Chapter 37 Induction of Ovulation
INTRODUCTION
This chapter reviews the underlying scientific basis and clinical guidelines regarding the use of different oral medications for induction of ovulation. It focuses on a discussion of oral agents, including clomiphene citrate, insulin sensitizers, and aromatase inhibitors. Other drugs for ovarian stimulation used for assisted reproductive technology (ART), including preantral medications (injectable gonadotropins and gonadotropin-releasing hormone [GnRH] analogues), and specific agents applied for particular medical disorders, such as prolactin-lowering agents in cases of hyperprolactinemic anovulation, are discussed in Chapters 22 and 38.
PHYSIOLOGIC BASIS OF FOLLICULAR DEVELOPMENT AND OVULATION
An understanding of normal physiology is an important basis for understanding ovulation induction. Ovulation is comprehensively discussed in Chapter 3. A brief description relevant to induction of ovulation is presented here.
Folliculogenesis
Ovarian folliculogenesis is regulated by both endocrine and intraovarian mechanisms that coordinate the processes of cell proliferation and differentiation. The main follicular component of the ovarian cortex is the primordial follicle, consisting of an oocyte arrested at the diplotene stage of the first meiotic division, surrounded by a few flattened cells that will develop into granulosa cells.1 In response to an unknown signal, primordial follicles are gradually and continuously recruited to grow.
Initial Follicular Growth
Initial follicular growth appears to be independent of pituitary gonadotropins. Follicle-stimulating hormone (FSH) does not appear to be essential for early follicle growth because in the absence of FSH follicles can still develop to the early antral stage. However, the involvement of FSH in the development of small follicles is supported by the presence of FSH receptors in granulosa cells.1 Also, FSH activates granulosa cell proliferation and differentiation and reduces the number of atretic follicles grown in vitro.2 Such mitogenic action is facilitated by locally produced growth factors; the production or action of these factors may be modified by FSH.3
Extrapolating from hypogonadal mice in which oocytes grow to normal size and can acquire developmental competence, gonadotropins may not be necessary for oocyte development per se. However, gonadotropins are believed to play a general role in supporting overall oocyte maturation by supporting follicular development and preventing degeneration.4
Early Follicular Development
During the early stage of follicular development, the oocyte grows and the granulosa cells proliferate to form a preantral follicle. At the preantral stage, theca cells begin to differentiate from the surrounding stroma, and once the follicle reaches a species-specific size, it forms a fluid-filled space called an antrum within the granulosa cell layers.5 Antral follicles become acutely dependent on gonadotropins for further growth and development. Follicular growth (from primordial to preantral follicle stage) is continuous. However, less than 1% of primordial follicles present at the time of birth will ever proceed to ovulation, with the majority of follicles degenerating by atresia.6
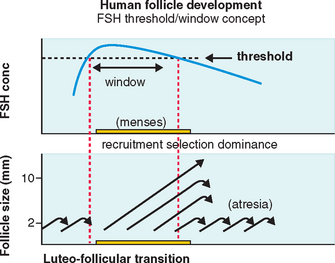
FSH Threshold
FSH concentrations must exceed a certain level before follicular development will proceed.7 When this FSH threshold is surpassed in the normal cycle, the growth of a cohort of small antral follicles is stimulated and ensures ongoing preovulatory follicular development (Fig. 37-1).8 The duration of this period in which the threshold is exceeded (the FSH window) is limited in the normal cycle by a decrease in FSH, which occurs in the early to mid-follicular phase because of negative feedback from rising estrogen levels.
Extending this window would allow the continuous development of more follicles. In the normal cycle, one follicle continues developing despite falling FSH levels because of an increased sensitivity to FSH8 while the remaining follicles respond to falling FSH levels by undergoing atresia, as shown in Figure 37-1.
When the follicle reaches a certain level of development, sustained high circulating levels of estradiol produced by the preovulatory follicle will initiate the midcycle surge in luteinizing hormone (LH) that will trigger ovulation. The surge initiates a gene cascade in granulosa cells, the products of which initiate luteinization, signal the egg to commence meiotic maturation, and lead to rupture of the follicle wall.9
Corpus Luteum Development
The preovulatory LH surge results in luteinization of granulosa and theca cells and alters the steroidogenic pathway so that progesterone is the primary steroid hormone produced after luteinization. However, the corpus luteum retains the ability to produce estrogen. Adequate luteal progesterone is secreted to allow maintenance of pregnancy until a conceptus-derived source of progesterone, the placenta, can produce adequate amounts of this steroid to support pregnancy.10
OVULATION INDUCTION
Ovulatory Dysfunction Classification
Clinically obvious anovulation or oligo-ovulation has been traditionally classified into three groups: World Health Organization (WHO) group I, II, and III anovulation.11
Medications for Ovarian Follicle Stimulation
Gemzell and his coworkers announced the first successful induction of ovulation using human pituitary gonadotropins in 1958 and the first pregnancy in 1960.12,13 One year later, Bettendorf and his group reported a similar experience.14 In 1961, Greenblatt and his coworkers published the first results of ovulation induction achieved by the use of clomiphene citrate (at that time known as MRL/41).15
CLOMIPHENE CITRATE
For more than 40 years, clomiphene citrate has been the most commonly used oral agent for induction of ovulation. Since the early 1960s, results of clomiphene citrate treatment have not changed appreciably, despite the advent of modern immunoassays for steroid hormones, advances in ultrasound technology for cycle monitoring, and the introduction of commercial ovulation predictor kits that allow accurate identification of the midcycle LH surge.
It is interesting to note that clomiphene citrate is considered pregnancy risk category X. Studies in rats and mice have shown a dose-related increase in some types of malformations and an increase in mortality. This is particularly important when considering the relatively long half-life of about 5 days to 3 weeks (depending on the isomer) and that clomiphene citrate may be stored in body fat.16–18 Fortunately, studies in humans have not found an association between clomiphene citrate and congenital defects.
Structure and Pharmacokinetics
Chemically, clomiphene citrate is a nonsteroidal triphenylethylene derivative and is chemically related to diethylstilbestrol and tamoxifen. Like the latter of these compounds, clomiphene can be considered to be a SERM that can exhibit both estrogen agonist and antagonist properties depending on the prevailing levels of endogenous estrogen. Estrogen agonist properties are manifest only when endogenous estrogen levels are extremely low. Otherwise, clomiphene citrate acts mainly as an antiestrogen.16
Clomiphene citrate is cleared through the liver and excreted in the stool. About 85% of an administered dose is eliminated after approximately 6 days, although traces may remain in the circulation for much longer.17 Clomiphene citrate is a racemic mixture of two distinct stereoisomers, enclomiphene and zuclomiphene, having different properties. Available evidence indicates that enclomiphene is the more potent antiestrogenic isomer and the one primarily responsible for the ovulation-inducing actions of clomiphene citrate.16–18
Levels of enclomiphene rise rapidly after administration and fall to undetectable concentrations after few days. Zuclomiphene, the transisomer of clomiphene citrate, is cleared far more slowly; levels of this less active isomer remain detectable in the circulation for more than 1 month after treatment and may actually accumulate over consecutive cycles of treatment.18
Pharmacodynamics and Mode of Action
Clomiphene binds to estrogen receptors throughout the body due to its structural similarity to estrogen. However, unlike natural estrogen, which only binds estrogen receptors for a period of hours, clomiphene citrate binds estrogen receptors for an extended period of time, weeks rather than hours. Such extended binding ultimately depletes estrogen receptor concentrations by interfering with the normal process of estrogen receptor replenishment.15
It is believed that the hypothalamus is the main site of action because in normally ovulatory women, clomiphene citrate treatment was found to increases GnRH pulse frequency.19 However, actions at the pituitary level may also be involved because clomiphene citrate treatment increased pulse amplitude, but not frequency in anovulatory women with PCOS, in whom the GnRH pulse frequency is already abnormally high.20
During clomiphene citrate treatment, levels of both LH and FSH rise, then fall again after the typical 5-day course of therapy is completed. In successful treatment cycles, one or more dominant follicles emerge and mature, generating a rising tide of estrogen that ultimately triggers the midcycle LH surge and ovulation.19,20
Clomiphene Citrate Administration
The effective dose of clomiphene citrate for most women ranges from 50 to 250 mg/day, although some women will be found to be sensitive to clomiphene citrate and only require doses as low as 12.5 to 25 mg/day. Most women will ovulate at the lower doses, with 52% ovulating after 50 mg/day and another 22% after treatment with 100 mg/day. Although higher doses are sometimes required, the ovulation rates are usually low at these doses, with only 12% ovulating after 150 mg/day, 7% after 200 mg/day, and 5% after 250 mg/day. Most women who fail to respond to 150 mg/day of clomiphene citrate will ultimately require alternative or combination treatments.21,22
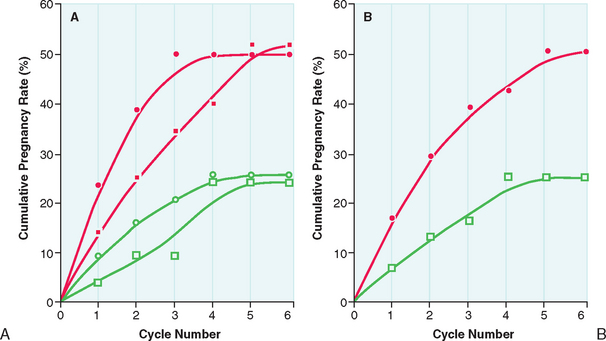
After the lowest ovulatory dose is determined, the same dose is repeated until pregnancy is achieved or a maximum number of approximately six cycles is reached. Pregnancy rates are highest during the first three cycles of clomiphene citrate treatment. The chances of achieving pregnancy are significantly lower beyond the third treatment cycle and uncommon beyond the sixth treatment cycle (Fig. 37-2). Therefore, it is rarely advisable to continue clomiphene citrate treatment beyond six treatment cycles.21
The recommendation regarding which dose to apply is clear in cases of anovulatory infertility (increments of 50 mg until ovulation is achieved, then the dose is maintained). However, this is not clear in cases with ovulatory infertility. There are no adequate studies regarding which dose or what response (number of mature follicles) would be optimal in these women. This uncertainty is related to the individual variability in response to clomiphene citrate treatment irrespective of the dose and to the absence of a clear correlation between pregnancy rates and the number of mature follicles or amount of clomiphene citrate administered. Moreover, the value of clomiphene citrate treatment in enhancing the chance of achieving pregnancy in cases with ovulatory infertility has been questioned22 and has not been proven by large controlled studies.
