobials excreted posteriorly, such as cefazolin;149 blocking agents such as probenecid may also increase the half-life of these drugs. The half-life for anteriorly excreted drugs such as gentamicin and amikacin is decreased by inflammation.150,151 A higher initial dose is preferred whenever possible to allow the drug to remain at levels greater than the MICs of common pathogens for a longer period. Known activity of the drug is also an important consideration in the choice of the antibiotics. If drugs are given in equivalent concentrations, the one with higher activity against suspected organisms should be chosen.
Route of administration
Intraocular administration of antibiotics is widely accepted as standard care in endophthalmitis. (Box 122.2) The major limitation of intraocular antimicrobials is the short duration of action. Most antibiotics studied have a drug level greater than the MICs for the common organisms, producing endophthalmitis for only 36–48 hours. Toxicity is also a significant problem. Injected antibiotics may create vascular shutdown (aminoglycosides),137,152,153 retinal damage, and retinal necrosis.154,155 Repeated injections of antibiotics are occasionally administered but may increase the potential for toxicity, as demonstrated by the combination of vancomycin and amikacin.154,155
Systemic antibiotics are recognized to be largely ineffective when used as the only route of administration with the exception of some cases of endogenous endophthalmitis. There is controversy over whether systemic antibiotics should be used, because of their poor penetration into the eye. One study demonstrated cures of endophthalmitis caused by Staphylococcus species and other more virulent organisms with use of IV antibiotics only.156 In the Endophthalmitis Vitrectomy Study, patients given IV antibiotics in conjunction with intraocular antibiotics did not have a better visual outcome than patients given intravitreal antimicrobials alone.3 Another recent study demonstrated that in an animal model of S. aureus endophthalmitis intravitreal vancomycin and amikacin were superior to intravenous imipenem alone, and combination treatment provided no additional benefit to intravitreal administration.157 The IV antibiotic given for Gram-positive coverage was amikacin, which has very poor penetration into the vitreous cavity after IV injection.158 Other antimicrobial drugs, such as vancomycin and cefazolin, gatifloxacin, or moxifloxacin which demonstrate better penetration, may be beneficial in some circumstances.141,143,145,159
Subconjunctival antibiotics, formerly recommended in endophthalmitis treatment124 are currently used as post-surgical prophylaxis. The levels achieved in the vitreous after subconjunctival injection, however, are insignificant in comparison to intravitreal injection and rarely reach therapeutic levels when given alone.125,126
Antibiotic administration as part of the infusion fluid has been recommended as part of vitrectomy procedure. This has the advantage of initiating antibiotic exposure to the organisms somewhat earlier than injection into the vitreous cavity at the close of the surgical procedure. Despite some concerns of retinal toxicity, one recommendation is to place gentamicin (8 mg/mL) into the infusion.160 Because this is approximately one-third of the concentration achieved by injecting 100 mg into a 4-mL eye (25 mg/mL), the peak dosage and effective duration of action are significantly reduced.
Antimicrobial agents
Cephalosporins
The cephalosporins are synthetic penicillins active against the bacterial cell wall. They are well tolerated systemically, and cefazolin has been established to be a relatively safe drug when 2.25 mg is injected intravitreally. All the cephalosporins have good broad-spectrum coverage for Gram-positive and some Gram-negative organisms, but the first-generation drugs are weak against enterococcus and meticillin-resistant staphylococcal organisms. Injection of cefazolin (2.25 mg) into the aphakic eye produces levels greater than the MICs for approximately 48 hours.149 Penetration in inflamed vitrectomized eyes can be achieved after repeated IV dosages, and cefazolin reaches levels well above the MICs for sensitive organisms.141 Ceftazidime is a promising antibiotic for Gram-negative coverage in endophthalmitis therapy because it has good cerebrospinal fluid penetration and excellent Pseudomonas coverage. In a study of 37 Gram-negative isolates from cases of endophthalmitis, 80% were susceptible to ceftazidime.22 Initial reports indicate an excellent therapeutic ratio after intraocular injection.161,162
Vancomycin
Vancomycin has been recommended as the antibiotic of choice for Gram-positive coverage.163–165 In a study of 246 Gram-positive isolates from cases of human endophthalmitis, 100% were susceptible to vancomycin.22 Its coverage is purely Gram-positive, but its spectrum includes all of the staphylococcal species, Bacillus, and P. acnes. The mechanism of vancomycin is inhibition of cell wall assembly, in addition to damaging protoplasts and inhibiting RNA synthesis. The intraocular therapeutic ratio for vancomycin is good, although the half-life suggests that therapeutic concentrations will be maintained for only about 48 hours after intravitreal injections.166,167 Vitreous sampling after intraocular injection in human infection has suggested that potentially therapeutic levels may persist for 3–4 days after initial injection depending on the initial dose.74,168 Systemic toxicity is seen after high IV dosages and is unfortunately additive with IV aminoglycosides. Penetration into the vitreous cavity of inflamed eyes after IV injection is sufficient to be above the MIC for most pertinent pathogens after repeated dosing in animal models,147 but produces variable concentrations in humans after a single dose.169
Aminoglycosides
Aminoglycosides have a spectrum that includes both Gram-positive and Gram-negative organisms. They are chosen particularly for their Gram-negative coverage in endophthalmitis. The mechanism of action for aminoglycosides is to inhibit protein synthesis. Unfortunately, the intraocular therapeutic ratio after intraocular injection is a source of problems.136,137,152,153 Retinal vascular infarction has been frequently reported after gentamicin,30 and it has also been noted after amikacin administration.136 Tolerated dosages may be higher for amikacin than for gentamicin, but all of the aminoglycosides cause retinal changes after higher intravitreal dosages.170–172 The half-life of amikacin is approximately 8 hours in inflamed, vitrectomized eyes.151 Because of the limitations in the amount given for the initial dosage, the concentration of these antibiotics remains above the MIC for only 24–36 hours after administration. The therapeutic ratio for treatment of ocular disease after IV administration is also unfavorable because of systemic toxicity. Penetration of gentamicin into the eye after IV administration has been studied in both rabbits173 and humans.174 It does not reach therapeutic levels in traumatized rabbit eyes,158 normal rabbit eyes, or human eyes with various ocular diseases after single doses.174
Fluoroquinolones
The quinolones are broad-spectrum antibiotics with both Gram-positive and Gram-negative coverage. Their mechanism of action is thought to be inhibition of DNA synthesis. The second-generation drugs are ciprofloxacin and ofloxacin, while levofloxacin is a third-generation agent. The fourth-generation drugs, gatifloxacin and moxifloxacin, have significant potential in the prophylaxis and treatment of endophthalmitis. Initial reports of the therapeutic ratio of ciprofloxacin after intraocular injection suggest that intraocular toxicity occurs at low dosage levels.175,176 Fluoroquinolones penetrate the blood–ocular barrier more readily than do several of the other classes of antimicrobials. Ciprofloxacin has reasonable penetration after oral administration, but many ocular pathogens have developed resistance to it.22 After two doses of oral administration levofloxacin achieves concentrations in the aqueous and vitreous above the MIC (90) for many Gram-positive and Gram-negative pathogens but not for Pseudomonas aeruginosa.175 Studies of penetration of gatifloxacin and moxifloxacin into noninflamed eyes undergoing vitreous surgery after oral administration of two doses demonstrated that the percentages of serum concentrations achieved in the vitreous and aqueous were 26.17% and 21.01%, respectively. These levels are above the MIC (90) for most of the pathogens producing human disease. These include: Staphylococcus epidermidis, S. aureus, Streptococcus pneumoniae, S. pyogenes, Enterococcus faecalis, Proteus mirabilis, Escherichia coli, and Propionibacterium acnes, among others. Notably, however, neither agent achieved vitreous MIC (90) for Pseudomonas aeruginosa and moxifloxacin did not reach the MIC (90) for Bacteroides fragilis.111,139,143 Because two genetic alterations in the target bacteria must occur for resistance to emerge to the fourth-generation drugs, the rapid development of resistance noted for ciprofloxacin may be avoided.
