Chapter 70 Infections in Anterior Cruciate Ligament Surgery
Introduction
The low prevalence of this complication limits the experience of any individual surgeon, and the relevant literature consists of few series with small numbers of patients treated with management protocols ranging in aggressiveness from arthroscopic irrigation to radical débridement with graft and hardware removal.1–9
Prevalence of Infection
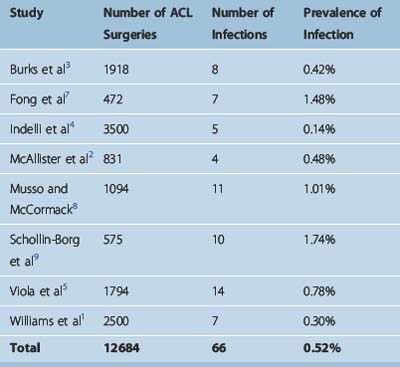
The prevalence of infection following ACL reconstruction is very low. In studies reporting on septic complications, the infection rate ranged from 0.14% to 1.74%1–5,7–9 (Table 70-1). Overall these eight studies reported 66 infections following 12,684 procedures, resulting in a mean infection prevalence of 0.52%.1–5,7–9
Matava et al surveyed directors of sports medicine fellowship programs about their experience with infections after ACL surgery.10 The 61 surgeons who responded performed on average 98 ACL reconstructions per year; 18 surgeons (30%) had treated an ACL infection within the past 2 years, and 26 (43%) had treated an infection within the past 5 years. Therefore even experienced surgeons have managed a limited number of cases in their career.
Pathogenesis: Predisposing Factors
Systemic Factors
The importance of host physiology in musculoskeletal infections has been emphasized in the literature.11 Systemic host factors include comorbidities, such as diabetes mellitus, malignancy, malnutrition, immunocompromised status, or other disease that may compromise the host defense against microbial pathogens. However, systemic host factors are not as prevalent in ACL surgery compared with other procedures because most patients undergoing ACL reconstruction are relatively young, active, and healthy. In the series with postoperative infections after ACL surgery presented in Table 70-1, the mean age of the patients ranged from 21 to 34 years, and no comorbidities were reported. A study on persistent infections reported comorbidities in three of five patients.6
Local Factors
Local risk factors for infection after ACL reconstruction include previous or concomitant secondary knee procedures.1–3 Williams et al1 reported that six of seven patients with infections had concomitant procedures performed, such as “outside-in” meniscal repair with polydiaxone (PDS) suture, medial collateral ligament reconstruction, and posterolateral corner reconstruction. In the series of McAllister et al,2 three of four patients had previous knee surgery and two of four patients had an “inside-out” meniscal repair. Burks et al3 reported that five of eight patients in their series had concomitant procedures performed at the time of ACL reconstruction. In the series of Musso and McCormack,8 five of nine patients also underwent meniscal procedures.
Potential explanations include the increased operative time, additional or larger incisions with more extensive dissection in cases where complex reconstructive surgery takes place, and implantation of foreign material such as suture. However, other authors did not report concomitant procedures in their infected ACL reconstructions.4 The role of secondary procedures in development of infection is not clear, as the existing studies have not performed a comparison between infected and control patients.
Contamination
Contamination of the operative site may occur from use of inadequately sterilized instruments or implantation of contaminated grafts. Contaminated in-flow cannulas have been identified as the source of infection. Viola et al reported a sudden increase in their infection rate from 0.1% in the period from 1991 to 1996 (2 in 1724 ACL reconstructions) to 14.2% in the period from December 1996 to February 1996 (10 of 70).5 “Sterile” sets of in-flow cannulas used for ACL reconstructions were found to be contaminated with coagulase-negative Staphylococcus. Following the discovery of the contaminated instruments, the infection rate dropped to 0.25% (1 in 400 cases). In another study, contamination with coagulase-negative Staphylococcus was present on supposedly sterile suture clamps on graft preparation boards.9 Inadequate disinfection of arthroscopic equipment12 and flash sterilization of meniscus repair cannulas with residual debris in the lumen13 have been reported as potential causes of septic arthritis following arthroscopy.
Undetected intraoperative contamination of the graft may take place as well. Hantes et al14 obtained culture specimens before implantation of autografts and reported that cultures were positive in 12% of cases (7 of 60). Diaz-de-Rada et al15 reported that allograft cultures were positive in 13% of cases (24 of 181). The source and significance of this contamination remain unclear. However, in both studies no clinical infections developed after a minimum 1-year follow-up. Contamination of allografts used in ACL reconstruction as a source of infection is discussed in detail later.
