Infectious and Inflammatory Disorders of the Gallbladder and Extrahepatic Biliary Tract
Jose Jessurun
Stefan Pambuccian
Introduction
The gallbladder is a common surgical pathology specimen obtained by laparoscopic cholecystectomy or laparotomy. Most surgical pathologists dedicate little time to the gross and microscopic examination of the gallbladder because they think that the information derived is not relevant to patient care. This persistent lack of interest has hampered understanding of the inflammatory conditions of this organ. This chapter describes the pathologic features of non-neoplastic disorders of the gallbladder and extrahepatic bile ducts, including the epidemiology and physiopathology of gallstones.
Gallstones
Gallstones are a common cause of morbidity worldwide. In the United States and Europe, an estimated 10% to 20% of the population have gallstones.1 Approximately 700,000 cholecystectomies are performed each year in the United States.2 Except for gastroesophageal reflux disease, gallbladder diseases account for the highest annual direct costs ($5.8 billion) of all gastrointestinal illnesses.3 If inpatient physician services and hospital costs are considered, cholelithiasis is one the most costly digestive disorders.4,5 Further improvements in therapy and prevention can be derived from a better understanding of the epidemiology and pathophysiology of gallstone formation.
Historical Perspective
Since antiquity, gallstones have been of interest to physicians. Historical accounts of Alexander the Great’s illness that preceded his death in 323 bc strongly suggest that he suffered from gallstones and cholecystitis. Galen, in his description of obstructive jaundice, mentioned small foreign bodies similar to grain, fig, and pomegranate seeds within the common bile duct.
The first description of gallstones as “dried up humors concreted like stones” and their relation to hepatic obstruction is ascribed to the Greek physician Alexander of Tralles (5th century ad). The 14th century physician Gentile da Foligno first postulated a relationship between cholecystitis and gallstones based on autopsy findings. Antonio Benivieni successfully diagnosed gallstone disease in a patient with abdominal pain. His clinical impression was confirmed at autopsy. However, it was Jean Fernel (1497-1558), physician to the King of France, who provided the most accurate clinical description of symptoms associated with cholelithiasis. In his masterpiece, De Sedibus et Causis Morborum per Anatomen Indagatis (1761), Giovanni Battista Morgagni described gallstones in great detail and noticed an increased frequency of this condition with age, occurrence in a preponderance of women, and an association with a sedentary lifestyle.
Gallstones were removed from a living patient for the first time in 1618 by the German surgeon Wilhelm Fabry. Two and a half centuries later, another German physician, Carl Langenbuch, performed the first cholecystectomy.
The composition of gallstones was essentially unknown until the end of the 18th century. It was through the excellent work of researchers such as Antonio Vallisneri, Francois Poulletier de la Salle, and Félix Vicq d’Azyr that the chemical composition and variability in the components of gallstones were determined.6
Gallstone Classification
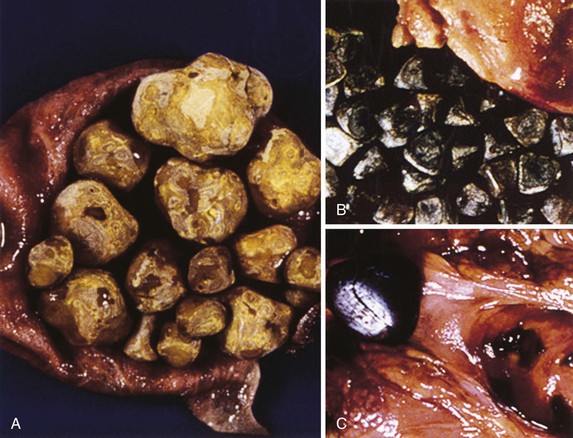
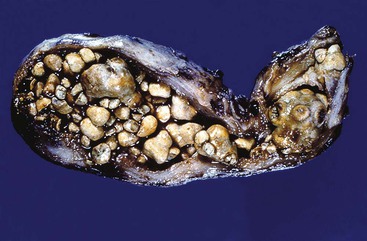
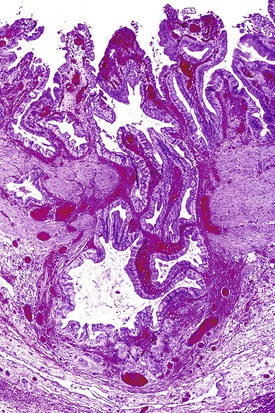
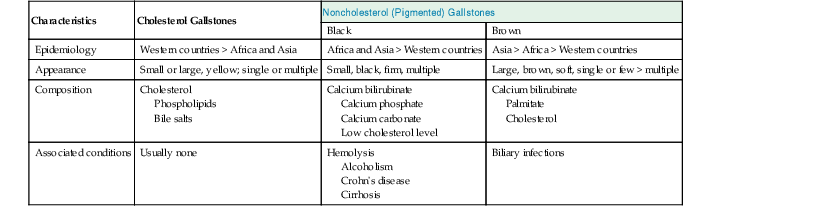
Gallstones are composed predominantly of cholesterol, bilirubin, and calcium salts, with lesser quantities of other constituents. The most widely used classification system is based on the relative amount of cholesterol in the stones. There are two main categories: cholesterol and noncholesterol (pigment) stones (Fig. 37.1). The latter are further classified as black or brown pigment stones.7–9 Cholesterol gallstones constitute more than 80% of stones in industrialized nations. They are composed predominantly of cholesterol crystals. Noncholesterol gallstones are far more common in other parts of the world, such as Asia.
Black pigment stones are formed from calcium salts of unconjugated bilirubin in a polymerized matrix. Brown pigment stones may form within bile ducts (i.e., primary bile duct stones) and contain the bacterial degradation products of biliary lipids, calcium salts of fatty acids, unconjugated bilirubin, and precipitated cholesterol. Because the pathogenesis and epidemiology of gallstones are considerably different, they are discussed separately (Table 37.1).
Table 37.1
Types of Gallstones
| Characteristics | Cholesterol Gallstones | Noncholesterol (Pigmented) Gallstones | |
| Black | Brown | ||
| Epidemiology | Western countries > Africa and Asia | Africa and Asia > Western countries | Asia > Africa > Western countries |
| Appearance | Small or large, yellow; single or multiple | Small, black, firm, multiple | Large, brown, soft, single or few > multiple |
| Composition | Cholesterol Phospholipids Bile salts |
Calcium bilirubinate Calcium phosphate Calcium carbonate Low cholesterol level |
Calcium bilirubinate Palmitate Cholesterol |
| Associated conditions | Usually none | Hemolysis Alcoholism Crohn’s disease Cirrhosis |
Biliary infections |
Cholesterol Gallstones
Pathogenesis
The major lipid components of bile are bile salts (67% of solutes by weight), phospholipids (22%), and cholesterol (4%). Hepatocytes express specific adenosine triphosphate (ATP)–binding cassette transport proteins (i.e., ABC transporters) for each of the three types of lipids at the canalicular membrane domain. The bile salt export pump is the ABCB2 transporter. The one for the major biliary phospholipid, phosphatidylcholine (i.e., lecithin) is the ABCB4 transporter. The one for cholesterol secretion is the obligate heterodimer ABCG5/ABCG8.1
Because cholesterol is insoluble in water, it requires a solubilizing system, which is provided by the detergent action of phospholipids and bile salts. After being cosecreted by hepatocytes, cholesterol and phospholipids form spherical structures composed of a double layer of phospholipids (mainly lecithin). These vesicles are soluble by virtue of the outward orientation of the hydrophilic (water-loving) choline groups, which allows cholesterol to be inserted into the hydrophobic (water-fearing) milieu provided by the fatty acid chains.9–11
Liver cells secrete bile acids through a different transport mechanism. Although soluble in water, bile salt monomers self-aggregate into simple micelles after they surpass the critical micellar concentration (≈0.5 to 5 mM). The amphophilic properties of bile acids produce an extremely water-soluble structure because of orientation of the hydrophobic portions of the molecules away from water and exposure of the hydrophilic surfaces to the aqueous environment. As detergents, bile acids can dissolve portions of vesicles and incorporate them into mixed micelles. The resulting structures are disks composed of cholesterol and phospholipids surrounded by bile acids.11–13
As the concentration of cholesterol increases, more of it is carried in vesicles. A higher cholesterol concentration increases cholesterol transfer from vesicles to micelles during the micellation process. The resulting cholesterol-enriched unilamellar vesicles are unstable and fuse into large, multilamellar vesicles. When the cholesterol-to-phospholipid ratio exceeds 1, cholesterol crystallizes at the surface. Crystallization is enhanced by the concentration of solutes in bile, because aggregation occurs more efficiently when cholesterol carriers are close to each other.10,12,14
Cholesterol is most soluble in a mixture of lipids that contains at least 50% bile acids and lesser amounts of phospholipids. Supersaturation occurs when a solution contains more cholesterol molecules than can be solubilized. Theoretically, bile supersaturation may be caused by hypersecretion of cholesterol, hyposecretion of bile acids, hyposecretion of phospholipids, or a combination of these mechanisms. An increase in biliary cholesterol output resulting from increased synthesis or increased uptake is the most common cause of supersaturation and subsequent stone formation. Increased uptake by hepatocytes may involve endogenous cholesterol (transported by low-density lipoprotein) or exogenous cholesterol (transported by chylomicrons).
Cholesterol supersaturation may result from bile acid hyposecretion. However, most patients with gallstones have normal biliary acid secretion. Adequate bile acid secretion depends on the integrity of the enterohepatic circulation. Approximately 90% of bile acids are resorbed from the terminal ileum and returned to the liver by the portal system 3 to 12 times per day. Bile acids are reused by hepatocytes after passive and active reuptake. Theoretically, interference with this recycling mechanism contributes to bile acid hyposecretion and subsequent cholesterol supersaturation.11–13
A study of first-degree relatives of gallstone carriers has provided clues that bile lipid secretion may be under genetic control.14 Most information is based on animal models. Knockout mice deficient in the multiple drug–resistant gene 2, maintain normal bile acid secretion but are incapable of secreting phospholipids and cholesterol into bile resulting from absence of a protein that flips phospholipids from the inner to the outer half of canalicular membranes.15 In other models, mice fed a lithogenic diet developed gallstones at a frequency that varied according to the presence of the Lith1, Lith2, or Lith3 genes and other genes.3
Supersaturation of cholesterol is necessary but not sufficient for the formation of cholesterol gallstones. For any degree of cholesterol saturation, patients with gallstones form cholesterol crystals more rapidly than individuals without gallstones. This observation led to the theory that stone formation probably involves a nucleation process. The tendency of bile to nucleate cholesterol depends on a balance between substances that promote and prevent nucleation. Pronucleating agents are mostly heterogeneous mucin gels. Other biliary proteins have been postulated as promoters (e.g., nonmucin glycoproteins, mainly immunoglobulins) or inhibitors (e.g., apolipoproteins A-I and A-II, other glycoproteins) of cholesterol precipitation in bile.16–21 However, their participation is most likely nonspecific, and their relevance remains controversial.
Abnormal gallbladder motility contributes to gallstone formation. By causing incomplete emptying of supersaturated and crystal-containing bile, gallbladder hypomotility promotes the formation of gallstones.22
Biliary sludge is a viscous gel composed of mucin and microscopic precipitates of multilamellar vesicles, cholesterol monohydrate, and calcium bilirubinate. Because mucin is at the center of almost all gallstones, it was suggested that the formation of biliary sludge precedes the formation of macroscopic cholesterol gallstones.16
Bacteria may contribute to the formation of gallstones. Using polymerase chain reaction (PCR) amplification, Swidsinski and colleagues identified bacteria in cholesterol gallstones.23 Pseudomonas and Escherichia coli were initially thought to be the main responsible organisms, but bile-resistant Helicobacter species and Helicobacter pylori are also thought to participate in the formation of mixed cholesterol gallstones. 24,25 Whether these organisms are innocent bystanders or play a role in the formation of gallstones awaits determination.
Epidemiology and Risk Factors
The prevalence of cholesterol gallstones depends on the age, gender, country of residence, and ethnicity of the population. Geographic differences are most likely related to interaction of genetic and environmental factors. In the Unites States, it is estimated that 20 to 30 million people have gallstones.
The prevalence increases with age. Cholelithiasis in children is rare. After 20 years of age, the prevalence of gallstones increases with each decade of life: 7% to 11% for those younger than 50 years, 11% to 30% for individuals between 60 and 70 years, and 33% to 50% for people older than 90 years of age.26 Gallstones develop more frequently in women than in men. For reproductive-age women, the risk of cholelithiasis is two to three times higher than for men. Increased risk of gallstones is associated with pregnancy, multiparity, estrogen replacement therapy, oral contraceptive use, obesity, and rapid weight loss.27 In addition to estrogens, other drugs that increase the risk of cholelithiasis are prednisolone, cyclosporine, azathioprine, octreotide (Sandostatin), clofibrate, and nicotinic acid.28 Whether diabetes predisposes to gallstone formation remains controversial. Substantial evidence suggests that alcohol intake protects against gallstone formation.29,30
The prevalence of cholelithiasis is influenced by the genetic composition of the population. Patients who have a relative who had gallstones have a two to four times higher rate of gallstones.28 In the United States, the highest prevalence of gallstones is observed among Native Americans, with a progressively lower risk among whites, blacks, and some Asian groups.31 The prevalence of gallstones among Mexican-American women is higher compared with other Hispanic women.32,33 Gallstones are extremely common in Chile and in Scandinavian countries, but the incidence is much lower in Asia and Africa.34,35
Epidemiologic data from North America suggest that populations with a high rate of gallstones carry dominant Amerindian lithogenic genes transmitted by common ancestral Asians who colonized America more than 20,000 years ago. In support of this hypothesis, an epidemiologic study from Chile found a positive correlation between Native American genes (measured by ABO blood group distribution and determination of mitochondrial DNA polymorphisms) and the prevalence of gallstones in young women.36 In this study, the highest prevalence of gallstone disease was detected among the indigenous Mapuche (35.2%), followed by residents of urban Santiago (27.5%) and the Maoris of Easter Island (20.9%).36 The high prevalence among Native-American and Mexican-American women also supports this hypothesis.