Treatment Monitoring
Monitoring is required to ensure that ovulation is occurring during a clomiphene citrate treatment cycle. For most patients treated with clomiphene citrate, ovulation monitoring can often be done with any one of a number of low-cost, highly convenient methods conducted in the setting of a general obstetrician and gynecologist practice. Objective evidence of ovulation and normal luteal function are keys to successful treatment. The choice may vary and should be tailored to meet the needs of the individual patient.23
Urinary LH Detection Kit
An ovulation prediction kit can identify the midcycle LH surge in urine and more precisely defines both the interval of peak fertility (day of surge detection and the next 2 days).24 Usually the LH surge is observed from 5 to 12 days after the last clomiphene citrate tablet.25 Ovulation will generally follow within 14 to 26 hours of detection of an LH surge. Consequently, timed coitus or intrauterine insemination (IUI) on the day after the first positive urinary LH test will have the highest pregnancy rates.
Midluteal Progesterone
BBT charts and urinary LH detection kits can confirm that ovulation has occurred but do not document the quality of luteal function, which requires further tests—classically, a serum progesterone determination or endometrial biopsy.
A progesterone level above 3 ng/mL is evidence of ovulation.26 Midluteal phase progesterone concentration (7 days after BBT shift or 7 to 9 days after urinary LH surge) offers more information. Levels exceeding 10 ng/mL are usually suggestive of an adequate luteal phase.27 Endometrial biopsy and “secretory” histology that results from the action of progesterone also provides evidence of ovulation. Endometrial “dating,” using established histologic criteria, has been traditionally suggested for diagnosing luteal phase adequacy.28 However, there is still much uncertainty about the importance of luteal phase defects diagnosed by serum progesterone levels or by endometrial biopsies, reflecting our poor state of knowledge regarding the actual mechanisms involved in endometrial receptivity and implantation.29,30
More sophisticated measures to monitor the outcome of clomiphene citrate treatment, including transvaginal follicular ultrasound scans and measurements of serum estradiol and LH levels, can usually be conducted in a subspecialist reproductive endocrinology practice. Because of the cost and logistic requirements involved, the method is generally reserved for patients in whom less complicated methods fail to provide the necessary information and for women who are at a high risk for developing serious complications such as severe ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy.23
In the past, it was generally recommended that patients receiving clomiphene citrate treatment be examined before starting each new treatment cycle to ensure that there was no significant residual ovarian enlargement or ovarian follicular cysts. However, it appears that small residual follicular cysts usually do not expand during continued treatment.31 Consequently, it is unnecessary to perform “baseline” ultrasound examinations before each new treatment cycle. However, it is reasonable to withhold treatment when symptoms suggest the presence of a large cyst or gross residual ovarian enlargement.23
Outcome of Clomiphene Citrate Treatment
Clomiphene citrate treatment has been reported to successfully induce ovulation in 60% to 80% of properly selected women. More than 70% of those who ovulate respond at the 50-mg or 100-mg dosage level. In young anovulatory women in whom anovulation is the sole reason preventing them from conceiving, cumulative conception rates between 60% and 70% are observed after up to three successfully induced ovulatory cycles, and 70% to 85% after five cycles. Overall, cycle fecundity is approximately 15% in women who ovulate in response to treatment.21
In the reality of daily clinical practice, clomiphene citrate induction of ovulation usually results in much lower pregnancy rates, particularly in a subspecialty referral infertility practice. Important factors that adversely affect treatment outcome are increased age (particularly older than age 35), presence of other treated or untreated infertility factors, and increasing duration of infertility.32 Amenorrheic women are more likely to conceive than oligomenorrheic women, probably because those who already ovulate, albeit inconsistently (oligomenorrheic), are more likely to have other coexisting infertility factors. Generally speaking, failure to conceive within six clomiphene citrate-induced ovulatory cycles is a clear indication to expand the diagnostic evaluation to exclude other factors, change the overall treatment strategy, or both.23
Adverse Effects and Risks of Clomiphene Citrate
Side Effects
Less specific side effects include breast tenderness, pelvic discomfort, and nausea, all observed in 2% to 5% of clomiphene citrate-treated women.33 In addition, there are relatively common reports of premenstrual syndrome-type symptoms in women on clomiphene citrate.34
Congenital Anomalies
There is no evidence that clomiphene citrate treatment increases the overall risk of birth defects or of any specific malformation. Several large series have examined the question and have drawn the same conclusion.35,36 Earlier suggestions that the incidence of neural tube defects might be higher in pregnancies conceived during clomiphene citrate treatment have not been confirmed by more recent studies.37 A small study of pregnancy outcome in women inadvertently exposed to clomiphene citrate during the first trimester also found no increase in the prevalence of congenital anomalies.38
Pregnancy Loss
More than one study has suggested that pregnancies resulting from clomiphene citrate treatment are at increased risk of spontaneous abortion compared to spontaneous pregnancies. A study reviewed outcomes of 1744 clomiphene pregnancies compared to outcomes of 3245 spontaneous pregnancies.39 In this study, pregnancy loss was classified as either clinical if a sac was seen on ultrasound or if it occurred after 6 weeks’ gestation, or preclinical if a quantitative human chorionic gonadotropin (hCG) was greater than or equal to 25 IU/L and no sac was seen or abortion occurred earlier. In this study, when preclinical and clinical were considered together, the overall incidence of spontaneous abortion was slightly higher for clomiphene pregnancies than for spontaneous pregnancies (23.7% versus 20.4%). When considered separately, preclinical spontaneous abortions were also increased by clomiphene citrate overall (5.8% versus 3.9%) and for women at least 30 years old (8.0% versus 4.9%), but not for those younger than age 30 (3.7 versus 3.0%). In contrast, when clinical abortions were considered separately, clomiphene citrate did not increase the overall rate (18.0% versus 16.4%), but the rate was increased for women younger than age 30 (15.9% versus 11.2%).
More recent studies looking at rate of spontaneous miscarriage in 62,228 clinical pregnancies resulting from ART procedures initiated in 1996 to 1998 in U.S. clinics also found that spontaneous miscarriage risk was increased among women who used clomiphene citrate.40,41 However, the results of these studies are not considered to be conclusive. The pregnancy loss seen in women after clomiphene treatment may actually be related to other comorbidities in these patients, such as insulin resistance, other genetic factors related to PCOS or unexplained infertility, endometriosis, and advancing maternal age.42
Antiestrogenic Effects and Fertility
These adverse effects are believed to explain the “discrepancy” between the ovulation and conception rates observed in clomiphene citrate-treated patients. These effects appear to be more apparent at higher doses or after longer durations of treatment. It has been suggested that treatment with exogenous supplemental estrogen may help to minimize or negate these effects.43,44 However, there is little compelling evidence to support any beneficial effect of estrogen replacement to reverse these peripheral antiestrogenic effects.23
Clomiphene citrate treatment has been shown to affect the quality and quantity of cervical mucus production as well as the structure of the endometrium.45–47 However, cervical mucus score (based on quantity and quality) has not been found to be of proven prognostic value on the treatment outcome (i.e., achievement of pregnancy).