Biofilm Formation
Biofilm formation is a key mechanism for persistence or recurrence of infection. The biofilm is an aggregation of microbial colonies enclosed within an extracellular polysaccharide matrix (glycocalyx) that adheres on the surface of implants or devitalized tissue.16,17 Gristina and Costerton18 reported that 59% (10 of 17) of orthopaedic biomaterial–related infections had positive findings of glycocalyx-enclosed organisms on electron microscopy. Presence of an avascular graft and metal fixation devices in ACL reconstruction create conditions conducive to biofilm development if a postoperative infection is not treated early and adequately.
The biofilm protects the organism from antibiotics and host defense mechanisms, such as antibody formation and phagocytosis; therefore infection may exist in a subclinical state and eventually recur. In chronic musculoskeletal infections, removal of the biofilm by removal of implants and débridement of devitalized tissue are necessary for successful treatment of infection.19
Diagnosis
Clinical Findings
An alterative presentation is with emergence of symptoms at a later time following a symptom-free interval. In some cases, the clinical picture may consist of mild pain, effusion, and difficulty performing physical therapy without the systemic signs of infection. As Burks et al3 warned, the surgeon should not interpret this relatively benign presentation as the absence of infection. A high index of suspicion is necessary, and patients who do not demonstrate steady postoperative improvement; present with increased pain, effusion, or stiffness following a symptom-free interval; or develop systemic symptoms (fever, chills, malaise) should be considered to have a septic knee until proven otherwise. Patients should be instructed to contact their physician immediately if knee symptoms develop postoperatively, which should be evaluated without delay.
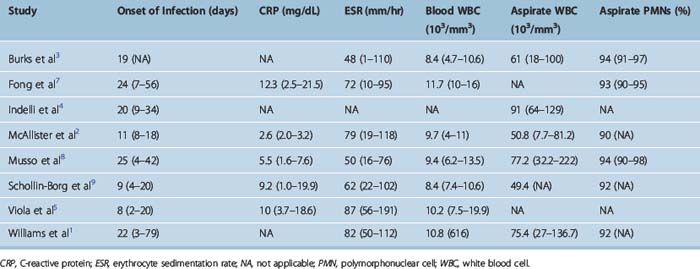
Infections have been classified as acute (presenting less than 2 weeks postoperatively), subacute (2 weeks to 2 months), and late (more than 2 months).1 The mean time for development of infection following ACL surgery ranged from 8 days5 to 25 days8 (Table 70-2).
Laboratory Findings
Peripheral white blood cell (WBC) count may be within normal limits. In contrast, markers of inflammation, such as the C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), are elevated and are helpful in the diagnosis. Elevated CRP has been invariably reported in patients with ACL postoperative infections; the mean CRP levels ranged from 2.6 mg/dL2 to 12.3 mg/dL7 in the existing studies (see Table 70-2). Elevated levels of ESR have been similarly reported in the literature, with mean values ranging from 48 mm/hr3 to 87 mm/hr.5
The anticipated increase of ESR and CRP in the immediate postoperative period may confound the diagnostic picture in the first week. Viola et al5 evaluated 15 patients with a normal postoperative course and reported that 5 days after ACL surgery they had elevated CRP levels with a mean of 2.7 mg/dL (range 0.6–12.3 mg/dL). Margheritini et al20 reported a postoperative increase of both CRP and ESR peaking on the third and seventh days, respectively. The CRP returned to nearly normal levels by postoperative day 15, which was faster than the ESR; the authors concluded that CRP is a more sensitive indicator of postoperative septic complications.20 Elevated levels of CRP beyond the postoperative day 15 strongly point toward a septic etiology for the patient’s symptoms.
Aspiration of the involved knee joint is necessary and yields turbid fluid that should be sent for Gram stain, WBC count and differential, and culture (both aerobic and anaerobic). The mean WBC count has ranged from 49,400 per mm3 in the study by McAllister et al2 to 91,000 per mm3 in the study by Indelli et al.4 Despite this variability in the absolute number of WBC in the joint aspirate, the differential count reveals mean values of 90% to 94% of polymorphonuclear (PMN) cells (see Table 70-2).
