Chapter 18 Infectious diseases
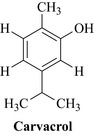
There are many reasons why plants are a valuable source of antimicrobial natural products and the most fundamental reason is that they contain intrinsically antimicrobial compounds such as carvacrol (Fig. 18.1 and see Chapters 6 and 7) from Thyme (Thymus vulgaris, Lamiaceae) which is a monoterpene and is present in the essential oil of this species.
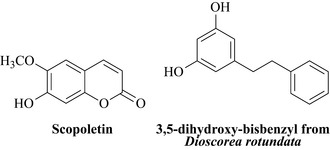
This phenolic monoterpene has a range of antibacterial and antifungal properties (Baser 2008) and may be produced by the plant to protect itself from attack from plant pathogenic microbes and insects that are present in its environment. This is an example of an intrinsic or latent antimicrobial natural product that the plant produces as a normal part of its chemistry which can be used medicinally. Plants also have the ability to produce antimicrobial natural products when they are under attack from microbes, herbivores and insects. These compounds are very quickly synthesized by the plant and are called phytoalexins which display antimicrobial properties to a wide range of bacteria and fungi. Examples of this phenomenon include the potato, which when inoculated with a fungus synthesizes the antimicrobial coumarin scopoletin (Fig. 18.2 and Chapter 6) and the bisbenzyl compound (3,5-dihydroxy-bisbenzyl) also depicted in Fig. 18.2, which is produced by a species of yam (Dioscorea rotundata, Dioscoreaceae).
This bisbenzyl is very strongly active against a range of Gram-positive and Gram-negative bacteria including Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa and Escherichia coli with minimum inhibitory concentrations of 10 mg/L (Fagboun et al 1987). This is astounding activity, particularly against Gram-negative bacteria such as E. coli and P. aeruginosa which are often impervious to plant antimicrobials.
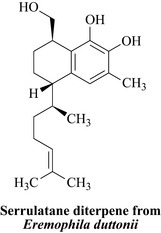
Plants are also used extensively as topical antimicrobials in many societies and there is an enormous body of primary literature in journals that specialize in ethnomedical research such as the Journal of Ethnopharmacology. In the North-Eastern part of Australia the indigenous peoples use the aerial parts of Eremophila duttonii (Myoporaceae) as a topical antibacterial preparation (Smith et al 2007) and the active constituent has been isolated and characterized as an unusual serrulatane diterpene (Fig. 18.3).
Probably the most important reasons that plants produce antibacterial natural products, and why they could be a valuable resource of antimicrobial materials, is that these chemicals are often exceptionally diverse, have stereochemical centres and have extensive functional group chemistry. These factors mean that the compounds will have very distinct shapes, developed by nature over millions of years to bind to protein and DNA targets, and consequently having an inherent biological activity. The readers are urged to consult a new and very important review on natural products highlighting this topic written by Professor Giovanni Appendino and colleagues (Appendino et al 2010). Plant antibacterials are very different in shape and chemistry to existing antibacterial chemotypes (Gibbons 2004, 2008), that are often microbially derived such as erythromycin (Fig. 6.11, Chapter 6) and tetracycline (Fig. 6.8, Chapter 6). This may mean that plant-derived antibacterials could function through a different and as yet undetermined mechanism of action. This would make them valuable where bacterial resistance to conventional antibiotics (beta-lactams, macrolides, tetracyclines) has arisen as these bacteria may be susceptible to these agents by working in a very different way. New agents that function by a different mechanism are currently needed, particularly in bacterial infections such as tuberculosis where the causative organism, Mycobacterium tuberculosis may possess multiple resistance to existing antibiotics of choice.