Antifungal agents
Amphotericin has been considered the gold standard in antifungal therapy. Its mechanism of action is the alteration of membrane permeability by combination with sterols and fungal cytoplasmic membranes. The intraocular therapeutic ratio has not been well studied, but the usual recommended dosage is 5 µg/mL.177 After IV administration, there are significant systemic complications, including renal toxicity. Penetration into the eye is also relatively poor. After intraocular injection, the half-life has been reported to be 9.1 days. The half-life is further decreased by inflammation and vitreous removal.177 Vitrectomy and oral fluconazole have been reported to treat Candida endophthalmitis successfully, with fewer side-effects.28 Fluconazole has significant penetration into the noninflamed eye after oral administration.178 Voriconazole is a triazole antifungal agent which is a second-generation synthetic derivative of fluconazole. It demonstrates a broad spectrum of action, including Aspergillus species, Candida species, and Paecilomyces, and has a low MIC (90) for many organisms. After oral administration, potentially therapeutic levels are achieved in aqueous and vitreous in noninflamed eyes.140 Uses of intravitreal voriconazole for fungal endophthalmitis have been reported.179,180
Pars plana vitrectomy
Pars plana vitrectomy plays a role in many phases of endophthalmitis therapy. As initial therapy it is validated by the Endophthalmitis Vitrectomy Study results only for acute post-cataract extraction infections in eyes presenting with vision of hand motions or less. This recommendation should not be generalized to infection associated with filtering blebs, endogenous endophthalmitis, posttraumatic endophthalmitis, or even chronic or delayed-onset endophthalmitis in which the clinical circumstances, and, most importantly, the causative organisms, are likely to be different.181 In addition to use as initial therapy in many of these clinical settings, pars plana vitrectomy should also be considered for eyes not responding to an original tap-and-inject strategy, and may be necessary to clear vitreous opacities in eyes cured of infection when spontaneous clearing does not occur.
Acute postoperative endophthalmitis after cataract surgery
The Endophthalmitis Vitrectomy Study was designed to address the issue of the relative efficacy of vitrectomy and intraocular antibiotic injection in the treatment of acute endophthalmitis after cataract surgery compared with initial diagnostic tap and injection of antibiotics alone.3,182 In this study, patients with acute postoperative endophthalmitis were randomized to one of the two strategies for initial management. Patients with a progressive downhill course after tap and injection were allowed to have a vitrectomy procedure. As a second randomization, patients in each group were assigned to either IV antibiotic therapy or no IV antibiotic therapy. A total of 420 patients with clinical evidence of endophthalmitis within 6 weeks of cataract surgery or secondary IOL implantation were included. Visual acuity was evaluated 9 months after the initial intervention.3
In this study, 30.7% of the eyes were culture-negative. Of the 291 culture-positive cases, the isolates identified were as follows: Gram-positive, coagulase-negative micrococci 70%; Staphylococcus aureus 9%; Streptococcus species 9%; Enterococcus species 2.2%; Gram-negative species 5.9%.51,52 The initial vision was an important determinant of outcome. In eyes presenting with vision of hand motions or better there was no difference in visual outcome regardless of whether an immediate vitrectomy was performed. In patients with initial vision of light perception, eyes treated by immediate vitrectomy had a threefold increase in the frequency of achieving 20/40 or better acuity (33% versus 11%), approximately a twofold chance of achieving 20/100 or better (56% versus 30%), and a 50% decrease in the frequency of severe visual loss (20% versus 47%) compared with an initial tap-and-inject strategy. Eyes treated with systemic antibiotics did not show improved outcomes compared with those not receiving them. Thus the study recommended that vitrectomy be reserved as an initial treatment strategy for those eyes presenting with light perception vision.3 Subsequent evaluation of the data suggested that patients with diabetes mellitus had a better outcome with an initial strategy of vitrectomy and antibiotic injection regardless of presenting vision. The conclusions of this retrospective analysis did not reach a level of statistical significance to allow the authors to make a firm recommendation that surgery should be the initial intervention in all diabetic patients.183
Traumatic endophthalmitis
Traumatic endophthalmitis accounts for approximately 25% of all cases of intraocular infection. These cases create difficult therapeutic problems because of the effects of the injury and the wider, more virulent spectrum of bacteria that are involved in more traumatic infections than in postoperative endophthalmitis.9,11–14,109,115
Bacillus species are commonly identified after injuries involving farm materials and may be the causative organism in approximately 25% of cases, depending on the environment of the injury.11 Rates of infection after trauma vary from 2–3% after penetrating injuries to 11–17% with industrial foreign bodies109,110 to 30% in injuries occurring in rural environments.11 Vitrectomy has been recommended because of the severity of the injuries, severity of infection, and the more adverse outcome reported in these cases.115 Vitrectomy allows treatment of the residual intraocular effects of the trauma, such as retained lens cortex, vitreous hemorrhage, and retinal breaks, as well as allowing removal of infected vitreous, bacteria, and toxins.
Chronic postoperative endophthalmitis
The syndrome of chronic or delayed-onset postoperative endophthalmitis has been increasingly recognized. Causative organisms of these cases include Propionibacterium acnes,15,17–20,134 fungal cases (particularly Candida parapsilosis),16,65 and nonvirulent forms of Staphylococcus epidermidis.16,54 The onset is usually days to weeks after surgery, and the clinical manifestation is one of chronic, indolent inflammation, often initially responding to suppression by topical corticosteroid therapy. P. acnes often produces a granulomatous inflammation, usually beginning 4–8 weeks after surgery. It characteristically manifests as a white plaque on the lens capsule. Fungal cases have less specific findings, and the diagnosis is often made by Gram stain, Giemsa stain, and culture. Cooperation with the microbiology department is important in these cases so that appropriate measures can be taken for the proper identification of the organisms. Cultures should be kept for at least 2 weeks, particularly for P. acnes because these organisms may grow slowly. Surgery is recommended in these cases because the slow growth of the organisms makes sterilization more likely after their surgical removal than intraocular antibiotic injection alone.
It is thought to be necessary to remove the white plaque on the lens capsule in cases of P. acnes, and in some cases the capsule itself along with the IOL.15,16,19 High rates of recurrence are noted when only intraocular antibiotics are injected, and persistent disease occurs in a significant percentage of patients even when the capsule is removed. Complete capsulectomy and IOL exchange is almost always successful in eradicating infection either as the initial intervention or as a secondary procedure.15,16,19 Recurrent inflammation and persistent infection are not uncommon, and secondary procedures are often necessary in both P. acnes and fungal infection some weeks after the initial surgery. Recommended antimicrobial therapy includes vancomycin for P. acnes and intraocular amphotericin for fungi; imidazoles, including ketoconazole, fluconazole, or voriconazole may be of benefit.15,16,140
Bleb-associated endophthalmitis
Bleb-associated endophthalmitis may be seen after cataract extraction or after filtering procedures.15,53,77,83,135 It typically occurs long after the initial surgery and is preceded by a period of irritation and redness of the eye. The classic initial finding is “white on red,” because the white bleb filled with inflammatory material is highlighted against the redness of the conjunctiva. Streptococcus is the infecting organism in as many as 60% of these cases. In general, bleb-associated endophthalmitis has a poor visual outcome, leading to a recommendation for initial vitrectomy in these cases.53,135 However, in some cases, particularly in phakic eyes, the initial infection may be confined to the anterior segment (“blebitis”), so systemic and intensive topical antibiotics may achieve good therapeutic levels in the anterior chamber and aqueous, thus curing the condition without vitrectomy surgery or intravitreal antimicrobial injection.184,185
Endogenous endophthalmitis
Endogenous endophthalmitis is often associated with significant systemic illness or IV drug use. In these cases it is important to search for the cause of the endophthalmitis because it is secondary to infection elsewhere and may be associated with a life-threatening condition. Repeated blood cultures and a multidisciplinary approach are often helpful for locating the source of infection.117,119–121,186 Systemic therapy may be sufficient in some cases if the vitreous cavity is not heavily involved. Vitrectomy has the advantage of both obtaining a reasonable amount of material for cytologic and microbiologic studies to make the diagnosis and allowing removal of the offending organisms. IV medication may penetrate at the site of invasion through the eye wall, but when organisms proliferate within the vitreous cavity, vitrectomy is more often needed. A literature review by Jackson et al reported a three times better chance of retaining useful vision and avoiding evisceration or enucleation if vitrectomy is performed.187
If fungal disease is strongly suspected, most authors41,129,188 concur that therapeutic vitrectomy is the treatment of choice if the vitreous is significantly involved, although systemic therapy may be sufficient in early endogenous disease. Vitrectomy has also been employed in chronic progressive inflammatory disorders that ultimately prove to be caused by fungus, such as Cryptococcus.189 In these instances the indications are diagnostic, as well as therapeutic.
Parasitic diseases may produce a chronic endophthalmitis with both acute components and secondary complications, such as retinal detachment, vitreous opacity, and cataract. In these instances the acute or active stages of the infection have been the indications for surgical intervention for some authors,190 although the chronic sequelae are more common indications for surgery in Toxocara canis and toxoplasmosis-related endophthalmitis.