Imaging Studies
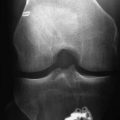
Magnetic resonance imaging (MRI) can help determine the extent of infection and the presence of any extraarticular fluid collections that otherwise could have been missed.21
Microbiology
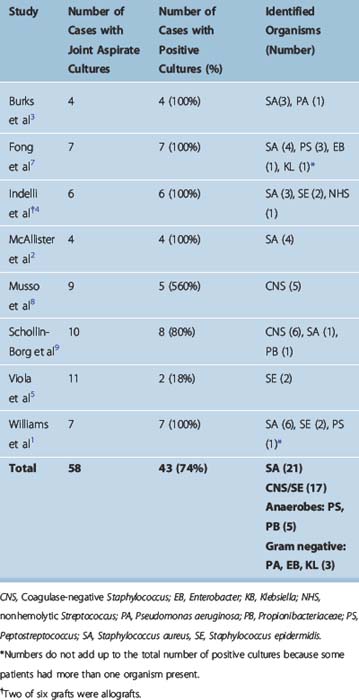
Forty-three (74%) of 58 joint fluid cultures were positive in studies reporting on postoperative autograft ACL infections (Table 70-3). Staphylococcus aureus was the most common pathogen, present in 21 of 43 cases with positive cultures (48.5%). Coagulase-negative Staphylococcus (including S. epidermidis) was cultured in 17 of 43 cases (39.5%). Overall, septic arthritis following ACL surgery was caused by staphylococcal species in the vast majority of cases (88%). Anaerobic infections (Peptostreptococcus, Propionibacteriaceae) were present in 11.5% of cases (5 of 43). Gram-negative bacteria (Pseudomonas aeruginosa, Enterobacter, Klebsiella) were relatively uncommon and were identified in 7% of cases (3 of 43).
Case reports of unusual infections following autograft ACL reconstruction have been reported, including infection with S. caprae,22Erysipelothrix rhusiopathiae,23 mucormycosis,24 and necrotizing fasciitis.25 Reports of infections following allograft ACL surgery are discussed later.
Management Protocol
Surgical Management
Surgical management of septic arthritis with irrigation and débridement of the knee joint is a critical component of the management protocol. Some investigators have suggested that initiation of antibiotics may suffice, and they have proposed an expectant policy, reserving surgical management for cases not responding to antibiotics.5,8 However, this approach has several disadvantages: evacuation of the purulent effusion is incomplete, débridement of the joint is not performed, and thereby an increased bacterial count remains, which may compromise eradication of the infection.
Therefore immediate surgical management has been proposed by several authors.1–3,7,9 In a survey of directors of sports medicine fellowship programs,10 98% of respondents (60 of 61) selected surgical irrigation as part of their management protocol in conjunction with intravenous antibiotics.
Initial Management
Irrigation and Débridement
Arthroscopic irrigation and débridement appear to be the most commonly used methods of initial management for the patient presenting with a septic knee following ACL surgery.1–5,7,9
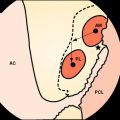
In addition to irrigation of the joint with copious amounts of saline, débridement of necrotic or inflamed tissue should be performed. Synovectomy has been proposed by some authors1,2 in order to decrease the bacterial count and aid in the resolution of infection. Particular attention should be paid to the graft; its stability and macroscopic appearance should be carefully evaluated, and débridement should include a fibrinous exudate that may be found covering the graft.
Any incisions from concomitant procedures should be opened and irrigated to avoid missing an extraarticular collection of fluid that could reseed the knee joint and lead to persistence of infection. Kohn26 described a case where the infection spread from the knee joint to the subcutaneous tissues of the operative wound and warned that in the presence of large surgical incisions, arthroscopic irrigation may spread purulent intraarticular material to adjacent extraarticular locations. Drains should be placed into the knee joint and in any incisions present; drains can be removed 48 hours later.
Graft Retention Versus Removal
Most authors have attempted to retain the graft in the initial management of septic arthritis after ACL surgery, but removal of the graft at a later time was necessary in some persistent cases.1–5,7,9
Williams et al1 removed acutely one of seven grafts because the graft appeared to be loose and nonfunctional. In three of the six knees with retained grafts, the infection persisted and a repeat procedure was performed; the graft was removed in another three cases, and, overall the graft was successfully salvaged in three of seven cases. Indelli et al4 attempted to retain all grafts; repeat procedures were needed in five of six patients and two grafts were subsequently removed, such that finally four of six grafts were retained. Other investigators were able to retain all implanted grafts.2,7
In contrast, Burks et al3 proposed an aggressive protocol that included graft removal at the initial irrigation and débridement procedure; the four patients in this series had no recurrence of infection, and all underwent repeat ACL reconstruction.
