CHAPTER 187 Infantile Posthemorrhagic Hydrocephalus
Intraventricular Hemorrhage in Preterm Infants
Epidemiology
The incidence of preterm birth continues to rise in the United States, with 12.7% of infants born before 37 weeks’ estimated gestational age (EGA), representing an almost 20% increase over the past 15 years.1 Preterm infants are stratified by birth weight or EGA, or both. For classification purposes, the EGA of preterm infants is not rounded up. For example, an infant born at 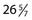 weeks is classified as a 26-week EGA infant.2 The survival of all preterm cohorts has risen dramatically over the past few decades owing to improvements in perinatal medicine, but many of these infants remain at risk for neurodevelopmental deficits. According to the 2007 report from the Institute of Medicine’s Committee on Understanding Preterm Birth, preterm infants account for 64% to 75% of infant mortality, 42% to 47% of children with cerebral palsy, 27% of children with cognitive deficits, 37% of children with visual impairments, and 23% of those with hearing impairments.3 Neurodevelopmental outcomes are discussed in more detail later.
weeks is classified as a 26-week EGA infant.2 The survival of all preterm cohorts has risen dramatically over the past few decades owing to improvements in perinatal medicine, but many of these infants remain at risk for neurodevelopmental deficits. According to the 2007 report from the Institute of Medicine’s Committee on Understanding Preterm Birth, preterm infants account for 64% to 75% of infant mortality, 42% to 47% of children with cerebral palsy, 27% of children with cognitive deficits, 37% of children with visual impairments, and 23% of those with hearing impairments.3 Neurodevelopmental outcomes are discussed in more detail later.
The prevalence of IVH varies, depending on the availability and quality of neonatal intensive care, and only a small subset of patients requires neurosurgical intervention. The incidence of IVH increases inversely with decreasing birth weight or EGA.4 In a study of infants born in the mid-1990s weighing less than 1500 g, 22% had IVH, and one fourth of those infants had progressive ventricular dilation.5 Among survivors with progressive ventricular dilation, one third required surgical intervention.5 Although infants born more recently continue to suffer IVH, fewer require surgical intervention. A recent collaborative study from the National Institutes of Health (NIH) Neonatal Research Network following more than 6000 preterm infants born at less than 1000 g between 1993 and 2002 showed that one third suffered IVH, and one third of those with IVH developed progressive ventricular dilation.6 Ten percent of those with IVH (3% of the total weighing <1000 g) required shunt insertion for symptomatic PHH, a decrease from 15% in older studies.6 IVH is graded on Papile’s scale from I to IV (Table 187-1).7 Although only 1% of infants with grade I or II IVH in this recent NIH study required shunt insertion, 18% of infants with grade III and 29% with grade IV IVH required a shunt.6 As perinatal care continues to improve, the proportion of preterm neonates who require shunt insertion will likely continue to decline.
TABLE 187-1 Papile’s Classification of Preterm Intraventricular Hemorrhage on Ultrasonography
| GRADE | DESCRIPTION |
|---|---|
| I | Isolated germinal matrix hemorrhage |
| II | Intraventricular hemorrhage without ventricular dilation |
| III | Intraventricular hemorrhage with ventricular dilation |
| IV | Intraparenchymal plus intraventricular hemorrhage |
Pathophysiology of Germinal Matrix Hemorrhage and Posthemorrhagic Infarction
The germinal matrix is a transient structure in the subependyma of the ventricular walls that generates neural cell progenitors for the overlying cortex, primarily from 8 to 28 weeks’ EGA. The germinal matrix typically completes involution by 32 weeks’ EGA. The proliferative germinal matrix is a metabolically active area with friable, immature vessels prone to hemorrhage.8 Hemorrhage can be limited to within the germinal matrix (grade I), but about 80% of germinal matrix hemorrhages rupture through the ventricular wall into the ventricle (grade II, III, or IV; see Table 187-1). Grade IV is also called periventricular hemorrhagic infarction.9 Venous infarction is believed to result from the germinal matrix hemorrhage rather than direct expansion of the hemorrhage into the parenchyma.