The specific genes associated with gallstone susceptibility have been partially characterized in animal models. Undoubtedly, the corresponding human genes and their products will be elucidated soon. Knowledge of the function of gene products involved in lithogenesis and the potential relevance of genetic polymorphism in their synthesis or functionality will elucidate their complex interactions with environmental (dietary) factors. Based on this information, specific prevention strategies can be tailored to populations with a high prevalence of cholesterol gallstones.
Pigment Gallstones
Pathogenesis
There are two types of pigment stones: black and brown. This distinction is important because they differ in their pathophysiology, associated clinical conditions, morphology, and chemical composition. Black stones are composed of calcium bilirubinate, phosphate and carbonate embedded in a glycoprotein and have a very low cholesterol concentration. Brown stones contain calcium salts of bilirubin and fatty acids (palmitate) in a glycoprotein matrix and have a higher concentration of cholesterol. Calcium carbonate and phosphate are usually absent.37 Black stones are small, black, and multiple. Brown stones are soft, brownish-green, and large.
Because it is a precursor of calcium bilirubinate, unconjugated bilirubin plays a central role in the formation of brown and black pigment stones. Unconjugated bilirubin is solubilized by bile salts in mixed micelles and then combines with calcium to form calcium bilirubinate. Any condition that results in elevated levels of unconjugated bilirubin may predispose to stone formation. Biliary infections that contribute to bile stasis are common causes of brown stones, because bacterial overgrowth generates hydrolases that form free bile acids from conjugated bile salts. Bacteria elaborate phospholipase A, which cleaves phospholipids to form lysolecithin and free fatty acids. Free fatty acids (mainly palmitic and stearic) combine with free bile salts generated by bacterial hydrolases and precipitate as calcium salts. It is therefore not surprising that bacteria are found within the matrix of most brown stones.37
Black pigment stones are not associated with bacterial infection. An increased concentration of unconjugated bilirubin originates from an increase in the secretion of bilirubin conjugates, as in patients with hemolysis and chronic alcoholism, followed by nonbacterial enzymatic or nonenzymatic hydrolysis. An analogous effect occurs if the secretion of bile salts is decreased, as in patients with cirrhosis, because these compounds are required to solubilize unconjugated bilirubin and buffer ionized calcium.16 Phospholipids also play an important role in pigment sludge formation. Calcium bilirubinate sludge contains an increased amount of phospholipids, and these compounds occur in the core of pigment gallstones. Carbohydrate-rich diets stimulate enzymes that are important in the synthesis of phospholipids, such as fatty acid synthetase. Increased activity of these enzymes helps to explain the higher hepatic bile phospholipid concentrations found in some clinical situations, such as total parenteral nutrition.
The gallbladder mucosa plays a role in lithogenesis. Biliary epithelium acidifies bile, increasing the solubility of calcium carbonate. Mucosal inflammation interferes with the ability of the epithelium to perform its acidifying role, which results in increased biliary pH and subsequent calcium carbonate precipitation. Reparative metaplastic changes in the mucosa (discussed later) increases the concentration of biliary glycoproteins, which promotes gallstone formation.38
In adult and fetal gallbladders, MUC1, MUC2, MUC3, MUC5AC, MUC5B, and MUC6 gene expression has been found; the most prominently expressed appears to be MUC5B.39,40 With the exception of MUC2, all of these mucin genes appear to be expressed in the gallbladder epithelium of gallstone patients. MUC5AC gene expression is elevated in patients with cholecystitis, particularly those with brown stones. Inflammation does not affect the expression of other mucins.41
Brown stones are strongly associated with infection, and they may be found within the bile ducts and gallbladder. The β-glucuronidase produced by bacteria deconjugates bilirubin to its water-insoluble form. Calcium salts of unconjugated bilirubin (i.e., deconjugated bile acids and saturated long-chain fatty acids) are generated, which leads to the formation of brown stones. Organisms implicated in the formation of these stones include E. coli, Clonorchis sinensis, Opisthorchis viverrini, and Ascaris lumbricoides.42
Epidemiology
Pigment gallstones occur in patients worldwide. Although they account for only 20% to 25% of gallstones in the United States, they are much more common in other parts of the world, such as Asia. Similar to cholesterol gallstones, pigment stones develop more frequently in women, and the incidence increases with age. Race is not a factor.
Clinical conditions associated with black gallstones include hemolytic anemia, cirrhosis, alcoholism, malaria, pancreatitis, total parenteral nutrition, and older age. Black pigment stones develop more frequently in patients with Crohn’s disease, particularly those with extensive ileitis and those who have had an ileal resection. The predilection for stone formation in patients with ileitis or in those who have had an ileal resection stems from decreased or absent functionality of the terminal ileum, which is the site of 90% of bile salt resorption. In normal individuals, unconjugated bilirubin precipitates in the colon as calcium bilirubinate or other bilirubinates. In contrast, impaired or absent resorptive function in the ileum in patients with Crohn’s disease leads to increased levels of bile salts in the colon, where salts solubilize unconjugated bilirubin.43 Subsequent increased resorption of unconjugated bilirubin in the colon leads to supersaturation of bile (as much as three times normal levels), and stone formation.
Cholecystitis
Inflammatory diseases of the gallbladder are a frequent cause of morbidity in Western countries. The term cholecystitis encompasses a group of disorders that have different pathologic, pathogenetic, and clinical characteristics. As in other organs of the gastrointestinal tract, most inflammatory diseases of the gallbladder produce nonspecific histologic features because they elicit a nondistinctive type of cellular inflammatory infiltrate. However, characterization of specific inflammatory patterns helps to establish pathologic diagnoses and provides insight into the pathogenesis of disease. Recognition of different patterns of inflammation can render clinically useful histologic diagnoses (Box 37.1).
Acute Cholecystitis
Clinically, acute cholecystitis is defined as an episode of acute biliary pain accompanied by fever, right upper quadrant tenderness, guarding, persistence of symptoms beyond 24 hours, and leukocytosis.44 Approximately 90% of cases are associated with gallstones. Ultrasonography often demonstrates thickening of the gallbladder wall or pericholecystic fluid. The diagnosis is also supported by failure to visualize the gallbladder during a hepatobiliary scintigram.45 Because of unique clinical and pathologic characteristics, the three types of acute cholecystitis—acute calculous cholecystitis, acute acalculous cholecystitis, and emphysematous cholecystitis— are discussed separately.
Acute Calculous Cholecystitis
Clinical Features
Most patients in whom acute calculous cholecystitis develops are women between 50 and 70 years of age. Typical symptoms are right upper quadrant pain of recent onset, accompanied by abdominal guarding and local tenderness. Anorexia, nausea, and vomiting are common. A history of similar episodes in the past that resolved spontaneously is frequently obtained. In the elderly, symptoms may be deceptively mild or even absent. Occasionally, an enlarged gallbladder may be palpated. Pain may be elicited on palpation of the right upper quadrant when the patient inhales deeply (i.e., Murphy sign). Some patients may be febrile. Rarely, jaundiced develops. Most patients have leukocytosis.
Because the clinical features are nonspecific, imaging techniques such as ultrasonography or cholescintigraphy are used to confirm the clinical diagnosis. Preoperative clinical findings of acute cholecystitis are highly reliable for predicting intraoperative gross findings. However, intraoperative findings of acute cholecystitis are commonly found in the absence of preoperative clinical signs. For unknown reasons, the correlation between pathologic and intraoperative findings is poor.45
A rare complication that results from compression of the common bile duct by gallstones lodged in the cystic bile duct is Mirizzi syndrome. Imaging techniques may help to confirm the extrinsic nature of the bile duct obstruction and help to plan the appropriate surgical procedure.
Pathogenesis
The main precipitating event in the development of acute calculous cholecystitis is occlusion of the neck of the gallbladder (i.e., cystic duct) by gallstones. The resulting increase in intraluminal pressure dilates the gallbladder and causes mural edema. However, outflow obstruction does not always cause acute cholecystitis. Animal models in which the cystic duct has been ligated or obliterated experience shrinkage of the gallbladder but not acute cholecystitis.46 Factors contributing to the development of acute cholecystitis include mucosal ischemia resulting from visceral distention and external compression of the cystic artery by an impacted stone.
Formation of inflammatory mediators, such as lysolecithin and prostaglandins, and mucosal damage by concentrated bile, cholesterol, or gallstones may also contribute to mucosal injury.47 Trauma to the mucosa caused by stones may release phospholipase from lysosomes that normally reside in mucosal epithelial cells. This enzyme converts lecithin to lysolecithin, an active detergent that is toxic to the mucosa.48 Phospholipids can damage biliary cells. Bile from patients with gallstones contains lysophosphatidylcholine, which induces mucosal necrosis and inflammation of the gallbladder.38
When bile cultures are obtained within 48 hours of onset, bacteria are identified in 42% to 72% of cases. The predominant organisms are E. coli, other gram-negative aerobic rods, enterococci, and anaerobes (20% of cases).49,50 Most authorities agree that infection is secondary and not the principal etiologic factor of acute cholecystitis.
Pathologic Features
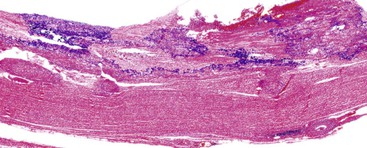
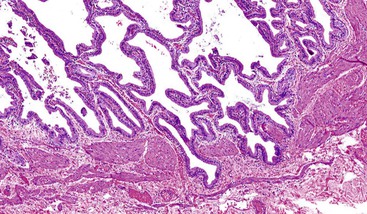
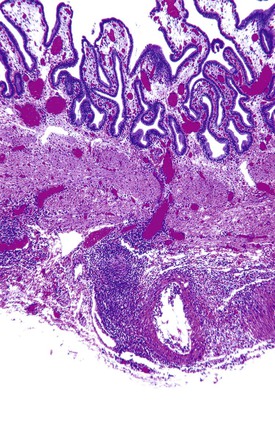
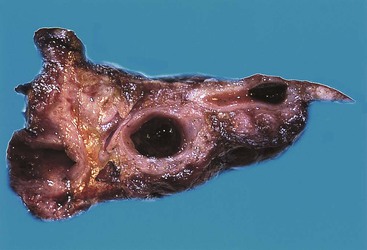
Acute cholecystitis may be identified at the time of laparoscopy or laparotomy by gross visualization of signs of acute inflammation, such as omental adhesions to the gallbladder wall, edema, friability, pericholecystic fluid, and frank gangrene. The gallbladder is usually enlarged and the wall thickened by edema, vascular congestion, and hemorrhage, or it may appear necrotic (Fig. 37.2). The serosa is usually dull and often covered by patches of fibrinopurulent exudate. A gallstone is frequently found obstructing the lumen of the cystic duct. Pus may fill the lumen, and it may be mixed with thick, cloudy bile. Depending on the severity of the inflammatory response, mucosal changes range from edema and congestion to widespread ulcers and necrosis.
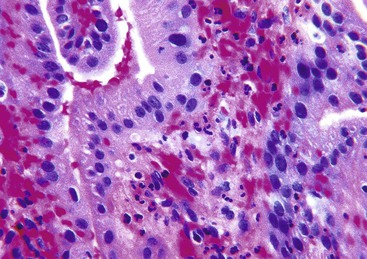
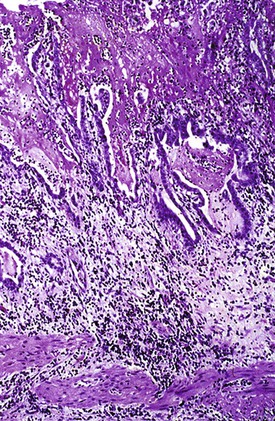
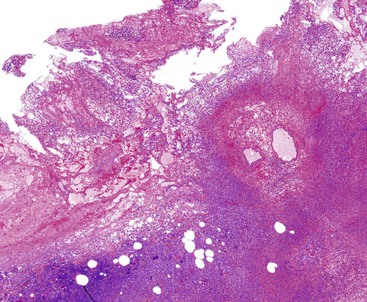
Histologic evaluation invariably identifies ischemic changes, which may be the predominant findings. An acute inflammatory reaction, characterized by edema, vascular congestion, hemorrhage, neutrophilic infiltration, and mucosal necrosis, predominates in specimens obtained early in the course of disease (Fig. 37.3). In the early phases, the inflammatory and necrotic changes are confined to the mucosa. As the pathologic process evolves, transmural inflammation, secondary acute vasculitis, and transmural necrosis follow. Fibrinous pseudomembranes (i.e., pseudomembranous cholecystitis) may develop over necrotic-appearing mucosa (Fig. 37.4). After the first week, lymphocytes, plasma cells, macrophages, and eosinophils appear. Granulation tissue and collagen then replace previously ulcerated or necrotic tissue.