In our opinion, preservation of the graft may be justified in acute postoperative infections that are diagnosed and treated without a delay. Graft removal during the initial procedure should be performed if the graft is loose and nonfunctional.1 Graft removal should be considered if there is a delay in presentation and an ongoing infection has been untreated for more than a few days, if the articular cartilage seems to be already affected, or if a virulent organism is present.4 Although unlikely, the presence of patient comorbidities is a factor in favor of graft removal because the defense mechanisms of the host may be compromised due to chronic disease. The type of graft is another consideration; allograft contamination has been reported, and surgeons are more prone to acutely remove an allograft compared with an autograft.10
Management of Persistent Cases
Initial management with arthroscopic irrigation and débridement, graft preservation, and antibiotic therapy may not control the infection in all cases. The infection recurrence rate was 83% (5 of 6 cases) in the series by Indelli et al,4 50% (3 of 6 cases) in the series of Williams et al,1 and 29% (2 of 7 cases) in the series by Fong et al.7 McAllister et al2 reported that, despite the acute presentation of infections (8 to 18 days) and the immediate (within 24 hours) intervention, two to four repeat surgical procedures were necessary in each patient to control the infection and restore range of motion of the knee.
Indelli et al4 have clearly established the goals of management in their manuscript; the first goal is to protect the articular cartilage, and the second goal is to protect the graft. The articular cartilage may undergo irreversible damage from an ongoing or inadequately treated infectious process, and currently options for restoring articular cartilage are very limited.
Persistent septic arthritis following failure of the arthroscopic irrigation and débridement procedure with graft retention to control the infection is of particular concern; the infectious process has not been controlled by the initial procedure, the articular cartilage has been exposed to the detrimental effects of a persistent infectious process for a prolonged period of time, and the avascular graft and hardware provide substrate for biofilm formation,17 which may prevent eradication of infection. McAllister et al2 were able to retain the graft by managing persistent infections with two to four subsequent débridement procedures. However, degenerative changes developed in all four of their patients at a mean follow-up of 36 months, possibly because of the adverse effect of ongoing infection on articular cartilage. On the contrary, Burks et al3 reported that the four patients managed by graft removal and repeat ACL reconstruction in their series had no joint space narrowing at a mean follow-up of 21 months.
Therefore persistent septic arthritis calls for a more aggressive approach to avoid articular cartilage damage and arthrofibrosis. Persistence of infection has been proposed as a reason for graft removal in the literature.1,3,4 However, a survey of directors of sports medicine fellowship programs showed no agreement regarding the fate of the graft and implanted hardware.10 In the event of a persistent infection unresponsive to the initial treatment, 36% of surgeons (22 of 61) would proceed with graft removal, whereas 64% would elect to retain the graft.
The treating surgeon may be reluctant to remove the graft in persistent infections because the knee joint will be destabilized and a subsequent procedure will be necessary for repeat reconstruction of the ACL. However, there are unique advantages to this approach. First, the articular cartilage is protected from permanent damage, which would adversely affect the final outcome. Second, removal of the graft does not preclude ACL reconstruction at a later stage; the treating surgeon can employ alternative autograft or allograft techniques to address the unstable knee following removal of an infected graft, resulting in a satisfactory outcome.3 Third, although the additional procedure appears to be a drawback, it may actually decrease hospitalization time and overall cost because graft retention has been associated with repeat surgeries in order to control the infection.1,2,4,7 In the series by McAllister et al,2 two to four subsequent procedures were needed per patient and the mean hospital stay was 12.5 days. In contrast, Burks et al reported a total hospitalization time of 4 days for management of infection and repeat ACL reconstruction in patients managed with an aggressive protocol.3
Authors’ Protocol for Persistent Septic Arthritis of the Knee
Our protocol for persistent infections is based on radical débridement consisting of the following elements: open arthrotomy, complete synovectomy, graft removal, removal of any interference screws or other implants, and curettage and débridement of both the femoral and tibial tunnel.6 Aerobic, anaerobic, mycobacterial, and fungal cultures are obtained from multiple sources: joint fluid, synovium, graft, and bone (from the vicinity of both the femoral and the tibial tunnels). Organism-specific antibiotic therapy is given for 6 weeks.
This protocol was used in five consecutive patients with persistent septic arthritis of the knee following arthroscopic ACL reconstruction.6 Patients had previously undergone one to three unsuccessful débridement procedures with recurrence of the infection and were referred to the senior author (M.J. Patzakis). The time elapsed from the initial diagnosis of infection to definitive management with radical débridement ranged from 11 days to 22 months. At a median follow-up time of 20 months (6 to 27 months), all patients were free from infection, but degenerative changes of the involved knee joint developed and one patient underwent total knee arthroplasty.