Broad-spectrum antimicrobial agents
Umckaloabo (pelargonium), pelargonium sidoides DC and P. reniforme curt (pelargonii radix)
Therapeutic uses and available evidence
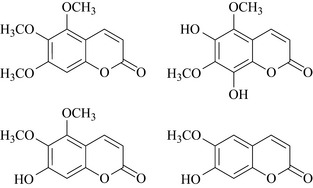
Extracts of Pelargonium species have been shown to inhibit the adherence of bacteria to cells of the mucous membrane and there is some published chemistry and biology on methoxylated coumarins (Fig. 18.4) from P. sidoides which have weak antibacterial activity (Kayser and Kolodziej 1997). They have also been shown to interfere with viral replication (Michaelis et al 2011) and to inhibit viral adherence to cells of mucous membrane and to loosen viscous mucus in the respiratory tract. It has also been postulated that these extracts have immuno-modulatory properties. There have been some small clinical trials on efficacy in reducing the symptoms associated with tonsillitis and bronchitis, particularly amongst children (Matthys et al 2007). These materials are not, however, a replacement for antibiotics, but they may be used as a supplement to ameliorate the symptoms associated with inflammation of the upper respiratory tract (URT).
There has also been a study on extracts demonstrating weak antibacterial activity which is due to the presence of the ubiquitous unsaturated fatty acids oleic and linoleic acid against fast-growing species of Mycobacterium; however these compounds are unlikely to be responsible for the ‘anti-TB’ activity of Steven’s cure (Seidel and Taylor 2004).
Lemon balm, melissa officinalis L. (melissae folium) 
There are a number of topical formulations which are marketed for Herpes simplex virus skin lesions and there are clinical data and some in vitro activity has been confirmed with the extracts of Melissa officinalis (Koytchev et al 1999). The herb is generally well-tolerated, although it has been suggested that long-term use may interfere with thyroid function.
Therapeutic uses and available evidence
Lemon balm is antimicrobial, carminative and sedative. Hot water extracts have antiviral properties, mainly due to the polyphenolic acids. Topical formulations are used for Herpes simplex virus skin lesions, the antiviral activity having been confirmed in vitro and also by clinical trial. Aqueous extracts also inhibit division of tumour cells, and tannin-free extracts inhibit protein biosynthesis (for review, see Ulbricht et al 2005). The herb is used as an ingredient of herbal teas, often with other herbs, for nervous disorders and insomnia (see Chapter 17). Lemon balm is well tolerated, although it should not be taken internally in high doses over a long period because of its reputed antithyroid activity.
Garlic, Allium sativum L. (Allii sativi bulbus) 
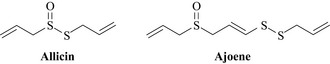
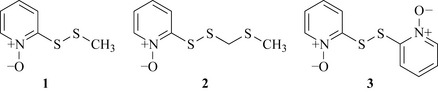
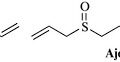
Garlic, and other Allium spp. (Alliaceae), have a very long history as both a topical and systemic material to treat various infections. The literature is full of in vitro studies showing efficacy of the extracts and oils of the bulbs of various Allium species with activity against various bacteria, fungi and viruses. The family has a long and rich usage as culinary herbs with onions, garlic, shallots and chives all producing antimicrobial sulphur containing natural products, typified by allicin and ajoene (Fig. 18.5). Garlic has been used clinically for the treatment of tuberculosis with some success in the United States in the 1940s and it has been referred to as Russian penicillin, as a result of its wide use in the former Soviet Union, again with some considerable success as an antibacterial (Bolton et al 1982). The widespread use of garlic has prompted recent investigation of other species which may harbour useful natural products and a number of interesting sulphur-containing antibacterials with very potent antibacterial activity have been isolated, such as the unusual pyridine-N-oxide natural products from Allium stipitatum (Fig. 18.6, O’Donnell et al 2009). These compounds displayed activity against slow- and fast-growing mycobacteria and a range of Staphylococcus aureus species some of which were methicillin-resistant and multidrug-resistant.
Therapeutic uses and available evidence
Garlic extracts have been shown to have antibiotic, expectorant and anti-thrombotic properties and many garlic preparations are marketed for their anti-blood clotting properties and to give some protection against atherosclerosis. Preparations from common garlic have also found much use in the treatment for respiratory tract infections, such as common cold, flu and bronchitis. The allyl sulphides, such as allicin and ajoene, are strongly antimicrobial having activity against Staphylococcus aureus, Streptococcus species and even some Gram-negative bacteria, such as Helicobacter pylori, the major bacterial causative agent of stomach ulcers (Harris et al 2001).
