Chapter 53C Infections of the Nervous System
Bacterial and Fungal
Bacterial Infections of the Central Nervous System
Meningitis
Etiology
Until recently, children had the highest incidence of meningitis, but with the development of an extremely effective vaccine against H. influenzae type b (Hib) and the heptavalent vaccine targeting invasive S. pneumoniae, adults now have the highest incidence of meningitis in developed countries, where it is 5 per 100,000 (Schut et al., 2008). The S. pneumoniae vaccine is not a meningitis vaccine but has decreased the incidence of meningitis by decreasing the incidence of otitis media in children. Though an absolute increase in number of cases of H. influenzae non-b and S. pneumoniae serotypes not in the vaccine (“replacement phenomena”) has been seen, this increase in absolute number is still small (Bender et al., 2010). The incidence of meningitis due to N. meningitidis has decreased with the tetravalent (serogroups A, C, W-135, and Y) meningococcal glycoconjugate vaccine, but the vaccine does not provide lasting immunity and does not include one of the major serotypes, serotype B, as the N. meningitidis group B polysaccharide capsule is poorly immunogenic. There is ongoing work to make the vaccine more efficacious (Riordan, 2010). L. monocytogenes accounts for approximately 8% of cases of acute bacterial meningitis. L. monocytogenes meningitis is uncommon in healthy children and adults. The most common predisposing factors for L. monocytogenes meningitis are age older than 50 years, diabetes, chronic illness, malignancy, and immunosuppressive therapy or an immunosuppressed state.
Table 53C.1 lists the most common bacterial causes of acute or chronic meningitis and diagnostic tests for identifying the organism.
| Organism | Blood | Cerebrospinal Fluid |
|---|---|---|
| Streptococcus pneumoniae | Culture | Gram stain: Gram-positive diplococci in pairs Culture |
| Listeria monocytogenes | Culture | Gram stain: Gram-positive rods Culture |
| Neisseria meningitides | Culture | Gram stain: Gram-negative diplococcus Culture |
| Haemophilus influenzae type b | Culture | Gram stain: Gram-negative coccobacillus Culture |
| Mycobacterium tuberculosis | 20-30 mL for AFB stain and culture; PCR | |
| Treponema palladium | RPR/VDRL; MHA-TPA | VDRL (non-traumatic tap) |
| Coxiella burnetii | Acute and convalescent serologies | |
| Brucella spp. | Culture: acute and convalescent serologies | Gram stain: Gram-negative coccobacillus Culture |
| Borrelia spp. | ELISA→ if equivocal or +, then IgG and IgM WB (follow CDC guidelines for + WB) | Antibody index: Anti-Borrelia IgG in CSF/anti-Borrelia IgG in serum to total IgG in CSF/total IgG in serum |
| Leptospira spp. | Acute and convalescent serologies (MAT only done in reference labs, ELISA and lateral flow dipstick less sensitive and specific) Culture: special media; may need to keep for 8-12 weeks |
Culture: Special media, fastidious |
AFB, Acid-fast bacilli; CDC, Centers for Disease Control and Prevention; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; MAT, microscopic agglutination test; MHA-TPA, microhemagglutination assay–Treponema antibody absorption test; PCR, polymerase chain reaction; RPR, rapid plasma reagin test; VDRL, Venereal Disease Research Laboratory test; WB, Western blot test.
Clinical Presentation
The classic symptoms of acute bacterial meningitis are fever, headache, meningismus, and a progressive decrease in the level of consciousness. In a study that evaluated the symptoms in 666 episodes of meningitis in adults, headache was the most common complaint (87%), followed by neck stiffness (83%), fever (77%), and altered mental status (69%). While no single symptom is particularly sensitive or specific, 95% of the patients had two of the four symptoms and only 1% had none. A petechial rash can also be present during acute bacterial meningitis, especially when N. meningitidis is the causative agent, although S. pneumoniae can produce a similar rash (van de Beek et al., 2004). It should be noted that in immunocompromised patients, fever and meningismus may not be present.
