Indwelling Vascular Devices
Emergency Access and Management
Indwelling vascular lines provide routes for short- and long-term infusion of antibiotics, antifungal agents, hyperalimentation fluids, chemotherapeutic agents, blood products, analgesics, and anesthetic agents. In addition, they provide access for lifesaving procedures such as hemodialysis (HD) and plasmapheresis. As of 2008, nearly 382,000 patients were receiving HD therapy, with 3 to 5 million central venous catheters being placed yearly in patients in the United States.1,2 For the purpose of this chapter, all implanted devices and intermediate- to long-term catheters for vascular access are considered vascular access devices (VADs). Arteriovenous (AV) fistulas and AV grafts are included because of their similarities to VADs.
Historical Perspective
A major advance that ultimately led to the development of several types of indwelling catheters was the introduction of Silastic (polymerized silicone rubber) in 1948 by the Dow Corning Corporation. This biocompatible material is an ideal substrate for intravenous (IV) catheters because it is chemically inert, antithrombogenic, rigid at room temperature, and pliable at body temperature. In 1973, Broviac and coworkers used this material to develop a 90-cm × 0.22-mm indwelling right atrial (RA) catheter for total parenteral nutrition (TPN).3 In 1979, Hickman and colleagues reported their experience with a 0.32-mm catheter that could be used for blood products and drug therapy in bone marrow transplant recipients.4 A totally implantable vascular access device (TIVAD) was described by Fortner and Pahnke in 1976.5 Since that time, TIVADs have become a mainstay of treatment in oncology patients. TIVADs allow less painful IV access and improve quality of life by permitting unrestricted mobility.
Temporary access for HD via an external AV shunt was pioneered by both Quinton and Scribner and their coworkers in 1960.6,7 This original shunt was composed of a loop of tubing lying on the volar aspect of the forearm that connected the radial artery to a wrist vein. Although it provided effective dialysis, it was associated with a high rate of infection, thrombosis, and restriction of patient activity. Brescia and colleagues then introduced the peripheral subcutaneous autogenous AV fistula in 1966.8 This Brescia-Cimino internal fistula used a side-to-side anastomosis (the current procedure of choice for long-term HD) to connect the radial artery to the cephalic vein in the nondominant hand. Erben and associates described the routine use of percutaneous cannulation of the subclavian vein for HD in 1969.9 In 1979, Uldall and coworkers reported the development of a single-needle, subclavian HD catheter.10
Indwelling VADs
VADs are typically chosen based on the least invasive, smallest catheter with the lowest risk for complications that will last as long as the length of therapy that is anticipated.11 Length of therapy is often the major consideration when choosing a device. Long-term VADs consist of cuffed, tunneled RA catheters and implantable ports. Medium-term VADs include midline catheters (lasting weeks), peripherally inserted central catheter (PICC) lines (lasting months), and Silastic subclavian or jugular catheters (percutaneous multilumen catheters). Short-term devices (see Chapter 21) include short peripheral IV, subcutaneous (butterfly), subclavian, and jugular catheters. Additional VADs include those used for dialysis, as well as AV fistulas and grafts.
Cuffed, Tunneled RA Catheters (Broviac, Hickman, Hemocath, Leonard, Raaf)
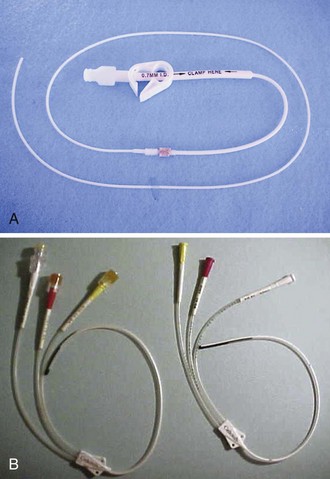
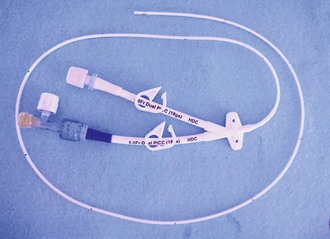
Several cuffed, tunneled RA catheters are available, each with differences tailored to specific applications (Fig. 24-1A). The Broviac is an all-Silastic single-lumen catheter with a 1.0-mm internal diameter (ID). It is 90 cm long with a thin intravascular segment (55 cm). The Hickman, also a Silastic catheter, has a 1.6-mm ID. It allows more frequent blood sampling without jeopardizing luminal patency.12 Single-, double- and triple-lumen variations exist. Hemocath/Permacath has the largest bore of the RA catheters, 2.2 mm ID. Quinton Instrument Company manufactures these catheters for HD, plasmapheresis, long-term nutritional support, and pain control. The main advantage of a cuffed catheter is that it can be used immediately, but it is not recommended for long-term access in patients undergoing dialysis if an AV fistula or graft is possible. Long-term dialysis with tunneled catheters has been associated with an increased risk for death, a 5- to 10-fold increase in the risk for infection, and a decreased likelihood of adequate dialysis.13 Ideally, these catheters should be used only as a bridge to longer-term devices.14
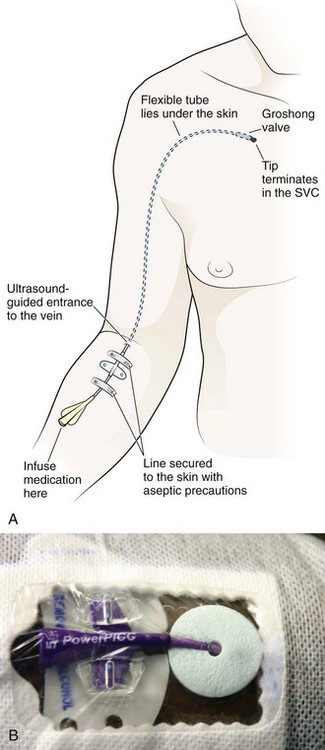
Nonemergency insertion of RA catheters is typically done in an operating room or interventional radiology suite. The device is introduced via the upper anterior chest wall and tunneled subcutaneously to enter the superior vena cava (SVC) system via the cephalic, subclavian, internal, or external jugular vein (Fig. 24-2). The distal tip of the flexible catheter is advanced to the distal SVC or into the mid-RA area. The subcutaneous tunnel isolates the venous puncture site from the skin and decreases the potential for bacterial contamination. Dacron cuffs (one near the venous entrance site and one near the skin exit site) anchor the catheter and are believed to inhibit colonization of the SVC by skin organisms.15 However, no study has been able to support this belief. Advantages of an RA catheter include ease of insertion and use, minimal interference with patient activity, low incidence of major complications or unintended dislodgment, ease of removal, and potential repair via a kit. Disadvantages include the need for regular maintenance and the potential for unacceptable cosmesis.
Groshong Catheter
In contrast to the Broviac and Hickman catheters, the Groshong catheter has slitlike openings just proximal to the end of the intravascular portion of the catheter (see Fig. 24-1B). This functions as a one-way valve to stop backbleeding and prevent air entry and embolism from the negative intrathoracic pressure. This feature obviates the need to use a heparin lock (saline may be used). In addition, external catheter clamping is not necessary. Disadvantages are its high cost and requirement for pressurized infusion systems.16
TIVADs/Ports (Port-A-Cath, Proport, Infuse-A-Port, Mediport)
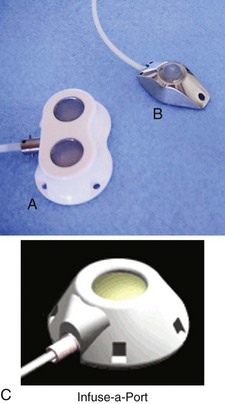
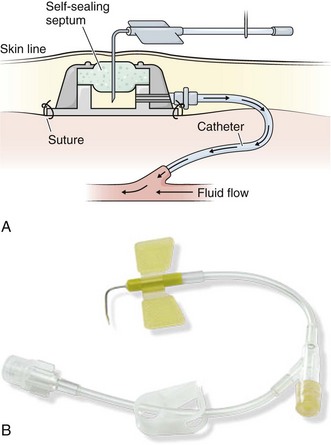
Since 1983, implanted ports have become the mainstay for long-term cancer therapy. TIVADs are tunneled RA catheters, but they differ from Broviac and Hickman catheters in that they have a subcutaneous titanium or plastic portal with a self-sealing septum (Fig. 24-3) that may be accessed by puncturing a specially designed needle (90-degree angled Huber needle) through intact skin (Fig. 24-4). Cosmetically, they are superior to external tunneled catheters, require less maintenance, and afford patients greater freedom of movement and activities such as swimming or bathing.