Multiple factors contribute to the germinal matrix’s propensity to hemorrhage, and the pathophysiology is incompletely understood. Because of their immaturity, preterm infants have impaired cerebral autoregulation. Systemic fluctuations in blood volume, flow, and pressure that commonly occur in preterm infants are thus transferred to the friable germinal matrix without the buffer of cerebral autoregulation. In addition, the germinal matrix has lower levels of fibronectin and other extracellular matrix components than do other areas of the late-gestation brain, and this likely contributes to the propensity to hemorrhage.10 An experimental study suggests that prenatal betamethasone treatment may enhance fibronectin levels and thus decrease the risk of hemorrhage. This correlates with the decreased risk of IVH observed clinically with prenatal betamethasone treatment.11 Although neonatologists have worked aggressively with obstetricians to minimize stress to preterm infants during the peripartum, the risk for IVH most likely reflects a combination of genetic and environmental factors. After IVH occurs, the absorption of cerebrospinal fluid (CSF) is impaired, likely caused by a combination of decreased absorption across the arachnoid villi into veins and from ependyma into parenchyma.8
IVH can occur in association with periventricular leukomalacia (PVL), injury to the developing white matter. PVL can occur as both deep cystic white matter lesions and diffuse noncystic loss of oligodendrocytes with associated astrocyte gliosis.12 Cystic PVL is visible on cranial ultrasonography (US), and most attention has focused on the white matter injury. Observations during the past decade from magnetic resonance imaging (MRI) and autopsy studies have shown that injury to the developing central nervous system affects both white and gray matter.13 The combined injury has been designated encephalopathy of prematurity, which more accurately reflects the widespread nature of the injury not only to the cerebral gray and white matter but also to the diencephalon, brainstem, and cerebellum.14 PVL is about 10-fold more common than IVH among infants weighing less than 1500 g at birth. At least half of these infants have imaging signs of PVL, but only 5% have IVH.14
Clinical Presentation and Diagnostic Evaluation
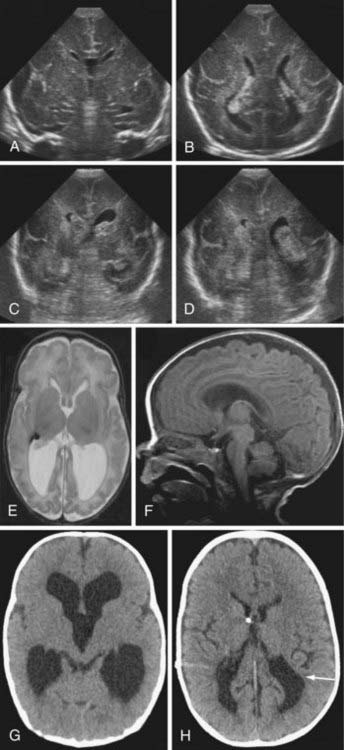
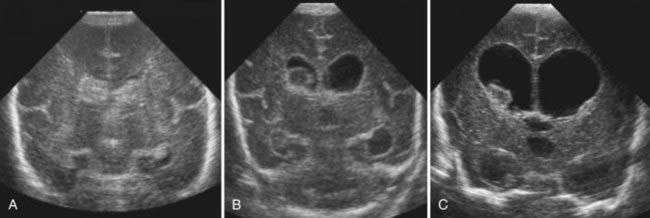
Most IVH occurs within the first 72 hours of life, when most preterm newborns are quite unstable (Fig. 187-1). IVH is readily diagnosed by bedside cranial US. Transport to obtain computed tomography (CT) or MRI can cause additional stress and potential injury in these very fragile patients. CT scans also involve radiation, and repeated exposure of the preterm brain to radiation should be minimized if possible. In general, CT scans should be reserved for the evaluation of diffuse, life-threatening cerebral hemorrhages when MRI is not feasible. US provides a reliable means of identifying ventricular dilation and IVH. If possible, all preterm newborns weighing less than 1500 g should have a cranial ultrasound examination within the first 48 hours of life, and then weekly or biweekly as needed. PVL can also be assessed by US. Although the interreader reliability for grades III and IV IVH is excellent, the reliability for grades I and II IVH and for PVL is less.15 These limitations of cranial US rarely present a problem for neurosurgical management (Fig. 187-2).