Three of five infections were polymicrobial. Interestingly, in all three polymicrobial cases different organisms grew from the multiple tissue samples that included joint fluid, synovium, graft, and bone. It has been proposed that different organisms may be preferentially growing in isolated microenvironments,27 and a study on chronic osteomyelitis evaluating cultures from multiple sites showed that the same organisms grew on culture of the specimens from every site in only 47% (14 of 30) of patients.28 Therefore multiple cultures from different sources may help identify additional pathogens that otherwise may have been undetected.
In persistent cases, the presence of an unusual organism may explain the poor response to therapy; aerobic, anaerobic, mycobacterial, and fungal cultures should be obtained, and tissue samples should be sent for pathology. Burke and Zych24 reported a case of persistent infection following ACL surgery that was diagnosed as mucormycosis approximately 7 months after the initial presentation, leading to osteomyelitis and destruction of the proximal tibia.
Allografts and Infections in Anterior Cruciate Ligament Surgery
Reconstruction of the ACL with autograft tissue, BPTB, or hamstrings, has been well described in the literature.29–31 Alternatively, allograft tissue can be used to provide a source of graft material in revision cases, preserve the extensor or flexor mechanisms, and decrease the operative time; however, allograft structural properties may be compromised by sterilization and storage procedures, incorporation may be slow and incomplete, an immunological response may take place, the cost is increased, and an infection risk is present.29,32,33 Contaminated allografts may result in transmission of viral disease or bacterial infections from the donor to the recipient.
Viral Disease
Viral disease, including human immunodeficiency virus (HIV) infection, hepatitis B, and hepatitis C, has been transmitted by transplantation of musculoskeletal allografts harvested from infected donors prior to implementation of a screening process.34
Therefore adherence to screening methods is critical to exclude grafts from infected individuals from being used. The Food and Drug Administration (FDA) initiated oversight of tissue banking in 1993 and requires that potential donors undergo a screening process that includes serologic tests for HIV-1, HIV-2, hepatitis B, and hepatitis C viruses.35 However, a time window exists from infection with one of these viruses to development of a detectable antibody response, and transmission of hepatitis B and C has been reported after allograft implantation for ACL reconstruction.36,37
Bacterial Infections
Implantation of contaminated allograft tissue has been reported as a source of unusual infections following ACL reconstruction and other knee procedures.32,38–42
The Centers for Disease Control and Prevention (CDC) in 2001 reported four cases of septic arthritis following ACL reconstruction associated with contaminated BPTB allografts; the report warned that when septic arthritis develops after allograft use, contamination of the allograft should be suspected.41 This is particularly important in polymicrobial, gram-negative, or anaerobic organism infections. Other reports included a patient who developed Clostridium sordellii septicemia and died within 1 week of receiving an osteochondral allograft,40,42 as well as a patient who developed an invasive Streptococcus pyogenes infection after ACL reconstruction with an allograft.38
As of March 2002, the CDC had identified 26 cases of bacterial infections associated with musculoskeletal allografts.39 Thirteen infections were caused by Clostridium species and 11 by gram-negative bacilli (5 were polymicrobial), and in two cases cultures were negative. Eighteen of these 26 infections (69%) occurred following allograft implantation for ACL reconstruction. Only 3 of 26 allografts (12%) were reported to have undergone gamma irradiation for sterilization.
Crawford et al43 investigated an outbreak of infections following ACL reconstruction in one outpatient surgical center. The infection rate was 3.3% (11 of 331), and all infections occurred in the subgroup of patients in whom aseptically processed—but not sterilized—allografts were used. The infection rate in this subgroup was 4.4% (11 of 250) compared with 0% (0 of 41) in the autograft group and 0% in the sterilized allograft group. Gram-negative organisms were identified in 6 of 11 cases and Candida glabrata in 2 of 11 cases.
These outbreaks of infections highlight the need for allograft sterilization. Aseptic processing and preservation of the graft without sterilization do not ensure patient safety because endogenous contamination of the allograft may exist at the time of harvesting.44,45
Deijkers et al44 evaluated the bacterial contamination of 1999 bone allografts retrieved from 200 cadaver donors under sterile operating conditions and reported that organisms of low pathogenicity (such as coagulase-negative staphylococci) were cultured from 50% of the allografts, whereas organisms of high pathogenicity (such as S. aureus, streptococcal species, Clostridium species, and Gram-negative organisms) were cultured from 3%. The authors described two mechanisms of contamination. Exogenous contamination, which was influenced by the procurement team, was considered mainly responsible for organisms of low pathogenicity; endogenous contamination, which was influenced by the status of the donor, was considered the probable source of virulent organisms. The risk of contamination with organisms of high pathogenicity was 3.4 times higher in donors with a traumatic cause of death.