TIVADs may be inserted on an outpatient basis under local anesthesia via a subcutaneous tunnel or open cutdown. The cutdown technique offers potential speed (mean placement time, 15 minutes), safety (negligible risk for pneumothorax), along with avoidance of early and late complications.17 Placement is typically in the nondominant arm with the portal in the upper part of the arm or chest unless a vein is occluded or radiation therapy is planned on that side.
Disadvantages of this type of device include increased cost, the need for a specific noncoring Huber access needle, and the small gauge (20 to 22) of the access needle, which limits fluid infusion rates.16
PICC (Nontunneled, Noncuffed)
PICC lines are centrally placed lines that were first described in the 1970s and originally developed for the neonatal population. Subsequently, their use expanded into the adult arena for prolonged antibiotic therapy, IV fluids, chemotherapy, TPN, and delivery of medications that are irritating to peripheral vessels. PICCs (Fig. 24-5) are made of two substances, either polyurethane (Intracath) or silicone (Intrasil), are radiopaque, and measure 50 to 60 cm in length with an outside diameter of 2 to 7 Fr. The catheter may have a single- or double-lumen configuration and can be open or closed ended or valved (e.g., Groshong). An open-ended PICC cannot prevent feedback of blood into the catheter and therefore must be flushed one or more times daily with heparinized saline. The Groshong valve reduces backup of blood into the catheter and therefore requires flushing as infrequently as once a week. The most common type of PICC line in use today is the 5-Fr, double-lumen, closed-ended catheter.
Selection of the device should be based on the number of lumens necessary for therapy. Selection of the access site depends on many factors, including the suitability of target vessels, body habitus, handedness, ability to manage self-care, comorbid conditions, desired infusion rate, number and compatibility of concurrent infusions, infusate characteristics, and the estimated duration of therapy. Infusate that is hyperosmolar (e.g., TPN) or vesicant requires rapid dilution. Accordingly, the tip must be in the SVC, where the estimated flow rate is 2000 mL/min. PICC lines are most frequently placed in the superficial veins proximal to the antecubital fossa (usually the basilic or cephalic vein) (Fig. 24-6). However, they may also be placed via a transhepatic or translumbar approach when the SVC is thrombosed or occluded.18
Advantages of PICC lines include usefulness in a wide variety of clinical situations, ease of placement, and ease of use and maintenance. They do not require surgical insertion and may be placed in an outpatient setting. A PICC line is an excellent vehicle for medium- to long-term IV therapy. With proper care, PICCs can remain in place for long periods, even months to years.19 To remove a PICC line, simply withdraw it from the vein and apply pressure and a sterile bandage.
HD VADs
Vascular access, which is often referred to as the Achilles heel of renal patients, remains problematic. From the moment that the first access is created, an ongoing process is started that will end with loss of all access sites if the patient survives long enough.20 Clinical practice guidelines of the National Kidney Foundation—Disease Outcomes Quality Initiative (NKF-DOQI) recommend early construction of an AV fistula and avoidance of catheters for permanent or prolonged vascular access,17,21 with less than 10% of permanent access being in the form of catheters.22 However, one study demonstrated that more than half the patients began dialysis with a central catheter because a well-developed AV fistula was not available.23 The risk of requiring three or more vascular access sites is almost double in patients who start HD with a central catheter.
Temporary Dialysis Catheters (Quinton, Mahurkar, Tessio, Vascath, Uldall)
Temporary vascular access catheters (Fig. 24-7) are used for emergency HD or for temporary HD access if a more permanent dialysis route (AV fistula or graft) is not available or has recently been placed and has not matured yet. The advantage of tunneled catheters is the ability to provide immediate access or temporary access while a more permanent structure matures, but this carries a long-term risk for infection, dysfunction, and central venous stenosis. The majority of bacteremia episodes in patients undergoing HD are caused by HD catheters, with an approximate 20% to 25% risk over the average duration of use.
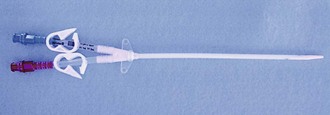
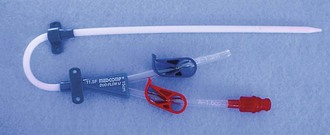
Figure 24-7 Mahurkar 11.5-Fr. × 16-cm Double-Lumen Catheter for temporary hemodialysis, apheresis, and infusion.
These large-bore catheters allow the necessary blood flow rate of 300 mL/min for dialysis. The Quinton catheter has two ports, one to deliver the patient’s blood to the dialysis machine and another to return the blood to the patient’s circulation (see Fig. 24-2B). These catheters are placed in a central vein, either the internal jugular, subclavian, or femoral. The right internal jugular approach is preferred, even if permanent access is to be created on the right side, because it has the lowest thrombosis rate. The NKF-DOQI recommends avoiding the subclavian vein unless no other options exist or the ipsilateral arm has no more permanent access sites. For patient comfort, a special 180-degree catheter can be used (Fig. 24-8). There are two avenues to place this catheter: percutaneously or surgically. Emergency percutaneous placement may be performed by the emergency clinician at the bedside. Using sterile technique and after injection of a local anesthetic, insert the catheter by following the same procedure for placing a central line into one of the central veins.
Chronic HD Vascular Access
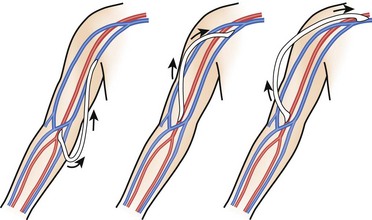
The goal of chronic HD vascular access is to provide safe, effective, and repeated easy access to the circulation. The three principal types of access are native AV fistulas, synthetic grafts, and double-lumen tunneled cuffed catheters (described above). The access types differ in failure rates, patency, complications, and other morbidity. In general, fistulas are preferred over grafts because of superior long-term patency and lower complication rates. However, fistulas are more likely than grafts to experience primary failure, defined as being unable to provide reliable access. Both fistulas and grafts must mature before they can be used for HD, a process that may take several weeks to several months. HD is often performed via a Quinton catheter during this hiatus, so a patient in the emergency department (ED) with both a catheter and a shunt either has a nonfunctioning fistula or graft or the access site is still immature. Grafts can usually be cannulated earlier than fistulas, often within weeks of placement. The minimum time for fistula maturation is at least 1 month. Complications common to both grafts and fistulas are thrombosis, infection, steal syndrome, aneurysms, venous hypertension, seromas, and local bleeding (Fig. 24-9). Overall, grafts are more likely to experience infection and thrombosis requiring thrombectomy or require other types of access intervention. The risk for infection with HD grafts is about 10% over prolonged use and 1% to 2% with HD fistulas. Graft infection requires complete excision to eradicate an infection of the foreign material, whereas fistula infections may resolve with IV antibiotic use.
