94 Infections of the Cardiovascular System
Infective Endocarditis
Pathogenesis
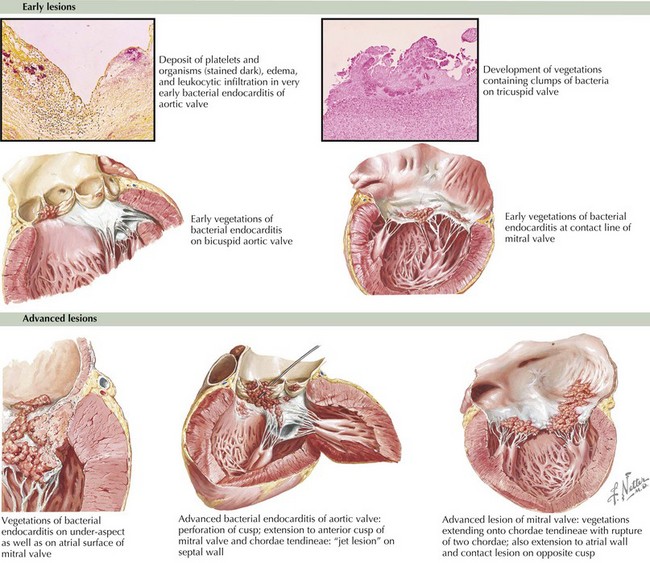
Underlying endothelial injury combined with bacteremia leads to the development of vegetations. Heart lesions with high-velocity jets predispose to endothelial injury. The resulting platelet–fibrin coagulum creates a rich environment for the deposition of bacteria and the growth of a vegetation. The bacteria often become fully enveloped by fibrin, allowing the pathogens to evade the host defenses (Figure 94-1). Congenital heart lesions most at risk include unrepaired cyanotic heart disease (including palliative shunts and conduits), any repaired congenital heart defect with prosthetic material or device in the first 6 months after the procedure, and repaired heart defects with residual defects at the site or adjacent to the site of the prosthesis.
Clinical Presentation
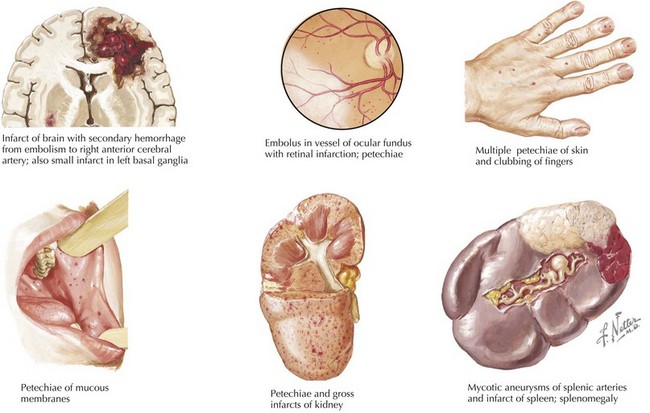
Symptoms of IE range from being asymptomatic, to congestive heart failure, to end-organ involvement with septic emboli. History taking should focus on fever, predisposing heart conditions, recent procedures at risk for bacteremia, the existence of indwelling catheters, and symptoms from end-organ involvement. The organs most commonly involved with septic emboli from left-sided IE include the central nervous system, eyes, kidneys, spleen, and skin. Septic pulmonary emboli are seen in cases of right-sided IE. Neurologic complications, which can occur in up to 33% of cases, include stroke, hemorrhage, seizure, abscess, and meningoencephalitis. The physical examination should include a thorough cardiac examination and a search for splenomegaly and for emboli of the fundus, conjunctiva, skin, and digits. Cardiac findings that are most likely to be present include new regurgitant murmurs and signs of congestive heart failure. Physical examination findings that support the diagnosis of IE but are not diagnostic include splinter hemorrhages, Janeway’s lesions, Osler’s nodes, and Roth’s spots (Figure 94-2). These peripheral stigmata of IE are less common in acute IE because patients often present at an earlier stage of disease than those with subacute IE. IE remains a disease with high morbidity and mortality. Cardiac complications occur in up to 30% to 50% of cases. Heart failure is the most common complication and the leading cause of death.