Martinez et al45 reported that positive blood cultures were present in 8.6% of “beating heart cadaver” donors compared with 38% of postmortem donors. This increase may be attributed to the postmortem dissemination of endogenous bacteria (such as normal intestinal flora) secondary to loss of the intestinal barrier. Microorganisms were isolated from the bones of 59% (118 of 201) of donors who had negative blood cultures; thus blood cultures alone are not useful indicators of sterility of the tissues recovered for transplantation.
Therefore, in addition to aseptic harvesting and processing, allograft tissue should undergo a sterilization process, such as ethylene oxide or gamma irradiation, to avoid transmission of infectious agents.46 However, concerns exist regarding the current processes; gamma irradiation may cause structural damage to the allograft,47,48 whereas ethylene oxide may penetrate tissue inadequately and cause inflammatory intraarticular reactions.49 New sterilization techniques are being developed with the aim of killing microorganisms and spores while at the same time preserving the biomechanical integrity of the processed tissues.46
Intraoperative Graft Contamination
Intraoperative contamination of the graft may occur by accidentally dropping the graft on the floor or by contacting the graft with a nonsterile object. A recent survey of 196 sports medicine fellowship directors showed that 49 of the responding surgeons (25%) had experienced contamination of 57 grafts; it occurred once in 43 surgeons (22%) and two to four times in six surgeons (3%).50 Another study reported that the graft was dropped on the operating room floor in four of 1038 ACL reconstruction cases, resulting in a 0.4% rate of accidental graft contamination.51
In this unfortunate event, the surgeon is faced with the dilemma of implanting a contaminated graft following a cleansing procedure or discarding the contaminated graft and employing an alternative one. Cleansing the graft carries the potential risk for septic arthritis. However, the alternatives are not without potential problems; use of an alternative autograft creates further morbidity for the patient, whereas an allograft increases the cost and may not be readily available. The survey by Izquierdo et al50 reported that cleansing of the graft is the most common practice; graft cleansing was used for 75% of contaminated grafts (43 of 57), whereas an alternative autograft was used in 18% of cases (10 of 57) and an allograft in 7% (4 of 57). Solutions of chlorhexidine gluconate, antibiotics, povidone-iodine, or combinations thereof were used for cleansing of the graft, and none of the 43 decontaminated grafts was associated with a postoperative infection.50 Casalonga et al51 sequentially soaked four contaminated grafts in rifamycin and gentamicin solutions, and no infections occurred at a mean follow-up of 2 years.
An in vitro study warned that soaking the graft for 15 minutes in an antibiotic solution (bacitracin and polymyxin B) will not sterilize the graft in 30% of cases (3 of 10).52 Another study found that soaking for 30 minutes in a 10% povidone-iodine or a triple-antibiotic solution (gentamicin, clindamycin, polymyxin) was not able to sterilize grafts contaminated with two different species of coagulase-negative staphylococci, whereas 4% chlorhexidine gluconate effectively decontaminated the grafts.53 The same study reported that when grafts were contaminated with five virulent organisms (S. aureus, Escherichia coli, P. aeruginosa, K. pneumoniae, and Enterococcus faecalis), 4% chlorhexidine gluconate was able to eliminate all organisms except K. pneumoniae. Using a triple-antibiotic solution after chlorhexidine gluconate eliminated this organism as well.53
Molina et al54 evaluated three antibacterial solutions for decontamination of ACL specimens harvested during total knee arthroplasty and dropped on the floor. Soaking in chlorhexidine gluconate solution for 90 seconds appeared to be the most effective with positive cultures in broth only in 1 of 50 specimens (2%). Grafts soaked in antibiotic solution of neomycin and polymyxin B had 3 of 50 specimens positive (15%), whereas grafts soaked in 10% povidone-iodine solution had 12 of 50 specimens positive (24%).
Burd et al55 reported that power irrigation with a 2% chlorhexidine gluconate was effective in decontaminating grafts within 10 to 12 minutes, thus expediting the decontamination process. It should be noted that chlorhexidine may cause articular cartilage damage.56 Subsequent soaking in an antibiotic solution and rinsing of the graft prior to implantation may be beneficial if a chlorhexidine solution has been used.
1 Williams RJIII, Laurencin CT, Warren RF, et al. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Diagnosis and management. Am J Sports Med. 1997;25:261-267.