AV Fistulas
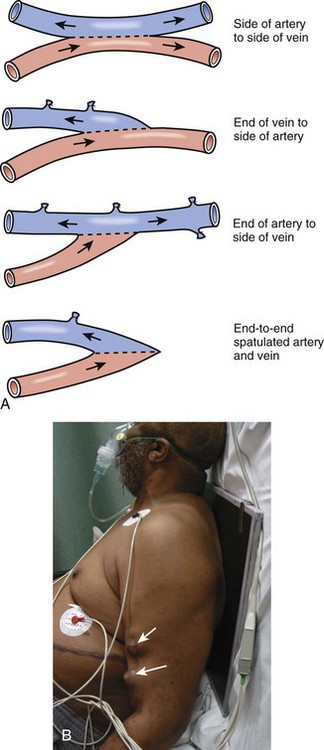
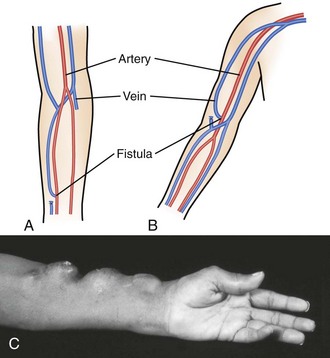
An AV fistula is a direct subcutaneous anastomosis of an artery and vein without prosthetic material and is the preferred means of vascular access for HD (see Fig. 24-9). Historically, the percentage of patients with AV fistulas fell well below the recommended goal, with most patients receiving AV grafts or long-term vascular access catheters. The most recent data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) show that from 1996 to 2007, AV fistula use in the United States almost doubled (24% to 47%) and AV graft use fell by 50%.24 Use of an autogenous fistula is associated with the longest period of patency with relative freedom from thrombotic and infectious complications. Once a fistula matures, long-term patency is high (48% of AV fistulas versus 14% of AV grafts at 5 years), with low infection rates relative to grafts.25
An autogenous AV fistula is constructed by anastomosing an artery to a vein (see Fig. 24-9), preferably a nearby one. Various configurations are possible, but AV fistulas are typically an end-to-side vein-to-artery anastomosis. The radial-cephalic (Brescia-Cimino forearm) fistula in the forearm is the most frequently used (Fig. 24-10), with the brachial-cephalic, brachial-basilic, and rarely the proximal part of the thigh being alternatives. Over time, the venous portion of the shunt is subjected to high pressure, and flow becomes arterialized (hypertrophied and dilated), which renders it suitable for repeated vascular access. Full epithelialization of the shunt does not occur for 3 to 6 months, thus necessitating anticipatory placement as renal function worsens.
AV Grafts
If a forearm radiocephalic fistula cannot be constructed or has failed, an AV bridge graft using a donor vein or synthetic material is a well-accepted alternative. Several synthetic materials are used for grafts. Polytetrafluoroethylene (PTFE) is the most commonly used but takes 3 weeks to mature. An available polyurethane graft (Vectra) has the ability to be accessed within 24 hours. A standard graft is 6 to 8 mm in diameter and usually positioned in a U-shaped subcutaneous tunnel in the forearm. The graft is attached by end-to-side anastomoses to the brachial artery and antecubital vein. If no suitable antecubital vein is available, a straight bridge graft between the brachial artery and either the axillary or the basilic vein is often used. Multiple configurations are possible (Fig. 24-11). The characteristic C shape of the graft can aid in differentiating a graft from a more tortuous fistula. A jump graft between opposite extremities with creation of a loop across the chest or anastomosis of the axillary artery to the iliac vein is a possibility if all other sites have been exhausted. When compared with AV fistulas, AV grafts have a significantly higher incidence of thrombosis, infection, pseudoaneurysm formation, and limb loss. PTFE grafts have a low primary patency rate (29% at 1 year according to one study).26 However, they have a low incidence of aneurysm formation and are comparatively easy to revise. The estimated life span of a PTFE graft in clinical practice is often less than 2 years.
Accessing Vads in the ED
The emergency need to administer parenteral medications to patients lacking other means of vascular access is the most common reason to access a VAD in the ED. Assuming that proper access methods are used to prevent infection, the greatest risk is sludging in the catheter with resultant occlusion. After administering medications, follow with a saline flush to clear the catheter and ensure that the medications reach the circulation. Do not administer medications concurrently that are known to be incompatible when mixed (e.g., calcium and bicarbonate), even through separate ports of multilumen catheters. Blood specimens, including samples for culture, can also be obtained via VADs. For phlebotomy specimens, stop the infusion, use appropriate catheter antisepsis, remove fluid occupying dead space in the catheter, and follow the blood draw with a flush of heparinized saline.27
Accessing Long-Term Venous Access Catheters
To accomplish phlebotomy, withdraw the dead space solution, reclamp it, and use a separate syringe to remove the desired amount of blood.28 If clots are withdrawn, continue withdrawing blood until it is clot free. Inject bolus medications and infuse IV solutions through the catheter, and clamp it whenever it is unattached. Deliver a 5-mL normal saline flush between medications through a 10-mL syringe. On completion of either blood withdrawal or medication infusion, inject 3 to 4 mL of saline to flush it and then inject 3 to 5 mL of heparin (1000 U/mL). Clamp the line and reposition the cap.28 Note that 1000 U/mL of heparin should be used; less concentrated solutions may promote clotting. Do not inject larger amounts because it can heparinize the patient systemically. Groshong catheters need not be flushed with heparin but instead may be flushed briskly with 5 to 10 mL of saline. Multilumen central catheters have one port for each lumen, so access each one in the same manner. After antiseptic preparation, gain access either by inserting a needle or a syringe into the protective cap or by removing the cap entirely. Flush with 5-mL normal saline or sterile water, and verify backflow before all subsequent procedures. Perform phlebotomy through the proximal lumen to prevent mixing with medications being delivered through the other ports. Deliver IV infusions in similar fashion, and inject a normal saline flush between medications. Terminate the procedure by flushing 3 to 5 mL of heparin (1000 U/mL) through each port.
Accessing TIVADs
The procedure for accessing TIVADs is unique because these devices are not external. Instead, a circular reservoir (cylinder) lies subcutaneously on the anterior chest wall. First, palpate the cylinder and then prepare the overlying skin with povidone-iodine solution. Fill a 10-mL syringe with normal saline and attach it to connecting tubing. Attach this to a 19- to 22-gauge, 90-degree tapered (Huber) needle. The Huber needle is a specialized needle designed for use with TIVADs to prevent damage to the portal septum. It has a 90-degree bend with a slightly curved tip and the opening on the side rather than on the end. Most importantly, the Huber is a noncoring needle. This avoids damage to the Silastic septum and allows up to 2000 punctures. Do not access the TIVAD with a standard needle unless an arrest is in progress and a Huber needle is not immediately available. Apply a clamp to the connecting tubing whenever the system is open. Expel the air and insert the Huber needle through the septum of the reservoir. Insert the needle slowly and steadily through the diaphragm until it contacts the back of the reservoir. Be aware that although incomplete perforation of the septum will block flow, substantial pressure may also damage the back of the device and bend the tip of the needle. Remove the clamp slowly, and inject 5 mL of solution to ensure patency. If patency is not easily demonstrated, consider using alteplase (recombinant tissue plasminogen activator) as a fibrinolytic agent for catheter occlusion.29
Once the solution has been injected, apply gentle negative pressure to demonstrate backflow of blood. Stabilize the Huber needle by building a 4- × 4-inch gauze pad about the needle and further reinforce it with 2.54-cm (1-inch) silk tape. First, remove 8 to 9 mL of blood with a separate syringe and waste it, and then perform phlebotomy through the extension tubing. If necessary, deliver IV solutions through extension tubing, but remember that the rate of flow will be limited by the smaller radius of the Huber needle. Deliver a 5-mL normal saline flush between medications. Complete the procedure with a 3- to 5-mL heparin (1000 U/mL) flush, and remove the Huber needle.28
Accessing AV Fistulas, Shunts, and HD Catheters
AV fistulas, shunts, and temporary dialysis catheters are placed in patients who require HD, and they represent the sole access for that purpose. Consequently, routine use of these sites for phlebotomy and fluid administration is strongly discouraged. In fact, venipuncture in the same extremity as a patent AV fistula is not recommended, except for the veins in the dorsum of the ipsilateral hand. When standard IV access cannot be obtained under emergency circumstances, however, fistulas, shunts, and catheters may all be used to administer IV solutions and medications (Fig. 24-12). Though avoided whenever possible, the option to access these sites is based on clinical judgement by the clinician. If possible, ascertain patency of the fistula by noting a bruit and palpable thrill, although these signs may not be appreciable if the patient is in extremis.