Preoperative evaluation
A careful and extensive history should be taken. Clinical details such as systemic infectious disease, type of eye injury, or previous surgery may hold important clues to the identity of the infecting organism. Particular attention should be paid to the length of time from the surgical insult or trauma to the onset of symptoms and to the time that has passed since symptoms began. Previous antibiotic or corticosteroid therapy should be noted. A thorough ocular examination should include a careful search for any possible route of entry for the infecting organism. The effects of the inflammation should also be noted: corneal clarity and thickness, condition of any surgical wound, degree of anterior-chamber reaction, hypopyon, clarity of the vitreous, visibility of the retina, and presence or absence of a red reflex. Standardized Ultrasonography can define the degree of condensation of the vitreous, determine whether the retina is attached, and identify choroidal swelling.191 Preoperative ERG findings may have a predictive value for postoperative visual result, but this has not yet been well defined.107,191
Surgical techniques
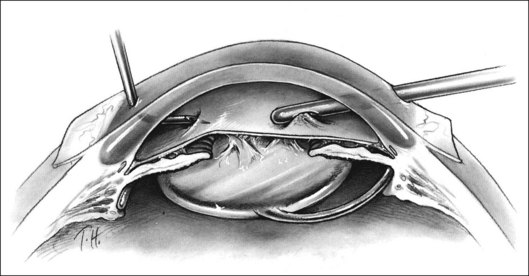
The anterior chamber often contains significant amounts of fibrin and hypopyon. Because the cornea invariably has some combination of epithelial edema, folds, and cells deposited on the posterior surface, the iris and central anterior vitreous are often impossible to visualize adequately. Initial incisions may be made in the limbus at approximately the 9.30 and 2.30 clock positions, modifying the location as necessary depending on the condition of the previous surgical wound and on the presence of a filtering bleb. Fluid is infused into the anterior chamber as inflammatory debris is removed with the suction and cutting instrument (Fig. 122.1). This may also be accomplished with a single incision and a small-gauge instrument combining infusion, cutting, and suction. The use of a single incision reduces the flexibility of the surgical approach, however. When two incisions are used and when it is necessary to switch the cutting instrument from one site to the other, it is useful to remove the cutting instrument from its site and then replace it with a second infusion on a blunt needle the same size as the cutting instrument. Only then is the initial infusion removed and the cutting instrument replaced in its site. This allows the pressure to be maintained at a constant level, minimizing the chances of hemorrhage and making passage of instruments through the limbal incisions easier.
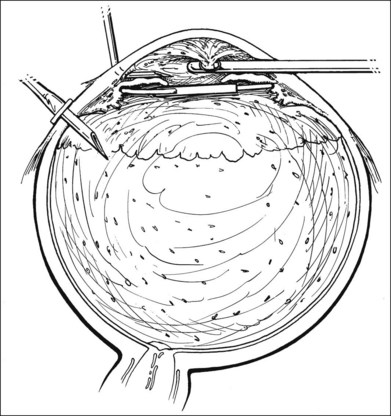
Fig. 122.1 Opaque material being removed from the anterior chamber; active infusion into the anterior chamber.
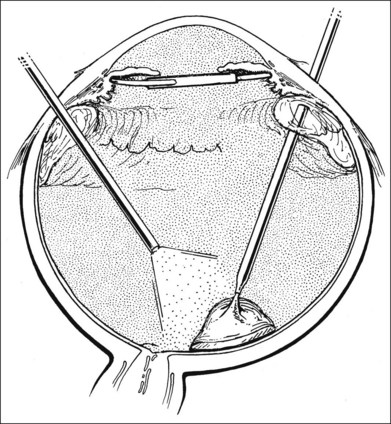
An inflammatory membrane usually extends continuously over the lens or pseudophakos and on to the surface of the iris. When a pseudophakos is present, the lens need not be removed; attempting to do so may increase the risk of bleeding. The inflammatory membrane, however, should be removed from its surface for better visualization of the posterior segment. It may be initially incised with a myringotomy blade or other sharp needle and then elevated for removal with a cutting instrument (Fig. 122.2). It may also be engaged with a hooked needle and rolled on to the needle. Removal of an inflammatory membrane from the crystalline lens should begin over the iris, close to the pupillary border, if it is believed that the lens can be spared. Often, because of poor dilation of the pupil and poor visualization of the internal structures, the lens in phakic eyes must be removed. The fastest way to accomplish this is with fragmentation through pars plana incisions, although young, soft lenses can often be removed with cutting instruments.
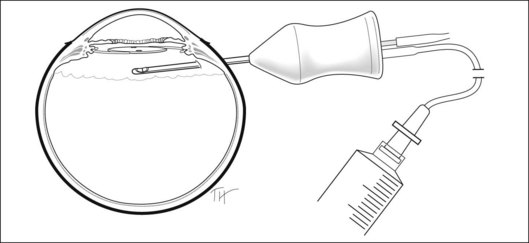
Material for culture and stain should be removed from the eye early in the case. Because anterior-chamber samples frequently do not render positive culture results, attention should be directed to obtaining an adequate vitreous sample. In most surgical setups, the tubing that comes from the suction–cutting portion of the instrument can be opened. Alternatively, a very short piece of tubing is attached to the egress port of the vitrectomy probe (Fig. 122.3). A sterile syringe is connected, and the vitreous is withdrawn with manual suction. Approximately 0.2 mL is removed before starting infusion into the eye to obtain an undiluted sample. The material is then immediately sent to the laboratory for Gram- and Giemsa-stain as well as cultures on blood agar, chocolate agar, brain–heart infusion, and Sabouraud’s media or broth and in thioglycolate broth. It is important to obtain the specimens for culture before any antibiotics are injected into the eye.
The vitrectomy is now progressively carried posteriorly. The vitreous removal is performed initially in the center of the vitreous cavity. Pockets of more heavily infiltrated vitreous are sometimes located; in the aphakic eye, peripheral depression may be used to bring these into view. Aggressive removal of all infiltrated vitreous in the basal area should not be attempted because this often results in retinal tears. The presence of a posterior vitreous detachment, on the other hand, allows more complete vitreous removal. If the vitreous is still attached, a judgment must be made about the amount of vitreous to be removed. The cutting of vitreous adjacent to inflamed or necrotic retina will often cause retinal breaks; these are difficult to seal and may result in failure of the case. In eyes with posterior vitreous detachment, a white mound of inflammatory debris may be visible over the posterior pole. This should be approached with care and may be gently aspirated into the cutting port. If the mound proves to be solid and adherent, small amounts can usually be removed, but in most cases it is unwise to attempt to remove large portions. In some instances the material is flocculent and equivalent to an unorganized hypopyon; this can be gently sucked up with vacuum techniques (Fig. 122.4).
Postoperative management
Not all infections are cured by a single dose of injected antimicrobial.3,133,192–197 If the inflammation appears to worsen or does not respond as well as expected, particularly if it is associated with persistent pain, the physician should suspect that the infectious process remains active. The index of suspicion should be highest for streptococcal species and Gram-negative organisms; Gram-positive coagulase-negative micrococci usually respond to initial therapy. A repeat tap and injection of antibiotics, chosen on the basis of the culture results, should be considered; if the media appears significantly opaque, or if the initial therapy was only injection of antibiotics, a vitrectomy may be considered, particularly if most of the vitreous was not removed during the initial procedure. If the initial culture sensitivities show that the organism is resistant to the antibiotic originally injected, injection of an appropriate antibiotic is strongly recommended. In the Endophthalmitis Vitrectomy Study 8% of eyes were subjected to early secondary intervention.3
IV aminoglycosides have not been demonstrated to be effective in improving outcome, but other antimicrobials with better penetration into the vitreous cavity after IV administration may be considered in some cases. Vancomycin,147 ceftazidime,143,145 cefazolin,140,145 or a fluoroquinolone139,143,147 may be useful when a longer duration of antimicrobial effect is desired than the 24–48 hours provided by intravitreal injection.
Second operations are frequent in patients with endophthalmitis. In the Endophthalmitis Vitrectomy Study, 35% of all eyes needed some secondary procedure.3,145 Opacities in the vitreous cavity may continue to interfere with vision, even if the eye responds well in terms of inflammatory signs. The retina should be monitored at regular intervals with ultrasound if the surgeon cannot be sure by indirect ophthalmoscopy that it remains attached. Removal of these opacities may be undertaken with a repeat vitrectomy as an elective procedure once the eye becomes quiet.