2 McAllister DR, Parker RD, Cooper AE, et al. Outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:562-570.
3 Burks RT, Friederichs MG, Fink B, et al. Treatment of postoperative anterior cruciate ligament infections with graft removal and early reimplantation. Am J Sports Med. 2003;31:414-418.
4 Indelli PF, Dillingham M, Fanton G, et al. Septic arthritis in postoperative anterior cruciate ligament reconstruction. Clin Orthop. 2002;398:182-188.
5 Viola R, Marzano N, Vianello R. An unusual epidemic of Staphylococcus-negative infections involving anterior cruciate ligament reconstruction with salvage of the graft and function. Arthroscopy. 2000;16:173-177.
6 Zalavras CG, Patzakis MJ, Tibone J, et al. Treatment of persistent infection after anterior cruciate ligament surgery. Clin Orthop Relat Res. 2005;439:52-55.
7 Fong SY, Tan JL. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Ann Acad Med Singapore. 2004;33:228-234.
8 Musso AD, McCormack RG. Infection after ACL reconstruction: what happens when cultures are negative? Clin J Sport Med. 2005;15:381-384.
9 Schollin-Borg M, Michaelsson K, Rahme H. Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: a case control study. Arthroscopy. 2003;19:941-947.
10 Matava MJ, Evans TA, Wright RW, et al. Septic arthritis of the knee following anterior cruciate ligament reconstruction: results of a survey of sports medicine fellowship directors. Arthroscopy. 1998;14:717-725.
11 Cierny G, Mader JT, Pennick H. A clinical staging system for adult osteomyelitis. Contemp Orthop. 1984;10:17-37.
12 Armstrong RW, Bolding F. Septic arthritis after arthroscopy: the contributing roles of intraarticular steroids and environmental factors. Am J Infect Control. 1994;22:16-18.
13 Blevins FT, Salgado J, Wascher DC, et al. Septic arthritis following arthroscopic meniscus repair: a cluster of three cases. Arthroscopy. 1999;15:35-40.
14 Hantes M, Basdekis G, Giotikas A, et al. Is there a potential for graft contamination during preparation for anterior cruciate ligament reconstruction?. Presented at the European Bone and Joint Society Meeting, June, 2005, Lisbon, Portugal. 2005.
15 Diaz-de-Rada P, Barriga A, Barroso JL, et al. Positive culture in allograft ACL-reconstruction: what to do? Knee Surg Sports Traumatol Arthrosc. 2003;11:219-222.
16 Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95-108.
17 Gristina AG, Costerton JW. Bacterial adherence and the glycocalyx and their role in musculoskeletal infection. Orthop Clin North Am. 1984;15:517-535.
18 Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg. 1985;67A:264-273.
19 Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13:417-427.
20 Margheritini F, Camillieri G, Mancini L, et al. C-reactive protein and erythrocyte sedimentation rate changes following arthroscopically assisted anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2001;9:343-345.
21 Papakonstantinou O, Chung CB, Chanchairujira K, et al. Complications of anterior cruciate ligament reconstruction: MR imaging. Eur Radiol. 2003;13:1106-1117.
22 Elsner HA, Dahmen GP, Laufs R, et al. Intra-articular empyema due to Staphylococcus caprae following arthroscopic cruciate ligament repair. J Infect. 1998;37:66-67.
23 Vallianatos PG, Tilentzoglou AC, Koutsoukou AD. Septic arthritis caused by Erysipelothrix rhusiopathiae infection after arthroscopically assisted anterior cruciate ligament reconstruction. Arthroscopy. 2003;19:E26.
24 Burke WV, Zych GA. Fungal infection following replacement of the anterior cruciate ligament: a case report. J Bone Joint Surg. 2002;84A:449-453.
25 Campion J, Allum R. Necrotising fasciitis following anterior cruciate ligament reconstruction. A case report. Knee. 2006;13:51-53.
26 Kohn D. Unsuccessful arthroscopic treatment of pyarthrosis following anterior cruciate ligament reconstruction. Arthroscopy. 1988;4:287-289.
27 Marrie TJ, Costerton JW. Mode of growth of bacterial pathogens in chronic polymicrobial human osteomyelitis. J Clin Microbiol. 1985;22:924-933.
28 Patzakis MJ, Wilkins J, Kumar J, et al. Comparison of the results of bacterial cultures from multiple sites in chronic osteomyelitis of long bones. A prospective study. J Bone Joint Surg. 1994;76A:664-666.
29 Beynnon BD, Johnson RJ, Abate JA, et al. Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med. 2005;33:1579-1602.
30 Prodromos CC, Han YS, Keller BL, et al. Stability results of hamstring anterior cruciate ligament reconstruction at 2- to 8-year follow-up. Arthroscopy. 2005;21:138-146.