Gangrenous cholecystitis is a severe form of acute cholecystitis that occurs most frequently in patients with comorbidities such as cardiovascular disease, diabetes, and trauma. The gallbladder is characteristically distended and has a hemorrhagic to black wall indicative of ischemia. Histologically, the mucosa and muscularis often are absent and replaced by necrotic debris, neutrophils, and granulation tissue.
Areas of perforation are commonly sealed off by the omentum, inducing formation of pericholecystic adhesions or abscesses. Severe complications include life-threatening bacteremia and sepsis.44 Correlation between the pathophysiologic events leading to acute cholecystitis and the pathologic patterns of injury are summarized in Table 37.2.
Table 37.2
Pathophysiologic and Pathologic Manifestations of Acute Cholecystitis
| Cause | Mechanism of Injury | Type of Injury |
| Obstruction of the outflow tract and/or compression of the cystic artery by a gallstone | Distention of the gallbladder | Ischemic necrosis |
| Mechanical and inflammatory injury to biliary cells | Formation of inflammatory mediators and chemical injury by detergents | Mucosal injury or necrosis and inflammation |
| Secondary bacterial infection | Inflammatory response | Mucosal injury or necrosis and inflammation |
Natural History and Treatment
Most patients treated medically have symptomatic remission within a few days. Cholecystectomy is the treatment of choice for patients with complications. Because most patients with acute cholecystitis experience at least one recurrence, surgical treatment is recommended for all patients when possible. Cholecystectomy should be performed, preferably within 2 to 3 days of the onset of symptoms, a time frame that is referred to as the “golden period.”51,52 After inflammation has persisted for more than 72 hours, the development of fibrous adhesions and transmural inflammation makes surgery more laborious and prone to complications.
Acute Acalculous Cholecystitis
Clinical Features and Pathogenesis
Acute acalculous cholecystitis occurs in approximately 2% to 15% of all patients who have undergone cholecystectomy.53,54 Affected individuals often have associated conditions, such as a history of trauma or a nonbiliary surgical procedure, sepsis, burns, parenteral nutrition, mechanical ventilation, multiple blood transfusions, or prior use of narcotics or antibiotics. However, this disorder may occur de novo in patients without any predisposing factors.55 In these cases, the pathogenesis is probably multifactorial. Visceral hypoperfusion, ischemia, reperfusion injury, and bile stasis have been postulated as possible mechanisms.27
Increased bile viscosity from stasis, with subsequent obstruction of the cystic duct, has been suggested as a contributing factor. It may help to explain the association of acalculous cholecystitis with a clinical history of fasting, narcotic use, dehydration, or recent anesthesia, all of which may result in bile stasis.
Mucosal ischemia plays a major role in patients with underlying cardiovascular disease and those who develop acute acalculous cholecystitis develops after trauma, sepsis, or a surgical procedure. There is a high mortality rate, as high as 45%, for this group of patients.
Prostanoid and bile salts may contribute to the development of acalculous cholecystitis. Prostaglandins are involved in gallbladder contraction, water absorption, inflammation, and pain associated with gallbladder disease. Prostaglandins have various roles in acute inflammatory conditions of the gallbladder. Prostaglandin E (PGE) levels correlate positively with the degree of inflammation. The levels of this prostaglandin are increased sevenfold in patients with acute acalculous cholecystitis. Tissue anoxia may result from shock, bacterial contamination and invasion, stasis, and changes in bile salt concentration, which can injure gallbladder mucosa. As a consequence, inflammation, distention, atonicity, and pain develop.55,56
In animal models, platelet-activating factor (PAF) plays a role in the induction of acute acalculous cholecystitis. PAF is released by basophils, eosinophils, neutrophils, macrophages, monocytes, mast cells, vascular endothelial cells, and smooth muscle cells. It increases vascular permeability and induces neutrophil aggregation and degranulation. Indirectly, PAF may cause acalculous cholecystitis by stimulating and releasing interleukin-1, tumor necrosis factor, and interleukin-6. PAF may also be associated with the development of arteriolar thrombosis and ischemia.56
Secondary infection of the gallbladder during sepsis may cause acute acalculous cholecystitis. This situation has been reported for patients with disseminated candidiasis, leptospirosis, chronic biliary tract carriage of typhoidal and nontyphoidal Salmonella, cholera, Campylobacter enteritis, tuberculosis, malaria, brucellosis, Q fever, and dengue fever. Hepatitis A and B and Epstein-Barr virus infections have been associated with this condition. Obstruction of extrahepatic bile ducts by ascariasis and echinococcal cysts also may cause acute alcalculous cholecystitis.57
Acalculous cholecystitis is the most common form of cholecystitis in children. Dehydration, acute bacterial infection, and viral illnesses contribute to its development in this group.58,59
Pathologic Features
The gallbladder is frequently distended, and the serosa appears congested. The appearance of the mucosa varies from normal to hyperemic and necrotic. By definition, gallstones are absent.
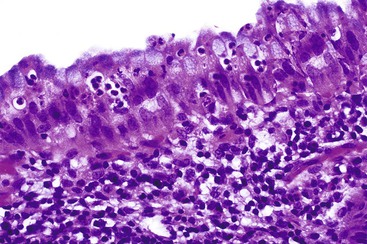
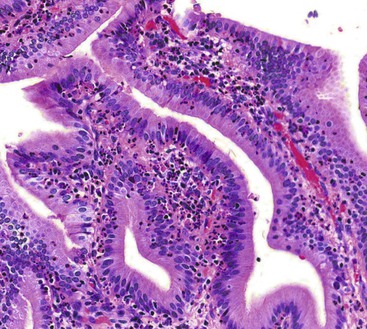
Common histologic features of acute acalculous cholecystitis include bile infiltration, leukocyte margination within blood vessels, neutrophilic and mononuclear cell infiltration of the lamina propria and biliary epithelium, edema, and lymphatic dilatation. Compared with calculous cholecystitis, bile infiltration of the gallbladder is typically wider and deeper, as is the extent of necrosis of the muscularis.27 Similar to calculous cholecystitis, mucosal ischemic changes are frequently observed (Fig. 37.5). Ischemic changes are particularly prominent in postsurgical patients and in those hospitalized for trauma or another type of critical illness.
Specific histologic differences between acute calculous cholecystitis and acalculous cholecystitis are lacking. A possible drug-induced injury should be suspected when there is a predominance of eosinophils within the lamina propria in the setting of acute cholecystitis.60
Acute Emphysematous Cholecystitis
Clinical Features and Pathogenesis
Acute emphysematous cholecystitis is an uncommon variant of acute cholecystitis caused by bacterial infection with gas-producing organisms. This condition is clinically indistinguishable from simple acute cholecystitis. The diagnosis is usually established with the use of radiographic studies. Abdominal radiographs are relatively insensitive for emphysematous cholecystitis. However, because of the regular use of ultrasonography for patients with suspected hepatobiliary disease, emphysematous cholecystitis is being diagnosed with increased frequency.61 Diagnostic delay results in a high incidence of complications, such as gangrene and perforation, which explains the high overall mortality rate for patients with this condition (15% versus 4.1% for acute calculous cholecystitis).
Approximately 50% of patients have a positive blood culture for clostridial organisms. Tests reveal E. coli, Bacteroides fragilis, Klebsiella species, and anaerobic streptococci infection in a lower percentage of patients.62 Occlusion of the cystic artery or its branches by atherosclerosis and small vessel disease (both frequent complications of diabetes mellitus) are major contributing factors. Other ischemic events, such as arterial embolism, vasculitis, and systemic hypoperfusion, also predispose to this condition.63–65
Pathologic Features
At the time of cholecystectomy, the gallbladder may appear distended, tense, or encased by omentum and may have fibrous adhesions or a pericholecystic abscess, or both. A necrotic, friable wall frequently is the cause of fragmentation of the gallbladder during an attempt at removal. On opening the gallbladder, gas and a foul-smelling purulent exudate may escape from the lumen. Gallstones, typically of the pigment type, are detected in 70% of cases. The mucosa usually appears necrotic, congested, and hemorrhagic.
Microscopically, necrotic and acutely inflamed mucosa often contains colonies of gram-positive bacilli. The inflammatory infiltrate is composed predominantly of neutrophils admixed with necrotic debris. Inflammation of the mural and blood vessels, which can include fibrinous necrosis of the wall, is common and should not be interpreted as a primary vasculitis. Gas bubbles occasionally occur within the wall of the gallbladder or subserosal connective tissue. Perforation and bile peritonitis occur in approximately 10% of cases (Fig. 37.6).
Chronic Cholecystitis
Chronic Calculous Cholecystitis
Clinical Features and Pathogenesis
Chronic cholecystitis is better defined by its gross and histologic features than by its clinical characteristics. There is uncertainty regarding symptoms associated with gallstone disease and chronic cholecystitis. Most patients with gallstones never experience attacks of pain. In some cases, the only symptom related to gallstones may be episodic, mild upper abdominal pain.66 Dyspeptic symptoms, belching, bloating, abdominal discomfort, heartburn, and food intolerances are frequently attributed by patients and physicians to cholelithiasis and chronic cholecystitis. However, most of these symptoms are probably unrelated to gallstone disease, and some persist after cholecystectomy. Because chronic cholecystitis typically is associated with cholelithiasis, the demographic characteristics of these patients and risk factors are the same as for patients with cholesterol gallstones.
The most common symptom is episodic, nonintermittent abdominal pain (erroneously referred to as biliary colic) that is typically located in the epigastrium or right upper quadrant. Pain may be precipitated by ingestion of food, but in most instances, it occurs spontaneously without an inciting event. On physical examination, mild to moderate tenderness may be elicited when palpating the gallbladder, particularly during a pain attack. Ultrasound examination of the gallbladder is the method of choice to demonstrate stones and abnormalities in the gallbladder wall resulting from inflammation or fibrosis, or both.
Chronic cholecystitis typically is associated with gallstones. The pathogenesis of this common disorder is poorly understood. It has been suggested that chronic cholecystitis results from recurrent attacks of mild acute cholecystitis, but few patients provide a clinical history that supports this hypothesis. The inflammatory and reparative changes may be explained in part by repetitive mucosal trauma and inflammation produced by gallstones, although other factors likely play a role.
Because of poor correlation between the severity of the inflammatory response and the number and volume of stones, it is possible that the intensity of the inflammatory response induced by gallstones in different populations is genetically determined.67 One hypothesis is that a copious inflammatory response provides a protective effect in some patient populations whose ancestors resided in geographic areas with a high incidence of parasitic biliary infections.
Some scientists have postulated that cholelithiasis and chronic cholecystitis are caused by an abnormal composition of bile, which leads to stone formation and chemical injury to the mucosa. At variance with a high percentage of positive bile cultures for patients with acute cholecystitis is that bacteria, mostly E. coli and enterococci, are cultured in less than one third of cases of chronic cholecystitis.68 DNA from Helicobacter species has been identified in biliary tract specimens from patients with cholecystitis.69 However, this association has not been confirmed in other studies.70
Pathologic Features
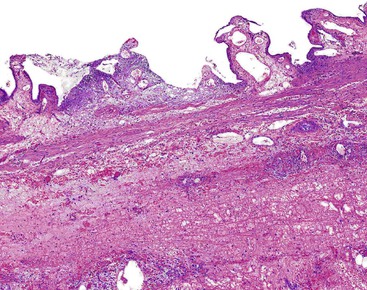
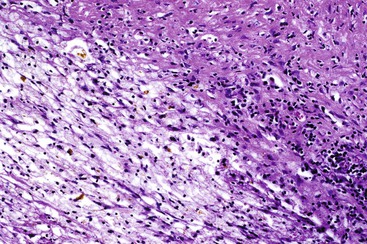
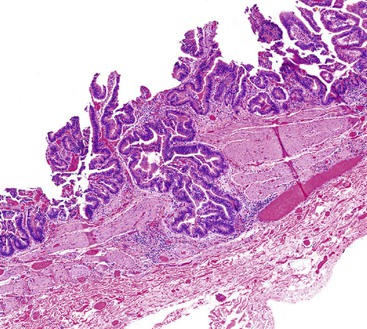
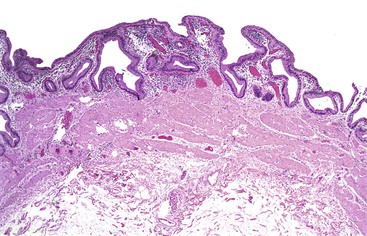
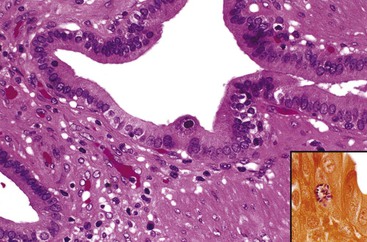
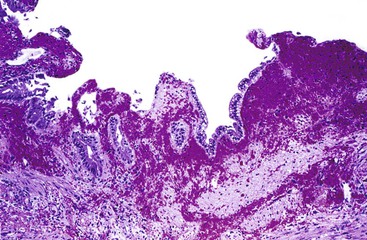
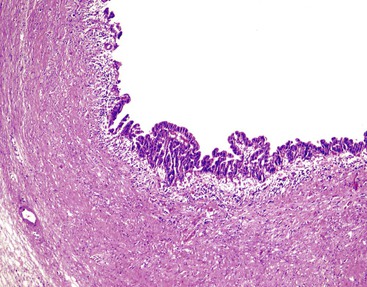
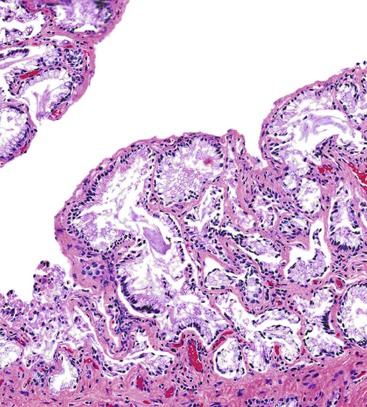
In chronic cholecystitis, the varied appearance of the gallbladder reflects the degree of inflammation and fibrosis. The gallbladder may be distended or shrunken and appear atrophic. Fibrous serosal adhesions suggest previous episodes of acute cholecystitis. On gross examination, the wall is usually thickened, but it may be thin in some cases. The mucosa may be intact with preservation or accentuation of its folds, or it may be flattened with outflow obstruction. Mucosal erosions or ulcers are frequently associated with impacted stones (Fig. 37.7).