Control of inflammation
Corticosteroid administration by other routes is often chosen as part of the therapeutic plan. All patients in the Endophthalmitis Vitrectomy Study received systemic prednisone 30 mg twice daily for 5–10 days.3 Systemically administered corticosteroids have been demonstrated to reduce inflammation independently of the effects of surgery in animal models of treatment of Staphylococcus epidermidis endophthalmitis.198,199 Intraocular corticosteroid administration was first advocated by Peyman and others200–202 in treatment of animal models of endophthalmitis. In treatment of a rabbit model of S. epidermidis endophthalmitis, Meredith et al.199 demonstrated a beneficial effect of intraocular corticosteroid administration that was equivalent but not superior to systemic administration. Histopathologic studies of treatment of a similar model by Maxwell et al.203 also demonstrated a beneficial effect of intraocular corticosteroid administration. When intraocular corticosteroids were administered after vitrectomy and intraocular antibiotic injection in a model of S. aureus endophthalmitis, however, there was an increase in inflammatory scores, corneal opacity, and the number of eyes developing retinal necrosis.192 These results suggest caution in intraocular corticosteroid use because a beneficial effect may not result in all cases of endophthalmitis. There is a number of prospective, randomized204–206 and retrospective207,208 human series that demonstrate earlier reduction of intraocular inflammation after injection of intravitreal dexamethasone but there appears to be no lasting benefit on visual outcome compared to controls. However, one retrospective series found a significantly reduced likelihood of achieving a three-line improvement in visual acuity among patients receiving intravitreal corticoteroids.207
Complications
Retinal detachment is a feared complication of vitrectomy for endophthalmitis. Retinal detachment occurred in 8.3% of eyes in the EVS.209 Tears that occur at the time of surgery are managed as outlined earlier. Unrecognized intraoperative tears, such as entry-site tears, can result in a detachment soon after surgery. Necrotic retina may also break down, creating an atrophic retinal break. Standard buckling procedures may help in many cases, but these may be difficult to perform because of the inability to see the fundus clearly on account of corneal opacity, poor dilation of the pupil, persistent opacity of the media, haze on the surface of an IOL, or opacification of the vitreous base. These retinal detachments can sometimes be repaired successfully, but they are reportedly the major cause of failure in most series.210 Anatomic success was achieved in 78% of the cases in the Endophthalmitis Vitrectomy Study, but the occurrence of detachment was correlated with a poor visual outcome.209 Proliferative vitreoretinopathy is a major risk in eyes with detachment; sympathetic ophthalmia has also been reported.29
Despite anatomic success, some eyes see poorly. A rough correlation exists between poor visual results and an abnormal ERG, suggesting that these eyes have sustained extensive damage to the retina.107
Postoperatively a small percentage of eyes injected with aminoglycosides at surgery develop whitening of the macular area with intraretinal hemorrhages in the posterior pole. Fluorescein angiography demonstrates shutdown of the capillaries and arterioles supplying the macula and vision is frequently poor.137,152 Histologic examination of similar-appearing lesions produced experimentally in primates by injection of gentamicin shows extensive destruction of the nerve fiber layer.170
Results
The outcome of treatment for postoperative endophthalmitis began to improve dramatically during the 1980s. Factors in this improved outcome include: (1) higher incidence of endophthalmitis produced by less virulent organisms;3,5,122 (2) earlier diagnosis and treatment; (3) widespread acceptance of intra-vitreal antibiotic therapy; (4) employment of vitrectomy surgery; and (5) control of the inflammation with corticosteroids.
At this time, the visual outcome after treatment of postoperative endophthalmitis is better than in other forms of the disease. Nine months after therapeutic intervention in the Endophthalmitis Vitrectomy Study, 53% of the patients achieved visual acuity of 20/40 or better and 74% achieved 20/100 or better; 15% of eyes were equal to or worse than 5/200 and 5% had no light perception. A significantly greater percentage of patients in the vitrectomy group had clear media by the 3-month follow-up visit (86% versus 75%).3,5 There was a significant difference in outcome depending on the infecting organism. The rates of achieving final visual acuity of 20/100 or better were as follows: Gram-positive, coagulase-negative micrococci, 84%; Gram-negative organisms, 14%; Staphylococcus aureus, 50%; Streptococci, 30%; and Enterococci, 14%. Both a positive Gram-stain and infection with species other than Gram-positive coagulase-negative micrococci were associated with a significantly worse outcome.3,51
A number of prognostic factors have been proposed, the most important of which is probably the virulence of the infecting organism, as demonstrated in the EVS. The percentage of eyes achieving at least 20/400 vision is relatively high in cases in which the culture obtained is negative (53–94%),1–4,7,51,68,116,211 when Staphylococcus epidermidis is the infecting organism (65–91%),2,8,23,107,135 or if Propionibacterium acnes or fungi is the infecting organism. In endophthalmitis caused by Gram-negative organisms and streptococci, vision of 20/400 or better is reported in 40–50% of cases. When Pseudomonas aeruginosa or Bacillus organisms are the infecting microbes, salvage of useful vision is almost never reported.12,13,21,104 Delay of therapy for more than 36 hours after onset of symptoms has been reported to be associated with poor visual outcome in one series, whereas delay of more than 24 hours after onset of symptoms in eyes, in which vitrectomy was performed, was associated with a worse outcome in others.114,212 In animal experiments, administration of antibiotics in a model of Pseudomonas endophthalmitis 24 hours after bacterial injection produced sterilization of the vitreous cavity, whereas later injection did not.193
Visual acuity at initial manifestation has also been correlated with outcome. In one series, initial vision of light perception infrequently improved to acuity of 20/400 or better (21% of cases), whereas 87% of cases with initial acuity of 20/400 or better had this level of vision after therapy. In the Endophthalmitis Vitrectomy Study 33% of eyes with initial vision of light perception achieved 20/40 when treated with immediate vitrectomy.3 Cases associated with other ocular diseases such as damage from trauma and proliferative vitreoretinopathy tend to have less frequent achievement of 20/400 vision postoperatively.104
Future directions
Improved control of inflammation initiated by the infection will be critical to improve the visual outcome. This will include expanded understanding of the role of corticosteroids in endophthalmitis treatment and a definition of the best route of administration. Other means of reducing intraocular inflammation, particularly in Bacillus and Pseudomonas infections, must be devised and may include repeat surgery or lavage. Blockage of toxins that mediate intraocular damage may be developed clinically.195 Finally, rapid diagnostic systems that allow immediate identification of bacteria and more specifically targeted antimicrobial therapy should further increase the efficacy of treatment.
1 Bode DD, Jr., Gelender H, Forster RK. A retrospective review of endophthalmitis due to coagulase-negative staphylococci. Br J Ophthalmol. 1985;69:915–919.
2 Driebe WT, Jr., Mandelbaum S, Forster RK, et al. Pseudophakic endophthalmitis: diagnosis and management. Ophthalmology. 1986;93:442–448.
3 Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol. 1995;113:1479–1496.
4 Ficker LA, Meredith TA, Wilson LA, et al. The role of vitrectomy in Staphylococcus epidermidis endophthalmitis. Br J Ophthalmol. 1987;72:386–389.
5 Kattan HM, Flynn HW, Pflugfelder SC, et al. Nosocomial endophthalmitis survey: current incidence of infection following intraocular surgery. Ophthalmology. 1991;98:227–238.
6 Valenton MJ, Brubaker RF, Allen HF. Staphylococcus epidermidis (albus) endophthalmitis. Arch Ophthalmol. 1973;89:94–96.
7 Weber DJ, Hoffman KL, Thoft RA, et al. Endophthalmitis following intraocular lens implantation: report of 30 cases and review of the literature. Rev Infect Dis. 1986;8:12–20.
8 Mao LK, Flynn HW, Jr., Miller D, et al. Endophthalmitis caused by streptococcal species. Arch Ophthalmol. 1992;110:798–801.
9 Hamady R, Zaltas M, Paton B. Bacillus-induced endophthalmitis: new series of 10 cases and review of the literature. Br J Ophthalmol. 1990;74:26–29.
10 Beecher DJ, Pulido JS, Barney NP, et al. Extracellular virulence factors in Bacillus cereus endophthalmitis: methods and implication of involvement of hemolysin BL. Infect Immun. 1995;63:632–639.