Hydrocephalus ex vacuo refers to ventricular dilation without increased intracranial pressure. In the mid-1990s, 24% of surviving infants with IVH had ventricular dilation without progression.5 Hydrocephalus ex vacuo reflects encephalomalacia, or a lack of adequate parenchymal brain growth; the implications for developmental outcomes are discussed later. Infants can have transient symptomatic hydrocephalus that spontaneously resolves. Distinguishing hydrocephalus ex vacuo from symptomatic hydrocephalus can be challenging. With continued observation, the type of hydrocephalus typically declares itself.
Treatment
Nonsurgical Treatment
A commonly used treatment paradigm begins with serial lumbar punctures. In many cases, serial lumbar punctures can be used as a temporizing measure until the infant is older and more medically stable and thus a better surgical candidate. Ideally, lumbar punctures should be initiated as soon as progressive ventricular dilation is observed.16 This may reduce the need for a permanent shunt. The fourth ventricle is sometimes difficult to visualize by cranial US, and the tentative diagnosis of aqueductal stenosis may be suggested by the radiologic interpretation. As long as there is no large posterior fossa hemorrhage, most preterm infants can safely undergo lumbar puncture. Fortunately, progressive ventricular dilation after IVH spontaneously resolves in a significant proportion of infants. Infants with transient hydrocephalus should be followed for the late development of symptomatic hydrocephalus. The chance of surgical intervention becoming necessary continues to decline for these infants over time. If serial lumbar punctures are inadequate and the patient is a relatively stable candidate for surgery, a temporary CSF diversion device is used to delay the need for definitive surgical treatment as long as possible. A meta-analysis of repeated lumbar or ventricular punctures failed to show that CSF removal improved outcomes compared with no intervention.17 This study, however, included ventricular taps, which have a higher rate of complications and poorer outcomes; it also included patients without progressive ventricular dilation. Serial CSF removal would not be expected to improve the outcome for children with hydrocephalus ex vacuo.
As observed in many areas of medicine in which definitive surgical treatment is not ideal, many nonsurgical interventions have been attempted to minimize the need for neurosurgical intervention. Unfortunately, these interventions have not been effective. Currently, medical therapy is not recommended for preterm infants with symptomatic hydrocephalus. Diuretic therapy, including acetazolamide and furosemide, is not effective in this population and may increase the risk of nephrocalcinosis and other complications.18 Likewise, intraventricular streptokinase is not effective in this population.19
Temporary Surgical Interventions
Some infants do not obtain adequate relief of symptomatic hydrocephalus with serial lumbar punctures, as defined by stabilization of the head circumference on a reasonable growth curve and stabilization or reduction of ventricular dilation on cranial US. In these cases, temporary shunts are used to treat symptomatic hydrocephalus and allow the infant to grow and recover from other complications of prematurity before undergoing definitive surgical therapy. Preterm infants have immature immune systems, fragile skin, and impaired wound healing. In addition, infants weighing less than 1500 g often have insufficient peritoneal absorptive capacity to handle the CSF volume. Also, in the subset of infants with transient symptomatic hydrocephalus that persists only for several weeks, temporary shunts avoid permanent shunt placement. Clinical judgment is crucial to balance the timing and risk of surgical intervention against the potential detriment of temporarily and inadequately treated hydrocephalus. For example, preterm infants with a history of bacterial infection of the blood or CSF are at higher risk of significantly poorer neurological outcomes than comparable infants without infection.20 The need to weigh the potential detriment of delaying surgery for several days to allow infection to be eradicated against the risks associated with symptomatic hydrocephalus is not uncommon for pediatric neurosurgeons and neonatologists.