Diagnosis
The diagnosis of acute bacterial meningitis depends on recognizing the clinical picture as one consistent with acute meningitis and performing a lumbar puncture (LP) to evaluate for meningeal inflammation and bacteria. The necessity of head computed tomography (CT) prior to performing an LP is often discussed. A head CT prior to LP is recommended in the patient with any of the following: an altered level of consciousness, a focal neurological deficit, new-onset seizure, papilledema (or other signs of increased intracranial pressure), or an immunocompromised state. The utility of imaging is twofold: (1) to evaluate for focal mass lesions and edema that put the patient at risk for uncal herniation and (2) to find those diseases that might mimic acute meningitis but in fact are quite distinct (bacterial abscess, tumor). The imaging modality to use in such patients is CT; the scans can be obtained quickly, and CT is sensitive enough to rule out lesions that predispose patients to herniation. Noncontrast imaging may show no abnormality; postcontrast images will often show diffuse meningeal enhancement. If it is determined that head imaging would be appropriate before LP, and acute bacterial meningitis is high in the differential, the most critical step to take is to obtain blood cultures and begin empirical antibiotics before the patient is sent for imaging. Starting empirical antibiotics quickly is critical because there is burgeoning evidence that a delay in initiating antibiotic treatment for acute bacterial meningitis leads to increased morbidity and mortality (Auburtin et al., 2006; Proulx et al., 2005). If antibiotics are not initiated prior to imaging, it is also clear that imaging prior to LP significantly delays the time to antibiotics (Proulx et al., 2005).
The gold standard for the diagnosis of acute bacterial meningitis is identification of the meningeal pathogen in Gram stain and/or culture of cerebrospinal fluid (CSF). The culture may take 48 to 72 hours to be positive. The organism may also be cultured from blood. Newer diagnostic techniques include polymerase chain reaction (PCR) assays for use on CSF. The real-time multiplexed PCR on CSF specifically determines whether S. pneumoniae, H. influenzae, or N. meningitides are present (Corless et al., 2001). The conserved-sequence bacterial 16S rRNA is a broad-based PCR that if positive requires a second step to identify the specific pathogen detected (Schuurman et al., 2004). Identification can be accomplished either by pathogen-specific PCR or by sequencing of the 16S rRNA band that is amplified. The advantages to PCR-based diagnostics are improved sensitivity and shorter times to diagnosis, but the disadvantages are that these tests are not routinely available, and antibiotic sensitivity data, which is essential, can only be obtained from culture. Thus, at this time in many hospitals, Gram stain and culture remain the best tools for diagnosing acute bacterial meningitis.
Diagnosis of chronic infectious meningitis is much more complicated, and the number of tests required to determine the specific etiology is often much higher. Lumbar puncture is important in documenting meningeal inflammation, although if there are clinical signs or symptoms consistent with increased intracranial pressure, neuroimaging should be done prior to LP. Unlike acute bacterial meningitis, in this scenario, magnetic resonance imaging (MRI) with and without contrast is the study of choice because of increased sensitivity of MRI compared to CT, and because there is less urgency to start empirical treatment and obtain CSF for diagnostic studies. Depending on the CSF abnormalities, certain etiologies may be more or less likely. For example, a mononuclear predominance with a mildly decreased glucose concentration and increased protein concentration suggests tuberculous meningitis, while a CSF pleocytosis with a mononuclear predominance and a normal glucose concentration and either a normal or mildly elevated protein concentration is more consistent with neurosyphilis. To distinguish between just these two possibilities, one would need to send serum and CSF tests for syphilis (see Table 53C.1) and high-volume CSF AFB smear, culture, and PCR for M. tuberculosis.
A noninfectious etiology of meningitis that has a clinical presentation and CSF and neuroimaging abnormalities similar to bacterial meningitis is neoplastic meningitis. Neoplastic meningitis is called carcinomatous meningitis when the leptomeninges are seeded with malignant cells from solid tumors, most commonly melanoma, breast or lung cancer, and leukemic or lymphomatous meningitis from hematological malignancies. The CSF in neoplastic meningitis can range from being completely normal in all routine parameters (cells, protein and glucose concentrations) to having a moderately elevated pleocytosis (<500 cells/mm3) and protein concentration as well as an impressive hypoglycorrhachia. The differences in these CSF parameters are often reflective of the extent of meningeal involvement, with normal CSF parameters occurring in those patients with a low burden of neoplastic meningeal disease. While the gold standard for the diagnosis of neoplastic meningitis is a positive conventional cytological analysis or flow cytometry for neoplastic cells, the sensitivity of these tests is poor. To increase the yield of CSF analytical cytologies, the following is recommended: send high volumes of CSF (>10 mL), send a minimum of two high-volume CSF samples (obtained at different time points), make sure the cytological evaluation can be done on the same day the sample is collected, and if possible, obtain the CSF from a source close to any abnormalities on neuroimaging (Chamberlain et al., 2009). While waiting to determine whether a chronic meningitis is neoplastic in origin, CSF should also be sent for bacterial and fungal smears and cultures, and PCR should be done to rule out common causes of viral meningitis.