11 Boldt HC, Pulido JS, Blodi CS, et al. Rural endophthalmitis. Ophthalmology. 1989;96:1722–1726.
12 Davey RT, Tauber WB. Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev Infect Dis. 1987;9:110–123.
13 O’Day DM, Smith RS, Gregg CR, et al. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838.
14 Schemmer GB, Driebe WT. Post-traumatic Bacillus cereus endophthalmitis. Arch Ophthalmol. 1987;105:342–344.
15 Aldave AJ, Stein JD, Deramo VA, et al. Treatment strategies for postoperative Propionibacterium acnes endophthalmitis. Ophthalmology. 1999;106:2395–2401.
16 Fox GM, Jooneph BC, Flynn HW. Delayed-onset pseudophakic endophthalmitis. Am J Ophthalmol. 1991;111:163–173.
17 Meisler DM, Palestine AG, Vastine DW. Chronic Propionibacterium endophthalmitis after extracapsular cataract extraction and intraocular lens implantation. Am J Ophthalmol. 1986;102:733–739.
18 Meisler DM, Zakov ZN, Bruner WE, et al. Endophthalmitis associated with sequestered intraocular Propionibacterium acnes. Am J Ophthalmol. 1987;104:428–429.
19 Winward KE, Pflugfelder SC, Flynn HW, et al. Postoperative Propionibacterium endophthalmitis. Treatment strategies and long-term results. Ophthalmology. 1993;100:447–451.
20 Zambrano W, Flynn HW, Jr., Pflugfelder SC, et al. Management options for Propionibacterium acnes endophthalmitis. Ophthalmology. 1989;96:1100–1105.
21 Eifrig CW, Scott IU, Flynn HW, Jr., et al. Endophthalmitis caused by Pseudomonas aeruginosa. Ophthalmology. 2003;110:1714–1717.
22 Benz MS, Scott IU, Flynn HW, et al. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42.
23 Irvine WD, Flynn HW, Jr., Miller D, et al. Endophthalmitis caused by Gram-negative organisms. Arch Ophthalmol. 1992;110:1450–1454.
24 Aguilar GL, Blumenkrantz MS, Egbert PR, et al. Candida endophthalmitis after intravenous drug abuse. Arch Ophthalmol. 1979;97:96–100.
25 Elliot JH, O’Day DM, Gutow GS. Mycotic endophthalmitis in drug abusers. Am J Ophthalmol. 1979;88:66–72.
26 Parke DW, II., Jones DB, Gentry LO. Endogenous endophthalmitis among patients with candidemia. Ophthalmology. 1982;89:789–795.
27 Stern WH, Tamura E, Jacobs RA, et al. Epidemic postsurgical Candida parapsilosis endophthalmitis: clinical findings and management of 15 consecutive cases. Ophthalmology. 1985;92:1701–1709.
28 Christmas NJ, Smiddy WE. Vitrectomy and systemic fluconazole for treatment of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1996;27:1012–1018.
29 Croxatto JO, Galentine P, Cupples HP, et al. Sympathetic ophthalmia after pars plana vitrectomy–lensectomy for endogenous bacterial endophthalmitis. Am J Ophthalmol. 1981;91:342–346.
30 Hagler WS, Pollard Z, Jarrett WH, et al. Results of surgery for ocular Toxocara canis. Ophthalmology. 1981;88:1081–1086.
31 Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005;20(3):129–141.
32 Meyers-Elliott RH, Dethlefs BA. Experimental Klebsiella-induced endophthalmitis in the rabbit. Arch Ophthalmol. 1982;100:1959–1963.
33 Davey P, Barza M, Peckman C. Spontaneous inhibition of bacterial growth in experimental Gram-negative endophthalmitis. Invest Ophthalmol Vis Sci. 1987;28:867–873.
34 Meredith TA, Trabelsi A, Miller MJ, et al. Spontaneous sterilization of experimental Staphylococcus epidermidis endophthalmitis. Invest Ophthalmol Vis Sci. 1990;31:181–186.
35 Peyman GA. Antibiotic administration in the treatment of bacterial endophthalmitis. II. Intravitreal injections. Surv Ophthalmol. 1977;21:332–346.
36 Beyer TL, O’Donnell FE, Gonclaves V, et al. Role of the posterior capsule in the prevention of postoperative bacterial endophthalmitis. Br J Ophthalmol. 1985;69:841–846.
37 Ormerod D, Koh K, Juarez RS, et al. Anaerobic bacterial endophthalmitis in the rabbit. Invest Ophthalmol Vis Sci. 1986;27:115–118.
38 Eifrig CW, Flynn HW, Jr., Scott IU, et al. Acute-onset postoperative endophthalmitis: review of incidence and visual outcomes (1995–2001). Ophthalmic Surg Lasers. 2002;33:373–378.
39 Bannerman TL, Rhoden DL, McAllister SK, et al. The source of coagulase-negative staphylococci in the Endophthalmitis Vitrectomy Study. A comparison of eyelid and intraocular isolates using pulsed-field gel electrophoresis. Arch Ophthalmol. 1997;115:357–361.
40 Speaker MG, Milch FA, Shah MK. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639–649.
41 Forster RK, Abbott RL, Gelender H. Management of infectious endophthalmitis. Ophthalmology. 1980;87:313–318.
42 Allen HF. Symposium: postoperative endophthalmitis. Introduction: incidence and etiology. Ophthalmology. 1978;85:317–319.
43 Bohigian G. A study of the incidence of culture-positive endophthalmitis after cataract surgery in an ambulatory care center. Ophthalmic Surg Lasers. 1999;30:295–298.
44 Lalwani GA, Flynn HW, Jr., Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996–2005). Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology. 2008;115(3):473–476.
45 Montan P, Lundstrom M, Stenevi U, et al. Endophthalmitis following cataract surgery in Sweden. The 1998 national prospective survey. Acta Ophthalmol Scand. 2002;80:258–261.
46 Sandvig KU, Dannevig L. Postoperative endophthalmitis: establishment and results of a national registry. J Cataract Refract Surg. 2003;29:1273–1280.
47 Packer AJ, Weingeist TA, Abrams GW. Retinal periphlebitis as an early sign of bacterial endophthalmitis. Am J Ophthalmol. 1983;96:66–71.
48 Mandelbaum S, Forster RK. Postoperative endophthalmitis. Int Ophthalmol Clin. 1987;27:95–106.
49 Ormerod LD, Becker LE, Cruise RJ, et al. Endophthalmitis caused by the coagulase-negative staphylococci 2. Factors influencing presentation after cataract surgery. Ophthalmology. 1993;100:724–729.
50 Wisniewski SR, Capone A, Kelsey SF, et al. Characteristics after cataract extraction or secondary lens implantation among patients screened for the Endophthalmitis Vitrectomy Study. Ophthalmology. 2000;107:1274–1282.
51 Endophthalmitis Vitrectomy Study Group. Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:830–846.
52 Han DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:1–17.
53 Mandelbaum S, Forster RK, Gelender H, et al. Late onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985;92:964–972.
54 Ficker L, Meredith TA, Wilson LA, et al. Chronic bacterial endophthalmitis. Am J Ophthalmol. 1987;103:745–748.
55 Beatty RF, Robin JB, Trousdale MD, et al. Anaerobic endophthalmitis caused by Propionibacterium acnes. Ophthalmology. 1986;101:114–116.
56 Wallin T, Parker J, Jin Y, et al. Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg. 2005;31:735–741.
57 Ormerod LD, Ho DD, Becker LE, et al. Endophthalmitis caused by the coagulase-negative staphylococci. 1. Disease spectrum and outcome. Ophthalmology. 1993;100:715–723.
58 Gelender H. Bacterial endophthalmitis following cutting of sutures after cataract surgery. Am J Ophthalmol. 1982;94:528–533.
59 Cooper BA, Holekamp NM, Bohigian G, et al. Case-control study of endophthalmitis after cataract surgery comparing scleral tunnel and clear corneal wounds. Am J Ophthalmol. 2003;136:300–305.
60 Oshika T, Hatano H, Kuwayama Y, et al. Incidence of endophthalmitis after cataract surgery in Japan. Acta Ophthalmol Scand. 2007;85(8):848–851.
61 Miller JJ, Scott IU, Flynn HW, Jr., et al. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139(6):983–987.
62 Nagaki Y, Hayasaka S, Kadoi C, et al. Bacterial endophthalmitis after small-incision cataract surgery. Effect of incision placement and intraocular lens type. J Cataract Refract Surg. 2003;29:20–26.
63 Scott IU, Flynn HW, Feuer WJ. Endophthalmitis after secondary intraocular lens implantation. Ophthalmology. 1995;102:1925–1931.
64 McCray E, Rampell N, Solomon SL, et al. Outbreak of Candida parapsilosis endophthalmitis after cataract extraction and intraocular lens implantation. J Clin Microbiol. 1986;24:625–628.