Prepare the area overlying the fistula with antiseptic solution and access the fistula with the smallest-gauge needle that is appropriate.30 Puncture 1 to 2 cm from the end of the anastomosis nearest the venous side, and avoid aneurysmal sites.30 Access AV shunts similarly by placing the smallest needle possible in the catheter and bridging the arterial and venous circulations. Apply local pressure for at least 5 minutes after the procedure is completed and monitor subsequently for hemorrhage. Access the Uldall and Mahurkar catheters in much the same way that multilumen central catheters are accessed. Remove or inject through the retaining cap on each arm. Up to 5000 units of heparin is present within the two lumens, and so it is imperative to aspirate before administering fluid or medications. After use, flush each catheter arm with 10 mL of normal saline and instill 1.5 mL of heparin solution (1000 U/mL) into each one.
Complications of VADs
VADs are now sufficiently commonplace that patients with these devices are seen in the ED on a regular basis. Given a complication rate of 4% to 10%,31 it is essential that emergency clinicians be aware of these complications and their management. The risk for complications associated with a VAD is dependent on several factors, including the type of catheter, underlying patient pathology and anatomy, the site chosen for placement, and the experience of health care workers obtaining access and caring for the device.32
Complications of VADs include (1) infection, (2) hemorrhage and coagulation abnormalities, (3) malfunction, and (4) miscellaneous problems (Box 24-1).
Infection
Infection is the most common complication leading to removal of VADs and potentially the most serious. Infection can also result in shunt thrombosis, rupture, or massive hemorrhage. An estimated 250,000 to 500,000 cases of nosocomial VAD-related bacteremia occur annually in the United States with an associated 10% mortality rate.33 Infectious complications include endocarditis, septic arthritis, pulmonary emboli, osteomyelitis, and spinal epidural abscess. The definitions of VAD-related infections are not uniform, which makes it difficult to compare the results of various investigations. However, it is generally agreed that risk factors for infection include the site of insertion, hospital size, duration of catheter placement, type of catheter, and patient factors. Most studies show a lower incidence of infection for TIVADs than for external systems, presumably because of lack of direct access by cutaneous organisms. Rates of VAD infection tend to be highest in the first 3 months after insertion, with skin flora being most common.31,34 The rate of infection decreases significantly and reaches a plateau after 5 to 6 months.34 The most common infecting organisms are listed in Box 24-2.
Draw two sets of blood for culture, with one set through the catheter itself and one from a peripheral site. Positive blood cultures for S. aureus, coagulase-negative staphylococci, or Candida species, in the appropriate patient setting and in the absence of another identifiable source of infection, should increase suspicion for catheter-related bloodstream infection.31 In most studies, blood obtained from a VAD yielding a colony count at least 5- to 10-fold greater than that for blood obtained from a peripheral site suggests a catheter-related source of infection.35 Similarly, catheter infection should be assumed if cultures of peripherally derived blood are negative and the VAD-derived cultures are positive.34 Not only is blood drawn from the catheter more likely to yield a positive culture, but it may also occur earlier in the course of infection. One study demonstrated high sensitivity and specificity for diagnosing a VAD-related infection if cultures of blood drawn from the access device became positive 2 or more hours before cultures of peripherally drawn blood.36
Infusate-related bloodstream infection is uncommon and defined as isolation of the same organism from both the infusate and separate percutaneous blood cultures, along with no other source of infection. When this diagnosis is suggested, cultures of the infusate should be performed in addition to catheter and peripheral blood cultures.31
When a catheter-related infection is suspected, an important management decision is required to determine whether to remove the catheter. Removal is recommended in patients who have a catheter placed solely for short-term use. In patients with long-term catheters, remove the catheter in the setting of severe sepsis, suppurative thrombophlebitis, endocarditis, or persistent positive blood cultures (longer than 72 hours) while on appropriate antibiotics. Also, remove the catheter if the infection is due to S. aureus, Pseudomonas aeruginosa, fungi, or mycobacteria. For patients who require long-term vascular access for survival or have no other vascular access options, salvage of the catheter may be attempted if the infecting agent is not Bacillus, Micrococcus, or propionibacteria, in addition to the previously listed organisms. If the catheter is left in place, obtain blood for repeated cultures and remove the catheter promptly if they remain positive following 72 hours of appropriate antibiotic therapy.37
If a VAD is removed, culture it to determine the offending organism. Place the catheter in a sterile tube (red top) and send it to the laboratory for culturing. The most widely used laboratory technique for the clinical diagnosis of catheter-related infection is the semiquantitative method, in which a segment of the catheter is rolled across the surface of an agar plate and colony-forming units (CFUs) are counted after incubation overnight. Quantitative culture of the catheter segment requires either flushing the segment with broth or vortexing in broth, followed by serial dilutions and surface plating on blood agar. A yield of 15 CFUs or more from a catheter by semiquantitative culture or a yield of 102 or more from a catheter by quantitative culture with accompanying signs of local or systemic infection is indicative of a catheter-related infection.31
HD catheters deserve special consideration. The process of HD requires several connections to the graft, thereby increasing the risk for infection. The rate of bacteremia in HD patients attributed to the graft varies from 48% to 73%. The incidence is highest when central venous dialysis catheters are used. Native AV fistulas carry the lowest risk for infection. Unfortunately, AV grafts are more commonly used in the United States.38 As with other VADs, initiate empirical antibiotic therapy based on epidemiologic and patient factors, followed by narrowed-spectrum therapy after isolation and determination of sensitivities. The most common infecting organism is S. aureus. There has been an association between nasal carriage of S. aureus in HD patients and catheter infection. Reducing carriage rates has resulted in a decreased incidence of bloodstream infections.31
Antimicrobial Therapy
Pending the results of culture, the initial choice of an antibiotic is empirical and depends on the clinical setting, site of infection, type of device, host factors (e.g., immunocompromised state), severity of illness, and whether the device has been removed. If a VAD-related bloodstream infection is suspected, vancomycin should be initiated empirically because coagulase-negative Staphylococcus is the most common infectious organism and the prevalence of methicillin-resistant S. aureus continues to increase.39 This should be followed by a semisynthetic penicillin or other appropriate antibiotics as guided by sensitivity studies. If the patient is severely ill, septic, or in an immunocompromised state or has a femoral catheter in place, additional coverage for gram-negative bacilli should be initiated.39 Institute a third-generation cephalosporin unless the organism is extended-spectrum β-lactamase positive, in which case a carbapenem is more appropriate.40 If there is concern for P. aeruginosa, initiate therapy with a fourth-generation cephalosporin (e.g., cefepime) or carbapenem, with or without an aminoglycoside. In addition to bacterial pathogens, fungemia should be suspected in septic patients who are receiving TPN, those with prolonged use of antibiotics, or those with a history of a hematologic malignancy or a bone marrow or solid organ transplant or if a femoral catheter is in place.39 If fungemia is suspected or confirmed, initiate an antifungal medication and remove the catheter. An echinocandin medication (e.g., micafungin, caspofungin) is the recommended empirical treatment of suspected fungal infection.40 Although there are no compelling data to specify a duration of antibiotic therapy, expert recommendation is to treat S. aureus infection for 14 days, gram-negative bacilli for 7 to 14 days, and candidal infection with antifungal therapy for 14 days after the first negative blood culture. Complicated infections (e.g., those with suppurative thrombophlebitis, endocarditis, or other similar infections) require 4 to 6 weeks of antibiotic treatment.40
Recommendations vary regarding exit site or tunnel or pocket infections. Jones recommended using aggressive local care in the early stages and a topical antibiotic ointment for short-term treatment.34 Long-term treatment should be avoided because of the risk for Candida colonization.34 Mermel and colleagues,31 however, recommended that in patients with complicated infections, such as tunnel infection or port abscess, the catheter be removed and antibiotics initiated for 7 to 10 days.