Management
Acute bacterial meningitis is treated initially with empirical antibiotics, which can be narrowed once the specific organism and its antibiotic sensitivities have been determined. Choosing the appropriate empirical antibiotic depends on the likely organism, which is dependent upon the patient’s age and risk factors. Table 53C.2 identifies which empirical antibiotics should be used for specific patient populations, and Table 53C.3 gives the appropriate CNS dosing for these antibiotics. Antibiotic therapy is modified when the antimicrobial sensitivity tests results are available.
Table 53C.2 Empiric Antibiotics for Bacterial Infections of the CNS
| Disease Entity | Organisms | Empiric Antibiotics |
|---|---|---|
| Bacterial Meningitis | ||
| Age < 50 and no risk factors for Listeria | S. pneumoniae, N. meningitidis | Vancomycin + ceftriaxone or cefotaxime or cefepime |
| Age > 50 and/or risk factors for Listeria | As above + L. monocytogenes | As above + ampicillin |
| Sinusitis, mastoiditis, or otitis predisposing cause of meningitis | As above (depending on age and risk factors) + anaerobes | As above (depending on age and risk factors) + metronidazole |
| Brain abscess | S. aureus, aerobic and anaerobic streptococci, oral and GI flora (including Bacteroides spp) | Vancomycin + ceftriaxone or cefotaxime or cefepime+ metronidazole |
| Nocardia | Trimethoprim-sulfamethoxazole | |
| Spinal epidural abscess | Staphylococcal spp, streptococcal spp, enteric gram negative bacilli | Vancomycin + ceftriaxone or cefotaxime or cefepime |
| Any of the above | M tuberculosis (high suspicion) | Four drug therapy (isoniazid, rifampin, ethambutal, pyrazinamide) |
Table 53C.3 Central Nervous System Dosages for Commonly Used Antibiotics
| Drug | Dose (Adult, Assuming a Normal Creatinine Clearance) |
|---|---|
| Vancomycin | 40-60 mg/kg/day, divided into q 6 h dosing |
| Ceftriaxone | 2 g, q 12 h |
| Cefepime | 2 g, q 8 h |
| Cefotaxime | 2 g, q 4-6 h |
| Ampicillin | 2 g, q 4 h |
| Metronidazole | 500 mg, q 6 h |
Whether or not to initiate steroids at the time of the administration of antibiotics has been a hotly debated topic for years. In 2002, a landmark prospective double-blinded, placebo-controlled randomized study was published which showed that adjunctive steroids at the time of initiation of empirical antibiotics significantly improved the overall mortality and morbidity of those with acute bacterial meningitis. Subgroup analysis clearly showed that all mortality and morbidity benefits were derived from the group that ultimately had S. pneumoniae meningitis, of which all tested strains were penicillin sensitive (de Gans et al., 2002). Thus, the current recommendations are to initiate adjunctive steroids just before or with the administration of empirical antibiotics. Based on animal model data, there was initially great concern that the addition of steroids to empirical antibiotics would decrease the CSF concentration of vancomycin, which would lead to undertreatment of penicillin-resistant S. pneumoniae meningitis—an issue not addressed by the 2002 study, as all of their isolates appeared to be penicillin sensitive. A small but well-done study in 2007 prospectively evaluated the CSF vancomycin concentration in patients with suspected S. pneumoniae meningitis who were placed on empirical antibiotics and steroids. Ultimately, over half of this group had penicillin-resistant S. pneumoniae, and all had CSF vancomycin concentrations at least fourfold higher than the minimum inhibitory concentration (MIC) of the cultured organism. In addition, on repeat LP, none of the patients had positive S. pneumoniae cultures, and the CSF vancomycin levels were proportional to the serum vancomycin levels (Ricard et al., 2007). Thus, it appears that concurrent administration of dexamethasone does not inhibit vancomycin CSF penetration in a clinically significant manner and that in these patients, serum vancomycin levels are similarly related to the CSF concentration as in patients without concomitant steroids. Another caveat to the study was that there were too few patients with N. meningitides or H. influenzae to determine whether or not steroids helped or harmed these patients. So although adjunctive steroid administration is recommended with the initiation of empirical antibiotics, it remains unclear whether or not to continue or stop the steroids if an organism other than S. pneumoniae is isolated.
