87 Infections of the Head and Neck
Cervical Lymphadenitis
Etiology and Pathogenesis
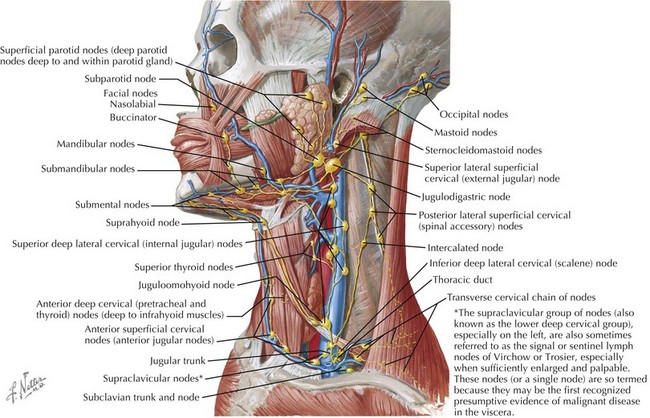
The cervical lymphatic system consists of a collection of both superficial and deep lymph nodes that protect the head, neck, nasopharynx, and oropharynx against infection (Figure 87-1). Lymph nodes can enlarge by either proliferation of normal cells intrinsic to the node such as lymphocytes or infiltration by cells extrinsic to the node such as neutrophils or malignant cells. The most common cause of cervical lymphadenopathy in children is reactive intranodal hyperplasia secondary to infection. The majority of lymphatics of the head and neck drain to the submandibular lymph nodes and the anterior and posterior cervical lymph node chains. Consequently, these nodes are involved in most children with cervical lymphadenitis.
Many different organisms have been implicated in cervical lymphadenitis (Box 87-1). The most common cause of cervical lymphadenitis is viruses that infect the upper respiratory tract, including adenovirus, respiratory syncytial virus, influenza, and parainfluenza. When bacterial in origin, cervical lymphadenitis may be a primary process or result from direct extension of a local infection such as pharyngitis or dental abscess. In the case of acutely inflamed and enlarged unilateral nodes, aspirates reveal infection by Staphylococcus aureus or Streptococcus pyogenes (group A β-hemolytic streptococci [GABHS]) in the majority of cases. Recent studies of suppurative lymphadenitis show the predominance of S. aureus and the increased prevalence of community-acquired methicillin-resistant S. aureus (CA-MRSA). More indolent causes of cervical lymphadenitis include Bartonella henselae, mycobacterial infections, and Toxoplasma gondii. The age of a child plays a role in predicting the infectious etiology of cervical lymphadenitis (Table 87-1).
| Age | Etiology |
|---|---|
| Infants | Staphylococcus aureus Group B streptococcus |
| Children age 1-4 y | S. aureus Group A streptococcus Nontuberculous mycobacterium Bartonella henselae |
| School-age children and adolescents | S. aureus Group A streptococcus Anaerobes B. henselae Toxoplasma gondii |
Clinical Presentation and Differential Diagnosis
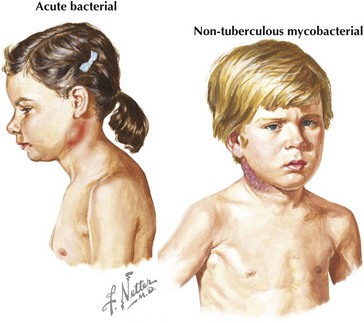
Acute unilateral cervical lymphadenitis is caused by S. aureus and S. pyogenes in the majority of cases. The onset may be associated with an upper respiratory tract infection, pharyngitis, or periodontal disease, and associated fever is common. Typically, the onset is acute with development of large, tender, erythematous, and warm lymph nodes that may become fluctuant over a few days (Figure 87-2). In addition, a cellulitis–adenitis syndrome caused by group B streptococcus in infants between 3 and 7 weeks of age is associated with irritability, fever, and unilateral facial or submandibular swelling with erythema and tenderness.
The most common causes of subacute or chronic lymphadenitis are mycobacterial infections, cat scratch disease, and toxoplasmosis. Lymph node enlargement is typically gradual in onset and progresses over weeks to months. The most common presentation of nontuberculous mycobacterium (NTM) disease in children is cervical lymphadenitis. The lymph nodes are large and indurated but nontender, and the overlying skin often becomes violaceous and thin (see Figure 87-2). Untreated lymphadenitis caused by NTM may resolve, but often it progresses to lymph node necrosis followed by fluctuance and spontaneous drainage. Cervical lymphadenitis caused by Mycobacterium tuberculosis has a similar presentation, but there are clinical and epidemiologic differences. NTM is uncommon in children older than 5 years of age compared with tuberculosis, which can occur at any age. With NTM, involvement is usually unilateral and associated with a normal chest radiograph and a normal or minimally indurated purified protein derivative (PPD). In contrast, children with tuberculous cervical lymphadenitis are more likely to have bilateral lymph node involvement, systemic symptoms, an abnormal chest radiograph, and an abnormal PPD result.
