2 Infection Control
Note 1: This book is written to cover every item listed as testable on the Entry Level Examination (ELE), Written Registry Examination (WRE), and Clinical Simulation Examination (CSE).
The listed code for each item is taken from the National Board for Respiratory Care’s (NBRC) Summary Content Outline for CRT (Certified Respiratory Therapist) and Written RRT (Registered Respiratory Therapist) Examinations (http://evolve.elsevier.com/Sills/resptherapist/). For example, if an item is testable on both the ELE and the WRE, it will be shown simply as (Code: …). If an item is testable only on the ELE, it will be shown as (ELE code: …). If an item is testable only on the WRE, it will be shown as (WRE code: …).
MODULE A
1. Hand washing or cleansing
2. Standard precautions
Standard (formerly called Universal) Precautions are designed for the care of all patients, regardless of their diagnosis or presumed infection status. Barriers such as gloves and masks and other procedures are used to prevent contact with body fluids. This approach to patient care has been adopted because of the concern of health care workers and the public that the human immunodeficiency virus (HIV), hepatitis B, or other deadly pathogens may be spread unknowingly by contact. Box 2-1 includes specific standard precaution guidelines established by the Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA).
BOX 2-1 Precautions to Prevent the Spread of Infection
EXCLUSION FROM PATIENT CONTACT
Any health care worker with exudative skin lesions should not work in the direct care of patients.
BARRIERS
NEEDLE AND INSTRUMENT PRECAUTIONS
PATIENT SPECIMENS
3. Respiratory hygiene/cough etiquette
4. Respiratory care equipment and procedures
5. Implement transmission prevention protocols
a. Airborne precautions
3. Respiratory protection
4. Eye protection
Health care workers should wear goggles or a face shield when within 3 feet of the patient.
b. Droplet precautions
1. Room placement
A private room is preferred, but patients with the same infection may be placed in the same room.
c. Contact precautions
1. Room placement
A private room is preferred, but patients with the same infection may be placed in the same room.
3. Gown
MODULE B
1. Pneumovax
In any immunocompetent person 2 years of age or older who fits the following conditions:
In any immunoincompetent person 2 years of age or older who has one of the following conditions:
2. Influenza
The flu vaccine is recommended for the following groups:
MODULE C
1. Choose the appropriate agent and method for disinfection and sterilization (ELE code: IIB1) [Difficulty: ELE: Ap, An]
a. Disinfection
Disinfection is a procedure that significantly reduces the microbial contamination of the equipment that has been processed. All disinfection processes destroy the vegetative form (the cell) of pathogenic organisms, including the vast majority of respiratory system pathogens. However, a few Bacillus-type bacteria are difficult to kill because they have a particularly tough cell wall or have spores for reproduction. Spores are analogous to seeds in that they grow into bacteria under the right conditions and are resistant to drying, heat, and many chemicals that kill the bacterial cell. Therefore a spore-forming organism may be able to reproduce itself after the cells have been killed. Obviously, disinfection can be used only on equipment that is not contaminated by spore-forming bacteria. Knowing what pathogen has infected the patient, if possible, can help the clinician determine the appropriate disinfection (or sterilization) method to be used on contaminated equipment. Some disinfecting agents kill different kinds of organisms, depending on the length of exposure time.
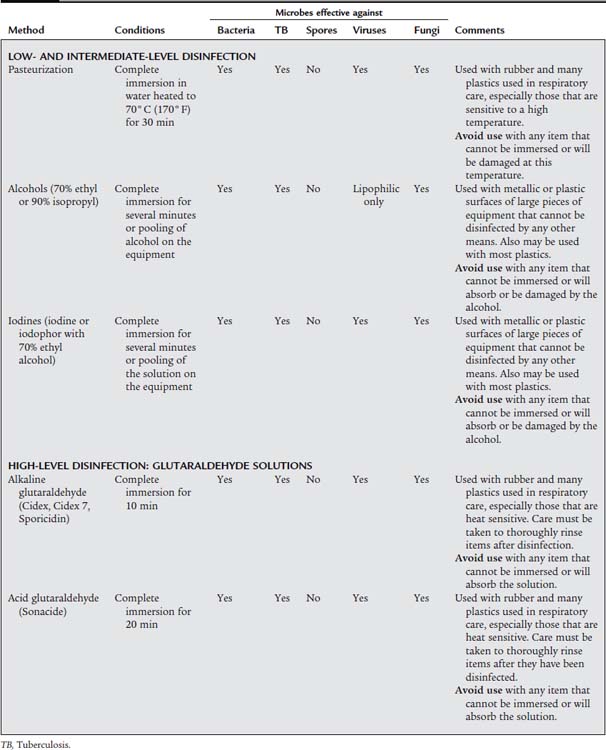
A third consideration in choosing the best disinfection method is the type of equipment that requires decontamination. Certain processes and agents can be used only on certain types of equipment. Table 2-1 lists the various ways of disinfecting reusable patient care equipment decontaminated in the hospital.