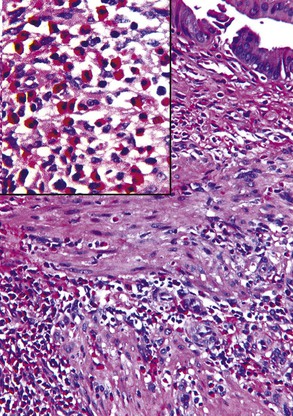
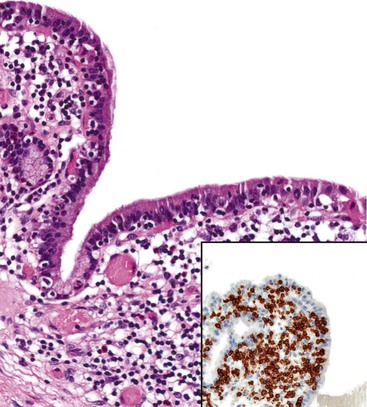
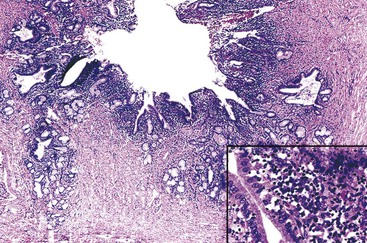
The presence of gallstones is neither necessary nor sufficient for a diagnosis of chronic cholecystitis. The diagnosis is based on three histologic characteristics: a predominantly mononuclear inflammatory infiltrate in the lamina propria, with or without extension into the muscularis and pericholecystic tissues; fibrosis; and metaplastic changes. The degree of inflammation varies. The infiltrate may be located exclusively in the mucosa, or it may extend into the muscularis and serosa. The inflammatory infiltrate may be distributed focally to diffusely. Commonly, lymphocytes predominate over plasma cells and histiocytes. Sparse, focally distributed lymphoid cells may be found in normal gallbladders obtained from healthy individuals who have died of trauma and whose livers were used for transplantation71 (Fig. 37.8). Occasionally, lymphoid follicles arise in a background of chronic inflammation. Most lymphoid follicles are located in the lamina propria, but they may be identified within the gallbladder wall. When diffuse, the term follicular cholecystitis has been used to describe this condition72 (Fig. 37.9). A minor component of eosinophils and neutrophils may be also seen. When neutrophils are predominantly found within the epithelium in the setting of chronic cholecystitis, the disorder should be called chronic active cholecystitis rather than mixed acute and chronic cholecystitis or subacute cholecystitis (Fig. 37.10).
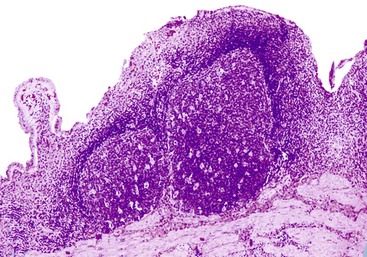
When bile penetrates into the subepithelial mesenchyme through mucosal ulcers or fissures, it frequently elicits an inflammatory reaction composed of closely packed histiocytes with pale cytoplasm containing abundant brown-pigmented granules (Fig. 37.11). In addition to its color, the ceroid pigment is characterized histochemically by acid fastness and diastase-resistant periodic acid–Schiff (PAS) positivity. A sparse lymphocytic reaction usually accompanies the histiocytes.73,74 Ceroid granulomas trigger a reparative response that often leads to deposition of dense collagen. Fibrosis eventually replaces areas previously involved by the inflammatory process and may replace the entire gallbladder. Dystrophic calcifications are often associated with this fibrous reaction, and when diffuse, they may produce a porcelain gallbladder. Although previously thought to be associated with a higher incidence of carcinoma than other forms of cholecystitis,75 studies do not support this contention.76
The term hyalinizing cholecystitis has been proposed for a type of chronic cholecystitis characterized by dense hyaline fibrosis with relatively sparse inflammation that transforms the gallbladder wall into a thin, uniform shell. Various degrees of dystrophic calcification are identified. This type of cholecystitis is thought to be associated with a higher incidence of carcinoma, particularly in cases with scant or no calcifications77 (Fig. 37.12).
In addition to ceroid granulomas, foreign body–type granulomas, characterized by aggregates of multinucleated giant cells and foamy histiocytes, may be seen around clefts that contain cholesterol crystals or concretions of bile. Foamy histiocytes are predominant in xanthogranulomas, which are associated with plasma cells, giant cells, or ceroid-containing histiocytes (Fig. 37.13). The cells can form a tumor-like aggregate that may be confused with a neoplasm (i.e., xanthogranulomatous cholecystitis).78–81 A granulomatous reaction caused by infection rarely occurs in the gallbladder (Fig. 37.14).
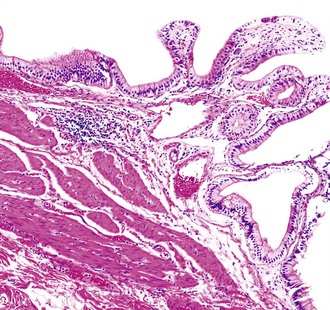
Rokitansky-Aschoff sinuses are pathologic herniations of the mucosa into or through the muscularis, analogous to intestinal diverticula (i.e., pseudodiverticula) (Fig. 37.15). They form in response to increased intraluminal pressure and are commonly associated with hypertrophic muscularis. In the absence of other features of chronic cholecystitis (i.e., inflammation or metaplastic changes), their occurrence is not considered sufficient to diagnose chronic cholecystitis. We prefer to document them as mucosal herniations, which are a consequence of outflow obstruction that is most frequently caused by gallstones. Rokitansky-Aschoff sinuses may be the only pathologic change detected in patients with biliary dyskinesia, a condition that is not normally associated with gallstones.71
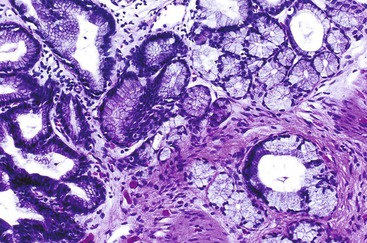
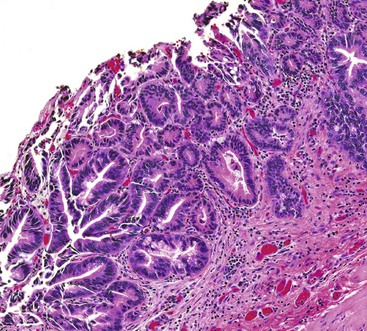
As in other organ systems, chronic injury to the gallbladder mucosa may cause a variety of metaplastic changes.82–85 The most common type of metaplasia—antral or pyloric metaplasia—is characterized by tubular glands in the lamina propria composed of clear cells with abundant mucin vacuoles. The glands are similar to those normally residing in the gastric antrum (Fig. 37.16). The surface epithelium frequently undergoes mucinous columnar metaplasia of the gastric type. This change is characterized by focal or diffuse replacement of the columnar epithelium of the gallbladder by tall, mucin-rich, PAS-positive columnar cells. When metaplastic pyloric glands proliferate and permeate smooth muscle fibers, their histologic appearance may be confused with an adenocarcinoma. Rarely, florid pyloric gland metaplasia may show features suggesting perineural or intraneural invasion. However, the lobular arrangement of the glands combined with the bland cytologic features helps to distinguish this reaction from adenocarcinoma.85 Less frequently, intestinal metaplasia may occur. It is identified as epithelium with an intestinal phenotype containing goblet cells, absorptive columnar cells, Paneth cells, and endocrine cells. Infrequently, squamous metaplasia may develop.
The differential diagnosis of invasive well-differentiated adenocarcinoma of the gallbladder and reactive atypia is summarized in Table 37.3. Dysplasia involving Rokitansky-Aschoff sinuses may also mimic invasive adenocarcinoma. Rokitansky-Aschoff sinuses usually have a perpendicular orientation to the surface, whereas adenocarcinoma glands proliferate haphazardly, are more abundant, and are frequently oriented parallel to the mucosa (Fig. 37.17). Although the finding of desmoplasia suggests a malignant process, dense fibrosis in common in the stroma surrounding inflamed Rokitansky-Aschoff sinuses. Cytologic atypia and mitoses favor a malignant tumor.
Table 37.3
Differential Diagnosis between Rokitansky-Aschoff Sinuses with Atypia and Well-Differentiated Adenocarcinoma
| Feature | RAS with Reactive Atypia | Well-Differentiated Adenocarcinoma |
| Orientation of the glands | Perpendicular to surface | Frequently parallel to surface |
| Glandular crowding | Usually absent | Often present |
| Glands adjacent to muscular vessels | Usually absent | May be present |
| Glandular necrotic debris | Absent | May be present |
| Dysplastic epithelium in the mucosa | Usually absent* | Frequently present |
| Presence of luminal bile | May be present | Absent |
| Inflammatory cells | Frequently present | Usually absent |
| Intravascular invasion | Absent | May be present |
| Perineural invasion | Absent† | May be present |
| Nuclear pleomorphism | Absent | Present, may be mild |
| Immunolabeling for CEA | Usually absent | May be present |
| Loss of DPC4 nuclear staining | Absent | Present in one half of cases |
* Dysplastic epithelium may extend into the RAS and should not be interpreted as representing invasive carcinoma.
† Although perineural invasion is not seen in relation to RAS, it may occur in chronic cholecystitis with extensive pyloric metaplasia and should not be interpreted as unequivocal evidence of malignancy.
CEA, Carcinoembryonic antigen; DPC4, mediator of intracellular signaling, with loss associated with a poor prognosis; RAS, Rokitansky-Aschoff sinuses.
Treatment
In the United States, laparoscopic cholecystectomy has become the preferred treatment for patients with cholelithiasis.86–88 The minimally invasive surgical procedure offers the advantage of shorter hospitalization, limited postoperative pain, diminished disability, and improved cosmesis. In most instances, the gallbladder is easily removed through the umbilical puncture wound, although difficulties may arise when bile or gallstones distend the gallbladder or when inflammation and fibrosis give rise to a thick, noncollapsible wall. This problem is usually solved by extending the umbilical incision or by removing bile and stones after the neck of the gallbladder has been pulled through the skin and amputated. Mechanical devices, ultrasound, or laser energy may be used to pulverize large stones.89
Chronic Acalculous Cholecystitis
Approximately 12% to 13% of patients with chronic cholecystitis do not have gallstones.90 In some instances, the inflammation is a consequence of postinflammatory stenosis or anatomic abnormalities of the cystic duct that may impede normal emptying of the gallbladder. Other patients with biliary symptoms do not show gallstones in imaging studies. The type of disorders associated with this condition varies. In some patients, the disease may be confined to the gallbladder, but in others, it may be a manifestation of a generalized extrahepatic bile duct disorder. Although the inflammatory pattern in some patients with chronic acalculous cholecystitis is nonspecific, judicious analysis of the type of inflammatory cells and their distribution in cholecystectomy specimens may provide clues about the nature of the disease in others. Three histologic patterns of chronic acalculous cholecystitis have recently been described (see later). These include lymphoeosinophic and eosinophilic cholecystitis, diffuse lymphoplasmacytic acalculous cholecystitis, and lymphocytic cholecystitis.
Some patients have a dysmotility disorder of the gallbladder known as biliary dyskinesia. Patients who may benefit from cholecystectomy are identified by a cholecystokinin provocation test. A positive test result consists of reproduction of pain within 5 to 10 minutes after an intravenous injection of cholecystokinin.91 Incomplete emptying of the gallbladder can be documented when this test is performed at the same time as oral cholecystography.
At the time of surgery, a normal, distended, or thickened gallbladder may be found. Microscopic examination may be unremarkable or demonstrate changes consistent with outflow obstruction or inflammation, or both. Rokitansky-Aschoff sinuses and thickening of the muscularis propria are characteristic of outflow obstruction. Gallbladders excised from patients with biliary dyskinesia may show abundant Rokitansky-Aschoff sinuses in the absence of inflammation, a condition referred to as microdiverticulosis or Rokitansky-Aschoff sinusosis71 (Fig. 37.18). Other patients may have a normal-appearing gallbladder or nonspecific chronic cholecystitis.
Lymphoeosinophilic and Eosinophilic Cholecystitis
Inflammatory infiltrates in patients with acalculous cholecystitis typically contain a higher percentage of eosinophils than in patients with gallstones. Lymphoeosinophilic cholecystitis is diagnosed when eosinophils comprise more than 50% of the total number of inflammatory cells. It has been hypothesized that abnormal biliary contents or certain hepatic metabolites evoke a hypersensitivity reaction that leads to recruitment of large numbers of eosinophils that cause mucosal damage and gallbladder dysmotility.92
True eosinophilic cholecystitis is rare and is characterized histologically by an inflammatory infiltrate composed almost exclusively of eosinophils (Fig. 37.19). In this condition, eosinophilic infiltrates commonly involve the extrahepatic bile ducts in addition to the gallbladder. At presentation, these patients often have obstructive jaundice that mimics a neoplasm.93 This disorder is discussed further under Eosinophilic Cholangitis.