If the VAD is retained, consider antibiotic lock therapy (ALT). ALT is a therapeutic option if the goal is to salvage the catheter. Most infections in tunneled catheters originate in the hub and spread to the catheter lumen. A biofilm can form and make eradication of the organism difficult because systemic antibiotics cannot achieve therapeutic levels there. ALT involves filling the catheter hub and lumen with a higher concentration of antibiotics and leaving them in place for extended periods. It is recommended that dwell times not exceed 48 hours before reinstilling the antibiotic solution.40,41 If it is a femoral catheter, dwell time should not exceed 24 hours. ALT should always be used in conjunction with systemic antibiotics, and the recommended duration of treatment is 7 to 14 days.40
Prophylactic Measures
Antibiotic Prophylaxis during Initial Line Insertion: Prophylaxis with vancomycin or teicoplanin during central line insertion has not consistently demonstrated a reduced incidence of catheter-related bloodstream infection. Based on the limited data available, Centers for Disease Control and Prevention (CDC) guidelines currently recommend against giving vancomycin prophylactically because it is an independent risk factor for the acquisition of vancomycin-resistant enterococci and staphylococci with reduced susceptibility to glycopeptides.42 Rather than using antibiotic prophylaxis, focus efforts on interventions designed to discourage the emergence of antimicrobial resistance, such as maximal barrier precautions.43
Impregnated Catheters: Using antimicrobial- or antiseptic-impregnated catheters or silver-impregnated collagen cuffs may be an effective intervention to reduce VAD-related bloodstream infection. A recent metaanalysis favored impregnated catheters in reducing central line–associated bloodstream infections (CLABSIs), but it was acknowledged that the overall methodology of the studies included was poor.44 Nonetheless, the CDC currently recommends the use of these devices if the catheter is anticipated to remain in place for more than 5 days and if the institution’s CLABSI rate is not decreasing after implementation of a comprehensive strategy to reduce catheter-associated infections.41
Routine Line Changing: Despite the incidence of infection and the potential complications, routine changing of VADs is not recommended.41 Cobb and coworkers found that replacement of VADs every 3 days did not prevent infection and that doing so over a guidewire actually increased the risk for bloodstream infection.45
Thrombus Formation
It has been estimated that 2% to 42% of VADs are associated with deep venous thrombosis (DVT).46 Risk factors for catheter-related DVT include the composition, diameter, and position of the VAD; elevated intraluminal pressure; turbulent blood flow; vascular calcification; endothelial injury; and increased levels of fibronectin.46,47 Polyurethane and silicone catheters have a lower rate of VAD-related DVT than do polyethylene or Teflon-coated catheters. A catheter with an external diameter of less than 2.8 mm has a lower incidence of DVT. Incorrect placement of the VAD in the SVC, as opposed to the junction of the SVC and right atrium, results in a higher incidence of catheter-related DVT.46
DVTs may be asymptomatic and not recognized because of the underlying condition of the patient. The incidence of central venous catheter–related DVT, as assessed by venography, has been found to range from 27% to 66%. The majority of these thromboses are asymptomatic. The incidence of clinically overt DVT has been found to range from 0.3% to 28.3%, with most studies finding the incidence to be less than 5%. The first 6 weeks after catheter insertion presents the greatest risk for thromboembolic complications.48
Most HD access failures (80%) are related to thrombosis, with greater than 90% of these thromboses associated with venous outflow stenosis.14 Histologically, hyperplasia of the endothelium and fibromuscular vessel wall occurs. Over time, fibrin deposits build up on the tip of the VAD. This may continue to the point of complete occlusion and prevent infusion or aspiration from the VAD. Early detection may be enhanced by maintaining a high index of suspicion and noting prolonged bleeding after withdrawal of the cannula or a change in the bruit over the device, or both. Prompt consultation with a nephrologist and vascular surgeon is indicated. Heparin or local thrombolytic agents, such as alteplase, may be tried (see the next section).
Catheter Occlusion
Occlusion of the catheter or low flow can be caused by improper positioning, kinking, or compression of the catheter; intraluminal thrombi; extraluminal thrombi; fibrin deposits at the tip of the catheter; or intraluminal precipitation of infusate. Pinch-off syndrome occurs when the line (most often a PICC) is compressed between the clavicle and the first rib. It is manifested clinically as difficulty injecting that is posturally related.16 Overzealous withdrawal of the syringe will collapse the catheter and cause occlusion. Chest radiographs may reveal scalloping of the catheter. Maneuvers to facilitate flow include the Valsalva maneuver, the reverse Trendelenburg position, slight tension on the catheter, placement of the catheter more laterally, IV hydration, and extension of the arms above the head.28,49
If these measures are unsuccessful, the occlusion may be caused by clot formation. The clinician should be familiar with local institutional recommendations for thrombolysis in the setting of recent VAD occlusion attributed to a fibrin plug. Admission and consultation are usually required, and maneuvers to address clotted or occluded catheters are not a standard ED procedure but may be performed under proper circumstances and according to local guidelines. Alteplase has been shown to be both effective and safe in treating central venous catheter occlusions caused by clot.50 If attempted, first withdraw any mobile clot by aspiration with a syringe. Instill 2 mL of alteplase (1 mg/mL) and allow it to dwell for up to 120 minutes. If it remains occluded, the dose may be repeated once.
Embolization
A serious but uncommon complication of VAD use is embolization with air, a catheter fragment, or thrombotic emboli.51–54 Air emboli develop when the lumen of the catheter is left open to air or the catheter is fractured, perforated, or cut. The one-way valve in the Groshong catheter prevents this complication. Be careful to maintain a closed system by clamping the catheter appropriately to prevent delivery of air into the venous circulation. Should the externalized portion of a catheter be damaged, immediately place an appropriate clamp between the damaged portion and the skin.28 Be suspicious for air embolism if an open or damaged catheter is found in association with tachypnea and hypotension.51 Place the patient in a left lateral decubitus and Trendelenburg position to reduce ventricular outflow obstruction by air pockets. Initiate supportive measures, including high-flow oxygen. If this is unsuccessful, attempt aspiration with the catheter advanced into the right ventricle, if possible. Emergency thoracotomy to aspirate air (see Chapter 18) and cardiothoracic surgical consultation may also be warranted.