TABLE 2-1 Methods of Disinfection Used in the Hospital Setting for Respiratory Care–Related Equipment
b. Sterilization
Sterilization is a procedure that destroys all living microbial organisms and renders them unable to reproduce. All sterilization procedures destroy the vegetative forms and spores of all microscopic organisms. Examples of spore-forming bacteria include Bacillus anthracis (anthrax), Clostridium botulinum (botulism), C tetani (tetanus), and C perfringens (gas gangrene). Any equipment or instruments that penetrate body tissue (e.g., a surgical scalpel) are listed as “critical” (high risk of spreading infection) and must be sterilized before use on another patient. As was discussed previously, the method of sterilization depends on the type of equipment under consideration. Table 2-2 lists various methods of sterilization for reusable supplies and patient care equipment decontaminated in the hospital.
TABLE 2-2 Methods of Sterilization Used in the Hospital Setting for Respiratory Care–Related Equipment
| Method | Conditions | Comments |
|---|---|---|
| Steam autoclave | Autoclave chamber with an internal steam pressure of 15 lb per sq in, 121° C (250° F), 15 min | Used with glass, cloth, bandages, unsharpened stainless steel instruments, ventilator bacteria filters. |
| Avoid use with many plastics used in respiratory care, rubber, dextrose solutions, sharpened stainless steel instruments, electrical devices, or machines. | ||
| Dry heat | Autoclave chamber at 160° C-180” C (320° F-356°F), 2-hr use | Used with glass or sharpened stainless steel instruments. |
| Avoid use with many plastics used in respiratory care, rubber, dextrose solutions, electric devices, or machines. | ||
| Ethylene oxide gas | Specific guidelines vary depending on the manufacturer of the chamber and the supplies or equipment being sterilized. In general, a gas concentration of 800-1000 mg/L must be kept for 3-4 hr at 50%-100% relative humidity and 49° C-57° C (120° F-135” F). Great care must be taken to pre-dry all items before gassing and to properly aerate them after sterilization. | Used with heat-sensitive and moisture-sensitive items, as are many plastics in respiratory care equipment. |
| Avoid use with supply pouches or plastic films, such as aluminum foil, nylon, thermoplastic resin (Saran), Mylar, cellophane polyamide, polyester, or other films that are not penetrated by the gas, or with PVC that has been sterilized previously by the manufacturer with gamma radiation. | ||
| GLUTARALDEHYDE SOLUTIONS | ||
| Alkaline glutaraldehyde (Cidex, Cidex 7, Sporicidin) | Complete immersion. Cidex products for 10 hr; Sporicidin for 6 hr and 45 min | Used with rubber and many plastics in respiratory care, especially those that are heat sensitive; care must be taken to thoroughly rinse items after they have been cleansed. |
| Avoid use with any item that cannot be immersed or that will absorb the solution. | ||
| Acid glutaraldehyde (Sonacide) | Complete immersion for 1 hr at 60° C (140° F) | Use with rubber and many plastics used in respiratory care, especially those that are heat sensitive; care must be taken to thoroughly rinse items after they have been cleansed. |
| Avoid use with any item that cannot be immersed or that will absorb the solution. | ||
PVC, Polyvinyl chloride
2. Disinfect or sterilize respiratory care equipment (ELE code: IIB1) [Difficulty: ELE: Ap, An]
Any department that processes its own equipment must have adequate facilities for receiving used equipment and for its disassembly, cleansing, packaging, and distribution. First, a “dirty” area must exist where contaminated equipment is brought for disassembly, scrubbing of secretions or blood, and rinsing. Equipment then is placed into the glutaraldehyde solution or a pasteurizing machine. After that, equipment is taken to a “clean” area to be rinsed, dried, reassembled, and placed into plastic bags for storage. Care must be taken not to recontaminate the equipment during this procedure. Items that must be sterilized usually are just processed through the dirty area before being sent to the central supply department, where equipment is sterilized according to the criteria shown in Table 2-2.