Ideally, the neurosurgical team begins interacting with the infant’s family when it is clear that serial lumbar punctures are failing. Because surgical intervention for this population is associated with a relatively high rate of complications, including potential shunt revisions and infections, the family needs to understand that all nonsurgical means were exhausted. Infants with symptomatic hydrocephalus have poorer neurodevelopmental outcomes than those without hydrocephalus.21 Therefore, the family needs to be advised that treatment of hydrocephalus is necessary to optimize brain development, but it does not mitigate the neurological deficits associated with preterm IVH.
Temporary ventricular CSF diversion can be achieved with either a ventricular access device22,23 or a ventriculosubgaleal CSF shunt.24,25 A ventricular access device consists of a reservoir that caps a ventricular catheter, and the reservoir is accessed via a needle through the scalp every 12 to 48 hours to withdraw enough CSF to maintain a stable head circumference. Ventriculosubgaleal shunts are also composed of a ventricular catheter and reservoir, with a 4- to 8-cm piece of shunt tubing exiting the reservoir and ending in a subgaleal pocket. CSF collects in the subgaleal scalp pocket and is slowly reabsorbed. When the subgaleal shunt is functioning, the CSF forms a large, sometimes tense pocket in the scalp, but the fontanelle is soft and flat, and the cranial sutures are not splayed.
Both temporary shunt methods are widely used, and some institutions may have slightly better results with one method than the other. With both procedures, meticulous technique is essential to minimize complications. No randomized multicenter trial comparing the two methods has been performed. Temporary shunts can be used for several months if necessary. Both methods can provide adequate temporary relief from symptomatic hydrocephalus, and both are prone to infection, wound dehiscence, and catheter occlusion.22,26 Some have proposed that subgaleal shunts provide more consistent ventricular decompression compared with the fluctuation in ventricular size that occurs with daily aspiration from a ventricular access device, but the daily fluctuation has not proved harmful. Specially trained nonphysician health care providers can safely and reliably perform shunt taps.
Direct ventricular taps with a needle through the fontanelle should generally be avoided as a routine treatment modality. Ventricular taps are reserved for life-threatening emergencies because they are associated with a markedly increased risk of infection and loculated hydrocephalus in childhood. Similarly, external ventricular drains have a much higher risk of complications in preterm infants than in older neurosurgical patients.27 They have a higher risk of infection than temporary internalized shunt devices and have the same risk of catheter occlusion from debris in the CSF. A randomized trial comparing standard treatment with aspiration through a ventricular access device and a more aggressive protocol consisting of drainage, irrigation, and fibrinolytic therapy showed no difference in the number of patients who died or required permanent shunts, and those receiving more aggressive therapy were much more likely to have a secondary IVH.28
Permanent Cerebrospinal Fluid Diversion Procedures
Permanent ventriculoperitoneal shunts are required in only a small minority of preterm infants who suffer IVH. As mentioned earlier, the family needs to understand that although shunts and other surgical procedures are lifesaving, they are not without risks and complications. These children are prone to cerebral palsy, epilepsy, cognitive delay, and behavioral abnormalities regardless of whether the CSF diversion is effective. Insertion of the permanent shunt is frequently delayed through the use of a temporary shunt as long as feasible. This likely decreases the risk of shunt infection.29 Permanent shunt insertion at an older age in the neonatal period also likely decreases the need for shunt revisions throughout childhood. Among 79 preterm infants with grade III or IV IVH born before 32 weeks’ EGA, weighing less than 1500 g, and followed for a mean of 10 years, the shunt revision rate for those with permanent shunts inserted at 3 to 5 months of age (uncorrected) was about half (55%) the shunt revision rate for infants with permanent shunts inserted before 3 months (Robinson and colleagues, unpublished results). Some have advocated that low-pressure valves be inserted initially in preterm infants. However, in a retrospective study of children with infantile hydrocephalus of all causes, medium- and high-pressure valves inserted during infancy were associated with a lower shunt revision rate during childhood.30 Many former preterm infants develop slit ventricle syndrome during childhood, which can be associated with more shunt revisions. The high-pressure valve may deter slit ventricle formation in some but not all patients.