Tea tree and tea tree oil, melaleuca alternifolia (maiden et betche.) cheel (melaleucae atheroleum) 
Constituents
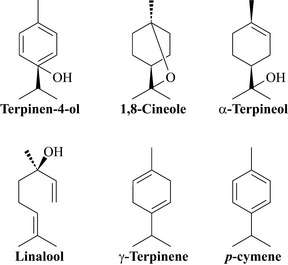
Tea tree oil is a highly complex mixture of monoterpenes and the major component is terpinen-4-ol (Fig. 18.7), that may be present at concentrations as high as 30%.
Therapeutic uses and available evidence
Tea tree oil is now used worldwide in the form of skin creams for pimples and acne, pessaries for vaginal thrush, as an inhalation for respiratory disorders and in pastilles for sore throats. It is also popular as a lotion for the treatment of lice and scabies infestations, and for dandruff and other hair and scalp disorders. The oil has broad-spectrum antimicrobial activity against Staphylococcus aureus, Escherichia coli and various pathogenic fungi and yeasts including Candida albicans, and also against the protozoa Leishmania major and Trypanosoma brucei. There have also been studies conducted at using preparations containing the oil to reduce the spread of MRSA in hospital units (Warnke et al 2009) and there has been much research into the use of this oil as an antiseptic for nursing staff. The most active purified compounds from this oil include terpinen-4-ol, γ-terpinene, α-terpineol and linalool with minimum inhibitory concentrations in the range of 0.125–0.25% v/v (Carson and Riley 1995, Cox et al 2001, Raman et al 1995). These compounds also demonstrated broad-spectrum activity towards Gram-negative bacteria. It was also shown that the non-oxygenated monoterpenes such as γ-terpinene and p-cymene (Fig. X.7) reduced the efficacy of terpinen-4-ol by reducing its aqueous solubility (Cox et al 2001). Clinical trials have supported many of the uses of tea tree oil, including for Herpes labialis, although most of the studies are rather small (see Carson et al 2006). Undiluted essential oils can cause skin irritation and tea tree oil should be used with care. It should only be taken internally in small doses.
Bearberry, arctostaphylos uva-ursi L. (uvae ursi folium) 
Constituents
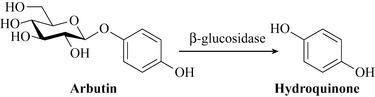
The main constituents are hydroquinone derivatives, notably the glycoside arbutin. This compound is hydrolysed in vivo by the enzyme β-glucosidase to give the diphenol, hydroquinone (Fig. 18.8). Other constituents include terpenoids such as α- and β-amyrin, flavonoids and tannins.
Therapeutic uses and available evidence
Hydroquinone is the main active component of this material and is a potent phenolic antiseptic. This compound is very active against many bacteria, but in particular those that are liable to cause urinary tract infections such as Escherichia coli and Pseudomonas aeruginosa. Activity has also been demonstrated against other species such as Bacillus subtilis and Staphylococcus aureus. Arbutin is hydrolysed by β-glucosidase to yield the active principle hydroquinone, which has antiseptic and astringent properties. Uva-ursi is also mildly diuretic and antilithuric (Beaux et al 1999). Uva ursi preparations such as Arctuvan require that the urine is alkaline for it to have antiseptic properties and, as such, acidic foods including cranberry juice (see below) should be avoided during treatment. Hydroquinone is a very reactive and biologically active compound and is cytotoxic and mutagenic. High doses and prolonged usage of bearberry products should be avoided and it should not be used during pregnancy or by anyone who has a kidney infection.
Cranberry juice, vaccinium macrocarpon aiton
Constituents
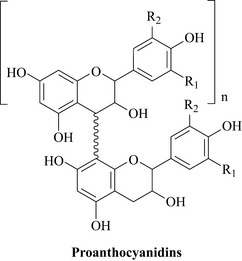
The chemistry of this plant is still not well understood because it contains many flavonoid polymers, in particular the proanthocyanidins that are believed to be important for the antibacterial activity of this species (Fig. 18.9).
These proanthocyanidins are exceptionally complex and vary in the number of flavonoid units in the polymer (n may vary considerably in Fig. 18.9), the way in which each of the units is connected and the functional groups present on each unit (R1 and R2 groups in the figure may be OH or OMe for example). This can give rise to a highly complex natural product mixture. These compounds are also polar and soluble in water, ethanol and methanol, which can make their analysis by conventional methods such as HPLC and HPLC-MS difficult.