Brain Abscess
Clinical Presentation
Most patients with brain abscess will present with the signs and symptoms of a space-occupying lesion, such as headache. They may also present with confusion, alterations in consciousness, seizure, or focal neurological deficits. Fever occurs in fewer than half of patients with brain abscess (Carpenter et al., 2007) and should not be used as a criterion to exclude brain abscess from the differential diagnosis. As in any space-occupying lesion, progressive nausea and vomiting can be seen as the mass expands and intracranial pressure increases. There are no signs or symptoms that definitively exclude or prove bacterial brain abscess in a patient presenting with a space-occupying lesion.
Diagnosis
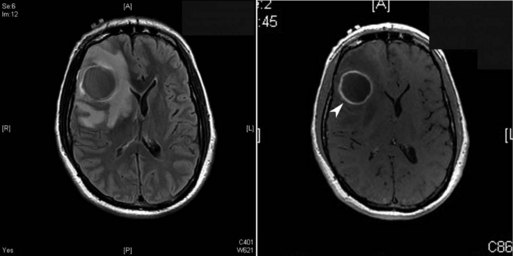
CNS imaging has highly improved the ability to diagnose bacterial brain abscesses and thus decreased mortality from 20% to 50% to less than 20% in most modern series (Seydoux and Francioli, 1992). Every patient with a suspected brain abscess needs a timely contrast-enhanced brain image. As seen in Fig. 53C.1, the contrast imaging will often show a ring-enhancing mass. Blood cultures can be useful in determining the etiology of a brain abscess, but they are rarely positive, compared to patients with acute bacterial meningitis. Predisposing factors such as otitis media or sinusitis can suggest the likely etiology, but definitive diagnosis requires either a culture from another source (such as blood in endocarditis or sputum in disseminated tuberculosis) or directly from the abscess itself. Given the improvements in neurosurgical techniques, including stereotactic aspiration, the ability to treat and definitively diagnose the etiological agent of the abscess has greatly improved. Ideally, if the patient is stable from a hemodynamic and neurological standpoint, and neurosurgical drainage and cultures can be obtained quickly (within 24-48 hours of presentation), empirical antibiotics should be avoided until after the abscess cultures have been obtained. Similar to acute bacterial meningitis, the 16S rRNA conserved-sequence broad-based bacterial PCR can prove whether the abscess is of bacterial etiology, but a second step is necessary to identify the pathogen, and culture is needed for antimicrobial sensitivity testing.
Notably, LP plays no role in the diagnosis of brain abscess. Lumbar puncture has the potential to lead to brain herniation if the abscess is large enough, and little diagnostic information is gained because it is unusual to culture the bacteria from the CSF, and there is nothing specific about the profile that suggests or confirms bacterial brain abscess (Seydoux and Francioli, 1992).
Management
Currently, CT-guided stereotactic aspiration of the brain abscess in combination with antibiotics is the standard of care. While there are reports of patients surviving after being treated with antibiotics alone (typically if the abscess is very small, very deep, or there are multiple abscesses), generally both antibiotics and surgical aspiration are recommended. As mentioned earlier, aspiration offers the opportunity for both diagnosis and decreasing the size of the abscess, allowing quicker resolution. Patients with small abscesses, who are poor surgical candidates, or who have abscesses in deep brain structures can be treated with medical management alone, although with improved surgical technique, even some deep brain structures can be drained (Wait et al., 2009).
Spinal Epidural Abscess
Etiology
Spinal epidural abscesses (SEA) are rare and are often initially misdiagnosed, leading to profound neurological deficits or death (Davis et al., 2004). A variety of organisms can cause SEA. The most common organism is Staphylococcus aureus (usually about 50%-60% of the isolated organisms in any series), with Streptococcus spp., coagulase-negative staphylococci, and enteric gram-negative rods making up the bulk of the non-tuberculous SEA (Curry et al., 2005; Davis et al., 2004; Reihsaus et al., 2000; Soehle and Wallenfang, 2002). In areas with endemic tuberculosis or in patients who have recently emigrated from endemic areas, M. tuberculosis as a cause of SEA associated with vertebral osteomyelitis should be high on the differential diagnosis.