Endoscopic Third Ventriculostomy
Endoscopic third ventriculostomy is a very effective treatment for noncommunicating hydrocephalus in children older than 2 years. Its use in young infants and in communicating hydrocephalus remains controversial, however. Several studies have shown that endoscopic third ventriculostomy is not effective in more than half of infants with nonobstructive hydrocephalus.31 In a few circumstances, despite the relatively low chance of success, it may still be the best option available.
Complications
Infection
In preterm infants, seeding of the shunt hardware from bacterial and fungal systemic infections is common, and the shunt may be contaminated when it is inserted or any time it is manipulated. CSF glucose levels are typically lower at baseline in preterm infants, and hypoglycorrachia, especially when mild, does not necessarily imply infection. Similarly, the CSF protein concentration may be higher than normal due to IVH. Markedly elevated white blood cells compared with red blood cells in the CSF suggests that infection is likely, but a moderate level of white blood cells may be present as a reaction to the resolving IVH. Elevated CSF white blood cell and protein levels may persist for weeks to months after the IVH. Ideally, CSF should be sent for culture every time any CSF is obtained. Unlike in older children, shunt infection can often be treated initially with antibiotics without removal of the hardware.22 After the infection has cleared, the temporary shunt can be replaced with a permanent shunt. As part of their immature immune systems, some infants produce insufficient immunoglobulins and benefit from immunoglobulin G infusions. Despite good surgical technique, reservoirs can migrate through the thin scalp, and any exposed shunt components are presumed to be infected. The scalp erosion tends to progress quickly, and the hardware typically must be removed. In some patients ventricular drainage at the time of shunt removal can provide temporary relief for several days before a new temporary shunt is inserted. This allows the CSF infection to be treated before inserting new hardware; rarely, the temporary shunt may not require replacement.
Neurological Outcome and Comorbidities
Preterm infants with IVH who require a shunt are more likely to have neurological deficits than those with IVH without PHH, suggesting that the need for a shunt is an independent risk factor for poor outcome.6 A small subset of preterm infants with PHH does not demonstrate significant neurological deficits at follow-up, and it is difficult to predict outcomes for children when they are infants. The likelihood of neurological deficits should be explained to the parents and caregivers to help them understand that the goal of a shunt is to optimize neurodevelopment. Shunt insertion and revision do not cause the neurological deficits associated with prematurity, but PHH and the need for a shunt clearly represent a more extensive central nervous system injury during development. For example, about two thirds (59% to 66%) of former preterm infants with grade IV IVH surviving at 2 years have motor deficits, and almost half (44% to 50%) have cognitive delay.32–34 A study from the Netherlands showed that 80% of children with grade IV IVH and a shunt had cerebral palsy.21 Children with shunts can be expected to have more deficits in all realms. Among 79 children who were born at less than 32 weeks’ EGA, weighed less than 1500 g, required shunt insertion for PHH, and were followed for an average of 10 years, only 29% performed at the expected grade level, ambulated without an assistive device, and were free of seizures (Robinson and colleagues, unpublished results).
An inherent part of using standardized techniques to assess outcomes in young children is that studies of preterm infants tend to focus on severe, well-defined deficits present at about 2 years of age. Although this allows an accurate comparison of various interventions, the more subtle deficits that can significantly impact a child’s achievement are not well documented. For example, 26% of former preterm (<1500 g) infants have symptoms strongly suggestive of autism spectrum disorders,35 yet these disorders are not routinely assessed in outcome studies. A recent study compared the intellectual function of children aged 6 to 16 years with shunted PHH who performed within 1 year of expected grade level and that of normal controls; the investigators found that the full-scale, verbal, and performance IQ in children with shunts averaged a full standard deviation below that of controls.36 This study likely overestimates the performance of former preterm children with shunts, because only half the patients in the study were born preterm, and those not performing near expected grade level were excluded.