Therapeutic uses and available evidence
In-vitro experiments with cranberry juice and proanthocyanidins have shown that they have the ability to affect the binding of the bacterium Escherichia coli, which is a major causative agent of urinary tract infections, to uroepithelial cells, therefore, inhibition adherence of this bacterium allowing its clearance. Cranberry juice is also thought to act by increasing levels of hippuric acid (a metabolite of benzoic acid) and, therefore, acidity of the urine. Clinical studies of cranberry juice have provided equivocal evidence for prevention or treatment of UTIs and the efficacy remains unproven (see Jepson and Craig 2008). Cranberry juice has a high calorific content, and patients with diabetes who wish to use cranberry juice should use sugar-free preparations. Reports that cranberry juice interacts with warfarin are controversial, and are now considered to be unproven (Zikria et al 2010). Cranberries have also been used for unrelated disorders such as kidney stones, and are frequently used in foods.
Single chemical entity (SCE) and novel plant antibacterials
So far we have looked at herbal products and their extracts as antibacterials, but there is a growing body of literature describing the activities associated with single compounds isolated in bioassay-guided fractionation studies on plants (Gibbons 2004, 2008). These plants may result from random screening, from a traditional antibacterial use of the plant or the plant material may have been treated prior to the study to elicit phytoalexins.
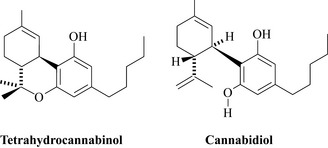
Cannabis sativa has a long history of use as a medicinal material having not only euphoriant, but also antiseptic properties. In Afghanistan there is anecdotal usage of cannabis resin to treat plague and as a topical antimicrobial preparation. There is in vitro evidence to support the antibacterial properties of cannabis as the major components, tetrahydrocannabinol and cannabidiol (Fig. 18.10) are highly active against Gram-positive bacteria such as Staphylococcus aureus and its methicillin-resistant (MRSA) variants (Appendino et al. 2008).
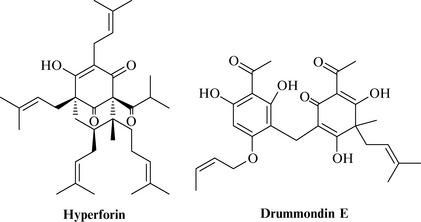
There has also been some work on the acylphloroglucinol group of natural products and one of the first members of this class to be elucidated was hyperforin (Fig. 18.11), from St John’s wort. This metabolite was studied due to its excellent activity against penicillin- and methicillin-resistant Staphylococcus aureus strains with MIC values being 0.1–1 mg/l (Schempp et al 1999).
The acylphloroglucinols are relatively complex natural products based on a cyclic aromatic-derived core with many prenyl groups, which may be either cyclized or oxidized to give a highly functional group rich and chiral class of products such as hyperforin. Other examples from this group include the drummondins (Fig. 18.11) from another species of Hypericum, H. drummondii, which had potent activity (MIC = 0.39 mg/l, Jayasuriya et al 1991).
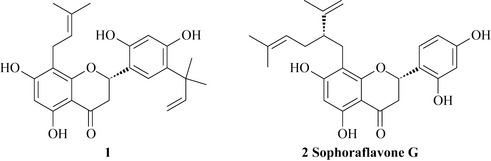
The flavonoids are probably the most intensively studied natural products in terms of their antimicrobial activity and the flavanones, for example compound 1 (Fig. 18.12), within this class have some very interesting levels of potency and action. Many of these natural products possess prenyl or geranyl groups that presumably contribute to the lipophilicity and membrane solubility of these compounds. This could improve their cellular uptake and enhance their ability to penetrate the bacterial cell.
Compound 1 has excellent potency toward MRSA strains with MIC values of 1.5 mg/l and compound 2 is sophoraflavanone G, which is antibacterial in its own right (MIC = 3.1 mg/l) and also has strong synergism in combination with the glycopeptide antibiotic vancomycin, which is used clinically to treat MRSA infections (Sakagami et al 1998). A combination of sophoraflavanone G with vancomycin could feasibly contribute to better treatment of an MRSA infection and it is conceivable that these lipophilic compounds could be formulated into a topical antiseptic preparation to help with decolonization.