The development of SEA can occur from direct spread (i.e., psoas abscess, vertebral osteomyelitis) or from hematogenous spread from a distant site. The cervical spine is less frequently involved than the thoracic or lumbar spine, which may be related to the extent of the epidural space and the venous drainage of the thoracic and lumbar spine (Reihsaus et al., 2000).
Clinical Presentation
Spinal epidural abscess is a progressive disease that usually begins with new-onset back pain or localized tenderness and progresses to radicular pain and ultimately to neurological deficits including bowel and bladder dysfunction. The classic triad of SEA is back pain or localized tenderness, fever, and progressive neurological deficits localized to the spinal cord. Like the classic triad of bacterial meningitis, this triad is highly specific but has low sensitivity, especially early in the disease course (Davis et al., 2004; Reihsaus et al., 2000). In most studies, back pain is a presenting feature in about 60% to 70% of patients, and “fever” is present in 50% to 60% of (although it is not always clear if this is a reported or documented fever). In addition, as one study noted, patients with back pain often use antiinflammatory medications which have antipyretic effects, making the presence or development of a fever even less likely in these patients (Davis et al., 2004).
When trying to determine whether a patient with new-onset back pain should be imaged to rule out SEA, one should assess the patient for any of the known risk factors for SEA. The most common risk factors for developing SEA are diabetes mellitus, intravenous drug use, alcohol abuse, immunocompromised state (including immunosuppressive drugs, AIDS, cancer patients), spinal surgery/procedure, trauma (spinal or extraspinal), and extraspinal infections (furunculosis/cellulites, psoas abscess, etc.) Which risk factor is most highly related to SEA depends on the study and likely reflects the different populations studied (Davis et al., 2004; Pradilla et al., 2009; Reihsaus et al., 2000). In every study there are a small number of patients without any risk factors, but in general, most patients have at least one of the previously listed risk factors (Davis et al., 2004; Reihsaus et al., 2000). In addition, while SEA can be located anywhere along the spinal axis, up to a third of patients will present with thoracic pain. Because the thoracic spine is an unusual place to present with mechanical back pain, new-onset back pain in this region should make one consider SEA sooner rather than later.
Diagnosis
Diagnosing SEA is difficult because the vast majority of patients with back pain (>99%) will not have SEA. Thus, in this group of patients, choosing whom to further evaluate, especially early on when the patient has no neurological deficits and may not have fever, is extremely difficult. As mentioned, using risk factors, fever, and location of pain can help narrow the number of appropriate patients for evaluation. One provocative retrospective study suggested that using risk factors to evaluate which patients were at high risk for an SEA had a sensitivity of 98% and a negative predictive value of 99%. Yet, given the high incidence of risk factors and back pain compared to SEA, 50 patients would need further evaluation for a single case of SEA to be found (Davis et al., 2004). Clearly, further research into reliable unique biomarkers for SEA is certainly needed. The one laboratory test that may have some role in helping to narrow the patients for evaluation would be erythrocyte sedimentation rate (ESR). The majority of patients with SEA have an ESR greater than 20 mm/h (Davis et al., 2004; Reihsaus et al., 2000). As with the risk factor assessment, the sensitivity of this test will be very good, but the specificity will be poor. Ultimately, determining which patient is at high risk for SEA will require the physician to integrate the risk factors of a given patient, the physical exam, and limited laboratory data.
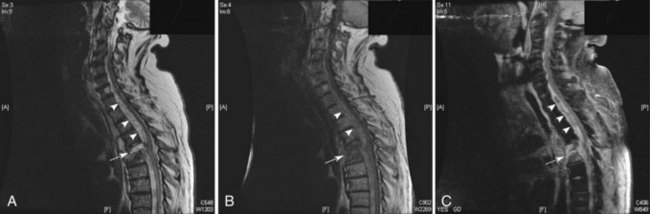
Once a patient is felt to have a high risk for SEA, MRI with gadolinium is the standard of care for evaluation. MRI has an excellent ability to evaluate the bones and discs for signs of infection (osteomyelitis and discitis) as well as to look for the presence and extent of an epidural abscess. Fig. 53C.2 is an example of a patient with a ventral epidural abscess with associated discitis. In patients who cannot undergo MRI, CT myelogram is the next best choice. Spinal epidural abscess is a neurological emergency. The urgency for diagnosing SEA is based on the suggestion that the outcome is better if patients are treated before the onset of neurological deficits or if neurological deficits have only been present for a short time period (<48-72 hours) (Khanna et al., 1996; Reihsaus et al., 2000). This assumes the deficits are from cord compression and not ischemia.