The endometrium is believed to be one of the most important targets of the antiestrogenic effect of clomiphene citrate and may explain a large part of the lower pregnancy rate and the possible higher miscarriage rate with clomiphene citrate. Successful implantation requires a receptive endometrium, with synchronous development of glands and stroma.48
Studies have reported conflicting effects of clomiphene citrate on the endometrium45–47,49–51 possibly due to different methodology used for endometrial assessment. However, a recent study has prospectively applied morphometric analysis of the endometrium, a quantitative and objective technique to study the effect of clomiphene citrate on the endometrium in a group of normal women. In this study, clomiphene citrate was found to have a deleterious effect on the endometrium, demonstrated by a reduction in glandular density and an increase in the number of vacuolated cells.51 In addition, a reduction in endometrial thickness below the level thought to be needed to sustain implantation was found in up to 30% of women receiving clomiphene citrate for ovulation induction or for unexplained infertility.45 This observation has been confirmed by other studies.46,47
Decreased uterine blood flow during the early luteal phase and the peri-implantation stage has been suggested to explain, at least in part, the poor outcome of clomiphene citrate treatment.51 Other investigators have suggested the presence of other unrecognized factors associated with clomiphene citrate treatment failure.52–54 Moreover, there is some evidence for a direct negative effect of clomiphene citrate on fertilization, and on early mouse and rabbit embryo development.55,56 In addition, there have been reports of low-quality oocytes associated with clomiphene citrate treatment. However, the negative effects of clomiphene citrate in some animal studies have not been confirmed by others,53,54 and at present it is unclear if there are adverse direct effects of clomiphene citrate on human oocytes and human embryo development.
Because of the above-mentioned evidence for adverse peripheral antiestrogenic effects of clomiphene citrate treatment, several investigators tried to reverse these effects by administering estrogen concomitantly with clomiphene citrate treatment. Some investigators have reported increased endometrial thickness and improved pregnancy rates with this approach57,58; others have reported no benefit44 or even a deleterious effect of estrogen administration.59 Another approach has been to administer clomiphene citrate earlier during the menstrual cycle rather than starting on day 5,60 in the hopes of allowing the antiestrogenic effect to wear off to some extent before ovulation and implantation. In view of the long half-life of clomiphene citrate isomers, this approach is unlikely to be successful. A third method has been to combine another SERM such as tamoxifen, which has more estrogen agonistic effect on the endometrium with clomiphene citrate or to use tamoxifen as an alternative to clomiphene citrate.61 However, none of these strategies have proved to be completely successful in avoiding the peripheral antiestrogenic effects of clomiphene citrate. A more recent publication has suggested that high-dose soy isoflavones may be able to overcome the antiestrogenic effect of clomiphene citrate on the endometrium.62 This report remains to be confirmed by other investigators.
To summarize, from the available evidence and accumulated clinical experience, it is difficult to conclude the exact significance of adverse antiestrogenic effects on pregnancy outcome in clomiphene citrate-treated women. It seems that individual variability exists regarding sensitivity to the peripheral antiestrogenic effects of clomiphene citrate, likely because of the complexity of estrogen receptor replenishment and activation, and individual differences in the pharmacokinetics of clomiphene citrate. The discrepancy in the success rates in achieving pregnancy between women with unexplained infertility and women with PCOS in response to clomiphene citrate treatment (higher rates in PCOS women) suggests that PCOS women may be less vulnerable to the antiestrogenic effects of clomiphene citrate on peripheral tissues. Recent findings of differences in the expression levels of receptors (androgen receptor, estrogen receptor α and β, progesterone receptor) and cofactors in human endometrium and myometrium in PCOS patients support this hypothesis.63 For example, the p160 family of steroid receptor coactivators, known to facilitate the action of steroid receptors throughout the menstrual cycle, was found to be overexpressed in the endometrium of women with PCOS.80 However, the presence of infertility factors, rather than an increased sensitivity to the antiestrogenic effects of clomiphene citrate, in non-PCOS women may explain the less favorable outcome of clomiphene citrate treatment.
FAILURE OF CLOMIPHENE CITRATE TREATMENT
Etiologies
Clomiphene Pregnancy Failure
The reasons for clomiphene pregnancy failure (women who ovulate in response to clomiphene citrate but do not achieve pregnancy) may be related to a wide variety of underlying infertility factors, such as male factor infertility, endometriosis, undiagnosed tubal factor, or endometrial receptivity factors. However, the success of many of these women in achieving pregnancy with alternative ovarian stimulation protocols using injectable gonadotropins or aromatase inhibitors supports the hypothesis that persistent antiestrogenic effects associated with clomiphene citrate might play a major role in the discrepancy between ovulatory rates and pregnancy rates.64–66
ALTERNATIVE APPROACHES FOR CLOMIPHENE FAILURES
Some women who are refractory to standard clomiphene citrate treatment regimens will ovulate in response to alternative regimens of clomiphene citrate, including longer duration or higher doses of clomiphene citrate treatment. For example, an 8-day treatment regimen or doses of 200 to 250 mg/day can be effective when shorter courses of therapy fail. However, longer treatment and higher doses are expected to be associated with more antiestrogenic effects and reduced chances for achieving pregnancy even though ovulation is achieved.23
Insulin-Sensitizing Agents
Insulin resistance and hyperinsulinemia are recognized features of PCOS. Hyperinsulinemia contributes significantly to hyperandrogenism and chronic anovulation. It is logical that agents that improve insulin action in the body (insulin sensitizers) will help infertile PCOS women with insulin resistance to achieve ovulation by lowering their hyperinsulinemia. This is particularly true in PCOS patients who fail to ovulate with clomiphene citrate treatment. De Leo and colleagues reviewed the use of insulin-lowering agents in the management of PCOS,67 with extensive discussion of the effect of these agents on endocrine, metabolic, and reproductive functions.
Although it is believed that the endocrine and reproductive benefits observed during treatment with insulin sensitizers in PCOS women are due to improving insulin resistance, insulin sensitizers may work through other mechanisms to achieve ovulation in these patients. One such mechanism is a direct effect on ovarian steroidogenesis. There is evidence that the insulin sensitizers, metformin and thiazolidinediones, can modulate steroid production through direct suppression of steroidogenesis enzymes. Troglitazone was found to inhibit progesterone production by human luteinized granulosa cells and to reduce androgen production by rat theca cells.68,69 It was also found to directly inhibit 3β-hydroxysteroid dehydrogenase, leading to decreased progesterone secretion by porcine granulosa cells.70 Troglitazone, and presumably other thiazolidinediones, inhibits cholesterol biosynthesis in Chinese hamster ovary cells71 and aromatase in human granulosa cells.72
Metformin
Several studies demonstrated that treatment alone with the insulin-sensitizing agent metformin could restore menses and cyclic ovulation in many amenorrheic PCOS women.16,73 This led some to advocate metformin as primary therapy in infertile PCOS women with insulin resistance (1000 to 2000 mg/day in divided doses) and add clomiphene citrate only in those who fail to respond. However, the relatively greater costs per cycle compared to clomiphene citrate, as well as the complexity of metformin treatment and the frequency of significant gastrointestinal side effects (e.g., nausea, vomiting, diarrhea) made others prefer to reserve metformin treatment for those who first prove resistant to clomiphene citrate. Caution should be used in prescribing these drugs to women with potential renal or liver disease. It is recommended that a creatinine level and liver function tests be obtained before starting therapy.73
Unfortunately, most of the studies published so far on the use of metformin in PCOS women were not randomized. Even the randomized trials included a small number of patients, which prevented definitive conclusions. However, meta-analysis of the combined results of prior clinical trials concluded that metformin is effective in achieving significant rates of ovulation and pregnancy in women with PCOS. It is important to note the difference between achieving ovulation and achieving a pregnancy.
Effectiveness of Metformin
A recent Cochrane meta-analysis showed that metformin is effective in achieving ovulation in women with PCOS with an odds ratio (OR) of 3.88 (confidence interval [CI], 2.25–6.69) for metformin versus placebo and 4.41 (CI, 2.37–8.22) for metformin and clomiphene citrate versus clomiphene citrate alone. An analysis of pregnancy rates suggests a significant treatment effect for metformin and clomiphene citrate (OR, 4.40; CI, 1.96–9.85). The authors concluded that metformin is an effective treatment for anovulation in women with PCOS, and its choice as a first-line agent seems justified.73
A more recent systematic review of metformin use in women with PCOS included cohort and randomized, controlled trials of metformin versus placebo, metformin versus clomiphene citrate, and metformin plus clomiphene citrate versus placebo plus clomiphene citrate. The authors found metformin to be 50% better than placebo for ovulation induction (RR, 1.50; 95% CI, 1.13–1.99). However, metformin alone was not of confirmed benefit versus placebo for achievement of pregnancy (RR, 1.07; CI, 0.20–5.74) whereas metformin plus clomiphene citrate was threefold superior to clomiphene citrate alone for ovulation induction (RR, 3.04; CI, 1.77–5.24) and pregnancy (RR, 3.65; CI, 1.11–11.99). The authors concluded that metformin is effective for ovulation and metformin plus clomiphene citrate appears to be very effective for achievement of pregnancy compared to clomiphene citrate alone.74 In a prospective, randomized, double-blind, controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with PCOS, it was shown that metformin 850 mg twice daily was associated with a higher pregnancy rate than clomiphene citrate 150 mg for 5 days from the third day of a progesterone withdrawal bleed (69% versus 34%).75 There was also a tend toward a higher birth rate. In a recent multicenter randomized placebo controlled trial, it was shown that metformin did not increase the probability of ovulation or pregnancy when added to clomiphene citrate as a first-line treatment of infertile women with PCOS.76 Therefore, in most cases clomiphene citrate is considered the drug of choice in the treatment of anovulatory infertility of PCOS. Metformin can be added if clomiphene fails to induce ovulation.