Embolization of a catheter fragment is a potentially life-threatening complication that can cause acute dyspnea, palpitations, atypical chest pain, hypoxia, and atrial fibrillation. As with any foreign-body embolus, sequelae include sepsis, lung abscess, dysrhythmias, vascular or cardiac perforation, and sudden death. Catheter fragments may be identified radiographically or potentially by ultrasonography and may be removed either surgically or by intravascular retrieval methods.52
Embolization of thrombi with potentially lethal sequelae may occur during routine flushing and injection of solutions through the catheter. Anderson and coworkers prospectively evaluated the size and frequency of catheter thrombi in 43 patients by aspirating after a urokinase flush.53 Clots were noted in 40 of 43 subjects and in 153 of 508 total specimens. Thrombi varied in size from small fragments to 5 cm in length. Burns and McLaren reported one case of a hemodynamically significant pulmonary embolus associated with PICC placement in a 77-year-old man.54
Hemorrhagic Complications
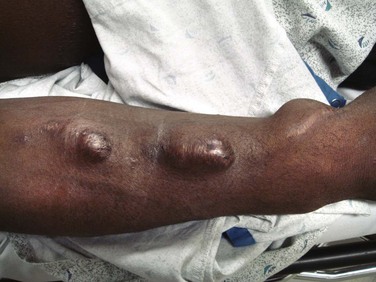
Bleeding may be seen with any indwelling VAD or AV fistula but more commonly occurs with nontunneled VADs or temporary dialysis catheters. It is often related to mechanical trauma, transient or preexisting thrombocytopenia, uremia- or drug-induced platelet dysfunction, and infection. Repeated cannulation of the fistula or graft weakens the wall of the device. Many fistulas will develop aneurysms from repeated use; synthetic grafts can develop pseudoaneurysms from frequent punctures (Fig. 24-13). Severe life-threatening hemorrhage from this high-pressure system may occur and necessitates rapid control. The primary goal should be to control bleeding and prevent exsanguination; however, take care to minimize damage to the VAD that would prevent future use. Use full universal precautions because there is an increased risk for communicable disease in this patient population (2.4% for hepatitis B virus and 7.4% for hepatitis C virus in the United States).55 When the bleeding stops, observe the patient for 2 hours for evidence of rebleeding and to detect graft thrombosis. Treatment can be divided into mechanical modalities and correction of coagulopathy.
Approach to Bleeding Complications
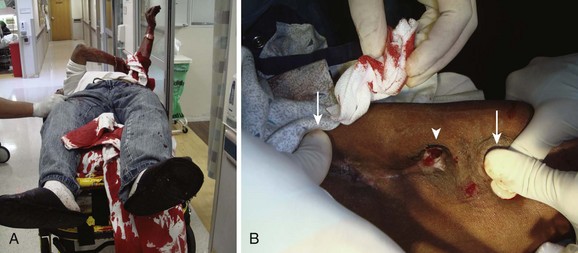
It is important to identify the specific bleeding site, which is challenging when hemorrhage is massive (Fig. 24-14). This is most easily accomplished by digital pressure over the feeding and draining vessels of the shunt above and below the bleeding site. The most easily controlled bleeding is that occurring immediately after dialysis, when simple measures are often effective. Spontaneous bleeding between dialysis treatments often signifies more serious problems, such as infection or mechanical problems with the access site. High pressure from venous stenosis secondary to long-term dialysis complicates the control of bleeding (Fig 24-15).
Direct Pressure: Begin management of bleeding with direct pressure. Always use sterile technique to lessen the chance of infecting a bleeding shunt. With gloved hands, apply direct pressure with fingers and a sterile gauze bandage. For bleeding AV fistulas and shunts secondary to cannulation, apply pressure focally over the site of cannulation. For tunneled catheters, apply pressure over the site of vascular entry of the catheter, not the subcutaneous exit site. Note that this is not possible with subclavian VADs. Hold the pressure for a minimum of 5 minutes and then reevaluate for hemostasis. Holding direct pressure for extended periods or maintaining more diffuse pressure may cause thrombosis and ultimate graft failure, which is a known and unfortunate risk. Wrapping the site with an elastic bandage for a few minutes is acceptable, but longer application in this broad manner may lead to the entire graft clotting.
Dialysis Clamps: Special self-retaining dialysis clamps may be applied over a specific bleeding site (Fig. 24-16). Be careful that the clamp does not slip off the bleeding site because the surface area of the clamp is small. Place sterile gauze or topical thrombin-impregnated gauze under the clamp. Ten to 15 minutes may be required to stop the bleeding, but it is best to avoid frequent checks during the first 5 to 8 minutes to avoid disrupting hemostasis.
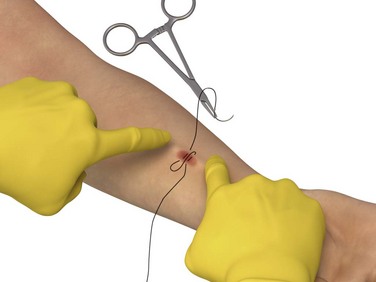
Suture: To adequately visualize the bleeding site, (1) apply concomitant digital pressure to the proximal and distal ends of the shunt or fistula (Fig 24-17), or (2) apply a pneumatic blood pressure cuff or tourniquet distal to the fistula or graft to impede distal-to-proximal arterial flow, unless it is a loop graft, in which case it is applied above (proximal to) the device. Inject the bleeding site subcutaneously with lidocaine with epinephrine. Place a figure-of-eight or horizontal mattress suture of 4-0 nonabsorbable polypropylene or nylon (noncutting needle) at the site of bleeding. Be careful to suture as superficially as possible to prevent damage to the underlying graft or fistula. The patient may require a venogram for evaluation of VAD patency before use. Remove the suture in a few days.
Thrombogenic Agents: If major bleeding has been controlled and oozing is minimal, oxidized cellulose hemostatic agents (Oxycel, Surgicel) may be used to achieve hemostasis. Apply these agents directly over the site of bleeding and hold them in place with gloved hands and a gauze bandage or clamp. The disadvantage of this approach is that the agents are costly and a potential nidus for infection.
Vasoconstrictive Agents: Subcutaneous injection of 2 to 4 mL of lidocaine (2%) with epinephrine to form a wheel around the site may decrease bleeding by both vasoconstriction and local pressure. This may be used in conjunction with chemical cautery.
Chemical Cautery: Silver nitrate (AgNO3), a mildly caustic and hemostatic agent, may stop residual bleeding. The agent needs to be protected from light until just before use. When ready, remove the wooden stick from the container and black plastic wrap and gently apply the gray-tipped end directly on the site. Do not apply it aggressively because this can result in dissolution or dislodgment of the formed clot.
Coagulopathy
Control of hemorrhage may require treatment of an underlying coagulopathy. HD patients are generally considered to be at increased risk for bleeding because of uremic platelet dysfunction. The incidence of major bleeding in these patients is increased with the concomitant use of anticoagulant medications.56 Perform laboratory testing for evaluation: complete blood count, blood urea nitrogen, prothrombin time, international normalized ratio (INR), partial thromboplastin time, and other tests depending on the patient’s medical history or signs. Consult nephrology, vascular surgery, or both if possible before reversing therapeutic anticoagulation given the potential for graft thrombosis and the need for close follow-up.
Uremic Platelet Dysfunction
If platelet dysfunction is suspected because of uremia, administer desmopressin (DDAVP) or cryoprecipitate to control the hemorrhage.30 Adult dosing of DDAVP to control uremic bleeding is 0.3 µg/kg intravenously as a single dose or every 12 hours. Onset occurs in 1 to 2 hours, and its duration is 6 to 8 hours after a single dose. Disadvantages include high cost and adverse reactions, including anaphylaxis, water intoxication or hyponatremia, and thrombotic events (rare). Use of DDAVP may be most appropriate if the platelet count and standard coagulation profile is otherwise normal.