Intraventricular Hemorrhage in Term Infants
Epidemiology
IVH in term infants is rare. Among the more than 2675 full-term infants admitted to a neonatal intensive care unit from 2003 to 2005, approximately 15% had peri- or intraventricular hemorrhage.37 At another institution, 66 full-term infants with any type of intracranial hemorrhage were identified during a 12-year period, and only 30% had IVH.38 In contrast to the minority of preterm infants who have low-grade IVH, 71.7% of term infants had a grade I lesion on cranial US, and 27.7% had grade II. Only two infants had grade III IVH, and one had grade IV.37
Pathophysiology
Similar to preterm infants, IVH appears to arise from both genetic and environmental factors in term infants. Male gender, poor Apgar score, respiratory distress syndrome, and hyperbilirubinemia increase the risk of IVH.37 Similarly, another study showed that forceps were used during delivery in 45% of infants with IVH, Apgar scores were less than 9 in 90% at 1 minute and in 60% at 5 minutes, and intubation after birth was required in 35%.38 Term infants are more likely to develop IVH from venous infarction due to hypercoagulopathy or from hemorrhage of the choroid plexus. In many cases a cause cannot be identified. An increased frequency of the PLA2 (platelet glycoprotein IIb/IIIa Leu33Pro polymorphism) allele was found in term infants with IVH compared with controls.39 The exact mechanism is likely to be poorly understood in most patients.
Clinical Presentation
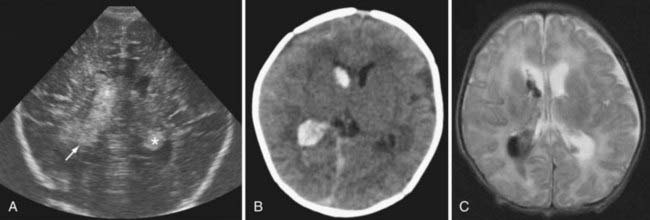
Term infants with IVH typically present with lethargy or seizures. A subset of infants presents with distress at birth, and the remainder often present within the first week. Some infants have already been discharged home from the newborn nursery and return through the emergency department. Magnetic resonance angiography or venography can sometimes visualize underlying abnormalities and suggest a diagnosis, but MRI rarely affects urgent surgical decisions (Fig. 187-3). These infants often have seizures that are difficult to control, and the timing of the MRI needs to be coordinated with the need for electroencephalographic monitoring. Most term infants with IVH benefit from a coagulation evaluation, but typically this cannot be completed conclusively until the infant is about 1 year old. Many infants have no or transient ventricular dilation in the period immediately after the hemorrhage. A significant proportion may eventually develop symptomatic hydrocephalus and require a shunt, usually during the first year of life.
Adams-Chapman I, Hansen N, Stoll B, Higgins R. Neurodevelopmental outcome of extremely low birth weight infants with post hemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:e1167-e1177.
Allen M. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21:123-128.
Khwaja O, Volpe J. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153-F161.
Pierson C, Folkerth R, Billiards S, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619-631.
Volpe J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110-124.
1 Martin J, Hamilton B, Sutton P, et al. Births: final data for 2005. National Vital Statistics Reports. 2007;56(6):1-3. & 21, 65, 79-80
2 Engle W, American Academy of Pediatrics Committee on Fetus and Newborn. Age terminology during the perinatal period. Pediatrics. 2004;114:1362-1364.
3 Allen M. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21:123-128.
4 Kadri H, Mawla A, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086-1090.
5 Murphy B, Inder T, Rooks V, et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37-F41.
6 Adams-Chapman I, Hansen N, Stoll B, Higgins R. Neurodevelopmental outcome of extremely low birth weight infants with post hemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:e1167-e1177.
7 Papile L-A, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. 1978;92:529-534.
8 Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14:305-311.
9 Volpe J. Intraventricular hemorrhage in the premature infant—current concepts. Part 1. Ann Neurol. 1989;25:3-11.
10 Xu H, Hu F, Sado Y, et al. Maturational changes in laminin, fibronectin, collagen IV, and perlecan in germinal matrix hemorrhage, cortex, and white matter and effect of betamethasone. J Neurosci Res. 2008;86:1482-1500.