31 Laxdal G, Kartus J, Hansson L, et al. A prospective randomized comparison of bone-patellar tendon-bone and hamstring grafts for anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:34-42.
32 Barbour SA, King W. The safe and effective use of allograft tissue—an update. Am J Sports Med. 2003;31:791-797.
33 Shelton WR, Treacy SH, Dukes AD, et al. Use of allografts in knee reconstruction: I. Basic science aspects and current status. J Am Acad Orthop Surg. 1998;6:165-168.
34 Tomford WW. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg. 1995;77A:1742-1754.
35 Human tissue intended for transplantation—FDA. Interim rule; opportunity for public comment. Fed Regist. 1993;58–238:65514-65521.
36 Hepatitis C virus transmission from an antibody-negative organ and tissue donor–United States, 2000–2002. MMWR Morb Mortal Wkly Rep. 2003;52-13:273-274. 276
37 Grafe M, Kurzweil P. Anterior cruciate ligament reconstruction with achilles tendon allografts in revisions and patients over 30. Presented at the Arthroscopy Association of North America Meeting, May, 2005, Vancouver, BC, Canada. 2005.
38 Invasive Streptococcus pyogenes after allograft implantation—Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52-48:1174-1176.
39 Update: allograft-associated bacterial infections—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51-10:207-210.
40 Update: Unexplained deaths following knee surgery—Minnesota, 2001. MMWR Morb Mortal Wkly Rep. 2001;50-48:1080.
41 Septic arthritis following anterior cruciate ligament reconstruction using tendon allografts—Florida and Louisiana, 2000. MMWR Morb Mortal Wkly Rep. 2001;50-48:1081-1083.
42 Unexplained deaths following knee surgery—Minnesota, November 2001. MMWR Morb Mortal Wkly Rep. 2001;50:1035-1036.
43 Crawford C, Kainer M, Jernigan D, et al. Investigation of postoperative allograft-associated infections in patients who underwent musculoskeletal allograft implantation. Clin Infect Dis. 2005;41:195-200.
44 Deijkers RL, Bloem RM, Petit PL, et al. Contamination of bone allografts: analysis of incidence and predisposing factors. J Bone Joint Surg. 1997;79B:161-166.
45 Martinez OV, Malinin TI, Valla PH, et al. Postmortem bacteriology of cadaver tissue donors: an evaluation of blood cultures as an index of tissue sterility. Diagn Microbiol Infect Dis. 1985;3:193-200.
46 Vangsness CTJr, Garcia IA, Mills CR, et al. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474-481.
47 Gibbons MJ, Butler DL, Grood ES, et al. Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res. 1991;9:209-218.
48 Fideler BM, Vangsness CTJr, Lu B, et al. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23:643-646.
49 Jackson DW, Windler GE, Simon TM. Intraarticular reaction associated with the use of freeze-dried, ethylene oxide-sterilized bone-patella tendon-bone allografts in the reconstruction of the anterior cruciate ligament. Am J Sports Med. 1990;18:1-10.
50 Izquierdo RJr, Cadet ER, Bauer R, et al. A survey of sports medicine specialists investigating the preferred management of contaminated anterior cruciate ligament grafts. Arthroscopy. 2005;21:1348-1353.
51 Casalonga D, Ait Si Selmi T, Robinson A, et al. [Peroperative accidental contamination of bone-tendon-bone graft for the reconstruction of the anterior cruciate ligament. Report of 4 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1999;85:740-743.
52 Cooper DE, Arnoczky SP, Warren RF. Contaminated patellar tendon grafts: incidence of positive cultures and efficacy of an antibiotic solution soak—an in vitro study. Arthroscopy. 1991;7:272-274.
53 Goebel ME, Drez DJr, Heck SB, et al. Contaminated rabbit patellar tendon grafts. In vivo analysis of disinfecting methods. Am J Sports Med. 1994;22:387-391.
54 Molina ME, Nonweiller DE, Evans JA, et al. Contaminated anterior cruciate ligament grafts: the efficacy of 3 sterilization agents. Arthroscopy. 2000;16:373-378.
55 Burd T, Conroy BP, Meyer SC, et al. The effects of chlorhexidine irrigation solution on contaminated bone-tendon allografts. Am J Sports Med. 2000;28:241-244.
56 van Huyssteen AL, Bracey DJ. Chlorhexidine and chondrolysis in the knee. J Bone Joint Surg. 1999;81:995-996.