65 Pettit TH, Olson RJ, Foos RY, et al. Fungal endophthalmitis following intraocular lens implantation: a surgical epidemic. Arch Ophthalmol. 1980;98:1025–1039.
66 Hoffmann KK, Weber DJ, Gergen MF, et al. Pseudomonas aeruginosa-related postoperative endophthalmitis linked to a contaminated phacoemulsifier. Arch Ophthalmol. 2002;120:90–93.
67 Chen JC, Roy M. Epidemic Bacillus endophthalmitis after cataract surgery II: chronic and recurrent presentation and outcome. Ophthalmology. 2000;107:1038–1041.
68 Olson JC, Flynn WH, Jr., Forster RK, et al. Results in the treatment of postoperative endophthalmitis. Ophthalmology. 1983;90:692–699.
69 Guss RB, Koenig S, De La Pena W, et al. Endophthalmitis after penetrating keratoplasty. Am J Ophthalmol. 1983;95:651–658.
70 Taban M, Behrens A, Newcomb RL, et al. Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol. 2005;123(5):605–609.
71 Confino J, Brown SI. Bacterial endophthalmitis associated with exposed monofilament sutures following corneal transplantation. Am J Ophthalmol. 1985;99:111–113.
72 Leveille AS, McMullan FD, Cavanagh HD. Endophthalmitis following penetrating keratoplasty. Ophthalmology. 1983;90:38–39.
73 Katz LJ, Cantor LB, Spaeth GL. Complications of surgery in glaucoma: early and late bacterial endophthalmitis following glaucoma filtering surgery. Ophthalmology. 1985;92:959–963.
74 Gan IM, van Dissel JT, Beekhuis WH, et al. Intravitreal vancomycin and gentamicin concentrations in patients with postoperative endophthalmitis. Br J Ophthalmol. 2001;85:1289–1293.
75 Gedde SJ, Scott IU, Tabandeh H, et al. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. 2001;108:1323–1327.
76 Stein JD, Ruiz D, Jr., Belsky D, et al. Longitudinal rates of postoperative adverse outcomes after glaucoma surgery among medicare beneficiaries 1994 to 2005. Ophthalmology. 2008;115(7):1109–1116.e7.
77 Poulsen EJ, Allingham RR. Characteristics and risk factors of infections after glaucoma filtering surgery. J Glaucoma. 2000;9:438–443.
78 Greenfield DS, Suner IJ, Schiffman J. Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996;114:943–949.
79 Higginbotham EJ, Stevens RK, Musch DC. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology. 1996;103:650–656.
80 Wolner B, Liebmann JM, Sassani JW. Late bleb-related endophthalmitis after trabeculectomy with adjunctive 5-fluorouracil. Ophthalmology. 1991;98:1053–1060.
81 Ciulla TA, Beck AD, Topping TM, et al. Blebitis, early endophthalmitis, and late endophthalmitis after glaucoma-filtering surgery. Ophthalmology. 1997;104:986–995.
82 Kangas TA, Greenfield DS, Flynn HW, et al. Delayed-onset endophthalmitis associated with conjunctival filtering blebs. Ophthalmology. 1997;104:746–752.
83 Song A, Scott IU, Flynn HW, Jr., et al. Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. Ophthalmology. 2002;109:985–991.
84 Ho PC, Tolentino FI. Bacterial endophthalmitis after closed vitrectomy. Arch Ophthalmol. 1984;102:207–210.
85 Blankenship GW. Endophthalmitis after pars plana vitrectomy. Am J Ophthalmol. 1977;84:815–817.
86 Chong LP, de Juan E, Jr., McCuen BW, et al. Endophthalmitis in a silicone oil-filled eye. Am J Ophthalmol. 1986;102:660–661.
87 Kunimoto DY, Kaiser RS, Wills Eye Retina Service. Incidence of endophthalmitis after 20- and 25-gauge vitrectomy. Ophthalmology. 2007;114:2133–2137.
88 Scott IU, Flynn HW, Jr., Dev S, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina. 2008;28:138–142.
89 Hu AY, Bourges JL, Shah SP, et al. Endophthalmitis after pars plana vitrectomy: a 20- and 25-gauge comparison. Ophthalmology. 2009;116:1360–1365.
90 Mason JO, III., Yunker JJ, Vail RS, et al. Incidence of endophthalmitis following 20-gauge and 25-gauge vitrectomy. Retina. 2008;28:1352–1354.
91 Shimada H, Nakashizuka H, Hattori T, et al. Incidence of endophthalmitis after 20- and 25-gauge vitrectomy: causes and prevention. Ophthalmology. 2008;115:2215–2220.
92 Wu L, Berrocal MH, Arévalo JF, et al. Endophthalmitis after pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina. 2011;31(4):673–678.
93 Eckardt C. Staphylococcus epidermidis endophthalmitis after pneumatic retinopexy. Am J Ophthalmol. 1987;103:720–721.
94 Schwartz SG, Flynn HW, Scott IU. Endophthalmitis after intravitreal injections. Expert Opin Pharmacother. 2009;10(13):2119–2126.
95 Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004;24:S3–S19.
96 Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791–796.
97 Recchia FM, Baumal CR, Sivalingam A, et al. Endophthalmitis after pediatric strabismus surgery. Arch Ophthalmol. 2000;118:939–944.
98 Salamon SM, Friberg TR, Luxenberg MN. Endophthalmitis after strabismus surgery. Am J Ophthalmol. 1982;93:39–41.
99 Apt L. Purulent anterior segment endophthalmitis following paracentesis. Ophthalmic Surg. 17, 1986. 526–526
100 Joondeph BC, Joondeph HC. Purulent anterior segment endophthalmitis following paracentesis. Ophthalmic Surg. 1986;17:91–93.
101 Aquavella JV, Rao GN, Brown AC, et al. Keratoprosthesis: results, complications, and management. Ophthalmology. 1982;89:655–660.
102 Nouri M, Terada H, Alfonso EC, et al. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119:484–489.
103 Gelender H, Flynn HW, Jr., Mandelbaum SH. Bacterial endophthalmitis resulting from radial keratotomy. Am J Ophthalmol. 1982;94:528–533.
104 Bohigian GM, Olk RJ. Factors associated with a poor visual result in endophthalmitis. Am J Ophthalmol. 1986;101:332–341.
105 Forster RK, Zachary IG, Cottingham AJ, Jr., et al. Further observations on the diagnosis, cause, and treatment of endophthalmitis. Am J Ophthalmol. 1976;81:52–56.
106 Rowsey JJ, Newson DL, Sexton DJ, et al. Endophthalmitis: current approaches. Ophthalmology. 1982;89:1055–1066.
107 Azad R, Ravi K, Talwar D, et al. Pars plana vitrectomy with or without silicone oil endotamponade in post-traumatic endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2003;241(6):478–483.
108 Gilbert CM, Soong HK, Hirst LW. A two-year prospective study of penetrating ocular trauma at the Wilmer Ophthalmological Institute. Ann Ophthalmol. 1987;19:104–106.
109 Brinton GS, Topping TM, Hyndiuk RA, et al. Posttraumatic endophthalmitis. Arch Ophthalmol. 1984;102:547–550.
110 Williams DF, Mieler WF, Abrams GW, et al. Results and prognostic factors in penetrating ocular injuries with retained intraocular foreign bodies. Ophthalmology. 1988;95:911–916.
111 Lemley CA, Han DP. Endophthalmitis: a review of current evaluation and management. Retina. 2007;27(6):662–680.
112 Lieb DF, Scott IU, Flynn HW, Jr., et al. Open globe injuries with positive intraocular cultures: factors influencing final visual acuity outcomes. Ophthalmology. 2003;110:1560–1566.
113 Peyman GA, Carroll CP, Raichand M. Prevention and management of traumatic endophthalmitis. Ophthalmology. 1980;87:320–324.
114 Puliafito CA, Baker AS, Haaf J, et al. Infectious endophthalmitis: a review of 36 cases. Ophthalmology. 1982;89:921–929.
115 Affeldt JC, Flynn HW, Jr., Forster RK, et al. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94:407–413.
116 Nobe JR, Gomez DS, Liggett P, et al. Post-traumatic and postoperative endophthalmitis: a comparison of visual outcomes. Br J Ophthalmol. 1987;71:614–617.