Alternative Insulin-Sensitizing Agents
Other insulin-sensitizing agents such as the thiazolidinediones (troglitazone, rosiglitazone, and pioglitazone) and chiroinositol77–84 have been used in small clinical trials to achieve ovulation in PCOS women with insulin resistance. However, because of the higher safety profile, longer clinical experience, and lower cost of metformin, the other insulin sensitizers should be reserved for cases resistant to metformin treatment.85 Finally, before concluding this section on the use of insulin sensitizers in infertile women with PCOS, it is necessary to stress the importance of other nonpharmacologic approaches to improve insulin action and reduce insulin resistance, including dietary factors, weight loss, and exercise. The use of insulin-sensitizing agents should be as an adjuvant to these nonpharmacologic approaches.
Human Chorionic Gonadotropin
A number of clinical studies have examined the efficacy of using exogenous hCG as a surrogate LH surge in women who fail to ovulate in response to clomiphene citrate alone.31,86,87 Clinical studies in anovulatory infertile women have demonstrated that on the day of the spontaneous LH surge in successful clomiphene citrate-induced ovulatory cycles, mean diameter of the lead follicle ranges between 19 and 30 mm (median diameter, 25 mm).87 Given that the average growth rate of the preovulatory follicle over the days immediately preceding ovulation is approximately 2 mm/day, the LH surge can occur during an interval of time of approximately 5 days. Normally, the preovulatory follicle triggers its own ovulation at the peak of maturity by generating and maintaining estrogen levels above the threshold required, inducing the LH surge. Timing of the spontaneous LH surge is therefore always optimal, and that of hCG administration can never be more than an educated guess.
In those uncommon instances in which hCG treatment may be effective and is necessary, it is probably best postponed until the lead follicle reaches or exceeds 20 mm in mean diameter.23 Occasionally, a couple may require both clomiphene citrate treatment and IUI because of anovulation and a coexisting male factor. Clearly, accurate timing of the insemination is crucial to the success of treatment.
Effectiveness
In a review of 2000 consecutive completed ovarian stimulation cycles with timed intercourse or IUI, the occurrence of an LH surge was associated with a higher pregnancy rate compared to hCG administration with clomiphene citrate treatment, but a lower pregnancy rate compared to hCG administration with gonadotropin treatment, suggesting that awaiting occurrence of the LH surge might be associated with a better outcome with clomiphene citrate treatment.88
Gonadotropins
Clomiphene citrate-resistant anovulatory women who ultimately require exogenous gonadotropins to achieve ovulation might benefit from a trial of sequential clomiphene citrate and gonadotropin therapy. Sequential clomiphene citrate and gonadotropin (hMG or FSH) therapy has become increasingly used to produce ovarian superovulation for patients who fail clomiphene citrate treatment.89,90
The value of adding clomiphene citrate during ovarian superovulation is to decrease the FSH dose required for optimum stimulation. However, care in monitoring must be taken because clomiphene citrate use may be associated with peripheral antiestrogenic effects, offsetting the FSH dose reduction benefit.91
Laparoscopic Ovarian Drilling
Ovarian drilling is reported to result in resumption of spontaneous ovulation or at least restore sensitivity to clomiphene citrate treatment. Formation of postoperative adhesions that may compromise fertility is common.92 Serum testosterone concentrations typically fall by approximately 40% to 50%, at least for a period of months. About 70% to 90% of properly selected candidates will ovulate after drilling, and 50% to 80% of those will conceive. Consequently, ovarian drilling is perhaps best reserved for clomiphene citrate-resistant women in whom cost or logistic considerations effectively preclude alternative treatments (e.g., exogenous gonadotropins).23 Ovarian drilling does not seem to improve the metabolic derangement of insulin resistance and lipid dysfunction.93
Aromatase Inhibitors
Aromatase Description
Aromatase is a microsomal member of the cytochrome P450 hemoprotein-containing enzyme complex superfamily (P450arom, the product of the CYP19 gene). Aromatase catalyzes the rate-limiting step in the production of estrogens, that is, the conversion of androstenedione and testosterone via three hydroxylation steps to estrone and estradiol, respectively.94 Aromatase activity is present in many tissues, such as the ovaries, the brain, adipose tissue, muscle, liver, breast tissue, and in malignant breast tumors. The main sources of circulating estrogens are the ovaries in premenopausal women and adipose tissue in postmenopausal women.95 Aromatase is a good target for selective inhibition because estrogen production is a terminal step in the biosynthetic sequence (Table 37-1).
Table 37-1 Potential Advantages of Using an Aromatase Inhibitor for Induction of Ovulation
| High pregnancy rates |
| Monofollicular ovulation in most anovulatory patients |
| High safety due to short half-life and few adverse effects |
| Reduced rate of multiple pregnancy |
| Reduced risk of severe ovarian hyperstimulation syndrome |
| Reduced FSH dose required for controlled ovarian stimulation |
| Improved response to FSH in poor responders |
| Low cost of treatment (average, $30–$100 per cycle) |
| Convenience of administration: oral route, different regimens, including single-dose regimen |
Types of Aromatase Inhibitors
A large number of aromatase inhibitors have been developed over the past 30 years with the most recent, third-generation aromatase inhibitors licensed mainly for breast cancer treatment in postmenopausal women.96 Aromatase inhibitors have been classified in different ways: into steroidal and nonsteroidal and, according to the stage of development, into first-, second-, and third-generation groups.
The first aromatase inhibitor to be used clinically was aminoglutethimide, which induces a medical adrenalectomy by inhibiting many other enzymes involved in steroid biosynthesis.97 Although aminoglutethimide is an effective hormonal agent in postmenopausal breast cancer, its use is complicated by the need for concurrent corticosteroid replacement, in addition to side effects such as lethargy, rashes, nausea, and fever that result in 8% to 15% of patients stopping treatment.98 The lack of specificity and unfavorable toxicity profile of aminoglutethimide led to the search for more specific aromatase inhibitors. In addition, the earlier aromatase inhibitors were not able to completely inhibit aromatase activity in premenopausal patients.
The third-generation aromatase inhibitors commercially available include two nonsteroidal preparations, anastrozole and letrozole, and a steroidal agent, exemestane.99–101 Anastrozole and letrozole are available for clinical use in North America, Europe, and other parts of the world for treatment of postmenopausal breast cancer. These triazole (antifungal) derivatives are reversible, competitive aromatase inhibitors with considerably greater intrinsic potency than aminoglutethimide (more than 1000 times), and at doses of 1 to 5 mg/day, inhibit estrogen levels by 97% to more than 99% down to concentrations below detection by most sensitive immunoassays. Aromatase inhibitors are completely absorbed after oral administration, with a mean terminal half-life of approximately 45 hours (range, 30 to 60 hours) with clearance from the systemic circulation mainly by the liver. Mild gastrointestinal disturbances account for most of the adverse events, although these have seldom limited therapy. Other adverse effects including asthenia, hot flashes, headache, and back pain are based on studies in postmenopausal women.99–101
Mechanism of Action for Ovulation Induction
Recently, success in using aromatase inhibitors for induction of ovulation has been reported.102–109 Several hypotheses have been postulated for the mechanisms underlying the effectiveness of these medications in ovulation induction.110
Central Hypothesis
It might be possible to mimic the action of clomiphene citrate, without depletion of the estrogen receptors, by administration of an aromatase inhibitor in the early part of the menstrual cycle. There is evidence that both circulating estrogen (produced by the ovaries) and locally produced estrogen (produced in the brain) exert negative feedback on the release of gonadotropins.111–114 Blocking estrogen production from all sources by inhibiting aromatization would release the hypothalamic-pituitary axis from estrogen-negative feedback, thereby increasing gonadotropin secretion and resulting in stimulation of ovarian follicles. The selective nonsteroidal aromatase inhibitors have a relatively short half-life (approximately 45 hours) compared to clomiphene citrate, and would be ideal for this purpose because they are eliminated from the body rapidly.115,116 In addition, no adverse effects on estrogen target tissues is expected, because no estrogen receptor down-regulation occurs, in contrast to the estrogen receptor depletion observed in clomiphene citrate-treated cycles.