Management
There are no randomized controlled trials to evaluate the efficacy of medical management with or without surgical treatment. The current standard of care is to employ both surgical (aspiration or evacuation of the abscess) and medical management (antibiotics). While there have been reports of patients doing equally well with medical management alone (Tang et al., 2002), there are similar reports showing that patients treated with surgery as well as antibiotics have better outcomes compared to those who only receive medical management (Curry et al., 2005). In addition, the reasons for choosing medical management alone are usually the lack of neurological deficits or poor surgical candidates—baseline differences that could have a huge impact on outcomes. At this time, the management of SEA relies on both surgical and medical management, with the appropriateness of only medical management being reserved for a small group of well-defined patients who will have excellent follow-up.
Empirical antibiotics for suspected or confirmed SEA must cover S. aureus, streptococci, coagulase-negative staphylococci, and enteric gram-negative bacilli. If M. tuberculosis is highly suspected, four-drug therapy should be initiated until further confirmation that M. tuberculosis is the organism or another organism is identified. Once the organism is isolated from the blood or the abscess itself, the antibiotics can be modified. Please see Table 53C.2 for suggested empirical antibiotics. The length of intravenous antibiotic treatment is not well defined, but usually 6 to 8 weeks is recommended, with some clinicians preferring to switch to oral medications for further treatment. M. tuberculosis vertebral osteomyelitis with or without associated epidural abscess is treated for 6 to 9 months (American Thoracic Society/Center for Disease Control/Infectious Disease Society of America, 2003).
Follow-up MRIs to guide decisions about treatment failure is a common practice, but clinical evidence to support this approach is limited. Two studies have addressed the utility of follow-up MRIs for SEA/vertebral osteomyelitis. While neither study was particularly large, neither found that routine follow-up MRIs were cost-effective predictors for clinical outcomes. Thus, both studies recommended not obtaining routine follow-up studies for determining response to therapy (Carragee, 1997; Kowalski et al., 2006) but rather limiting follow-up imaging to those patients who are clinically worse (Kowalski et al., 2006).
Fungal Infections of the Central Nervous System
Meningitis
Clinical Presentation
The clinical presentation of fungal meningitis is similar to bacterial meningitis except that the time course is usually subacute to chronic rather than acute, and fever tends to be less common, especially in immunocompromised patients. In a recent review of 71 cases of CNS coccidioidomycosis in which less than half were immunocompromised, 77% of the patients presented with headache, but only 28% presented with fever (Drake and Adam, 2009). Altered mental status is also a relatively common presentation for fungal meningitis. The alteration in mentation may be due directly to the infectious process or to secondary complications such as hydrocephalus or vasculitis.
Diagnosis
In general, the CSF profile of fungal meningitis resembles that of M. tuberculosis meningitis, with a mononuclear pleocytosis and an elevated protein concentration, but the CSF glucose concentration in fungal meningitis is moderately decreased, while in tuberculous meningitis, the CSF glucose concentration is only mildly decreased. Certain CSF findings suggest specific etiologies. For example, a neutrophilic pleocytosis in a patient from the Mississippi and Ohio river basins suggests Blastomyces meningitis, whereas an eosinophilic meningitis in a patient from the San Joaquin Valley in California is consistent with Coccidioides meningitis (Bariola et al., 2010; Drake and Adam, 2009). Imaging studies often show basilar meningitis with contrast enhancement or unexplained hydrocephalus. Determining the exact fungal etiology is highly dependent on the suspected fungal species. For Cryptococcus spp., a serum and/or CSF cryptococcal antigen test is recommended. For Coccidioides immitis, serum and CSF enzyme-linked immunosorbent assay (ELISA) and CSF complement fixation test are recommended. For Histoplasma capsulatum, a CSF Histoplasma polysaccharide antigen test is the test of choice (Chayakulkeeree and Perfect, 2006; Kauffman, 2006). CSF fungal smears (India ink) may demonstrate the organism, but as a rule, cultures of large volumes (20-30 mL) of CSF from the lumbar space are needed. If negative, CSF from a high cervical puncture will have the highest yield.