117 Binder MI, Chua J, Kaiser PK, et al. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore). 2003;82:97–105.
118 Essman TF, Flynn HW, Jr., Smiddy WE, et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1997;28:185–194.
119 Okada AA, Johnson RP, Liles WC, et al. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology. 1994;101:832–838.
120 Schiedler V, Scott IU, Flynn HW, Jr., et al. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004;137:725–731.
121 Jackson TL, Eykyn SJ, Graham EM, et al. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423.
122 Leopold IH. Management of intraocular infection. Trans Ophthalmol Soc UK. 1971;91:575–610.
123 Barza M. Factors affecting the intraocular penetration of antibiotics. The influence of route, inflammation, animal species and tissue pigmentation. Scand J Infect Dis. 1978;14:S151–S159.
124 Baum JL. Antibiotic administration in the treatment of bacterial endophthalmitis. I. Periocular injections. Surv Ophthalmol. 21, 1977. 332–0
125 Barza M, Kane A, Baum J. Oxacillin for bacterial endophthalmitis: subconjunctival, intravenous, both, or neither? Invest Ophthalmol Vis Sci. 1980;19:1348–1354.
126 Barza M, Kane A, Baum J. Ocular penetration of subconjunctival oxacillin, methicillin, and cefazolin in rabbits with staphylococcal endophthalmitis. J Infect Dis. 1982;145:899–903.
127 Baum J, Peyman GA, Barza M. Intravitreal administration of antibiotic in the treatment of bacterial endophthalmitis. III. Consensus. Surv Ophthalmol. 1982;26:204–206.
128 Von Sallmann L, Meyer K, DiGrandi J. Experimental study on penicillin treatment of ectogenous infection of vitreous. Arch Ophthalmol. 1944;32:179–189.
129 Irvine AR. The role of vitrectomy in endophthalmitis. Trans Pac Coast Otoophthalmol Soc. 1977;58:185–188.
130 Peyman GA, Raichand M, Bennett TO. Management of endophthalmitis with pars plana vitrectomy. Br J Ophthalmol. 1980;64:472–475.
131 Maylath FR, Leopold IH. Study of experimental intraocular infection. I. The recoverability of organisms, inoculated into ocular tissues and fluids. II. The influence of antibiotics and cortisone, alone and combined, on intraocular growth of these organisms. Am J Ophthalmol. 1955;40:86–101.
132 Barza M, Kane A, Baum J. Pharmacokinetics of intravitreal carbenicillin, cefazolin, and gentamicin in rhesus monkeys. Invest Ophthalmol Vis Sci. 1983;24:1602–1606.
133 Mao LK, Flynn HW, Miller D, et al. Endophthalmitis caused by Staphylococcus aureus. Am J Ophthalmol. 1993;116:584–589.
134 Rousell TJ, Culbertson WW, Jaffe NS. Chronic postoperative endophthalmitis associated with Propionibacterium acnes. Arch Ophthalmol. 1987;105:1199–1201.
135 Mandelbaum S, Forster RK. Endophthalmitis associated with filtering blebs. Int Ophthalmol Clin. 1987;27:107–111.
136 Campochiaro PA, Conway BP. Aminoglycoside toxicity: a survey of retinal specialists. Arch Ophthalmol. 1991;109:946–950.
137 Conway BP, Campochiaro PA. Macular infarction after endophthalmitis treated with vitrectomy and intravitreal gentamicin. Arch Ophthalmol. 1986;104:367–371.
138 El Baba FZ, Trousdale MD, Gauderman WJ, et al. Intravitreal penetration of oral ciprofloxacin in humans. Ophthalmology. 1992;99:483–486.
139 Hariprasad SM, Mieler WF, Holz ER. Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch Ophthalmol. 2003;121:345–350.
140 Hariprasad SM, Mieler WF, Holz ER, et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004;122:42–47.
141 Martin DF, Ficker LA, Aguilar HA, et al. Vitreous cefazolin levels after intravenous injection. Effects of inflammation, repeated antibiotic doses, and surgery. Arch Ophthalmol. 1990;108:411–414.
142 O’Day DM, Head WS, Robinson RD, et al. Intraocular penetration of systemically administered antifungal agents. Curr Eye Res. 1985;4:131–134.
143 Hariprasad SM, Shah GK, Mieler WF, et al. Vitreous and aqueous penetration of orally administered moxifloxacin in humans. Arch Ophthalmol. 2006;124(2):178–182.
144 Wilson L. Endophthalmitis. Trans Ophthalmol Soc UK. 1986;105:56–59.
145 Aguilar HE, Meredith TA, Shaarawy A, et al. Vitreous cavity penetration of ceftazidime after intravenous administration. Retina. 1995;15:154–159.
146 Meredith TA, Mandell BA, Aguilar EA, et al. Amikacin levels after intravitreal injection: effects of inflammation and surgery. Invest Ophthalmol Vis Sci. 33, 1992. 747–747
147 Meredith TA, Aguilar HE, Shaarawy A, et al. Vancomycin levels in the vitreous cavity after intravenous administration. Am J Ophthalmol. 1995;119:774–778.
148 Meredith TA. Antimicrobial pharmacokinetics in endophthalmitis treatment; studies of ceftazidime. Trans Am Ophthalmol Soc. 1993;91:653–699.
149 Ficker L, Meredith TA, Gardner S, et al. Cefazolin levels after intravitreal injection. Effects of inflammation and surgery. Invest Ophthalmol Vis Sci. 1990;31:502–505.
150 Kane A, Barza M, Baum J. Intravitreal injection of gentamicin in rabbits: effect of inflammation and pigmentation on half-life and ocular distribution. Invest Ophthalmol Vis Sci. 1981;20:593–597.
151 Mandell BA, Meredith TA, Aguilar E, et al. Effects of inflammation and surgery on amikacin levels in the vitreous cavity. Am J Ophthalmol. 1993;115:770–774.
152 Campochiaro PA, Lin JI, Aminoglycoside Study Group. Aminoglycoside toxicity in the treatment of endophthalmitis. Arch Ophthalmol. 1994;112:48–53.
153 Conway BP, Tabatabay CA, Campochiaro PA, et al. Gentamicin toxicity in the primate retina. Arch Ophthalmol. 1989;107:107–112.
154 Oum BP, D’Amico DJ, Kwak HW, et al. Intravitreal antibiotic therapy with vancomycin and aminoglycoside: examination of the retinal toxicity of repetitive injections after vitreous and lens surgery. Graefes Arch Clin Exp Ophthalmol. 1992;230:56–61.
155 Oum BS, D’Amico DJ, Wong KW. Intravitreal antibiotic therapy with vancomycin and aminoglycoside. An experimental study of combination and repetitive injections. Arch Ophthalmol. 1989;107:1055–1060.
156 O’Day DM, Jones DB, Patrinely J, et al. Staphylococcus epidermidis endophthalmitis: visual outcome following noninvasive therapy. Ophthalmology. 1982;89:354–360.
157 Engelbert M, Miño de Kaspar H, Thiel M, et al. Intravitreal vancomycin and amikacin versus intravenous imipenem in the treatment of experimental Staphylococcus aureus endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2004;242(4):313–320.
158 El-Massry A, Meredith TA, Aguilar HE, et al. Aminoglycoside concentrations in the vitreous cavity after intravenous administration. Am J Ophthalmol. 1996;121:684–689.
159 Hariprasad SM, Mieler WF, Holz ER. Vitreous penetration of orally administered gatifloxacin in humans. Trans Am Ophthalmol Soc. 2002;100:153–159.
160 Peyman GA. Aminoglycoside toxicity. Arch Ophthalmol. 1992;110:1683–1685.
161 Campochiaro PA, Green WR. Toxicity of intravitreal ceftazidime in primate retina. Arch Ophthalmol. 1992;110:1625–1629.
162 Meredith TA, Abdala C, Aguilar HE, et al. Toxicity of intravitreal ceftazidime and vancomycin. Invest Ophthalmol Vis Sci. 1995;36:3647.
163 Davis JL, Koidou-Tsiligianni A, Pflugfelder SC, et al. Coagulase-negative staphylococcal endophthalmitis: increase in antimicrobial resistance. Ophthalmology. 1988;95:1404–1410.
164 Flynn HW, Jr., Pulido JS, Pflugfelder SC. Endophthalmitis therapy: changing antibiotic sensitivity patterns and current therapeutic recommendations (letter). Arch Ophthalmol. 1991;109:175–176.
165 Smith MA, Sorenson JA, D’Aversa G, et al. Treatment of experimental methicillin-resistant Staphylococcus epidermidis endophthalmitis with intravitreal vancomycin and intravitreal dexamethasone. J Infect Dis. 1997;175:462–466.