Diffuse Lymphoplasmacytic Acalculous Cholecystitis
Some cases of chronic cholecystitis, which are characterized by diffuse lymphoplasmacytic infiltrates confined to the lamina propria with or without active lesions (i.e., intramucosal neutrophilic infiltrates) (Fig. 37.20), occur in patients with extrahepatic bile duct obstruction.94 In the absence of gallstones, this form of chronic cholecystitis was thought to be relatively specific for primary sclerosing cholangitis (PSC).94 However, comparative studies that included controls with various types of extrahepatic bile duct obstruction showed that diffuse lymphoplasmacytic acalculous chronic cholecystitis was specific for extrahepatic biliary tract disorders but did not differentiate primary from secondary cholangiopathies.95,96
Acalculous cholecystitis with superficial or deep lymphoplasmacytic infiltrates has been described in patients with autoimmune pancreatitis.97,98 As in the pancreas, there typically are numerous plasma cells that express immunoglobulin G4 (IgG4). This condition is discussed further under Inflammatory Disorders of the Extrahepatic Bile Ducts.
Lymphocytic Cholecystitis
During the years, we have encountered four cases with a unique inflammatory pattern of cholecystitis characterized by an increased number of intraepithelial CD3-positive lymphocytes within biliary cells. All cases were accompanied by an inflammatory process within the lamina propria, similar to the diffuse lymphoplasmacytic cholecystitis seen in patients with PSC. The inflammatory changes primarily affected the infundibulum and were associated with epithelial hyperplasia. Because of its resemblance to lymphocytic colitis or gastritis, we proposed the term lymphocytic cholecystitis for this condition. All patients were adults and none had cholelithiasis. In three patients, cholecystitis was the predominant disease. The other patient had other medical problems (Fig. 37.21).
Xanthogranulomatous Cholecystitis
Xanthogranulomatous cholecystitis, an uncommon form of chronic cholecystitis, typically is associated with gallstones and is frequently accompanied by some degree of fibrosis. The incidence ranges from 0.7% to 1.8% of examined excised gallbladders, although one study reported an incidence of 9.0% in Japan and India.99–101
The pathogenesis of this condition is uncertain. It has been proposed that xanthogranulomas form as a reaction to penetration of bile into the gallbladder wall from mucosal ulcers or ruptured Rokitansky-Aschoff sinuses in conjunction with outflow obstruction by calculi and infection.101,102 Bile cultures are positive, mostly for enterobacteria, for about 50% of patients.
Xanthogranulomatous cholecystitis may be difficult to distinguish from other forms of cholecystitis clinically. However, in contrast to chronic cholecystitis, a history of at least one previous episode of acute cholecystitis is obtained from most patients. Some patients have a clinical picture that suggests acute cholecystitis. Imaging studies demonstrate a thickened wall. Gallstones are found in most patients.
This condition is confused clinically with carcinoma because the inflammatory process frequently extends to adjacent organs and the serum CA 19-9 level may be elevated. The molecular events and chemokines that explain this inflammatory pattern have been published.102 Pathologically, the areas involved by the xanthogranulomatous process may appear as firm, yellow masses that resemble carcinoma clinically and macroscopically.
Histologic examination shows round to spindle-shaped, lipid-laden macrophages, plasma cells, and fibrosis. Cholesterol clefts, foreign body– and Touton-type giant cells, and other types of inflammatory cells (i.e., lymphocytes, eosinophils, and neutrophils) are commonly found. Frequently, the xanthogranulomatous reaction occupies only a limited portion of the gallbladder, whereas the remainder shows conventional chronic cholecystitis, often with lymphoid follicles.
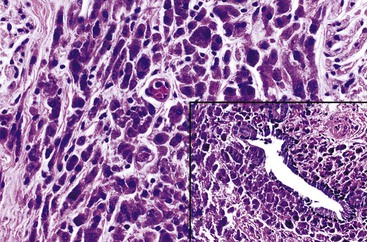
Xanthogranulomatous inflammation should be differentiated from malakoplakia, which has been reported rarely in the gallbladder.103 The characteristic microscopic findings of malakoplakia consist of a diffuse proliferation of histiocytes with abundant eosinophilic granular cytoplasm, some of which contain spherules (i.e., Michaelis-Gutmann bodies) that are positive for PAS and von Kossa (calcium) stains (Fig. 37.22).
Cholecystitis in Patients with Acquired Immunodeficiency Syndrome
Chronic acalculous cholecystitis may occur as a complication of human immunodeficiency virus (HIV) infection.104–108 Cryptosporidium is the most common cause of acquired immunodeficiency syndrome (AIDS)–related infection of the extrahepatic bile ducts and gallbladder. This organism has been found in the bile ducts or stools in as many as 62% of patients with symptoms of AIDS-related cholangitis. Cryptosporidium colonizes but does not invade biliary cells and elicits an inflammatory response of variable intensity, but it is mostly mild in cases not associated with other organisms.
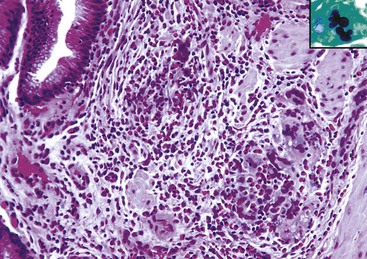
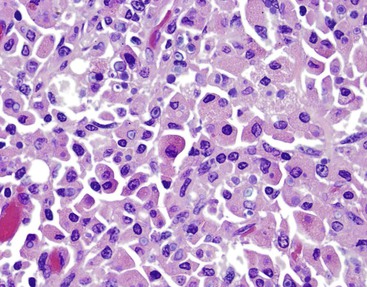
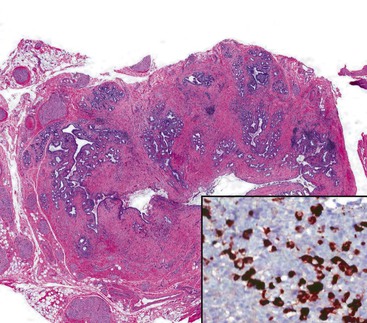
Cytomegalovirus (CMV) infection is the second most common type of infection in AIDS-related cholecystitis. Biliary involvement develops in as many as 10% of AIDS patients with CMV.104 CMV infection is associated with mucosal ulcers and mixed inflammatory infiltrates. Intranuclear and intracytoplasmic inclusions are found in endothelial and epithelial cells. Occasionally, CMV coexists with other infectious organisms in the same gallbladder (Fig. 37.23).
Rare instances of infection with Mycobacterium avium-intracellulare complex have been reported. The diffuse histiocytic proliferation in this condition may mimic xanthogranulomatous cholecystitis and malakoplakia. Other organisms in HIV patients that may involve the gallbladder include intracellular parasites of the Microsporida order, particularly Enterocytozoon bieneusi, and Isospora protozoa.109
Parasitic Infestation
Infestation of the gallbladder with Fasciola hepatica and the liver flukes C. sinensis and O. viverrini causes chronic infection of the bile ducts and may cause acute acalculous cholecystitis. These organisms may induce an inflammatory response rich in lymphocytes and eosinophils, usually accompanied by hyperplasia of metaplastic pyloric-type glands. Granulomatous cholecystitis has been described in association with the ova of Schistosoma mansoni, Paragonimus westermani, and A. lumbricoides.110
Polyarteritis Nodosa and Other Types of Vasculitis
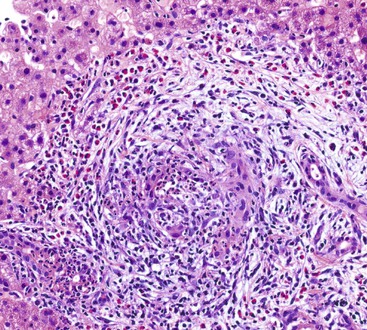
Histologic changes of classic polyarteritis nodosa may be detected in the gallbladder in two main clinical settings: in patients with isolated gallbladder involvement and in patients with systemic disease, such as scleroderma, systemic lupus erythematosus, or antiphospholipid syndrome.111–114 The localized form of polyarteritis nodosa may rarely progress to systemic disease, especially in patients with serum autoantibodies (e.g., rheumatoid factor, antinuclear antibodies). Other forms of vasculitis include granulomatous vasculitis of the gallbladder in patients with Churg-Strauss syndrome or temporal giant cell arteritis. Rarely, idiopathic lymphocytic phlebitis may be confined to the gallbladder, which shows histologic features similar to those reported in cases of idiopathic enterocolic lymphocytic phlebitis.115 Lymphocytic vasculitis may be identified in patients with Behçet disease (Fig. 37.24).
Cholesterolosis
Cholesterolosis is characterized by aggregates of lipid-containing macrophages in the lamina propria of the gallbladder. Autopsy and surgical studies have demonstrated a prevalence of 12% and 9% to 26%, respectively.116,117
The cause and pathogenesis of cholesterolosis are poorly understood. Accumulation of cholesterol esters and triglycerides may reflect increased hepatic synthesis of lipids or an increased absorption and esterification by the gallbladder mucosa. The normal gallbladder absorbs free and nonesterified cholesterols from the bile. Cholesterol is esterified in the endoplasmic reticulum and forms lipid droplets that are released into the intercellular space, where they are phagocytized by macrophages.118 Patients with cholesterolosis and patients with cholesterol stones have supersaturated bile; as expected, these conditions frequently coexist. Cholesterolosis likely results from increased cholesterol uptake from supersaturated bile.
Almost a century after the first description of this entity, the clinical relevance of cholesterolosis remains a subject of debate. Some studies have suggested that cholesterolosis is associated with symptoms in patients who have acalculous biliary disease, with colicky abdominal pain and selective food intolerance as the most common complaints. Some patients with biliary dyskinesia have cholesterolosis, in which case resolution of symptoms after is more likely caused by eradication of dyskinesia than removal of cholesterolosis. Evidence suggests a possible relationship between cholesterolosis and acute pancreatitis. Temporary impaction of cholesterolosis polyps at the sphincter of Oddi may produce recurrent attacks of acute pancreatitis.119
If the prevalence of cholesterolosis derived from autopsy studies reflects the frequency in the general population, symptoms do not develop in most individuals with cholesterolosis. This condition is more prevalent among individuals with morbid obesity.120 A negative association between cholesterolosis and gallbladder carcinoma has been reported.121
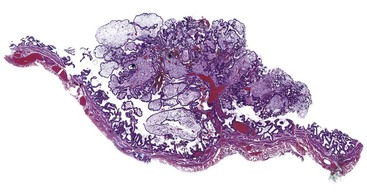
On gross examination, lipid deposits appear as yellow flecks against a dark green background, earning the sobriquet strawberry gallbladder. When extensive, lipid deposits may form polypoid excrescences that project into the lumen. Commonly referred to as cholesterol polyps but more properly called cholesterolosis polyps, these lesions are usually small but may be large enough to be detected by radiologic imaging. Cholesterol gallstones are associated with cholesterolosis in approximately 50% of surgical cases and 10% of autopsies (Fig. 37.25).
Microscopically, the diagnostic feature of cholesterolosis is accumulation of foamy macrophages within an expanded lamina propria, resulting in the development of thickened folds or polyps, or both (Fig. 37.26). The adjacent mucosa may be normal or inflamed. However, inflammation occurs almost exclusively in patients with coexistent stones.
Hydrops and Mucocele
Gallbladders distended by clear, watery fluid (i.e., hydrops) or mucus (i.e., mucocele) account for 3% of cholecystectomy specimens in adults.122 In this age group, the most common cause is an impacted stone in the neck of the gallbladder or cystic duct. Less common causes include cystic fibrosis, tumors, fibrosis, kinking of the cystic duct, and external compression by an inflammatory or neoplastic mass. In children, these conditions are usually acute and associated with infectious or inflammatory disorders of unknown origin, such as streptococcal infection, mesenteric adenitis, typhoid, leptospirosis, viral hepatitis, familial Mediterranean fever, or Kawasaki syndrome. Symptoms may resolve with conservative treatment.122,123
Hydropic gallbladders show considerable distention and may contain more than 1500 mL of fluid or inspissated mucin. When associated with numerous stones, the wall is usually thickened. The wall is thin when there is a single stone that obstructs the cystic duct or in acute childhood cases. Microscopic examination usually reveals flattened mucosa lined by low columnar or cuboidal cells. As a result of increased intraluminal pressure, Rokitansky-Aschoff sinuses may be plentiful. In some cases, mucin spills into the peritoneal cavity, simulating a mucinous adenocarcinoma. Inflammatory cells may be sparse or abundant. Acute cholecystitis, with edema of the lamina propria and abundant neutrophils, occurs in some patients with Kawasaki syndrome.123
Diverticular Disease
Congenital and traction diverticula are the two types of true diverticula that occur in the gallbladder. Congenital lesions are discussed in Chapter 36. Constituents of the wall of diverticula differentiate them from the more common acquired pseudodiverticula. True diverticula contain all elements of the normal gallbladder wall, whereas pseudodiverticula contain little or no smooth muscle.
Traction diverticula are caused by the pulling action of postinflammatory fibrous adhesions, which anchors the serosa of the gallbladder to adjacent structures. Erosion by stones, healing fistulas, widespread peritonitis of any cause, or previous intraabdominal surgery precede their formation. Traction diverticula are distinguished from congenital outpouchings principally by their relationship with other intraabdominal lesions in the vicinity of the gallbladder and by the predominance of serosal (rather than mucosal) inflammation and fibrosis. In some cases, however, distinction between the two types of true diverticula may be impossible.