In addition to the primary response of gonadotropins to diminished estrogen-negative feedback during aromatase inhibitor use, another centrally mediated mechanism of action may be possible independent of GnRH-mediated gonadotropin release. Accumulating evidence suggests that estrogen can modulate the activin/inhibin/follistatin system,117–120 leading to FSH production through a direct effect on the pituitary gonadotropes. Activins are produced by a wide variety of tissues, including the pituitary gland,121 and stimulate synthesis of FSH by a direct action on the gonadotropes.122 Follistatin, also produced by the pituitary gland, is an activin-binding protein and may decrease FSH synthesis by sequestering activin.123 Estrogen, through activin suppression,124 was found to selectively suppress FSH, but not LH, production through a GnRH-independent mechanism.124–126
In women with PCOS, relative oversuppression of FSH may be the result of excessive androgen produced from the ovary being converted to estrogen by aromatization in the brain. The aromatase inhibitors suppress estrogen production in both the ovaries and the brain. In the case of PCOS, therefore, aromatase inhibitors should result in a robust increase in FSH release and subsequent follicle stimulation and ovulation. The actual FSH release is likely to be blunted by the high circulating inhibin found in PCOS patients127–130 that would not be altered by aromatase inhibition. The raised concentrations of inhibin A and B is probably due to the increased number of small antral follicles.129 In addition, because aromatase inhibition does not antagonize estrogen receptors in the brain, the initiation of follicle growth accompanied by increasing concentrations of both estradiol and inhibin results in a normal secondary feedback loop that limits FSH response, thereby avoiding the risk of high multiple ovulation and OHSS.
Peripheral Hypothesis
A second hypothesis to explain the mechanism of action of the aromatase inhibitors in ovarian stimulation involves an increased follicular sensitivity to FSH. This may result from temporary accumulation of intraovarian androgens, because conversion of androgen substrate to estrogen is blocked by aromatase inhibition. Recent data support a stimulatory role for androgens in early follicular growth in primates.131 Testosterone was found to augment follicular FSH receptor expression in primates, suggesting that androgens promote follicular growth and estrogen biosynthesis indirectly by amplifying FSH effects.132,133 Also, androgen accumulation in the follicle stimulates insulin-like growth factor I (IGF-I), which may synergize with FSH to promote folliculogenesis.134–137 It is likely that women with PCOS already have a relative aromatase deficiency in the ovary, leading to increased intraovarian androgens,138,139 which leads to the development of multiple small follicles responsible for the polycystic morphology of the ovaries. The androgens may also increase FSH receptors, making these PCOS ovaries exquisitely sensitive to an increase in FSH either through exogenous administration of gonadotropins (hence the high risk of OHSS) or through endogenous increases in FSH as a result of decreased central estrogen feedback induced by aromatase inhibition. In the latter case, a relatively small rise in FSH, because of a normal inhibin/estrogen feedback loop, generally leads to monofollicular ovulation, thus avoiding the occurrence of OHSS.
Another part of the peripheral hypothesis involves estrogen receptors in the endometrium. It is possible that aromatase inhibition, with suppression of estrogen concentrations in the circulation and in peripheral target tissues, results in up-regulation of estrogen receptors in the endometrium, leading to rapid endometrial growth once estrogen secretion is restored. Estrogen has been shown to decrease the level of its own receptor by stimulating ubiquitination of estrogen receptors, resulting in rapid degradation of the receptors. In the absence of estrogen, ubiquitination is decreased, allowing up-regulation of the estrogen receptor and increasing sensitivity to subsequent estrogen administration.140 This could increase endometrial sensitivity to estrogen, resulting in more rapid proliferation of endometrial epithelium and stroma and improved blood flow to the uterus and endometrium.141 As a result, normal endometrial development and thickness occur by the time of follicular maturation, even in the face of the observed lower than normal estradiol concentrations in aromatase inhibitor-treated cycles.102–109
Potential Advantages Compared to Clomiphene Citrate
The significantly shorter half-life (approximately 45 hours) of the third-generation aromatase inhibitors compared to clomiphene citrate allows rapid elimination from the body.115,116 Because no estrogen receptor down-regulation occurs, no adverse effects on estrogen target tissues are observed, as is frequently the case with clomiphene citrate. Aromatase inhibitors are, therefore, an interesting alternative to clomiphene citrate, particularly in cases with recurrent clomiphene citrate failure, as explained earlier in this chapter. However, in the case of clomiphene citrate resistance (failure to ovulate) due to severe insulin resistance or the use of clomiphene citrate for inappropriate indications (e.g., hypothalamic amenorrhea or ovarian failure), the use of an aromatase inhibitor is also unlikely to be successful. The correction of insulin resistance with an insulin sensitizer is the logical approach in patients with insulin resistance. Alternative treatments should be considered for other problems, such as exogenous gonadotropin injection in hypothalamic anovulation patients and oocyte donation for cases with ovarian failure.
In rcent years, evidence supporting the success of aromatase inhibitors in infertility treatment has been accumulating.102–109 Most of the studies used letrozole. However, anastrozole, another third-generation aromatase inhibitor similar to letrozole, was used in other studies.107,142,143 It is currently not known whether there are any clinically significant pharmacologic differences between letrozole and anastrozole.144 Recent safety studies have found anastrozole to have no teratogenic or clastogenic effects in animal embryo development.145,146
Aromatase Inhibitors and Exogenous Gonatrotropins
A major advantage of an aromatase inhibitor used alone is the ability to achieve restoration of monofollicular ovulation in anovulatory infertility (e.g., PCOS) with either multiple102,103,105 or single-dose147 regimens of aromatase inhibitor early in the menstrual cycle. A single-dose regimen has the benefit of convenience but the potential disadvantage of increasing side effects from administration of a larger dose. However, single doses several times higher than the doses reported for ovulation induction have been administered with no adverse effects observed.148,149
An aromatase inhibitor may also be used in conjunction with FSH injections to increase the number of preovulatory follicles that develop and to improve the outcome of treatment. Giving an aromatase inhibitor together with FSH resulted in a significant reduction in the dose of FSH required for optimum ovarian stimulation105 even in poor responders.106 The additional effect of aromatase inhibitors to reduce the supraphysiologic levels of estrogen seen with the development of multiple ovarian follicles may also improve treatment outcome.110
To summarize, the aromatase inhibitor when used alone results in a predictable response with the development of one or two mature follicles and a significantly reduced risk for ovarian hyperstimulation and multiple gestations.150 To achieve higher-order multiple ovulation, the addition of FSH to the aromatase inhibitor seems to be necessary.
Aromatase Inhibitors for Endometriosis-Related Infertility
The exciting data on the expression of aromatase enzyme in endometriotic tissues with the significant role of locally produced estrogen on endometriosis progression151,152 suggests a benefit of aromatase inhibitors in endometriosis-related infertility. The inhibition of local estrogen production in endometrial implants and the lower peripheral estrogen levels associated with the use of aromatase inhibition for ovulation induction are expected to protect, to some degree, against progression of endometriosis during infertility treatment.
Concerns About Aromatase Inhibitors
There are concerns about using aromatase inhibitors for induction of ovulation, including potential adverse effects of aromatase inhibitors resulting from low follicular estrogen levels and a temporary increase in ovarian androgens. In clinical use, nonsteroidal aromatase inhibitors are generally well tolerated. The main adverse events observed are hot flushes, gastrointestinal events (nausea and vomiting), and leg cramps. Overall, very few patients withdrew from first- or second-line comparative Phase III trials because of drug-related adverse events with aromatase inhibitors.153,154 These adverse effects were observed in older women with advanced breast cancer who were given the aromatase inhibitors on a daily basis over several months. Fewer adverse effects would be expected in the usually healthy younger women administered a short course of aromatase inhibitor for induction of ovulation.
The question whether low or very low intrafollicular estrogen is compatible with follicular development, ovulation, and corpus luteum formation has been reviewed before.155 Markedly reduced to absent intrafollicular concentrations of estrogen are known to be compatible with follicular “expansion,” retrieval of fertilizable oocytes, and apparently normal embryo development.156–160 The rapid clearance of the aromatase inhibitors, the reversible nature of enzyme inhibition, and elevated levels of FSH, which induces new expression of aromatase enzyme, are factors that limit accumulation of androgens and result in increasing estrogen production.
ADVERSE EFFECTS AND CONCERNS REGARDING ORAL OVULATION INDUCTION AGENTS
Multiple Gestations
In the past decade, the significant increase in the incidence of multiple births in most countries is almost entirely the result of the use of gonadotropins and other agents for induction of ovulation.161 According to the National Vital Statistics Reports for 2001, the rate of twinning has increased 33% since 1990 and 59% since 1980. Furthermore, the rate of triplets and higher-order multiples has increased more than 400% since 1980.162 In 2001, 1.6% of singletons, 11.8% of twins, 36.7% of triplets, 64.5% of quadruplets, and 78.6% of quintuplets were delivered before 32 completed weeks of gestation.162 Even more noteworthy, the incidence of extreme prematurity (delivery before 28 weeks) for both triplets and quadruplets may be as high as 14%.163,164
The increased incidence of maternal and neonatal complications associated with multiple pregnancies has been well documented.165,166 Review of data from the National Center for Health Statistics reveals that twins are 4 times more likely than singletons to die within the first month of life, and triplets are 10 times more likely to die in this time.