166 Aguilar HE, Meredith TA, el Massry A, et al. Vancomycin levels after intravitreal injection. Effects of inflammation and surgery. Retina. 1995;15:428–432.
167 Pflugfelder SC, Hernandez E, Fleisler SJ, et al. Intravitreal vancomycin. Arch Ophthalmol. 1987;105:831–837.
168 Haider SA, Hassett P, Bron AJ. Intraocular vancomycin levels after intravitreal injection in post cataract extraction endophthalmitis. Retina. 2001;21:210–213.
169 Ferencz JR, Assia EI, Diamantstein L, et al. Vancomycin concentration in the vitreous after intravenous and intravitreal administration for postoperative endophthalmitis. Arch Ophthalmol. 1999;117:1023–1027.
170 D’Amico DJ, Libert J, Kenyon KR, et al. Retinal toxicity of intravitreal gentamicin: an electron microscopic study. Invest Ophthalmol Vis Sci. 1984;25:564–572.
171 D’Amico DJ, Caspers-Velu L, Libert J, et al. Comparative toxicity of intravitreal aminoglycoside antibiotics. Am J Ophthalmol. 1985;100:264–275.
172 Talamo JH, D’Amico J, Kenyon KR. Intravitreal amikacin in the treatment of bacterial endophthalmitis. Arch Ophthalmol. 1986;104:1483–1485.
173 Yoshizumi MO, Leinwand MJ, Kim J. Topical and intravenous gentamicin in traumatically lacerated eyes. Graefes Arch Clin Exp Ophthalmol. 1992;230:175–177.
174 Rubinstein E, Goldfarb J, Keren G, et al. The penetration of gentamicin into the vitreous humor in man. Invest Ophthalmol Vis Sci. 1983;24:637–639.
175 Slana VS, Marchese AL, Jay WM. Ocular toxicity of intravitreal ciprofloxacin injection in pigmented rabbit eyes. Invest Ophthalmol Vis Sci. 33, 1992. 727–727
176 Fiscella RG, Shapiro MJ, Solomon MJ, et al. Ofloxacin penetration into the eye after intravenous and topical administration. Retina. 1997;17:535–539.
177 Doft BH, Weiskopf J, Nillson-Ehle I, et al. Amphotericin clearance in vitrectomized versus non-vitrectomized eyes. Ophthalmology. 1985;92:1601–1605.
178 O’Day DM, Foulds G, Williams TE, et al. Ocular uptake of fluconazole following oral administration. Arch Ophthalmol. 1990;108:1006–1008.
179 Breit SM, Hariprasad SM, Mieler WF, et al. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005;139(1):135–140.
180 Gao H, Pennesi ME, Shah K, et al. Intravitreal voriconazole: an electroretinographic and histopathologic study. Arch Ophthalmol. 2004;122(11):1687–1692. [Erratum in: Arch Ophthalmol 2005;123(1):130]
181 Sternberg P, Jr., Martin DF. Management of endophthalmitis in the post-endophthalmitis vitrectomy study era. Arch Ophthalmol. 2001;119:754–755.
182 Doft BH. The Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 1991;109:487–489.
183 Doft BH, Wisniewski SR, Kelsey SF, et al. Diabetes and postoperative endophthalmitis in the endophthalmitis vitrectomy study. Arch Ophthalmol. 2001;119:650–656.
184 Brown RH, Yang LH, Walker SD, et al. Treatment of bleb infection after glaucoma surgery. Arch Ophthalmol. 1994;112:57–61.
185 Reynolds AC, Skuta GL, Monlux R, et al. Management of blebitis by members of the American Glaucoma Society: a survey. J Glaucoma. 2001;10:340–347.
186 Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: a contemporary reappraisal. Surv Ophthalmol. 1986;31:81–101.
187 Jackson TL, Eykyn SJ, Graham EM, et al. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48(4):403–423. Review
188 Verbraeken H, Van Laethem J. Treatment of endophthalmitis with and without pars plana vitrectomy. Ophthalmologica. 1985;191:1–3.
189 Henderly DE, Liggett PE, Rao NA. Cryptococcal chorioretinitis endophthalmitis. Retina. 1987;7:75–79.
190 Rodriguez A. Early pars plana vitrectomy in chronic endophthalmitis of toxocariasis. Graefes Arch Clinical Exp Ophthalmol. 1986;224:218–220.
191 Diamond JG. Intraocular management of endophthalmitis: a systematic approach. Arch Ophthalmol. 1981;99:96–99.
192 Aguilar HE, Meredith TA, Drews CD, et al. Treatment of experimental S. aureus endophthalmitis with vancomycin, cefazolin and corticosteroids. Invest Ophthalmol Vis Sci. 1990;31:S140.
193 Davey PG, Barza M, Stuart M. Dose response of experimental Pseudomonas endophthalmitis to ciprofloxacin, gentamicin, and imipenem: evidence of resistance to “late” treatment of infections. J Infect Dis. 1987;155:518–523.
194 Forster RK. Experimental postoperative endophthalmitis. Trans Am Ophthalmol Soc. 1992:505–559.
195 Jett BD, Jensen HG, Atkuri RV, et al. Evaluation of therapeutic measures for treating endophthalmitis caused by isogenic toxin-producing and toxin-nonproducing Enterococcus faecalis strains. Invest Ophthalmol Vis Sci. 1995;36:9–15.
196 Shaarawy A, Grand MG, Meredith TA, et al. Persistent infection after intravitreal antibimicrobial therapy. Ophthalmology. 1995;102:382–387.
197 Stern GA, Engel HM, Driebe WT. Recurrent postoperative endophthalmitis. Cornea. 1990;9:102–107.
198 Baum JL, Barza M, Lugar J, et al. The effect of corticosteroids in the treatment of experimental bacterial endophthalmitis. Am J Ophthalmol. 1975;80:513–517.
199 Meredith TA, Aguilar HE, Miller MJ, et al. Comparative treatment of experimental Staphylococcus epidermidis endophthalmitis. Arch Ophthalmol. 1990;108:857–860.
200 Coats ML, Peyman GA. Intravitreal corticosteroids in the treatment of exogenous fungal endophthalmitis. Retina. 1992;12:46–51.
201 Graham RO, Peyman GA. Intravitreal injection of dexamethasone: treatment of experimentally induced endophthalmitis. Arch Ophthalmol. 1974;92:149–154.
202 Peyman GA, Herbst R. Bacterial endophthalmitis: treatment with intraocular injection of gentamicin and dexamethasone. Arch Ophthalmol. 1974;91:416–418.
203 Maxwell DP, Jr., Brent BD, Diamond JG, et al. Effect of intravitreal dexamethasone on ocular histopathology in a rabbit model of endophthalmitis. Ophthalmology. 1991;98:1370–1375.
204 Das T, Jalali S, Gothwal VK, et al. Intravitreal dexamethasone in exogenous bacterial endophthalmitis: results of a prospective randomised study. Br J Ophthalmol. 1999;83:1050–1055.
205 Gan IM, Ugahary LC, van Dissel JT, et al. Effect of intravitreal dexamethasone on vitreous vancomycin concentrations in patients with suspected postoperative bacterial endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2005;243(11):1186–1189.
206 Albrecht E, Richards JC, Pollock T, et al. Adjunctive use of intravitreal dexamethasone in presumed bacterial endophthalmitis: a randomised trial. Br J Ophthalmol. 2011;95:1385–1388.
207 Shah GK, Stein JD, Sharma S, et al. Visual outcomes following the use of intravitreal steroids in the treatment of postoperative endophthalmitis. Ophthalmology. 2000;107:486–489.
208 Hall EF, Scott GR, Musch DC, et al. Adjunctive intravitreal dexamethasone in the treatment of acute endophthalmitis following cataract surgery. Clin Ophthalmol. 2008;2(1):139–145.
209 Doft BM, Kelsey SF, Wisniewski SR. Retinal detachment in the endophthalmitis vitrectomy study. Arch Ophthalmol. 2000;118:1661–1665.
210 Nelsen PT, Marcus DA, Bovino JA. Retinal detachment following endophthalmitis. Ophthalmology. 1985;92:1112–1117.
211 Rowsey JJ, Stonecipher KG, Jensen H, et al. Culture and sensitivities of infectious endophthalmitis: a microbiological analysis of 302 intravitreal biopsies. Invest Ophthalmol Vis Sci. 33, 1992. S745–S745
212 Laatikainen L, Tarkkanen A. Early vitrectomy in treatment of postoperative purulent endophthalmitis. Acta Ophthalmol. 1987;65:455–460.