Acquired pseudodiverticula are mucosal herniations within the smooth muscle of the gallbladder wall that should be regarded as prominent Rokitansky-Aschoff sinuses. Acquired pulsion diverticula typically are associated with stones and chronic cholecystitis or with outflow obstruction. Analogous to diverticular disease of the colon, the intervening smooth muscle is usually hypertrophic. The mucosal outpouchings combined with prominent smooth muscle hypertrophy may form a localized tumor-like lesion, called an adenomyoma (i.e., localized adenomyomatous hyperplasia), or it may diffusely thicken the gallbladder wall (i.e., diffuse adenomyomatous hyperplasia).124,125 The epithelial lining of mucosal herniations is usually normal but may rarely have gastric foveolar metaplasia or dysplastic or neoplastic changes. Perineural involvement has rarely been observed in cases of adenomyomatous hyperplasia and should not be confused with adenocarcinoma.85
Ischemic Diseases
Deprivation of arterial blood flow or obstruction to venous drainage may result in infarction of the gallbladder. Atherosclerosis and thrombosis are the usual causes of deficiency of arterial blood flow. Emboli may occur as a complication of valvular heart disease or bacterial endocarditis. Rarely, dissecting aneurysms may extend into the celiac artery, occluding the origin of the hepatic artery. External compression of the arteries or interference of the venous drainage may result from impingement on blood vessels by gallstones or tumors, or iatrogenic injury may be caused by surgical ligation.126
Gallbladders with a high degree of mobility (i.e., floating gallbladders) may twist on their pedicle and cause torsion or volvulus. Floating gallbladders lack a firm attachment to the liver and are completely surrounded by peritoneum (see Congenital Abnormalities in Chapter 8). Torsion in developmentally normal gallbladders may also result from loosening of the suspensory connective tissue, as in normal aging, or from shrinkage of the liver (i.e., cirrhosis), leading to detachment of the gallbladder from its bed and visceroptosis.122 Ischemic disease may also result from vasculitis. Most cases of ischemic cholecystitis occur in patients older than 60 years. The correct clinical diagnosis is rarely established before surgery, because the symptoms mimic those of acute cholecystitis.
The gallbladder wall in ischemic disease is usually thickened, congested, and hemorrhagic. Microscopic examination may reveal partial or complete loss (e.g., necrosis) of the epithelium, edema, or hemorrhage in the lamina propria (Fig. 37.27). When occlusion to venous outflow predominates, there is usually extensive and often transmural hemorrhagic infarction. Ischemic lesions associated with primary vasculitis are often focal and typically confined to the mucosa. In patients with calculous cholecystitis, superimposed ischemic damage, caused by secondary vasculitis or small vessel thrombosis, is often found. Healed ischemic lesions may cause fibrous replacement and dystrophic calcifications of the gallbladder wall.
Traumatic Conditions and Chemical Cholecystitis
The gallbladder is seldom damaged by abdominal trauma because it is partially protected by the ribs and liver. Occasionally, blunt abdominal trauma may disrupt a distended gallbladder, causing contusion, laceration, torsion, avulsion, or intraluminal hemorrhage.127 Penetrating wounds may damage the gallbladder, usually in association with injury to adjacent organs.
Iatrogenic injury can result from liver biopsy or percutaneous transhepatic cholangiography. Acute cholecystitis with mucosal necrosis and fibrosis may result from repeated infusion of chemotherapeutic agents through a catheter placed in the hepatic artery.128
Biliary Fistulas
In most cases, fistulas between the biliary tract and adjacent organs are a consequence of gallstone-associated necrosis with inflammation of the gallbladder or bile ducts, or both. Inflammatory adhesions precede fistula formation. These lesions may form a mass lesion that can resemble a neoplasm. Cholecystectomy in the setting of a fistula carries a high risk of injury to the bile ducts. The most common fistulas are cholecystoduodenal, followed by cholecystocolic and choledochoduodenal.124 Biliobiliary fistulas form between the gallbladder and the common bile duct. This complication should be suspected in patients with cholelithiasis and jaundice.129
Metachromatic Leukodystrophy
Metachromatic leukodystrophy is an inborn error of metabolism associated with a deficiency of aryl sulfatase. The disease is characterized by diffuse breakdown of myelin in the central and peripheral nervous systems. Microscopic changes in the gallbladder consist of papillary hyperplasia (i.e., papillomatosis) and expansion of the lamina propria by macrophages containing abnormal metachromatic material (i.e., sulfatides). The metachromatic material also is found within epithelial cells, and it may be responsible for the hyperplastic epithelial changes.130,131
Inflammatory Disorders of the Extrahepatic Bile Ducts
With the exception of PSC, the pathologic features of most types of inflammatory conditions of the bile ducts have been poorly studied. Clinically, these conditions mimic an obstructive tumor, because silent cholestasis is a common complication.
One useful clinical and pathologic classification of cholangitis provides seven categories: (1) simple obstructive cholangitis, (2) primary hepatolithiasis, (3) PSC, (4) IgG4-associated cholangitis, (5) follicular cholangitis and pancreatitis, (6) eosinophilic cholangitis, and (7) other conditions. The features of these disorders are summarized in Table 37.4
Table 37.4
Comparison of Types of Cholangitis with Lymphoplasmacytic Inflammation
| Features | Simple Obstructive Cholangitis | Primary Hepatolithiasis | Primary Sclerosing Cholangitis | IgG4-Related Cholangitis | Follicular Cholangitis and Pancreatitis |
| Epidemiology | All ethnic groups Women > men |
Predominantly Asians No gender predilection |
Predominantly whites Men > women |
All ethnic groups Men > women |
No gender or ethnic predilection |
| Presence and type of gallstone | Cholesterol | Pigmented | Usually none | None | None |
| Associated conditions | Cholelithiasis; obstructive conditions | Infections? Malnutrition? |
Ulcerative colitis | Pancreatitis; other IgG4-related conditions | Pancreatitis |
| Predominant site of obstruction | Common bile duct | Intrahepatic ducts | Intrahepatic and extrahepatic ducts | Intrapancreatic segment of common bile duct and hilar or large intrahepatic bile ducts | Hilar and intrapancreatic bile ducts |
| Pathologic aspects | NSI and PIF | NSI and PIF Adenomatous hyperplasia |
Mucosa-based NSI and PIF | Deep > mucosal inflammation IgG4-positive plasma cells OP, SF |
Inflammation with abundant lymphoid follicles and PIF Few IgG4 plasma cells No OP or SF |
| Predisposition to dysplasia and cholangiocarcinoma | Yes | Yes | Yes | No | Unknown |
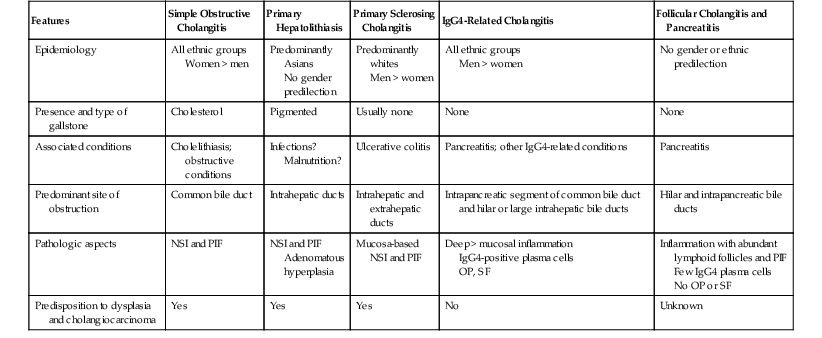
IgG4, Immunoglobulin G4; NSI, nonspecific inflammation; PIF, postinflammatory fibrosis; OP, obliterative phlebitis; SF, storiform fibrosis.
Simple Obstructive Cholangitis
Clinical Features
The classic symptoms associated with acute obstructive cholangitis are known as the Charcot triad: intermittent abdominal pain, fever, and jaundice. This triad of symptoms is identified in 20% to 70% of patients. Most patients have leukocytosis and abnormal liver function test results, mainly hyperbilirubinemia, with an elevated alkaline phosphatase level and a mild to moderate elevation of the levels of aminotransferases. Serum concentration of amylase is increased in 40% of patients and does not necessarily indicate concomitant pancreatitis. Blood cultures positive for enteric organisms suggest biliary sepsis.
A severe form of illness with a high mortality rate known as acute suppurative or toxic cholangitis develops in a few patients. The typical symptoms associated with this condition constitute the Reynolds pentad: abdominal pain, fever, jaundice, shock, and delirium. The risk of progression to toxic cholangitis is increased in patients who do not respond to antibiotic management and in those who have a congenital or malignant obstruction. Acute renal failure and intrahepatic abscesses are the two most common complications of acute cholangitis. Renal failure may develop as a result of hypoperfusion from sepsis, endotoxemia, and tubular injury from bile pigments.132–134
Risk Factors and Pathogenesis
Biliary obstruction is caused by disorders that primarily affect the bile ducts and diseases of other organs that produce secondary obstruction. Among the former are choledocholithiasis, bile duct cysts, diverticula, tumors, fistulas, and complications from previous surgical procedures (e.g., nonabsorbable sutures, metal clips, plugged T-tubes, stents). Extrinsic obstruction of the bile ducts may be a complication of pancreatic tumors, chronic pancreatitis, ampullary lesions, and gallstones within the cystic duct with secondary compression of the common bile duct (i.e., Mirizzi syndrome).
In Western countries, migration of cholesterol gallstones from the gallbladder into the bile ducts is the most frequent cause of obstruction.135 Most cholesterol gallstones do not harbor bacteria and are not associated with infected bile. In a few cases, the organisms most commonly found are enteric bacteria, such as E. coli, Streptococcus faecalis, Clostridium, Klebsiella, Enterobacter, Pseudomonas, and Proteus.135 The route by which bacteria colonize the biliary system remains unknown. Contamination from an infected gallbladder, duodenal reflux, and lymphatic, hepatic arterial, or portal venous bacteremia has been proposed.
Approximately 15% of patients with cholelithiasis have stones within the bile ducts; 15% to 20% are asymptomatic. Because severe symptomatic diseases such as acute cholangitis, chronic obstruction with hepatic fibrosis, and gallstone pancreatitis may develop in patients with ductal stones, stone removal is recommended at the time of cholecystectomy by endoscopic stone extraction.
Gallstones that form within the bile duct (i.e., primary bile duct stones) are usually brown pigment stones. At variance with the sterile nature of most cholesterol gallstones, brown stones are frequently associated with infection of the biliary tract. In Asia, primary bile duct stones often are associated with a high incidence of intrahepatic bile duct infections. Conditions that predispose to primary stone formation include obstructive diseases such as fibrosis resulting from prior biliary surgery, iatrogenic strictures, biliary-enteric anastomosis, sclerosing cholangitis, stenosing papillitis, periampullary duodenal diverticula, parasitic infections, Caroli disease, indwelling biliary endoprostheses, and use of nonabsorbable suture material or metal clips.136 The bacteria most commonly found are β-glucuronidase–producing, gram-negative organisms, such as E. coli and B. fragilis. Biliary sludge within the bile ducts has the same clinical significance as bile duct stones and predisposes to recurrent choledocholithiasis.137 A xanthogranulomatous reaction similar to xanthogranulomatous cholecystitis may be encountered in bile ducts in the absence of other lesions. This type of cholangitis may be confused with a malignant tumor on imaging studies or cytology.138,139
Pathologic Features
In acute cholangitis, examination of the extrahepatic bile ducts reveals edema, a predominantly neutrophilic infiltrate in the lamina propria, and focal infiltration of the epithelium (Fig. 37.28). Ulcers and erosions containing fragments of gallstones may be seen. The biliary epithelium usually shows degenerative and regenerative changes. Extravasation of bile into the lamina propria elicits an intense histiocyte-rich inflammatory reaction that may be followed by fibrosis. As the disorder evolves, lymphocytes and plasma cells become more abundant.
Treatment
Most symptomatic stones may be removed endoscopically with or without mechanical lithotripsy. Shock wave and laser lithotripsy have been used before endoscopic removal of large stones. The role of medical therapy with ursodeoxycholic acid remains controversial.140
Primary Hepatolithiasis
Clinical Features
Most cases of primary hepatolithiasis (i.e., recurrent pyogenic cholangitis or Oriental cholangiohepatitis) are diagnosed in patients 20 to 40 years of age. There is no sex predilection. There is a strong association with lower socioeconomic class. A history of recurrent attacks is common, and cases are characterized by abdominal pain, nausea, vomiting, fever, shaking chills, and jaundice. The findings on physical examination include epigastric tenderness and rigidity, enlargement of the liver, and a palpable gallbladder.
Laboratory findings include leukocytosis and an elevated serum alkaline phosphatase level. Most patients have bile cultures positive for enteric bacteria. Imaging studies demonstrate a characteristic pattern of ductal dilation with tight proximal stenosis and subsequent dilation and parenchymal atrophy, producing an arrowhead sign on computed tomography (CT).141
Epidemiology and Pathogenesis
Recurrent attacks of ascending cholangitis caused by intrahepatic stone formation characterize the syndrome of recurrent pyogenic cholangitis, also known as Oriental cholangiohepatitis, intrahepatic pigment stone disease, biliary obstruction syndrome of the Chinese, and primary hepatolithiasis. Primary hepatolithiasis is the preferred term because it distinguishes this form of hepatolithiasis from the Western type of cholangitis that complicates cholecystitis and cholelithiasis.