Hospital costs for each twin or triplet infant can be twice or three times that of a singleton, and lifetime costs to the healthcare system and community may be 100 to 200 times that of a singleton.167 After controlling for variables known to affect hospital charges, the predicted total charges to the family in 1991 for a singleton delivery were $9,845, as compared with $37,947 for twins ($18,974 per baby) and $109,765 for triplets ($36,588 per baby).167 Unfortunately, there is substantial pressure from patients in infertility treatment programs to increase their success rates. Among the strategies used are increasing the number of ovarian follicles matured by the administration of exogenous gonadotropins during induction of ovulation and increasing the number of embryos or gametes transferred in cases of IVF. Each of these strategies aims at increasing the percentage of successful pregnancies but also substantially increases the likelihood of multiple pregnancies. That increase, in turn, drives obstetric and neonatal charges higher. Many fear that decreasing the number of follicles stimulated or the number of embryos transferred to reduce the number of multiple pregnancies will lead to lower success rates and an increase in the number of cycles of treatment needed to achieve a pregnancy. However, the European experience with limiting the number of embryos transferred to two or even to single embryo transfer in Scandinavia in good-prognosis patients has not demonstrated a substantial drop in pregnancy rates while reducing the twin rate to less than 10%.168
More difficult is the problem of multiple pregnancy after ovulation induction with IUI. In the United States reported rates of multiple pregnancies greater than 30% result from ovarian stimulation, most commonly associated with IUI.169 The incidence of multiple pregnancy ranges from 10% to 15% with gonadotropins alone and from 15% to 29% with gonadotropins and IUI.170 With clomiphene citrate treatment, multifollicular development is relatively common and the risk of multiple gestation is clearly increased (approximately 8%). The overwhelming majority of multiple pregnancies that result from clomiphene citrate treatment are twin gestations; triplet and higher-order pregnancies are rare but may indeed occur.171 Several recent national studies of the increasing incidence of multiple pregnancies indicate that the vast majority of higher-order multiple gestations (63% to 80%) are a direct result of ovarian stimulation with gonadotropins, mainly FSH with IUI rather than with IVF with embryo transfer.165,172 There has been a consistent decrease in the number of embryos transferred per cycle and a drop in the percentage of pregnancies with three or more fetuses since 1997 in the United States.172
It is obvious that the best strategy is prevention of the occurrence of multiple pregnancies rather than dealing with the problem after its occurrence (e.g., multifetal pregnancy reduction). Selective fetal reduction presents an ethical problem for many couples, entails the risk of losing all fetuses being carried, and increases psychological stress for couples who have struggled through years of infertility.173,174 Other strategies that have focused on the prevention of premature delivery through intensive antenatal monitoring and interventions have been only partially successful in controlling the complications of multiple pregnancies.175 Each of these proposed methods of improving the outcome of multiple pregnancies relies on post hoc measures used after multiple gestation has been established. As mentioned above, a preferable and more economical solution would be to avoid the conception of multiple fetuses.
Several studies have shown that the number of multiple pregnancies can be decreased by the more judicious use of ovarian stimulation agents, by increased monitoring,176,177 and even by the use of less common but safer agents to induce ovulation, such as pulsatile GnRH.170 These strategies aim at reducing the incidence of multiple pregnancies by reducing multiple ovarian follicular development and by a more aggressive cancellation policy, or conversion to IVF.169 De Geyter and coworkers used ultrasound-guided aspiration of supernumerary follicles as a way of avoiding multiple-gestation pregnancies without canceling cycles.178 A study by Pasqualotto179 found that the total number of follicles was not predictive of the occurrence of multiple gestation. However, another study demonstrated a relationship between multiple gestation and the number of follicles of 15 mm or more.180 A safe alternative to ovarian follicular aspiration or cancellation may be to convert an IUI cycle to IVF, thereby giving clinicians control over how many embryos are introduced into the uterus. Conversion also prevents the complete financial loss and frustration of a canceled cycle by maintaining a pathway toward possible pregnancy, although the added costs of IVF are incurred.181 In conclusion, none of the methods suggested to reduce the risk of multiple pregnancies after ovarian stimulation and IUI proved to be effective without reducing the success rate significantly or adding significant cost by conversion to IVF. An ovulation induction agent that would achieve ovarian monofollicular development in most cycles with comparable success rate to ovarian stimulation with FSH would be an exciting approach for ameliorating the burden of multiple pregnancies after ovarian stimulation with IUI.
Ovarian Hyperstimulation Syndrome
The incidence of OHSS in clomiphene citrate-treated women and in association with mild gonadotropin stimulation for IUI is difficult to determine, because definitions of the syndrome vary widely among studies. Mild OHSS (moderate ovarian enlargement) is relatively common but does not require active management. When clomiphene citrate induction of ovulation proceeds in the recommended incremental fashion designed to establish the minimum effective dosage, the risk of severe OHSS (massive ovarian enlargement, progressive weight gain, severe abdominal pain, nausea and vomiting, hypovolemia, ascites, and oliguria) is remote.23 This complication is discussed in more detail in Chapter 40.
Ovarian Cancer
There is an uncertain association between ovarian cancer and clomiphene citrate treatment, derived from two epidemiologic studies published early in the past decade. The first was a case-control study concluding that ovarian cancer risk was increased nearly threefold overall in women receiving various infertility treatments, including clomiphene citrate.182 The study methodology was widely criticized for several reasons, the most important being that it compared infertile treated women to fertile women rather than to infertile untreated women, even though infertility and nulliparity have long been recognized as risk factors for ovarian cancer. There was no apparent increase in ovarian cancer risk in treated women who conceived. The second study was a cohort study concluding that risk of ovarian tumors was increased in women treated with clomiphene citrate.183 Comparisons within the clomiphene citrate-treated cohort showed no increase in risk with less than 12 cycles of treatment. This study too was widely criticized, primarily because it included cancers of varying types and tumors of low malignant potential (e.g., epithelial, germ cell, stromal); the pathophysiology of each is likely very different.
The results of subsequent studies have been reassuring, but the question of whether treatment with ovulation-inducing drugs increases risk of ovarian tumors or cancer remains unsettled and cannot be summarily dismissed.184–187 Certainly, no causal relationship between ovulation-inducing drugs and ovarian cancer has been established. Patients with concerns should be counseled that an increase in risk is possible but not established. In addition, carrying a pregnancy to term and using oral contraceptives for 2 or more years have both been demonstrated to significantly reduce overall ovarian cancer risk188–190 and could offset any potential increase in risk from fertility therapy. No change in prescribing practices is warranted, but prolonged treatment with clomiphene citrate is generally futile and should, therefore, be avoided, primarily because it offers little hope of success.23
1 Bao B, Garverick HA. Expression of steroidogenic enzyme and gonadotropin receptor genes in bovine follicles during ovarian follicular waves: A review. J Anim Sci. 1998;76:1903-1921.
2 Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783-790.
3 Zeleznik A, Hillier SG. The ovary: Endocrine function. In: Hillier SG, Kitchener HC, Neilson JP, editors. Scientific Essentials of Reproductive Medicine. Philadelphia: WB Saunders; 1996:133-147.
4 Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: Follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445-1453.
5 Gosden RG, Telfer E. Scaling of follicular sizes in mammalian ovaries. J Zool. 1987;211:157-168.
6 Erickson BH. Development and senescence of the postnatal bovine ovary. J Anim Sci. 1966;25:800-805.
7 Brown JB. Pituitary control of ovarian function—concepts derived from gonadotrophin therapy. Aust NZ J Obstet Gynaecol. 1978;18:46-54.
8 Fauser BCJM, van Heusden AM. Manipulation of human ovarian function: Physiological concepts and clinical consequences. Endocr Rev. 1997;18:71-106.
9 Richards JS, Russell DL, Robker RL, et al. Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol. 1998;145:47-54.
10 Niswender GD, Juengel JL, Silva PJ, et al. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80:1-29.
11 Breckwoldt M, Peters F, Geisthovel F. Classification and diagnosis of ovarian insufficiency. In: Insler V, Lunenfeld B, editors. Infertility: Male and Female. New York: Churchill Livingstone; 1986:191-212.
12 Gemzell CA, Diczfalusy E, Tillinger KG. Clinical effects of human pituitary follicle stimulating hormone FSH. J Clin Endocrinol Metab. 1958;18:138-148.
13 Gemzell CA, Diczfalusy E, Tillinger KG. Human pituitary follicle stimulating hormone. 1. Clinical effects of partly purified preparation. Ciba Found Colloq Endocrinol. 1960;13:191.