3. Monitor the sterilization process to ensure its effectiveness (ELE code: IIB1) [Difficulty: ELE: Ap, An]
The term surveillance describes the monitoring of equipment to ensure that the disinfection or sterilization process was successful and that in-use equipment is not a source of patient contamination. Processing (chemical) indicators are used to ensure that disinfection or sterilization was done correctly. Examples include special tapes used to hold the wrapping around packages of equipment being autoclaved or placed into ethylene oxide. These tapes turn color when the autoclave has reached the proper temperature, or when the correct concentration of ethylene oxide has been reached. The color change shows the user that the package was processed correctly; therefore the package contents are probably sterile.
Equipment held in storage or being used in patient care also is randomly sampled for contamination. A sample is taken in three ways for culturing of possible organisms in three ways. The first involves wiping a sterile swab onto an equipment surface, and then rubbing it over a plate of growth medium or placing it into a tube of liquid broth. The second, which is used to check inside lengths of tubing, necessitates pouring a liquid broth through the tube and into a sterile container. The third involves sampling the aerosol that a nebulizer produces. A hose usually is attached to the outlet of the nebulizer. The other end of the hose is connected to a funnel, which is attached to a culture plate, where the droplets impact. In all three examples, the growth of any organism in the growth medium indicates a form of contamination. The laboratory then determines whether the organism is pathogenic. If so, measures must be taken to improve the disinfection or sterilization process.
MODULE D
American Association for Respiratory Care (AARC). Guidelines for the prevention of nosocomial infections. Dallas: AARTimes, Sept. 1983.
American Respiratory Care Foundation (ARCF). Guidelines for disinfection of respiratory care equipment used in the home. Respir Care. 1988;33:801.
Carter C, Stone MK. Respiratory microbiology, infection, and infection control. In: Hess DR, MacIntyre NR, et al, editors. Respiratory care principles and practice. Philadelphia: WB Saunders, 2002.
Centers for Disease Control (CDC). Interim recommendations for infection control in health-care facilities caring for patients with known or suspected avian influenza. http://www.cdc.gov/flu/avian/professional/infect-control.htm, May 21, 2004.
Chatburn RL, Kallstrom TJ, Bajaksouzian S. A comparison of acetic acid with a quaternary ammonium compound for disinfection of hand-held nebulizers. Respir Care. 1988;33(3):179-187.
Eubanks DH, Bone RC. Comprehensive respiratory care, ed 2. St Louis: Mosby, 1990.
Fink JB. Infection control and safety. In: Fink JB, Hunt GE, editors. Clinical practice in respiratory care. Philadelphia: Lippincott Williams & Wilkins, 1999.
Gordon SM. Principles of infection control. In Wilkins RL, Stoller JK, Kacmarek RM, editors: Egan’s fundamentals of respiratory care, ed 9, St Louis: Mosby, 2009.
Johnson & Johnson Medical: Cidexplus, 28-day solution, Arlington, TX, 1999.
Merck & Co., Inc: Pneumovax 23 (pneumococcal vaccine polyvalent), Whitehouse Station, NJ, July 2008.
OSF Saint Anthony Medical Center, Infection control precautions, Rockford, IL, 1998.
Pagana KD, Pagana TJ. Mosby’s manual of diagnostic and laboratory tests. St Louis: Mosby, 1998.
U.S. Department of Health and Human Services: Public health guidance for community-level preparedness and response to severe acute respiratory syndrome (SARS), version 2: Supplement I: Infection control in healthcare, home, and community settings, January 8, 2004.
U.S. Food and Drug Administration (FDA). Influenza: Vaccination still the best protection. http://www.fda.gov/fdac/features/2006/506_influenza.html, September-October 2006.
Washington JA. Infectious disease aspects of respiratory therapy. In Burton GG, Hodgkin JE, Ward JJ, editors: Respiratory care, ed 4, Philadelphia: Lippincott-Raven, 1997.
SELF-STUDY QUESTIONS FOR THE ENTRY LEVEL EXAM See page 583 for answers
SELF-STUDY QUESTIONS FOR THE WRITTEN REGISTRY EXAM See page 606 for answers