First described in Chinese patients in Hong Kong, the disorder is recognized as a serious health problem in China, Taiwan, Japan, Korea, Singapore, Vietnam, Malaysia, and the Philippines.141 It is the main cause of acute abdominal pain in Hong Kong emergency rooms.142 Sporadic cases have been reported in Europe, South Africa, and Australia. In the United States and Canada, the disease is largely limited to Asian immigrants.143,144 The estimated incidence in the Far East is 4% to 52%, whereas in the West, it is 0.6% to 1.3%. With the exception of Korea, the incidence of this disorder appears to be decreasing in recent decades.144
The cause of the disease is unknown. The two most popular theories are infection and malnutrition. The former postulates that the inflammatory and fibrosing changes result from chronic infestation of the biliary tract with endemic parasites, such as C. sinensis and A. lumbricoides. Interference with bile flow caused by adult flukes or eggs, producing stagnation that leads to secondary bacterial infection, pigment stone formation, and pyogenic cholangitis.145 Demonstration of ova or fragments of parasites within stones provides support for this theory. However, patients with recurrent cholangitis have only a slightly higher rate of infestation by C. sinensis than the general population in endemic areas, where numerous individuals are infected with liver flukes without ever experiencing cholangitis. In other areas where parasitic infection is much less common, such as Taiwan, the incidence of recurrent cholangitis and hepatolithiasis is high. In Japan, primary hepatolithiasis still occurs despite eradication of intestinal parasites.146
The second theory postulates that recurrent infectious gastroenteritis in malnourished people causes frequent episodes of portal bacteremia. Calcium bilirubinate precipitates formed by deconjugation of bilirubin glucuronide by β-glucuronidase produced by E. coli infection initiates the formation of brown stones. A protein-poor diet is thought to be associated with a deficiency of glucaro-1:4-lactone, a natural inhibitor of the enzyme in bile. The formation of biliary sludge converts an otherwise innocuous portal bacteremia into an infection. This theory explains the much higher incidence of disease in patients from low socioeconomic classes and in rural areas. However, it does not explain why this disorder is uncommon in other geographic areas, where the population also suffers from chronic malnourishment.
Pathologic Features
The intrahepatic ducts, which are proximal to the confluence of the right and left hepatic ducts, and the extrahepatic bile ducts may be affected. The left lateral segmental duct is more frequently affected, followed by the posterior segmental duct of the right lobe. This distribution is thought to be related to the angulation of these ducts, which is more marked than in other segments.144 The liver may be enlarged, display irregular scarring, and have capsular adhesions. After multiple attacks, it may become shrunken, especially the lateral segment of the left lobe. The intrahepatic bile ducts show alternating areas of stricture and dilation (Fig. 37.29). An unusual feature is abrupt tapering toward the periphery of the dilated segments. This contrasts sharply with diffuse dilation seen in patients with other causes of obstruction. Pigment stones and secretions are usually found within the lumen.
In most cases, the extrahepatic ducts are not stenotic, except for the most distal segment, where repeated passage of stones through the sphincter of Oddi may cause inflammation and postinflammatory strictures of the papilla, a condition known as stenosing papillitis. The dilated segments are not strictly related to the location of the stones.147 Bile duct stones occur in 75% to 80% of cases. Histologic changes in the extrahepatic ducts include a mixed inflammatory infiltrate, sometimes with the formation of lymphoid follicles, some degree of fibrosis, and epithelial changes that range from loss of cells to adenomatous hyperplasia and dysplasia. The term chronic proliferative cholangitis is sometimes used for the latter type of lesions. The neutral and acid mucins produced by metaplastic glands participate in the formation of the stones.
Portal tracts in the liver show a characteristic cluster of changes of bile duct obstruction: proliferation of bile ducts, inflammatory cells (mainly neutrophils), and some degree of edema. Periductal fibrosis is frequently identified. The hepatic parenchyma adjacent to intrahepatic stones is commonly atrophic. However, postobstructive cirrhosis is rare.
Cholangiocarcinoma develops in 2.4% to 4.9% of patients with recurrent pyogenic cholangitis.148 It has been suggested that continuous inflammation caused by the concerted action of persistent infection and mechanical irritation by stones leads to adenomatous hyperplasia, dysplasia, and cholangiocarcinoma149 (Fig. 37.30).
Treatment
Several treatment options are available. Ideally, complete removal of stones and elimination of bile stasis and infection should be attempted to eliminate the inflammatory process and prevent the development of cholangiocarcinoma. Nonsurgical procedures such as percutaneous transhepatic cholangioscopic lithotripsy and extracorporal shock wave lithotripsy have been tried with suboptimal results due to a high prevalence of residual and recurrent stones. Poor results have also been reported with conservative surgical treatments, such as biliary-enteric anastomosis or T-tube insertion. Hepatic resection provides a definitive solution for patients who do not have bilateral disease.150
Primary Sclerosing Cholangitis
Clinical Features
Most patients with PSC are male and younger than 45 years. One half of patients also have a history of inflammatory bowel disease, usually ulcerative colitis. Other diseases associated with PSC include thyroiditis, pancreatitis, insulin-dependent diabetes mellitus, celiac disease, thymoma, sicca syndrome, retroperitoneal and mediastinal fibrosis, Peyronie disease, pseudotumor of the orbit, sarcoidosis, histiocytosis X, angioimmunoblastic lymphadenopathy, Weber-Christian disease, rheumatoid arthritis, autoimmune hemolytic anemia, systemic lupus erythematosus, and a variety of immunodeficiency syndromes.
Approximately 25% of patients with PSC are asymptomatic at presentation. They are diagnosed by abnormal liver test results and retrograde cholangiopancreatography. Typically, symptoms appear insidiously, and the most common are fatigue, pruritus, and jaundice. Cholangitis may occur with the development of advanced liver disease and ductal stenosis. Liver function tests typically reveal a cholestatic profile. The alkaline phosphatase level is always elevated, usually at least three to five times over its normal value. Serum aminotransferase and bilirubin levels are usually only mildly elevated at presentation but increase with disease progression.
No consistent serologic markers are useful for the diagnosis of this disorder. Less than 50% of patients have positive test results for antinuclear and smooth muscle antibodies. Antineutrophilic cytoplasmic antibodies are found in 87% of patients. However, they are not disease specific.
Cholangiographic examination demonstrates multifocal strictures involving in most instances intrahepatic and extrahepatic ducts. Strictures are typically short, annular, and alternate with normal or dilated segments.
Risk Factors and Pathogenesis
PSC occurs primarily in young men and has an estimated prevalence of 20 to 60 cases per 1 million people.151 The disorder is characterized by persistent chronic inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, with a gradual onset of progressive fatigue and pruritus, followed by jaundice and slow progression to cirrhosis.
PSC usually has a cholestatic biochemical profile. Approximately 70% of patients with PSC have or eventually experience ulcerative colitis.151 It is less commonly associated with Crohn’s disease. Inflammatory bowel disease can precede PSC by 1 to 63 years (median, 9 years). When PSC is the presenting disorder, inflammatory bowel disease may develop 0 to 32 years after. Most patients in whom PSC develops have ulcerative colitis with diffuse pancolitis or Crohn’s disease with colonic involvement.152
Designation of PSC as an autoimmune disease is based on its common association with an autoimmune haplotype (HLA-A1-B8-DR3) and the presence of autoantibodies against peptides shared by the colon and biliary epithelial cells.153 Additional evidence is provided by the finding of antineutrophilic cytoplasmic antibodies, at high titer in most patients with PSC and its strong association with ulcerative colitis and other autoimmune disorders (e.g., insulin-dependent diabetes mellitus, systemic lupus erythematosus, Sjögren syndrome, celiac disease).154
Chronic portal bacteremia may play a role in the pathogenesis of this disease. Chronic absorption of toxins and bacterial products in patients with ulcerative colitis may cause release of inflammatory cytokines in the biliary epithelium. Observations in animal models support this hypothesis. Whether some of these findings have direct pathogenic importance must be determined.155
Pathologic Features
The intrahepatic and extrahepatic bile ducts are affected in most patients with PSC. The disorder remains confined to the intrahepatic biliary tract in approximately 20% of patients. The intrahepatic and extrahepatic bile ducts have alternating areas of stricture and normal lumen or slightly dilated segments, producing a characteristic beaded pattern on cholangiographic studies. Diverticulum-like outpouchings are found in approximately 25% of cases.
Microscopically, cross section of the bile ducts shows expansion of the lamina propria by a plasma cell–rich infiltrate that is diffuse and seen even in segments that are grossly normal (Fig. 37.31). This diffuse type of mucositis mimics the morphology of the colonic mucosa in ulcerative colitis. The resemblance is further reinforced by active lesions that are characterized by neutrophilic infiltration of the biliary epithelium and glands, with formation of microabscesses, erosions, and ulcers. Changes in the biliary epithelium range from atrophy to regenerative hyperplasia and postinflammatory dysplasia (Fig. 37.32).156 Cholangiocarcinoma develops in 4% to 20% of patients with PSC.156
Fibrosis is typically found in strictured segments. Biliary stones are common, and if the clinical and pathologic features support a diagnosis of PSC, the stones should not be used to exclude the diagnosis. Cholelithiasis is usually absent, except in patients with cirrhosis. The inflammatory pattern described in the bile ducts often occurs in the gallbladder, a disorder known as diffuse lymphoplasmacytic acalculous cholecystitis (see Chronic Acalculous Cholecystitis). However, this inflammatory pattern is not specific for PSC.
Natural History
The natural history of PSC has not been clearly defined. The clinical course of patients with PSC varies. Some remain stable for many years, whereas the disease in others evolves rapidly to liver failure. In most instances, disease progresses to biliary cirrhosis within 10 to 15 years. The average survival time is 10 to 12 years. The most common cause of death is liver failure, followed by cholangiocarcinoma.151,152,157
Treatment
No effective medical treatment is available. Novel ursodeoxycholic acid derivatives and monoclonal antibodies against integrins that prevent mucosal homing of T lymphocytes are awaiting clinical trials. Dilation of dominant strictures with or without stent placement is recommended for symptomatic patients with complications. Liver transplantation is offered to patients with end-stage liver failure, refractory pruritus, or recurrent bacterial cholangitis. PSC recurs in transplanted livers in as many as 20% of patients after a median of 4.6 years.152
Immunoglobulin G4–Associated Cholangitis and Cholecystitis
IgG4-associated cholangitis and cholecystitis have clinical and pathologic features that separate them from the disorders discussed earlier in this chapter. They are included among the expanding group of fibroinflammatory conditions referred to as IgG4-related diseases. The prototype of these disorders is autoimmune pancreatitis, but IgG4-related conditions have been described in other organs, such as the biliary tract, gallbladder, salivary glands, periorbital tissues, kidneys, lungs, lymph nodes, meninges, aorta, breast, prostate, thyroid, pericardium, and skin.158
Clinical Features
IgG4-associated cholangitis predominantly affects patients older than 50 years of age. It has a male-to-female predominance of approximately 2.8 to 1.
Affected bile ducts may or may not be associated with autoimmune pancreatitis.159 The predominant clinical manifestation of IgG4-associated cholangitis is obstructive jaundice resulting from hilar bile duct strictures or a pancreatic head mass mimicking carcinoma. At presentation, most patients have hyperbilirubinemia; mild transaminasemia; elevated levels of alkaline phosphatase, γ-glutamyltransferase, and CA 19-9; and bile duct or pancreatic duct strictures seen on imaging studies.160 Elevated IgG and IgG4 serum levels and autoantibodies (e.g., rheumatoid factor, antinuclear antibodies) are common but not always found.
PSC tends to occur in younger patients (30 to 40 years old), and the clinical presentation usually is less symptomatic, with disease progression occurring during several years. Patients with IgG4-associated cholangitis are a decade or two older and have an acute presentation with a shorter duration of symptoms. Extrabiliary disease, especially pancreatic and kidney disorders, supports the diagnosis of an IgG4-related condition.161
Pathologic Features
The most frequently affected segments of the biliary tract are the intrapancreatic portion of the common bile duct and the hilar or intrahepatic large bile ducts. The common hepatic duct and the extrapancreatic portion of the common bile duct usually are spared. This anatomic distribution of disease probably reflects the abundance of peribiliary glands in these locations.162 Examination of the involved bile ducts reveals thickening of the wall, which is partially replaced by a white, fleshy tissue that merges with periductal tissues, and luminal stenosis with dilation of the proximal segments.