Management
Compared to bacterial meningitis, fungal meningitis is much more indolent and thus requires a much longer course of therapy. There are very comprehensive guidelines written by the Infectious Disease Society for America for the following fungi that cause meningitis: Blastomyces dermatitidis, C. immitis, Cryptococcus neoformans, and Sporothrix schenckii. These guidelines are written by experts in the field, are updated every few years, and grade the recommendations. In general, management will consist of several phases of treatment: induction, consolidation, and maintenance. The agents used and the length of each phase depends on the specific pathogen and the patient population. Table 53C.4 summarizes recommended treatment as determined by the fungus identified or suspected.
Table 53C.4 Suggested Treatment for Specific Fungal Diseases of the Central Nervous System
| Infection | Treatment |
|---|---|
| Aspergillosis |
Induction: Amphotericin B + flucytosine (can substitute liposomal amphotericin) × 2 weeks (minimum)
Consolidation: Oral fluconazole 400 mg/day × 8 weeks (minimum)
Maintenance: Oral fluconazole 200 mg/day × 1 year (minimum)
Induction: Lipid-formulation amphotericin + flucytosine × 2 weeks (minimum)
Consolidation: Oral fluconazole 400-800 mg/day × 8 weeks
Maintenance: Oral fluconazole 200-400 mg × 6-12 months
Induction: Amphotericin B + flucytosine × 4 weeks
Consolidation: Oral fluconazole 400-800 mg/day × 8 weeks
Maintenance: Oral fluconazole 200-400 mg × 6-12 months
If ICP ≥ 25 cm H2O and symptomatic, then remove cerebrospinal fluid (CSF) via lumbar puncture (LP) to closing pressure of ≤ 20 mm H2O or ≤ 50% of opening pressure (OP) if OP very high
Recheck OP daily until stable × 2 days; consider temporary ventriculostomy or lumbar drain if requiring daily LP of ICP management
From Chapman, S.W., Dismukes, W.E., Proia, L.A., et al., 2008. Clin Infect Dis 46, 1801-1812; Galgiani, J.N., Ampel, N.M., Blair, J.E., et al., 2005. Clin Infect Dis 41, 1217-1223; Kauffman, C.A., Bustamante, B., Chapman, S.W., et al., 2007. Clin Infect Dis 45, 1256-1265; Pappas, P.G., Kauffman, C.A., Andes, D., et al., 2009. Clin Infect Dis 48, 503-535; Perfect, J.R., Dismukes, W.E., Dromer, F., et al., 2010. Clin Infect Dis 50, 291-322; Walsh, T.J., Anaissie, E.J., Denning, D.W., et al., 2008. Clin Infect Dis 46, 327-360; Wheat, L.J., Freifeld, A.G., Kleiman, M.B., et al., 2007. Clin Infect Dis 45, 807-825.
Brain Abscess
Etiology
In general, fungal brain abscesses are found in the immunocompromised organ transplant patients, hematological malignancy patients, and acquired immunodeficiency syndrome (AIDS) patients, with Candida and Aspergillus spp. topping the list of etiologies (Leventakos et al., 2010). Candidal abscess are usually secondary to disseminated disease. Aspergillus involvement can be secondary to hematogenous dissemination from invasive pulmonary disease or can be direct extension from paranasal sinuses. Zygomycetes (Rhizopus spp., Mucor) generally involve the CNS by direct extension from paranasal sinuses. This disease occurs in classically immunosuppressed patients, though diabetes mellitus is often the most commonly cited risk factor. P. boydii is a rare cause of brain abscess but one associated with near-drownings or trauma (Kantarcioglu et al., 2008). Finally, the fungi mentioned in the proceeding section can also be associated with isolated brain abscesses but are more commonly associated with meningitis or meningoencephalitis. In general, these infections are extremely difficult to treat, often because they occur in the most immunosuppressed patients, and thus they carry a high mortality rate.
Management
The suspected organism determines the ultimate antimicrobial used, but an azole or a lipid formulation of amphotericin B are the most common agents. See Table 53C.4 for a summary of recommended treatments for suspected or isolated fungi. It is important to recognize the Achilles heels of the different antimicrobial agents. For example, voriconazole is the drug of choice to treat CNS aspergillosis, but is not effective against zygomycetes. Thus, in a patient with sinus disease and infectious extension into the brain, until a final diagnosis is determined, empirical treatment with amphotericin is appropriate. Despite new antifungal agents with more potency against fungi, the morality of CNS fungal abscesses remains high.