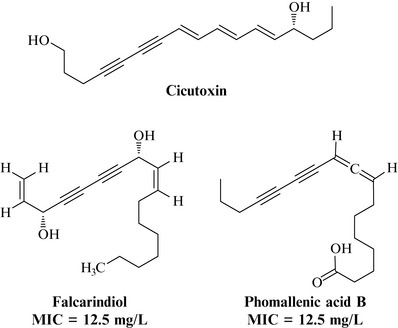
Plants within the Apiaceae family, which includes carrot, coriander, parsnip, caraway and dill, are known to produce polyacetylenic natural products. These compounds have conjugated triple bonds (acetylenes). Some of these metabolites are deadly poisonous, such as cicutoxin from Cowbane (Cicuta virosa), whereas others such as falcarindiol (Fig. 18.13) are present in the roots of these plants and are probably synthesized as a protection against microbes in their environment.
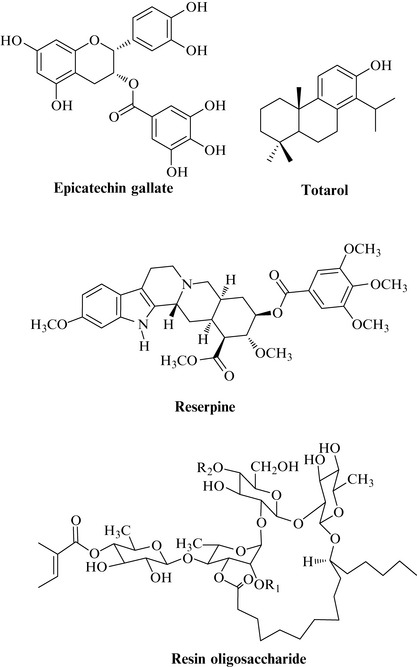
We have already seen that some natural products such as sophoraflavanone G have the ability to potentiate the action of existing antibacterial agents. Bacteria have the ability to remove antibiotics from their cells by a process known as efflux that can make the antibiotic inactive against that strain. Such effluxing strains have proteins on their cell membranes which transport the antibiotics out of the bacterial cell. Some natural products (Fig. 18.14) have been found to inhibit these proteins and stop them from removing the antibiotic and such an action has the potential to restore antibiotic activity.
Plant natural products with the ability to inhibit these processes include tea catechins such as epicatechin gallate, simple diterpenes like totarol, alkaloids such as reserpine and even complex resin oligosaccharides (Fig. 18.14) (Stavri et al 2007).
Antiprotozoal agents
Cinchona, cinchona spp. (cinchonae cortex) 
Trees of the genus Cinchona (Rubiaceae) are used as a source of quinine (for structure, see Chapter 1). Red cinchona, ‘cinchona rubra’, is C. pubescens (= C. succirubra Pavon); yellow cinchona, ‘cinchona flava’, is C. calisaya Wedd., or C. ledgeriana Moens. et Trim. Other species and hybrids of the genus Cinchona are also used. It has been called Peruvian bark, from the country of origin, and also Jesuit’s bark, since it was originally introduced into Europe by Jesuit missionaries. It is native to mountainous regions of tropical America, and cultivated in South-East Asia and parts of Africa. The bark is found in commerce as quills or flat pieces. The external surface is brownish-grey, usually fissured, and lichens and mosses may be seen as greyish-white or greenish patches.
Constituents
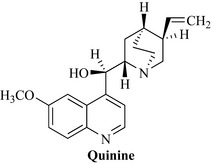
The actives are quinoline alkaloids, the major being quinine (Fig. 18.15), with quinidine, cinchonine, cinchonidine, epi and hydro derivatives of these, quinamine and others. The total alkaloid content of the bark should be not less than 6.5%, with 30–60% being of the quinine type. Identification is by thin-layer chromatography (TLC). The alkaloids are fluorescent.