14 Bettendorf G, Apostolakis M, Voigt KD: Darstellung hochaktiver Gonadotropinfraktionen aus menschlichen Hypophysen und deren anwendung bei Menschen. Proceedings, International Federation of Gynecology and Obstetrics, Vienna, 1961, p 76.
15 Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. JAMA. 1961;178:101-105.
16 Practice Committee of the American Society for Reproductive Medicine. Use of clomiphene citrate in women. Fertil Steril. 2004;82(Suppl 1):S90-S96.
17 Mikkelson TJ, Kroboth PD, Cameron WJ. Single dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil Steril. 1986;46:392-396.
18 Young SL, Opsahl MS, Fritz MA. Serum concentrations of enclomiphene and zuclomiphene across consecutive cycles of clomiphene citrate therapy in anovulatory infertile women. Fertil Steril. 1999;7:639-644.
19 Kerin JF, Liu JH, Phillipou G, et al. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab. 1985;61:265-268.
20 Kettel LM, Roseff SJ, Berga S, et al. Hypothalamic-pituitary-ovarian response to clomiphene citrate in women with polycystic ovary syndrome. Fertil Steril. 1993;59:532-538.
21 Agarwal SK, Buyalos RP. Clomiphene citrate with intrauterine insemination: Is it effective therapy above the age of 35 years? Fertil Steril. 1996;65:759-763.
22 Athaullah N, Proctor M, Johnson NP. Oral versus injectable ovulation induction agents for unexplained subfertility. Cochrane Database Syst Rev. 3, 2002. CD003052.
23 Usadi RS, Fritz MA. Induction of ovulation with clomiphene citrate. In Schiarra JJ, editor: Obstetrics and Gynecology, 2nd ed., Philadelphia: Harper and Row, 1976.
24 Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation: Effects on the probability of conception, survival of the pregnancy, and sex of the baby. NEJM. 1995;333:1517-1521.
25 Ho Yuen B, Pride SM, Sime MO. Successful induction of ovulation and conception with pulsatile intravenous administration of human menopausal gonadotropins in anovulatory infertile women resistant to clomiphene and pulsatile gonadotropin-releasing hormone therapy. Am J Obstet Gynecol. 1984;148:508-512.
26 Wathen NC, Perry L, Lilford RJ, et al. Interpretation of single progesterone measurement in diagnosis of anovulation and defective luteal phase: Observations on analysis of the normal range. BMJ. 1984;288:7-9.
27 Hull MG, Savage PE, Bromham DR, et al. Value of a single serum progesterone measurement in the miduteal phase as a criterion of a potentially fertile cycle derived from treated and untreated conception cycles. Fertil Steril. 1982;37:355-360.
28 Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262-263.
29 Jordan J, Craig K, Clifton DK, Soules MR. Luteal phase defect: The sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril. 1994;62:54-62.
30 Coutifaris C, Myers ER, Guzick DS, et alfor the NICHD National Cooperative Reproductive Medicine Network. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264-1272.
31 Hoff JD, Quigley ME, Yen SSC. Hormonal dynamics at midcycle: A reevaluation. J Clin Endocrinol Metab. 1983;7:792-796.
32 Imani B, Eijkemans MJC, Velde ER, et al. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligomenorrheic infertility. J Clin Endocrinol Metab. 1999;84:1617-1622.
33 Purvin V. Visual disturbance secondary to clomiphene citrate. Arch Ophthalmol. 1995;113:482-484.
34 Maruncic M, Casper RF. The effect of luteal phase estrogen antagonism on luteinizing hormone pulsatility and luteal function in women. J Clin Endocrinol Metab. 1987;64:148-152.
35 Hack M, Brish M, Serr DM, et al. Outcome of pregnancy after induced ovulation: Follow-up of pregnancies and children born after clomiphene therapy. JAMA. 1972;220:1329-1333.
36 Correy JF, Marsden DE, Schokman FC. The outcome of pregnancy resulting from clomiphene induced ovulation. Austral NZ J Obstet Gynecol. 1982;22:18-21.
37 Whiteman D, Murphy M, Hey K, et al. Reproductive factors, subfertility, and risk of neural tube defects: A case-control study based on the Oxford Record Linkage Study Register. Am J Epidemiol. 2000;152:823-828.
38 Carlier P, Choulika S, Efthymiou ML. Clomiphene-exposed pregnancies: Analysis of 39 information requests including 25 cases with known outcome. Therapie. 1996;51:532-536.
39 Dickey RP, Taylor SN, Curole DN, et al. Incidence of spontaneous abortion in clomiphene pregnancies. Hum Reprod. 1996;11:2623-2628.
40 Schieve LA, Tatham L, Peterson HB, et al. Spontaneous abortion among pregnancies conceived using assisted reproductive technology in the United States. Obstet Gynecol. 2003;1015(Pt 1):959-967.
41 Hsu CC, Kuo HC, Wang ST, Huang KE. Interference with uterine blood flow by clomiphene citrate in women with unexplained infertility. Obstet Gynecol. 1995;866:917-921.
42 Hakim RB, Gray RH, Zacur H. Infertility and early pregnancy loss. Am J Obstet Gynecol. 1995;1725:1510-1517.
43 Bateman BG, Nunley WC, Kolp LA. Exogenous estrogen therapy for treatment of clomiphene citrate-induced cervical mucus abnormalities: Is it effective? Fertil Steril. 1990;54:577-579.
44 Ben-Ami M, Geslevich Y, Matilsky M, et al. Exogenous estrogen therapy concurrent with clomiphene citrate: Lack of effect on serum sex hormones and endometrial thickness. Gynecol Obstet Invest. 1994;37:180-182.
45 Gonen Y, Casper RF. Sonographic determination of an adverse effect of clomiphene citrate on endometrial growth. Hum Reprod. 1990;5:670-674.
46 Nelson LM, Hershlag A, Kurl RS, et al. Clomiphene citrate directly impairs endometrial receptivity in the mouse. Fertil Steril. 1990;53:727-731.
47 Li TC, Warren MA, Murphy C, et al. A prospective, randomised, cross-over study comparing the effects of clomiphene citrate and cyclofenil on endometrial morphology in the luteal phase of normal fertile women. BJOG. 1992;99:1008-1013.
48 Hammond M, Halme J, Talbert L. Factors affecting the pregnancy rate in clomiphene citrate induction of ovulation. Obstet Gynecol. 1983;62:196-202.
49 Fritz MA, Holmes RT, Keenan EJ. Effect of clomiphene citrate treatment on endometrial estrogen and progesterone receptor induction in women. Am J Obstet Gynecol. 1991;165:177-185.
50 Yeko TR, Nicosia SM, Maroulis GB, et al. Histology of midluteal corpus luteum and endometrium from clomiphene citrate-induced cycles. Fertil Steril. 1992;57:28-32.
51 Sereepapong W, Triratanachat S, Sampatanukul P, et al. Effects of clomiphene citrate on the endometrium of regularly cycling women. Fertil Steril. 2000;73:229-287.
52 Oktay K, Berkowitz P, Berkus M, et al. The re-incarnation of an old question: Clomid effect on oocyte and embryo? Fertil Steril. 2000;74:422-423.
53 Branigan EF, Estes MA. Minimal stimulation IVF using clomiphene citrate and oral contraceptive pill pretreatment for LH suppression. Fertil Steril. 2000;733:587-590.
54 Zayed F. Outcome of stimulated in vitro fertilisation (SIVF) using clomiphene citrate and human menopausal gonadotropin in different infertility groups. Clin Exp Obstet Gynecol. 1999;263/264:227-279.
55 Schmidt GE, Kim MH, Mansour R, et al. The effects of enclomiphene and zuclomiphene citrates on mouse embryos fertilized in vitro and in vivo. Am J Obstet Gynecol. 1986;1544:727-736.
56 Yoshimura Y, Hosoi Y, Atlas SJ, Wallach EE. Effect of clomiphene citrate on in vitro ovulated ova. Fertil Steril. 1986;456:800-804.
57 Shimoya K, Tomiyama K, Hashimoto K, et al. Endometrial development was improved by transdermal estradiol in patients treated with clomiphene citrate. Gynecol Obstet Invest. 1999;474:251-254.
58 Gerli S, Gholami H, Manna A, et al. Use of ethinyl estradiol to reverse the antiestrogenic effects of clomiphene citrate in patients undergoing intrauterine insemination: A comparative, randomized study. Fertil Steril. 2000;73:85-89. 1
59 Bateman BG, Nunley WCJr, Kolp LA. Exogenous estrogen therapy for treatment of clomiphene citrate-induced cervical mucus abnormalities: Is it effective? Fertil Steril. 1990;54:577-579.
60 Wu CH, Winkel CA. The effect of therapy initiation day on clomiphene citrate therapy. Fertil Steril. 1989;52:564-568.
61 Saleh A, Biljan MM, Tan SSSL, Tulandi T. Effects of tamoxifen (Tx) on endometrial thickness and pregnancy rates in women undergoing superovulation with clomiphene citrate (CC) and intrauterine insemination (IUI). Fertil Steril. 2000;74(Suppl1):S90. (Abstract.)