Heparin-Associated Coagulopathy
Heparin administration is not routine during dialysis, and if it is used, small doses are infused (3000 to 5000 units). Heparin is usually stopped about 1 hour before the end of dialysis, and hence heparin-related bleeding is unusual in the ED. If uncontrollable bleeding occurs in the context of recent heparin use, reversal of heparin’s effect with protamine sulfate may be necessary. Administering 1 mg of protamine sulfate will effectively reverse the anticoagulant effect of 100 units of unfractionated heparin. Protamine is less efficacious in the reversal of low molecular weight heparins. Nonetheless, current recommendations are to administer 1 mg of protamine for every 1 mg of low-molecular-weight heparin that the patient received.57
Warfarin-Associated Coagulopathy
The use of warfarin predisposes patients to potentially serious bleeding complications. In an actively bleeding patient with an elevated INR, immediate reversal of the medication’s effect can be achieved with fresh frozen plasma or prothrombin complex concentrates (PCCs). PCCs have the advantage of requiring less total volume to achieve reversal of anticoagulation and therefore less risk for volume overload.57 Vitamin K should also be administered to the patient (10 mg by slow IV infusion).
Catheter Displacement, Migration, or Malposition
Catheter displacement may occur accidentally secondary to patient movement, iatrogenically, or both. The catheter should be firmly secured with sutures, sterile tape strips (with care taken to avoid direct contact with the catheter itself), and premanufactured devices.19 Even with these measures, migration may still occur. With regard to TIVADs, malposition of the intravascular portion may occur as a result of incorrect initial positioning or secondarily from forceful flushing, neck flexion, obesity, severe coughing, emesis, or upper extremity movement. Malposition of the port body may occur intentionally or by unintentional manipulation of the port (twiddler’s syndrome). Obtain a computed tomography scan of the chest to diagnosis this condition. Surgical intervention is usually necessary.58
Catheter Fracture
Fracture of a VAD can occur either subcutaneously or in the externalized portion.59 It may take place with certain physical activities that involve repetitive and excessive motion, such as golfing, swimming, and weight lifting. Subcutaneous fractures cause pain and swelling and require removal of the line. Fractures in the externalized portion may be repaired with commercially available kits and do not always have to be replaced.
Steal Syndrome
Vascular steal syndrome is an uncommon (1% to 3% incidence) but serious complication of AV fistulas that is difficult to predict and often leads to access failure. It occurs because of preferential flow through the low-resistance fistula at the expense of the distal circulation. The syndrome is manifested by classic arterial insufficiency symptoms with exacerbation during dialysis: pain, pallor, numbness, motor weakness, and diminished or absent pulses distal to the fistula.60 Steal syndrome must be differentiated from diabetic or uremic neuropathy because it may lead to the development of irreversible neuromuscular dysfunction and tissue necrosis. Prompt recognition and correction of hand ischemia lead to increased salvage and use of functioning fistulas. Steal syndrome usually requires ligation or removal of the VAD with placement of a new access device in the opposite extremity.
Aftercare Instructions
Before release from the ED, instruct the patient regarding proper catheter care to prolong the device’s lifetime and to decrease morbidity and mortality.28 Patients may bathe and swim normally after maturation of the site; they should avoid direct pressure on the reservoir and report bruising or bleeding immediately. Long-term venous access catheters and multilumen catheters should be dressed in sterile fashion and observed daily for bleeding and signs of infection, including fever, pain, redness, swelling, and purulent drainage. VADs should be gently flushed on a routine basis.28 Heparin flushes are essential to prevent thrombosis (Table 24-1; see p. 454). Tunneled (e.g., Hickman) and nontunneled (e.g., PICC) catheters require flushing twice weekly with 5 mL of heparin (10 U/mL). TIVADs require flushing with heparin every 4 weeks and after use. Generally, Groshong catheters are flushed with 5 mL of saline once weekly. Mahurkar and Uldall catheters are “flushed” during dialysis. Mahurkar catheters are also used for apheresis, in which case they are treated three times a week with normal saline and heparin, as outlined earlier. Patients should not allow phlebotomy or infusion into an extremity with a VAD or have blood pressure measured in that extremity. Signs of infection and any inability to flush an indwelling catheter should be reported immediately to a clinician.
TABLE 24-1
Standard Intravenous Catheter Flushes*
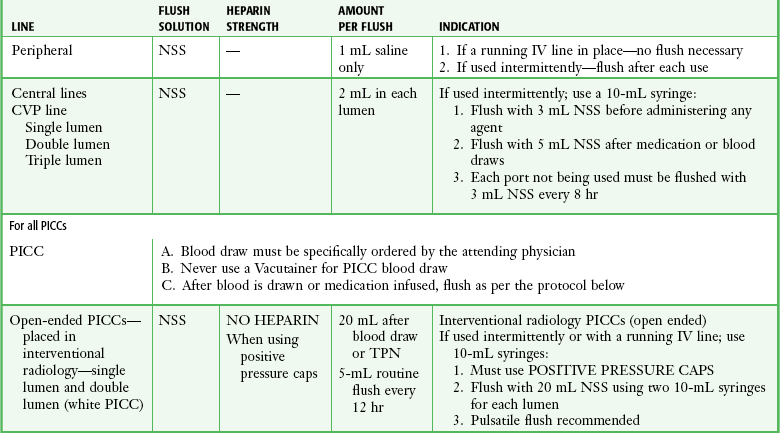
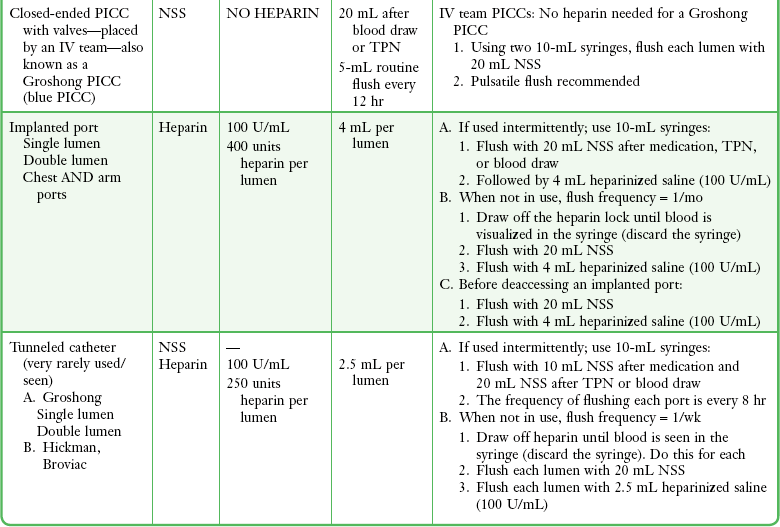
*If an indwelling catheter is used or manipulated in the emergency department, a flush solution is instilled to maintain patency of the catheter.
From Blackburn P, Kokotis K. BARD access systems: vascular access device selection, insertion, and management. In: Weinstein SM, ed. Plumers’ Principles and Practice of Intravenous Therapy. 9th ed. Baltimore: Lippincott; 2001; Infusion Nurses Society. Infusion Therapy in Clinical Practice. 2nd ed. Philadelphia: Saunders; 2001.
References
1. Collins, AJ, Foley, RN, Herzog, C, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(suppl 1):e1–e526.
2. Chugh, TD, Khan, ZU. Intravascular device–related infections: antimicrobial catheters as a strategy for prevention. J Hosp Infect. 2001;49:1.
3. Broviac, JD, Cole, JJ, Scribner, BH. A silicone rubber atrial catheter for prolonged parenteral alimentation. Surg Gynecol Obstet. 1973;136:602.
4. Hickman, RO, Buckner, CD, Clift, RA, et al. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet. 1979;148:871.
5. Fortner, JG, Pahnke, LD. A new method for long-term intrahepatic chemotherapy. Surg Gynecol Obstet. 1976;143:979.
6. Quinton, WE, Dillard, DH, Scribner, BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960;6:140.
7. Scribner, BH, Buri, R, Caner, JEZ. The treatment of chronic uremia by means of intermittent hemodialysis: a preliminary report. Trans Am Soc Artif Intern Organs. 1960;6:114.
8. Brescia, MJ, Cimino, JE, Appel, K, et al. Chronic hemodialysis using venipuncture and a surgically created AV fistula. N Engl J Med. 1966;275:1089.