Therapeutic uses and available evidence
Quinine was primarily used as an antimalarial before the advent of semi-synthetics, which have improved efficacy, especially against resistant strains, different pharmacokinetic profiles and reduced toxicity. The bark was formerly used as a febrifuge, tonic, orexigenic, spasmolytic and astringent, but it is only used now for the extraction of the alkaloids, quinine and its isomer quinidine. Both quinine and quinidine have antimalarial activity, although quinine is more widely used. Both are cardiac anti-arrhythmic agents (see Chapter 15), which limits their usefulness as antimalarials, and quinidine is still used clinically for this purpose. Quinine salts are used for the prevention of night cramps (the dose for this purpose is 200–300 mg of quinine sulphate or bisulphate) and in low doses is an ingredient of some analgesic and cold and flu remedies. Chronic overdosage can result in the condition known as cinchonism, which is characterized by headache, abdominal pain, rashes and visual disturbances. Cinchona and quinine should not be taken in large doses during pregnancy except for treating malaria.
Lapacho (taheebo, pau d’arco), tabebuia spp
Constituents
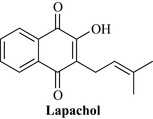
The active constituents are naphthoquinones, the most important being lapachol (Fig. 18.16), with deoxylapachol, α- and β-lapachone and others. It also contains anthraquinones, benzoic acid and benzaldehyde derivatives.
Therapeutic uses and available evidence
Antimicrobial effects are documented against Candida, Brucella and Staphylococcus spp., and for several viruses. Lapacho is also becoming popular as an anticancer treatment; antitumour activity has been shown in vitro and in vivo and a few uncontrolled trials have been carried out. The evidence available so far is inconclusive and this botanical drug should not be recommended for the treatment of cancer. Semi-synthetic derivatives of lapachol are being prepared in order to increase activity and reduce toxicity. Lapachol is cytotoxic in large doses, and inhibits pregnancy in mice; however, there is little evidence of toxicity for the herb when used in normal doses. For review, see Gómez Castellanos et al 2009.
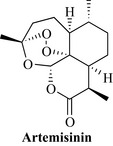
Sweet wormwood (syn. qinghaou), artemisia annua L
Constituents
The herb contains sesquiterpene lactones, the most important of which is artemisinin (qinghaosu; Fig. 18.17), as well as the arteannuins A–O, artemisitine, artemisinic acid, hydroarteannuin, and others. There is also a volatile oil containing artemisia ketone, cadinene and others, and flavonoids including artemetin.
Therapeutic uses and available evidence
Artemisinin is one of the most rapidly acting antiplasmodial compounds known. Several more stable and effective derivatives, such as artemether, arteether and artesunate have been developed and are being used clinically for both the prophylaxis and treatment of malaria (see Cui and Su 2009). The herb appears to be fairly non-toxic, although cytotoxicity in vitro and teratogenic effects have been observed in mice. There is evidence that the whole herb extract may be superior to isolated artemisinin, since the flavonoids present in the leaves have been linked to suppression of CYP450 enzymes responsible for altering the absorption and metabolism of artemisinin in the body, and also to a beneficial immunomodulatory activity in subjects afflicted with parasitic and chronic diseases (Ferreira et al 2010).
Insecticidal agents
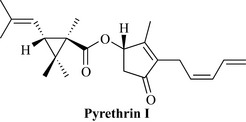
Pyrethrum (insect) flowers, chrysanthemum spp
Constituents
All species contain pyrethrins, which are esters of chrysanthemic and pyrethric acids and are the actives. They are known as pyrethrins I (Fig. 18.18) and II, cinerins I and II and jasmolins I and II.
Appendino G., Gibbons S., Giana A., et al. Antibacterial phytocannabinoids: a structure-activity study. J. Nat. Prod.. 2008;71:1427-1430.
Appendino G., Fontana G., Pollastro F. Natural products drug discovery. In: Comprehensive Natural Products II. London: Elsevier Ltd; 2010:205-236. (Chapter 3.08)
Baser K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des.. 2008;14:3106-3119.
Beaux D., Fleurentin J., Mortier F. Effect of extracts of Orthosiphon stamineus Benth, Hieracium pilosella L., Sambucus nigra L. and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother. Res.. 1999;13:222-225.
Bolton S., Null G., Troetel W.M. The medical uses of garlic–fact and fiction. Am. Pharm.. 1982;22:40-43.
Carson C.F., Riley T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol.. 1995;78:264-269.