Evaluation and Management
The Duke classification remains the most reliable way to diagnose IE (Box 94-1). A definitive diagnosis of IE requires direct histologic evidence of IE, culture of specimens from surgery or autopsy, two major criteria, one major criteria and three minor criteria, or five minor criteria. Blood culture results may be negative in up to 15% of cases. The most common reason for a negative culture result is pretreatment with antibiotics, but one must also consider organisms that are difficult to isolate such as nutritionally variant streptococci, HACEK organisms, fungi, and intracellular bacteria (Coxiella burnetii, Bartonella spp., Chlamydia spp., and Tropheryma whippelii). If blood culture results are negative but clinical suspicion remains high, specific culture media or adsorbent resins may be used to isolate these organisms. Cultures should be maintained for up to 2 weeks to allow slower growing organisms to replicate. Serologic testing may be useful for detecting C. burnetii and Bartonella spp. and should be performed if cultures are negative. Polymerase chain reaction (PCR) testing from the blood or infected tissue may also prove useful in cases of culture negative IE. Transesophageal echocardiography (TEE) remains more sensitive than transthoracic echocardiography (TTE) and should be used in high-risk groups. For routine screening in low-risk patients, TTE is acceptable, but if clinical suspicion remains high despite a normal finding, TEE may be required.
Box 94-1
Modified Duke Criteria for the Diagnosis of Infective Endocarditis
Major Criteria
Minor Criteria
From Li JS: Proposed modifications of the Duke Criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633-638, 2000.
The recommendations for antibiotic prophylaxis for procedures have recently changed. Current recommendations for prophylaxis focus on conditions at the highest risk for an adverse outcome, not an overall increased lifetime risk of IE (Box 94-2). Prophylaxis is only recommended for certain dental procedures and is no longer recommended for respiratory, gastrointestinal, or genitourinary procedures unless contact with infected tissue is expected.
Box 94-2
Recommendations for Prophylaxis for Infective Endocarditis
Prophylaxis Recommended
From Nishimura RA, Carabello BA, Faxon DP, et al: ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. J Am Coll Cardiol 52:676-685, 2008.
Infectious Myocarditis
Pathogenesis
IM is mainly caused by viruses, although bacteria, fungi, protozoa, and parasites have been implicated as well (Box 94-3). The most common causes include enteroviruses, adenovirus, human herpes virus-6 (HHV-6), Epstein-Barr virus (EBV), cytomegalovirus (CMV), parvovirus B19, and HIV. Coxsackievirus B was initially the most commonly discovered virus associated with myocarditis, but this shifted to include other enteroviruses and adenovirus in the late 1990s and more recently to HHV-6 and parvovirus B19. Borrelia burgdorferi (Lyme disease) should be considered in patients with a history of tick bite, especially if conduction abnormalities are present. Myocarditis is commonly seen in HIV infection—by some reports, up to 50% at autopsy. It is unknown if this is solely because of the viral infection or the effects of long-term antiretroviral therapy.
Evaluation and Management
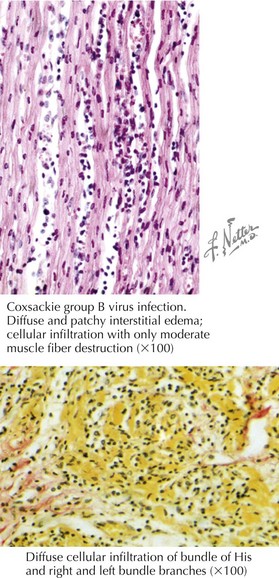
There is much debate on how to accurately diagnose myocarditis. Historically, diagnosis has relied on endomyocardial biopsy and the Dallas criteria, which state that an inflammatory cell infiltrate with associated myocyte necrosis be present (Figure 94-3). The sensitivity of the Dallas criteria is reported to be as low as 10% to 22%, and to increase sensitivity to 80%, an estimated 17 ventricular biopsy specimens are needed. The low sensitivity can be explained by a number of reasons. The inflammation in myocarditis is often patchy, leading to endomyocardial biopsy sampling error. There is also much variability in the interpretation of histopathologic samples, with differences in opinion among pathologists in interpreting the same specimen. Often, biopsy is not performed because it is thought to be too invasive, and the diagnosis of myocarditis is made on clinical grounds after other cardiac processes are ruled out.
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association-Executive Summary: endorsed by the Infectious Disease Society of America. Circulation. 2005;111:3167-3184.
Moreillon P, Que Y. Infective endocarditis. Lancet. 2004;363:139-149.
Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. J Am Coll Cardiol. 2008;52:676-685.
Paterick TE, Paterick TJ, Nishimura RA, Steckelberg JM. Complexity and subtlety of infective endocarditis. Mayo Clin Proc. 2007;82(5):615-621.
Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132(3):1025-1035.
Hartling RJ, Vandermeer B, Klassen TP: Intravenous immunoglobulin for presumed viral myocarditis in children and adults (review). The Cochrane Collaboration, 2009, Issue 2.
Cooper LTJr. Myocarditis. N Engl J Med. 2009;360:1526-1538.
Cox GF, Sleeper LA, Lowe AM, et al. Factors associated with establishing a causal diagnosis for children with cardiomyopathy. Pediatrics. 2006;118(4):1519-1531.
Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64(10):1235-1245.
Magnani J, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876-890.
Mahrholdt H, Wagner A, Deluigi CC, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114(15):1581-1590.