Noninfectious causes of lymphadenitis in children are less common but should always be considered in the differential diagnosis. Congenital cysts such as branchial cleft cysts, cystic hygromas, and thyroglossal duct cysts can mimic lymphadenitis, especially when infected. Malignancies such as lymphoma, leukemia, neuroblastoma, and rhabdomyosarcoma can present as cervical lymphadenopathy. Malignancy should be considered in cases of indolent lymphadenopathy, especially with a history of weight loss, fevers, night sweats, or lymphadenitis that is unresponsive to antibiotic treatment. Other causes of cervical neck masses should be included in the differential diagnosis of cervical lymphadenitis (Box 87-2).
Deep Neck Infections
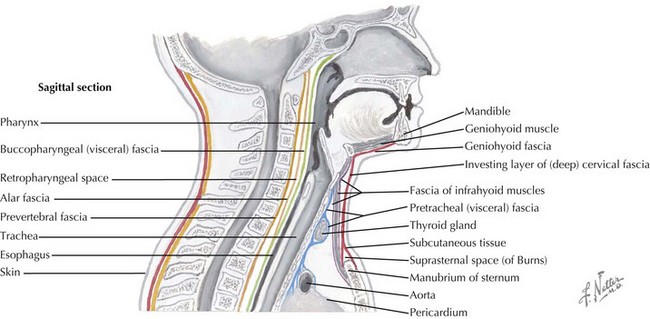
Multiple layers of cervical fascia encase the contents of the neck, creating three clinically important spaces: the peritonsillar area, retropharyngeal space, and parapharyngeal space (Figure 87-3). Peritonsillar, retropharyngeal, and parapharyngeal infections are among a group of potentially life-threatening deep neck infections in children that can present significant diagnostic challenges. All of these infections have the potential to progress from cellulitis to organized phlegmon (pre-abscess stage) and then to mature abscess. The initial step in the evaluation of a child with a potential deep neck space infection is rapid assessment for upper airway obstruction and need for emergent airway management. Therefore, prompt diagnosis of these conditions is essential for successful treatment and prevention of complications.
Clinical Presentation and Differential Diagnosis
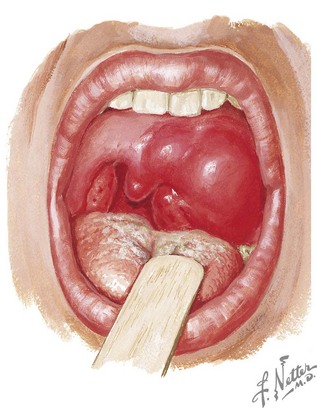
Patients with peritonsillar or retropharyngeal abscess present with fever, severe sore throat, neck pain, odynophagia, and decreased oral intake. Throat pain is more severe on the affected side and is often referred to the ipsilateral ear. Other associated findings include trismus, cervical lymphadenopathy, and pooling of saliva or drooling. With peritonsillar abscess, the physical examination usually reveals a patient with a partially opened mouth speaking in a muffled or “hot potato” voice. The oropharynx is erythematous with fullness or bulging of the superior pole of the tonsil. The uvula and tonsil may be deviated to the opposite side by the abscess (Figure 87-4). With retropharyngeal abscess, the physical examination may reveal neck swelling or torticollis. Bulging of the posterior pharyngeal wall lateral to the midline is suggestive of retropharyngeal abscess but is not consistently present. If left untreated, a peritonsillar or retropharyngeal abscess can spread through the deep tissues and produce complications such as airway compromise, mediastinitis, thrombophlebitis, and aspiration pneumonia if rupture occurs.
It can be difficult to differentiate between the early findings of peritonsillar, retropharyngeal, and parapharyngeal infections. Therefore, these diagnoses should all be considered in the differential diagnosis of deep neck infections. Patients with retropharyngeal or parapharyngeal abscesses may be misdiagnosed with meningitis because of neck pain and stiffness. Other diagnostic considerations include epiglottitis secondary to the signs of drooling and respiratory distress and laryngotracheobronchitis if the patient presents with stridor. The differential diagnosis of peritonsillar abscess includes severe tonsillopharyngitis and peritonsillar cellulitis. The lack of trismus and fluctuance in tonsillopharyngitis help to distinguish it from a peritonsillar abscess. It can be difficult to distinguish peritonsillar cellulitis from peritonsillar abscess clinically; however, with peritonsillar cellulitis, the uvula usually remains in the midline, and there is a lack of purulent material during a needle aspiration. A list of other differential diagnoses appears in Box 87-3.