Carson C., Hammer K.A., Riley T.V. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev.. 2006;19:50-62.
Cox S.D., Mann C.M., Markham J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol.. 2001;91:492-497.
Cui L., Su X.Z. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti Infect. Ther.. 2009;7:999-1013.
Fagboun D.E., Ogundana S.K., Adesanya S.A., Roberts M.F. Dihydrostilbene phytoalexins from Dioscorea rotundata. Phytochemistry. 1987;26:3187-3189.
Ferreira J.F., Luthria D.L., Sasaki T., Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135-3170.
Gibbons S. Anti-staphylococcal plant natural products. Nat. Prod. Rep.. 2004;21:263-277.
Gibbons S. Phytochemicals for bacterial resistance – strengths, weaknesses and opportunities. Planta Med.. 2008;74:594-602.
Gómez Castellanos J.R., Prieto J.M., Heinrich M. Red Lapacho (Tabebuia impetiginosa) – a global ethnopharmacological commodity? J. Ethnopharmacol.. 2009;121:1-13.
Harris J.C., Cottrell S.L., Plummer S., Lloyd D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol.. 2001;57:282-286.
Jayasuriya H., Clark A.M., McChesney J.D. New antimicrobial filicinic acid derivatives from Hypericum drummondii. J. Nat. Prod.. 1991;54:1314-1320.
Jepson R.G., Craig J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev.. 23(1), 2008. Jan (I)001321
Kayser O., Kolodziej H. Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med.. 1997;63:508-510.
Koytchev R., Alken R.G., Dundarov S. Balm mint extract (Lo-701) for topical treatment of recurring herpes labialis. Phytomedicine. 1999;6:225-230.
Luna L.A.Jr., Bachi A.L. Immune responses induced by Pelargonium sidoides extract in serum and nasal mucosa of athletes after exhaustive exercise: Modulation of secretory IgA, IL-6 and IL-15. Phytomedicine. 2011;18:303-308.
Matthys H., Kamin W., Funk P., Heger M. Pelargonium sidoides preparation (EPs 7630) in the treatment of acute bronchitis in adults and children. Phytomedicine. 2007;6:69-73.
Michaelis M., Doerr H.W., Cinatl J.Jr. Investigation of the influence of EPs® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18:384-386.
O’Donnell G., Poeschl R., Zimhony O., et al. Bioactive pyridine-N-oxide sulphides from Allium stipitatum. J. Nat. Prod.. 2009;72:360-365.
Raman A., Weir U., Bloomfield S.F. Antimicrobial effects of tea-tree oil and its major components on Staphylococcus aureus. Staph. epidermidis and Propionibacterium acnes. Lett. Appl. Microbiol.. 1995;21:242-245.
Sakagami Y., Mimura M., Kajimura K., et al. Anti-MRSA activity of sophoraflavanone G and synergism with other antibacterial agents. Lett. Appl. Microbiol.. 1998;27:98-100.
Schempp C.M., Pelz K., Wittmer A., Schöpf E., Simon J.C. Antibacterial activity of hyperforin from St John’s wort against multiresistant Staphylococcus aureus and Gram-positive bacteria. The Lancet. 1999;353:2129.
Seidel V., Taylor P.W. In vitro activity of extracts and constituents of Pelargonium against rapidly growing mycobacteria. Int. J. Antimicrob. Agents. 2004;23:613-619.
Smith J.E., Tucker D., Watson K., Lloyd Jones G. Identification of antibacterial constituents from the indigenous Australian medicinal plant Eremophila duttonii F. Muell. (Myoporaceae). J. Ethnopharmacol.. 2007;112:386-393.
Stavri M., Piddock L.J.V., Gibbons S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother.. 2007;59:1247-1260.
Ulbricht C., Brendler T., Gruenwald J., et al. Lemon balm (Melissa officinalis L.): an evidence-based systematic review by the Natural Standard Research Collaboration. J. Herb. Pharmacother.. 2005;5:71-114.
Warnke P.H., Becker S.T., Podschun R., et al. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J. Craniomaxillofac. Surg.. 2009;37:392-397.
Zikria J., Goldman R., Ansell J. Cranberry juice and warfarin: when bad publicity trumps science. Am. J. Med.. 2010;123:384-392.