11 Lee B, Stoll B, McDonald S, Higgins R. Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics. 2006;117:1503-1510.
12 Khwaja O, Volpe J. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153-F161.
13 Pierson C, Folkerth R, Billiards S, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619-631.
14 Volpe J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110-124.
15 Hintz S, Slovis T, Bulas D, et al. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150:575-577.
16 DeVries L, Liem K, van Dijk K, et al. Early versus late treatment of posthemorrhagic ventricular dilatation: results of a retrospective study from 5 neonatal intensive care units in The Netherlands. Acta Paediatr. 2002;91:212-217.
17 Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database Syst Rev. 2001;1:1-13.
18 Whitelaw A, Brion L, Kennedy C, Odd D. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst Rev. 2001;2:1-22.
19 Whitelaw A, Odd D, Brion L, Kennedy C. Intraventricular streptokinase after intraventricular hemorrhage in newborn infants. Cochrane Database Syst Rev. 2007;4:1-11.
20 Adams-Chapman I, Stoll B. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19:290-297.
21 Brouwer A, Groenendaal F, van Haastert I-L, et al. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152:648-654.
22 Hudgins R, Boydston W, Gilbreath C. Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device. Pediatr Neurosurg. 1998;29:309-313.
23 McComb J, Ramos A, Platzker A, et al. Management of hydrocephalus secondary to intraventricular hemorrhage in the preterm infant with a subcutaneous ventricular catheter reservoir. Neurosurgery. 1983;13:295-300.
24 Fulmer B, Grabb P, Oakes W, Mapstone T. Neonatal ventriculosubgaleal shunts. Neurosurgery. 2000;2000:80-84.
25 Perret G, Graf C. Subgaleal shunt for temporary ventricle decompression and subdural drainage. J Neurosurg. 1977;47:590-595.
26 Tubbs R, Banks J, Soleau S, et al. Complications of ventriculosubgaleal shunt in infants and children. Childs Nerv Syst. 2005;21:48-51.
27 Gurtner P, Bass T, Gudeman S, et al. Surgical management of posthemorrhagic hydrocephalus in 22 low birth weight infants. Childs Nerv Syst. 1992;8:198-202.
28 Whitelaw A, Evans D, Carter M, et al. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007;119:e1071-e1078.
29 Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22:696-697.
30 Robinson S, Kaufman B, Park T. Outcome analysis of initial neonatal shunts: does the valve make a difference? Pediatr Neurosurg. 2002;37:287-294.
31 Peretta P, Ragazzi P, Carlino C, et al. The role of ommaya reservoir and endoscopic third ventriculostomy in the management of post-hemorrhagic hydrocephalus of prematurity. Childs Nerv Syst. 2007;23:765-771.
32 Bassan H, Limperopoulos C, Visconti K, et al. Neurodevelopmental outcomes in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007;120:785-792.
33 O’Shea T, Kuban K, Allred E, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:e662-e669.
34 Roze E, Kerstjens J, Maathuis C, et al. Risk factors for adverse outcome in preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2008;122:e46-e52.
35 Limperopoulos C, Bassan H, Sullivan N, et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics. 2008;121:758-765.
36 Lacy M, Pyykkonen B, Hunter S, et al. Intellectual functioning in children with early shunted posthemorrhagic hydrocephalus. Pediatr Neurosurg. 2008;44:376-381.
37 Baumert M, Brozek G, Paprotny M, et al. Epidemiology of peri/intraventricular haemorrhage in newborn infants at term. J Physiol Pharmacol. 2008;59:67-75.
38 Jhawar B, Ranger A, Steven D, Del Maestro R. Risk factors for intracranial hemorrhage among full term infants. Neurosurgery. 2003;52:581-590.
39 Havasi V, Komlosi K, Bene J, Melegh B. Increased prevalence of glycoprotein IIb/IIIa Leu33Pro polymorphism in term infants with grade I intracranial haemorrhage. Neuropediatrics. 2006;37:67-71.