American Thoracic Society/Center for Disease Control/Infectious Disease Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
Auburtin M., Wolff M., Charpentier J., et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: The PNEUMOREA prospective multicenter study. Crit Care Med. 2006;34:2758-2765.
Bariola J.R., Perry P., Pappas P.G., et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50:797-804.
Bender J.M., Cox C.M., Mottice S., et al. Invasive Haemophilus influenza disease in Utah children: an 11-year population-based study in the era of conjugate vaccine. Clin Infect Dis. 2010;50:e41-e46.
Carragee E.J. The clinical use of magnetic resonance imaging in pyogenic vertebral osteomyelitis. Spine. 1997;2:780-785.
Carpenter J., Stapleton S., Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1-11.
Chamberlain M.C., Glantz M., Groves M.D., et al. Diagnostic tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol. 2009;36:S35-S45.
Chayakulkeeree M., Perfect J.R. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507-544.
Corless C.E., Guiver M., Borrow R., et al. Simultaneous detection of Neisseria meningitides, Haemophilus influenza, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Micro. 2001;39:1553-1558.
Curry W.T., Hoh B.L., Amin-Hanjani S., et al. Spinal epidural abscess: clinical presentation, management, and outcome. Surgical Neurol. 2005;63:364-371.
Davis D.P., Wold R.M., Patel R.J., et al. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004;26:285-291.
de Gans J., van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549-1556.
Drake K.W., Adam R.D. Coccidioidal meningitis and brain abscesses: analysis of 71 cases at a referral center. Neurology. 2009;73:1780-1786.
Kantarcioglu A.S., Guarro J., de Hoog G.S. Central nervous system infections by members of the Pseudallescheria boydii species complex in healthy and immunocompromised hosts: epidemiology, clinical characteristics, and outcome. Mycoses. 2008;51:275-290.
Kauffman C.A. Endemic mycoses: blastomycosis, histoplasmosis, and sporotrichosis. Infect Dis Clin North Am. 2006;20:645-662.
Khanna R.K., Malik G.M., Rock J.P., et al. Spinal epidural abscess: evaluation of factors influencing outcomes. Neurosurgery. 1996;39:958-964.
Kowalski T.J., Berbari E.F., Huddleston P.M., et al. Do follow-up imaging examinations provide useful prognostic information on patients with spine infection? Clin Infect Dis. 2006;43:172-179.
Leventakos K., Lewis R.E., Kontoyiannis D.P. Fungal infections in leukemia patients: how do we prevent and treat them? Clin Infect Dis. 2010;50:405-415.
Pradilla G., Ardila G.P., Hsu W., et al. Epidural abscesses of the CNS. Lancet Neurol. 2009;8:292-300.
Proulx N., Frechette D., Toye B., et al. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. Q J Med. 2005;98:291-298.
Reihsaus E., Waldbaur H., Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;232:175-204.
Ricard J.D., Wolff M., Lacherade J.C., et al. Levels of vancomycin in cerebrospinal fluid of adult patients receiving adjunctive corticosteroids to treat pneumococcal meningitis: a prospective multicenter observational study. Clin Infect Dis. 2007;44:250-255.
Riordan A. The implications of vaccines for prevention of bacterial meningitis. Curr Opin Neurol. 2010;23:319-324.
Schut E.S., de Gans J., van de Beek D. Community-acquired bacterial meningitis in adults. Pract Neurol. 2008;8:8-23.
Schuurman T., de Boer R.F., Kooistra-Smid A.M.D., et al. Prospective study of the use of PCR amplification and sequencing of 16S ribosomal DNA from cerebrospinal fluid for diagnosis of bacterial meningitis in a clinical setting. J Clin Microbiol. 2004;42:734-740.
Seydoux C., Francioli P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394-401.
Soehle M., Wallenfang T. Spinal epidural abscess: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002;51:79-87.
Tang H.J., Lin H.J., Liu Y.C., et al. Spinal epidural abscess- experience with 46 patients and evaluation of prognostic factors. J Infect. 2002;45:76-81.
van de Beek D., de Gans J., Spanjaard L., et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849-1859.
Wait S.D., Beres E.J., Nakaji P. Bacterial abscess of the medulla oblongata. J Clin Neurosci. 2009;16:1082-1084.