9. Erben, J, Krasnick, J, Bastecky, J, et al. Experience with routine use of subclavian vein cannulation in hemodialysis. Proc Eur Dial Transplant Assoc. 1969;8:59.
10. Uldall, PR, Dyck, RF, Woods, F, et al. A subclavian cannula for temporary vascular access for hemodialysis or plasmapheresis. Dial Transplant. 1979;8:963.
11. Poole, SM. Quality issues in access device management. J Intraven Nurs. 1999;22:526.
12. Bjeletich, J, Hickman, R. The Hickman indwelling catheter. Am J Nurs. 1980;80:62.
13. Rehman, R, Schmidt, RJ, Moss, AH. Ethical and legal obligation to avoid long-term tunneled catheter access. Clin J Am Soc Nephrol. 2009;4:456.
14. Roy-Chaudhury, P, Kelly, B, Melhem, M, et al. Vascular access in hemodialysis: issues, management, and emerging concepts. Cardiol Clin. 2005;23:249.
15. Umphrey, J, Tarrand, J, Raad, I, A clinical indicator for pediatric oncology patients: prospective monitoring for infections associated with Hickman/Broviac catheters and implantable ports. In: Proceedings of the 19th Annual Conference of American Practitioners of Infection Control. San Francisco, May 31-June 4, 1992.
16. Galloway, S, Bodenham, A. Long-term central venous access. Br J Anaesth. 2004;92:722.
17. DiCarlo, I, Cordio, S, LaGreca, G, et al. Totally implantable venous access devices implanted surgically. Arch Surg. 2001;136:1050.
18. Weeks, S. Unconventional venous access. Tech Vasc Interv Radiol. 2002;5:114.
19. Gorski, L, Czaplewski, L. Peripherally inserted central catheters and midline catheters for the homecare nurse. J Infus Nurs. 2004;27:399.
20. Vanholder, R. Vascular access: care and monitoring of function. Nephrol Dial Transplant. 2001;16:1542.
21. National Kidney Foundation Clinical Practice Guidelines and Clinical Practice Recommendations, 2006 Update for Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. 2006.
22. Schwab, SJ, Besarab, A, Beathard, G, et al. NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation—Dialysis Outcomes Quality Initiative. Am J Kidney Dis. 1997;30(suppl 3):S150.
23. Rodriquez, JA, Armadans, L, Ferrer, E, et al. The function of permanent vascular access. Nephrol Dial Transplant. 2000;15:402.
24. Ethier, J, Mendelsshon, DC, Elder, SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23:3219.
25. Woo, K, Doros, G, Ng, T, et al. Comparison of the efficacy of upper arm transposed arteriovenous fistulae and upper arm prosthetic grafts. J Vasc Surg. 2009;50:1405.
26. Mansilla, AV, Toombs, BD, Vaughn, WK, et al. Patency and life-spans of failing hemodialysis grafts in patients undergoing repeated percutaneous de-clotting. Tex Heart Inst J. 2001;28:249.
27. Hadaway, L. Technology of flushing vascular access devices. J Infus Nurs. 2006;29:129.
28. Taylor, JP, Taylor, JE. Vascular access devices: uses and aftercare. J Emerg Nurs. 1987;13:160.
29. Zacharias, JM, Weatherston, CP, Spewak, CR, et al. Alteplase versus urokinase for occluded hemodialysis catheters. Ann Pharm. 2003;37:27.
30. Hodde, LA, Sandroni, S. Emergency department evaluation and management of dialysis patient complications. J Emerg Med. 1992;10:317.
31. Mermel, LA, Farr, BM, Sheretz, RJ, et al. Guidelines for the management of intravascular catheter-related infections. J Intraven Nurs. 2001;24:180.
32. Polderman, KH, Girbes, ARJ. Central venous catheter use part 1: mechanical complications. Intensive Care Med. 2002;28:1.
33. Safdar, N, Kluger, DM, Maki, DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters. Medicine (Baltimore). 2002;81:466.
34. Jones, GR. A practical guide to evaluation and treatment of infections in patients with central venous catheters. J Intraven Nurs. 1998;21:5134.
35. Fan, ST, Teoh-Chan, CH, Lau, KF. Evaluation of central venous catheter sepsis by differential quantitative blood culture. Eur J Clin Microbiol Infect Dis. 1989;8:142.
36. Raad, I, Hanna, HA, Alakech, B, et al. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med. 2004;140:18.
37. Weber, DJ, Rutala, WA. Central line–associated bloodstream infections: prevention and Management. Infect Dis Clin North Am. 2011;25:77.
38. Nassar, GM, Ayus, JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60:1.
39. Han, Z, Liang, SY, Marschall, J. Current strategies for the prevention and management of central line–associated bloodstream infections. Infect Drug Resist. 2010;3:147.
40. Mermel, LA, Allon, M, Bouza, E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter–related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1.
41. O’Grady, NP, Alexander, M, Burns, LA, et al. Guidelines for the prevention of intravascular catheter–related infections. for the Healthcare Infection Control Practices Advisory Committee. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
42. Grohskopf, LA, Maki, DG, Sohn, AH, et al. Reality check: should we use vancomycin for the prophylaxis of intravascular catheter–associated infections? Infect Control Hosp Epidemiol. 2001;22:176.
43. Mermel, LA. Prevention of intravascular catheter–related infections. Ann Intern Med. 2000;132:391.
44. Hockenhull, JC, Dwan, KM, Smith, GW, et al. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: a systematic review. Crit Care Med. 2009;37:702.
45. Cobb, DK, High, KP, Sawyer, RG, et al. A controlled trial of scheduled replacement of central venous and pulmonary artery catheters. N Engl J Med. 1992;327:1062.
46. Luciani, A, Clement, O, Halimi, P, et al. Catheter-related upper extremity deep vein thrombosis in cancer patients: a prospective study based on Doppler US. Radiology. 2001;220:655.
47. Brattich, M. Vascular access thrombosis: etiology and prevention. ANNA J. 1999;26:537.
48. Verso, M, Agnelli, G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21:3665.
49. Karrei, I. Hickman catheters: your guide to trouble-free use. Can Nurs. 1982;78:25.
50. Semba, CP, Deitcher, SR, Resnansky, L, et al. Treatment of occluded central venous catheters with alteplase: results in 1,064 patients. J Vasc Interv Radiol. 2002;13:1199.
51. Clark, D, Plaizier, E. Devastating cerebral air embolism after central line removal. J Neurosci Nurs. 2011;43:193–196. [quiz 197-198].
52. Surov, A, Wienke, A, Carter, JM, et al. Intravascular embolization of venous catheter—causes, clinical signs, and management: a systematic review. JPEN J Parenter Enteral Nutr. 2009;33:677.
53. Anderson, AJ, Krasnow, SH, Boyer, MW, et al. Hickman catheter clots: a common occurrence despite daily heparin flushing. Cancer Treat Rep. 1987;71:651.
54. Burns, KEA, McLaren, A. Catheter-related right atrial thrombus and pulmonary embolism: a case report and systematic review of the literature. Can Respir J. 2009;16:163–165.
55. Goodkin, DA. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis. 2004;44(5 suppl 2):16.
56. Holden, RM, Harman, GJ, Wang, M, et al. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105.
57. Levi, M. Emergency reversal of antithrombotic treatment. Intern Emerg Med. 2009;4:137.
58. Viale, P. Complications associated with implantable vascular access devices in the patient with cancer. J Infus Nurs. 2003;26:97.
59. Gryn, J, Sacchetti, A. Emergencies of indwelling venous catheters. Am J Emerg Med. 1992;10:254.
60. Khalil, LM, Livingston, DH. The management of steal syndrome occurring after access for dialysis. J Vasc Surg. 1988;7:572.