Histologically, the classic triad of IgG4-related diseases—dense lymphoplasmacytic inflammation, fibrosis, and obliterative phlebitis—is seen, although the phlebitis is sometimes absent. Lymphoid follicles and tissue eosinophils may be observed. The inflammatory infiltrate tends to be more abundant in the peribiliary glands than in the lamina propria, the latter of which tends to be relatively spared (Fig. 37.33). The distribution of inflammation contrasts sharply with PSC, in which the infiltrate predominates in the luminal portion of the mucosa. In PSC, the biliary epithelium is inflamed, shows degenerative changes, and is frequently ulcerated. Extension of the inflammatory process into the soft tissues surrounding the bile ducts is common in IgG4-associated cholangitis. Immunohistochemically, most plasma cells express IgG, and most are positive for IgG4. The specificity of this type of infiltrate is good when the IgG4-to-IgG ratio is 0.47 or higher and the number of IgG4-positive plasma cells exceeds 10 per high-power field.163
The gallbladder is universally involved in patients with IgG4-associated cholangitis. The distribution and characteristics of the inflammatory infiltrate is similar to those of PSC. Diffuse lymphoplasmacytic inflammatory infiltrates expand the lamina propria. Active lesions (i.e., neutrophils within the epithelium) are common in patients with bile duct obstruction. However, one noticeable difference is an increase in transmural infiltration in IgG4-associated cholangitis, which contrasts with most cases of PSC in which the inflammation is mainly mucosal. In addition to the identification of IgG4-positive plasma cells, important findings are phlebitis and inflammatory nodules, particularly when located in perivesical tissues.163,164
IgG4-positive plasma cells may be observed in other conditions, such as suppurative granulation tissue, pancreatic carcinoma, and cholangiocarcinoma, PSC, and autoimmune hepatitis.165 A diagnosis of carcinoma in patients with a biliary or pancreatic mass should not be excluded solely because cholecystectomy or biliary biopsy specimens show a histologic pattern of injury that mimics IgG4-associated cholangitis, including abundant IgG4-positive plasma cells.
Treatment
Differentiating this type of cholangitis from PSC is important because IgG4-associated cholangitis is steroid responsive, whereas PSC usually is not.158 Spontaneous resolution has been described. Disease may recur during the tapering phase or after discontinuation of steroid therapy. Most relapses occur within the first 3 years. Increased alkaline phosphatase and IgG4 levels and proximal bile duct involvement have been associated with disease relapse. Other immunosuppressive drugs have been used when the disorder recurs or is refractory to steroid therapy.166 Patients with IgG4-associated cholangitis do not appear to have an increased risk of cholangiocarcinoma, as do those with PSC.
Eosinophilic Cholangitis
Clinical Features
Inflammatory diseases of the bile ducts account for approximately 10% of patients who undergo surgery for hepatic hilar strictures. One condition that closely mimics the presentation of a biliary tumor is eosinophilic cholangitis. This uncommon condition has a slight male predominance (1.6 to 1), and the mean age at presentation is 35.4 years.
Most patients have abdominal pain followed by jaundice, and 70% have peripheral eosinophilia. Ultrasound and contrast-enhanced CT studies commonly show thickening of the bile duct wall with or without biliary dilation. Brush cytology and biopsies may help to diagnose this condition and exclude cholangiocarcinoma.167 The gallbladder is frequently involved. This condition may be confined to the biliary tract, or it may be a component of a systemic disorder with eosinophilia, such as the idiopathic hypereosinophilic syndrome or eosinophilic gastroenteritis.
Pathologic Features
In patients with eosinophilic cholangitis, segmental thickening of the bile duct wall results from a white, fleshy tissue and various degrees of obliteration of the lumen. Microscopic examination reveals a dense inflammatory cell infiltrate composed almost exclusively of eosinophils within the mucosa and muscular layer and some degree of fibrosis and luminal obliteration. Liver biopsy specimens show an obstructive pattern with abundant eosinophils (Fig. 37.34).
Treatment
The disease may resolve after surgery or with corticosteroid therapy. A diagnostic trial of oral corticosteroids may provide support for the diagnosis of eosinophilic cholangitis before surgical intervention.168
Follicular Cholangitis and Pancreatitis
Clinical Features
Follicular cholangitis and pancreatitis constitute a specific condition. It affects adult elderly patients without gender predilection. The predominant clinical manifestations are related to hilar bile duct strictures or a bulky pancreatic head mass that mimics carcinoma. Most patients have hyperbilirubinemia; mild transaminasemia; elevated levels of alkaline phosphatase, γ-glutamyltransferase, and CA 19-9; and bile duct or pancreatic duct strictures seen on imaging studies.169
Pathologic Features
Gross examination shows bile duct wall thickening or an ill-circumscribed mass in the head of the pancreas. Histologically, there is a duct-centered lymphoplasmacytic infiltrate with many lymphoid follicles containing mitotic figures and tangible body macrophages within germinal centers. Nonstoriform collagen is deposited in the bile ducts or pancreatic ducts, or both. Within the interfollicular areas, there is a dense plasmacytic infiltrate with occasional eosinophils. Lymphoid follicles are usually found in the gallbladder, mimicking follicular cholecystitis. Nonobliterative phlebitis may be identified. IgG4-positive plasma cells are usually seen, but they represent only 2% to 15% of the plasma cell population.
Based on the absence of numerous IgG4-positive plasma cells, obliterative phlebitis, storiform-type periductal fibrosis, and granulocytic epithelial lesions (seen in type 2 autoimmune pancreatitis), it has been proposed that this entity is probably different from or represents a variant of autoimmune pancreatitis or cholangitis.169
Parasitic Infections of the Biliary Tract
Clonorchis sinensis
Worldwide, an estimated 19,000,000 persons are infected with C. sinensis; most live in the Far East.170 Infected persons can harbor the organism inside the biliary system for as long as 30 years. Routine stool screening of Chinese immigrants to the United States has demonstrated active infection in 25%.171
Two intermediate hosts are needed for transmission to humans: various species of snails (not found in the United States) and freshwater fish. The infection is acquired by eating raw freshwater fish infected with the metacercariae of this parasite. In humans, the metacercariae excyst in the duodenum, and the worms migrate into the bile ducts and lodge in the peripheral bile ducts, where they mature.
Symptoms are related to the burden of flukes. Persons with fewer than 100 flukes are usually asymptomatic, whereas those with 100 to 1000 flukes usually have anorexia, nausea, epigastric pain, and diarrhea. A higher fluke load is accompanied by biliary colic and right upper quadrant tenderness. On cholangiographic studies, the presence of wavy, filamentous filling defects in the bile ducts is pathognomonic for clonorchiasis.
The pathologic findings for patients with C. sinensis infection vary. Because the parasite does not invade bile ducts, little inflammatory response is elicited. Initially, the biliary mucosa is edematous and has an intact or desquamated epithelium. With persistent infection, the epithelium undergoes mucinous metaplasia and becomes hyperplastic (Fig. 37.35). Periductal fibrosis is the hallmark of long-standing infection. At this stage, it is common for organisms to be absent and for mucinous metaplasia to disappear. Uncomplicated lesions contain few or no inflammatory cells. When complicated by pyogenic cholangitis, a heavy neutrophilic response suggests bacterial superinfection, usually by E. coli. As a result of disruption of the eggs by inflammatory cells, granulomas rich in eosinophils may form. Secondary biliary cirrhosis is a long-term consequence of chronic cholangitis and fibrosis. Adenomatous hyperplasia may evolve into biliary dysplasia and adenocarcinoma.
Ascaris lumbricoides
The nematode A. lumbricoides is the most prevalent type of helminthic infestation in the world. As much as 60% of the general population may be infected in endemic areas of South Africa, Asia, India, and South America. In the United States, A. lumbricoides accounts for less than 1% of all helminthic infections. After ingestion of Ascaris eggs, the larva hatches in the jejunum, penetrates the lymphatic vessels and portal circulation, and then migrates through the liver to the lungs. The larvae are then swallowed and mature to adult worms in the gut. The worms may migrate from the intestine into the bile ducts, producing obstruction. Bacterial superinfection may cause recurrent cholangitis. The worms are easily visualized by imaging techniques, such as cholangiogram and ultrasound.172,173
Fasciola hepatica
Fasciola hepatica is another parasite that can produce biliary obstruction and cholangitis and rarely cause cholecystitis. Infection occurs more frequently in Europe, Asia, Africa, and South America and is acquired by ingestion of water plants that contain encysted metacercariae. In the duodenum, metacercariae hatch and migrate through the wall of the intestine, penetrate the Glisson capsule, burrow through the parenchyma of the liver, and invade bile ducts and occasionally the gallbladder. In the acute phase, an inflammatory infiltrate rich in eosinophils may be seen, followed by regenerative hyperplasia of the biliary epithelium and fibrosis.172,173
AIDS-Related Lesions
The therapeutic efficacy of current antiretroviral therapy has dramatically improved outcomes for HIV-infected patients. The biliary problems that afflict these individuals on therapy are identical to those that occur in immunocompetent patients. The most prevalent condition in this population is cholelithiasis, and when it is associated with biliary colic, cholecystectomy may result in pain relief if HIV cholangiopathy is absent. The term HIV cholangiopathy refers to several types of bile duct disorders unrelated to gallstones, malignant disease, or previous surgery. These conditions are seen mostly in severely immunosuppressed patients and include papillary stenosis, a sclerosing cholangitis–like disorder, a combination of papillary stenosis and sclerosing cholangitis, and long extrahepatic bile duct strictures.174
Patients with HIV-associated cholangiopathy experience right upper quadrant or epigastric pain and fever. Most have an elevated serum alkaline phosphatase level, but only 15% have elevated bilirubin levels. Endoscopic retrograde cholangiopancreatography frequently demonstrates duct irregularities and a beaded appearance. In patients with papillary stenosis, relief of pain typically follows endoscopic sphincterotomy. However, because of ongoing intrahepatic duct disease, serum alkaline phosphatase levels usually continue to increase.
The pathogenesis of AIDS-related cholangitis, although unknown, may be similar to that suggested for PSC. The altered immune status of HIV-infected patients may contribute to the pathogenesis of bile duct damage, as in some patients with congenital immunodeficiencies. Particularly attractive is the hypothesis that enteric infections in AIDS patients may lead to portal bacteremia, bile duct injury, and destruction. Some cases may be related to CMV or Cryptosporidium infection. However, by itself, Cryptosporidium is an unlikely candidate, because it usually elicits only a very mild inflammatory response. Histologic changes associated with CMV infection vary from mild to severe. In the neonate, this organism may infect bile duct epithelium and cause obliterative cholangitis and paucity of bile ducts.175 Its propensity to infect endothelial cells may induce vasculitis and ischemic damage. Infection of the biliary tract with E. bieneusi is associated with and may be a cause of AIDS-related cholangitis.176–178
Pathologic lesions that characterize these conditions have not been adequately studied. In most cases, the extrahepatic bile ducts show nonspecific mixed inflammatory infiltrates and fibrosis. In most instances, organisms are not identified. The organisms that have been reported in the extrahepatic bile ducts are CMV, Cryptosporidium, Isospora, M. avium complex, Pneumocystis carinii, and E. bieneusi. E. bieneusi organisms may be seen on sections stained with hematoxylin and eosin within the cytoplasm of biliary cells, but they are easily missed, even by experienced pathologists. A Giemsa stain may help in their identification, and in some cases, electron microscopy is required.
In the liver, the portal tracts show increased fibrous tissue and sparse lymphocytic inflammation. Interlobular bile ducts are absent or show degenerative epithelial changes. Inflammatory cells are characteristically sparse. Lymphoma and Kaposi sarcoma are rare complications that may cause cholangitis.174
Cholangiopathy
Ischemic Cholangitis
Damage to the blood vessels that nourish bile ducts may result in necrosis and fibrous occlusion. Unlike the dual (portal and arterial) blood supply of the hepatic parenchyma, the biliary system is nourished exclusively by arterial blood flow. As in other segments of the gastrointestinal tract, ischemia may be related to luminal occlusion of the blood vessels (e.g., thrombosis, vasculitis), external compression, or hypoperfusion. Ischemic cholangitis accounts for about 2% to 19% of nonanastomotic strictures in liver transplant recipients.179 Posttraumatic ischemia of the bile ducts causes jaundice in patients hospitalized for life-threatening trauma or blunt abdominal injury and in patients who suffer inadvertent damage to blood vessels during abdominal surgery. Another important cause is hepatic artery infusion of chemotherapeutic agents such as floxuridine, 5-fluorouracil, mitomycin C, and cis-platinum.180
Ischemic changes may be confined to one segment of the bile duct or affect discontinuous segments. Injury to the biliary epithelium varies according to the cause of hypoperfusion. When the ischemic event occurs abruptly, edema and hemorrhage of the lamina propria and sloughing of necrotic biliary epithelial cells obliterate the lumen of the duct. Necrotic biliary cells admixed with fibrinous exudate and bile components form a biliary cast. Full-thickness necrosis of the bile duct wall may occur with spillage of bile into the liver parenchyma, peritoneum, or retroperitoneum. In patients with less severe hypoperfusion or progressive arterial occlusion, fibrosis of the bile ducts may obliterate the lumen. Inflammatory cell infiltrates are usually sparse except in cases of superimposed infection.
Other Non-neoplastic Biliary Strictures
More than 80% of bile duct strictures occur after cholecystectomy. Two cases of this complication occur per 1000 operations. Inadvertent damage of the bile ducts may result from failure to recognize unexpected anatomic variations or poor visualization because of encasement of the ducts by an abscess or fibrous tissue. Attempts to gain hemostasis because of bleeding from the cystic or hepatic arteries may lead to bile duct injury. Even in expert hands, the extreme friability of acutely inflamed tissue makes dissection of the Calot triangle a formidable task. Cholecystectomy in the setting of a biliobiliary fistula carries a high risk of injury to the right or common hepatic duct. Other conditions that affect the biliopancreatoduodenal area that may give rise to postinflammatory bile duct strictures include subhepatic abscesses, chronic duodenal ulcers, chronic pancreatitis, and granulomatous lymphadenitis.
The location of the stricture influences the choice of surgical technique and likely outcome of the repair.181–183 Uncommon causes of biliary strictures include Langerhans cell granulomatosis (i.e., histiocytosis X), sarcoidosis, amyloidosis, congenital biliary anomalies, and cystic fibrosis.180 Extrinsic compression of the bile ducts from cirrhotic nodules, cysts, tumors, and adhesions may mimic a primary biliary disorder.