Periorbital and Orbital Cellulitis
Etiology and Pathogenesis
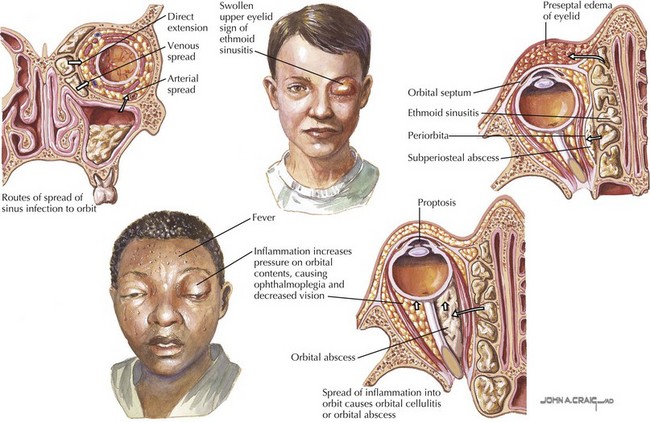
In contrast to the periorbital area, the orbit is surrounded by the paranasal sinuses. Many of the bony walls of the sinuses are thin and porous and allow spread of infection into the orbit. Orbital cellulitis most commonly results from extension of sinusitis with the ethmoid sinuses involved most frequently (Figure 87-5). Pathogens may also gain access to the orbit through direct trauma from a fracture or foreign body. In addition, spread of infection from adjacent structures can occur, such as an odontogenic infection extending into the maxillary sinus and subsequently into the orbit. Infection can also travel through the valveless venous system that drains the orbit and sinuses. These veins drain into the cavernous sinus, which can allow hematogenous spread of sinus infections into the orbit and result in complications such as thrombosis of the cavernous sinus, compression of the optic nerve, meningitis, or intracranial abscess.
Clinical Presentation and Differential Diagnosis
Periorbital and orbital cellulitis are both characterized by unilateral eyelid erythema, edema, induration, and tenderness. Only orbital cellulitis is associated with proptosis, impairment of extraocular movements, pain with extraocular movements, chemosis, or decreased visual acuity. The presence of fever or toxic appearance is variable, but both are more common with orbital cellulitis. Tearing may occur, but significant conjunctival injection and purulent drainage are not common unless there is associated conjunctivitis. Because the majority of patients with orbital cellulitis have concomitant sinusitis, a history of a recent upper respiratory tract infection or symptoms of sinusitis such as headache or sinus tenderness may be present (see Chapter 33). The clinician should inquire about recent trauma, insect bites, and skin or eye infections.
Evaluation and Management
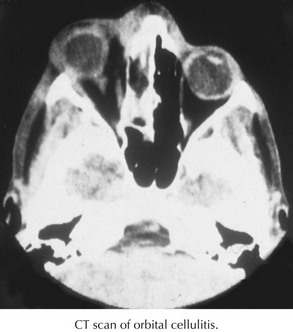
Most cases of periorbital cellulitis can be diagnosed clinically. A CT scan should be performed in any patient with suspected orbital cellulitis to evaluate the extent of infection in the orbit, detect coexisting sinus disease, and identify an orbital or subperiosteal abscess (Figure 87-6). Leukocytosis with neutrophil predominance is often present in patients with periorbital and orbital cellulitis, so obtaining a complete blood count does not necessarily help to differentiate between the two diagnoses. Inflammatory markers are usually elevated, but these tests are neither sensitive nor specific. Blood cultures are typically negative but worth considering in an ill-appearing patient. Drainage aspirates of sinuses or abscesses associated with orbital cellulitis should be sent for culture when surgery is indicated.
Friedman AM. Evaluation and management of lymphadenopathy in children. Pediatr Rev. 2008;29:53-59.
Leung AK, Davies HD. Cervical lymphadenitis: etiology, diagnosis, and management. Curr Infect Dis Rep. 2009;11:183-189.
Nield LS, Kamat D. Lymphadenopathy in children: when and how to evaluate. Clin Pediatr. 2004;43:25-33.
Peters TR, Edwards KM. Cervical lymphadenopathy and adenitis. Pediatr Rev. 2000;21:399-404.
Basel A, Salleen HB, Hagr A, et al. Retropharyngeal abscess in children: 10-year study. J Otolaryngol. 2004;33:352-355.
Brooks I. Microbiology and management of peritonsillar, retropharyngeal, and parapharyngeal abscesses. J Oral Maxillofac Surg. 2004;62:1545-1550.
Craig FW, Schunk JE. Retropharyngeal abscess in children: clinical presentation, utility of imaging, and current management. Pediatrics. 2003;111:1394-1398.
Johnson RF, Stewart MG, Wright CC. An evidence-based review of the treatment of peritonsillar abscess. Otolaryngol Head Neck Surg. 2003;128:332-343.
Rafei K, Lichenstein R. Airway infectious disease emergencies. Pediatr Clin North Am. 2006;53:215-242.
Periorbital and Orbital Cellulitis
Givner LB. Periorbital versus orbital cellulitis. Pediatr Infect Dis J. 2002;21:1157-1158.
McKinley SH, Yen MT, Miller AM, et al. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144:497-501.
Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
Wald ER. Periorbital and orbital infections. Infect Dis Clin North Am. 2007;21:393-408.