62 Unfer V, Casini ML, Costabile L, et al. High dose of phytoestrogens can reverse the antiestrogenic effects of clomiphene citrate on the endometrium in patients undergoing intrauterine insemination: A randomized trial. J Soc Gynecol Invest. 2004;11:323-328.
63 Vienonen A, Miettinen S, Blauer M, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Invest. 2004;112:104-112.
64 Franks S, Adams J, Mason H, Polson D. Ovulatory disorders in women with polycystic ovary syndrome. Clin Obstet Gynaecol. 1985;12:605-632.
65 Hull MGR. The causes of infertility and relative effectiveness of treatment. In: Templeton AA, Drife JO, editors. Infertility. London: Springer-Verlag; 1992:33-62.
66 Wysowski DE. Use of fertility drugs in the United States, 1979 through 1991. Fertil Steril. 1993;60:1096-1098.
67 De Leo V, la Marca A, Petraglia F. Insulin-lowering agents in the management of polycystic ovary syndrome. Endocr Rev. 2003;245:633-667.
68 Mitwally MFM, Witchel SF, Casper RF. Troglitazone: A possible modulator of ovarian steroidogenesis. J Soc Gyn Invest. 2002;9:163-167.
69 Veldhuis JD, Zhang G, Garmey JC. Troglitazone, an insulin-sensitizing thiazolidinedione, represses combined stimulation by LH and insulin of de novo androgen biosynthesis by thecal cells in vitro. J Clin Endocrinol Metab. 2002;873:1129-1133.
70 Bodenburg Y, Nagamani M, Green A, Urban RJ. Troglitazone inhibits progesterone production in porcine granulosa cells. Endocrinology. 1998;139:4962-4966.
71 Wang M, Wise SC, Leff T, Su TZ. Troglitazone, an antidiabetic agent, inhibits cholesterol biosynthesis through a mechanism independent of peroxisome proliferator-activated receptor-γ. Diabetes. 1999;48:254-260.
72 Mu YM, Yanase T, Nishi Y, et al. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun. 2000;271:710-713.
73 Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol for polycystic ovary syndrome. Cochrane Database Syst Rev. 2003. CD003053.
74 Kashyap S, Wells GA, Rosenwaks Z. Insulin-sensitizing agents as primary therapy for patients with polycystic ovarian syndrome. Hum Reprod. 2004;1911:2474-2483.
75 Palomba S, Orio FJr, Falbo A, et al. Prospective parallel randomized double blind double dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068-4074.
76 Moll E, Bossuyt P, Korevaar JC, et al. Effect of clomiphene citrate and placebo on induction of ovulation in women with newly-diagnosed polycystic ovary syndrome: Randomized double-blind clinical trial. BMJ. 2006;332:1485-1493.
77 Dunaif A, Scott D, Finegood D, et al. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81:3299-3306.
78 Ehrmann DA, Schneider DJ, Sobel BE, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2108-2116.
79 Hasegawa I, Murakawa H, Suzuki M, et al. Effect of troglitazone on endocrine and ovulatory performance in women with insulin resistance-related polycystic ovary syndrome. Fertil Steril. 1999;71:323-327.
80 Mitwally MFM, Kuscu NK, Yalcinkaya TM. High ovulatory rates with use of troglitazone in clomiphene-resistant women with polycystic ovary syndrome. Hum Reprod. 1999;14:2700-2703.
81 Azziz R, Ehrmann D, Legro RS, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: A multicenter, double blind placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626-1632.
82 Ghazeeri GC, Haas D, Ke RW, et al. The use of rosiglitazone in ovulation induction in clomiphene-resistant obese women with polycystic ovary syndrome. Fertil Steril. 2001;76(3S1):S36-S37.
83 Mitwally MFM, Greenblatt EM, Casper RF. Rosiglitazone improves endocrine and reproductive performance in women with polycystic ovary syndrome (PCOS) who failed to respond to metformin. Fertil Steril. 2001;76:S206. (Abstract.)
84 Cataldo NA, Abbasi F, McLaughlin TL, et al. Rosiglitazone in insulin-resistant women with polycystic ovary syndrome PCOS: Effects on ovarian function and metabolism. Fertil Steril. 2002;78:S36. (Abstract.)
85 Norman RJ, Wang JX, Hague W. Should we continue or stop insulin sensitizing drugs during pregnancy? Curr Opin Obstet Gynecol. 2004;163:245-250.
86 Zreik TG, Garcia-Velasco JA, Habboosh MS, et al. Prospective, randomized, crossover study to evaluate the benefit of human chorionic gonadotropin-timed versus urinary luteinizing hormone-timed intrauterine inseminations in clomiphene citrate-stimulated treatment cycles. Fertil Steril. 1999;71:1070-1074.
87 Opsahl MS, Robins ED, O’Connor DM, et al. Characteristics of gonadotropin response, follicular development, and endometrial growth and maturation across consecutive cycles of clomiphene citrate treatment. Fertil Steril. 1996;66:533-539.
88 Mitwally MF, Abdel-Razeq S, Casper RF. Human chorionic gonadotropin administration is associated with high pregnancy rates during ovarian stimulation and timed intercourse or intrauterine insemination. Reprod Biol Endocrinol. 2004;2:55.
89 Kemmann E, Jones JR. Sequential clomiphene citrate–menotropin therapy for induction or enhancement of ovulation. Fertil Steril. 1983;39:772-779.
90 Rose BI. A conservative, low-cost superovulation regimen. Int J Fertil. 1992;37:339-342.
91 Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotrophin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;188:1588-1597.
92 Greenblatt EM, Casper RF. Adhesion formation following laparoscopic ovarian cautery for polycystic ovarian syndrome: Lack of correlation with pregnancy rate. Fertil Steril. 1993;60:766-770.
93 Lemieux S, Lewis GF, Ben-Chetrit A, et al. Correction of hyperandrogenemia by laparoscopic ovarian cautery in women with polycystic ovarian syndrome is not accompanied by improved insulin sensitivity or lipid-lipoprotein levels. J Clin Endocrinol Metab. 1999;8411:4278-4282.
94 Cole PA, Robinson CH. Mechanism and inhibition of cytochrome P-450 aromatase. J Med Chem. 1990;33:2933-2944.
95 Santen RJ, Manni A, Harvey H, Redmond C. Endocrine treatment of breast cancer in women. Endocrine Rev. 1990;11:1-45.
96 Buzdar A, Howell A. Advances in aromatase inhibition: Clinical efficacy and tolerability in the treatment of breast cancer. Clin Cancer Res. 2001;7:2620-2635.
97 Santen RJ, Lipton A, Kendall J. Successful medical adrenalectomy with aminoglutethimide: Role of altered drug metabolism. JAMA. 1974;230:1661-1665.
98 Newsome HH, Brown PN, Terz JJ, et al. Medical and surgical adrenalectomy in patients with advanced breast carcinoma. Cancer. 1977;39:542-546.
99 Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: Status report 2002. J Clin Oncol. 2002;2015:3317-3327.
100 Buzdar A, Jonat W, Howell A, et al. Anastrozole, a potent and selective aromatase inhibitor, versus megestrol acetate in postmenopausal women with advanced breast cancer: Results of overview analysis of two phase III trials. J Clin Oncol. 1996;14:2000-2011.
101 Brueggemeier RW. Update on the use of aromatase inhibitors in breast cancer. Expert Opin Pharmacother. 2006;7:1919-1930.
102 Mitwally MFM, Casper RF. Aromatase inhibition: A novel method of ovulation induction in women with polycystic ovarian syndrome. Reprod Technol. 2000;10:244-247.
103 Mitwally MFM, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305-309.
104 Mitwally MFM, Casper RF. Single dose administration of an aromatase inhibitor for ovarian stimulation. Fertil Steril. 2005;83:229-231.
105 Mitwally MF, Casper RF. Aromatase inhibition reduces the dose of gonadotropin required for controlled ovarian hyperstimulation. J Soc Gynecol Invest. 2004;11:406-415.
106 Mitwally MFM, Casper RF. Aromatase inhibition improves ovarian response to follicle-stimulating hormone in poor responders. Fertil Steril. 2002;774:776-780.
107 Al-Omari WR, Sulaiman WR, Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2004;853:289-291.
108 Fatemi HM, Kolibianakis E, Tournaye H, et al. Clomiphene citrate versus letrozole for ovarian stimulation: A pilot study. Reprod Biomed Online. 2003;75:543-546.
109 Healey S, Tan SL, Tulandi T, Biljan MM. Effects of letrozole on superovulation with gonadotropins in women undergoing intrauterine insemination. Fertil Steril. 2003;806:1325-1329.
110 Mitwally MF, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;2:61-78.
111 Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab. 2002;133:122-128.
112 Naftolin F, MacLusky NJ. Aromatization hypothesis revisited. In: Serio M, editor. Differentiation: Basic and Clinical Aspects. New York: Raven Press; 1984:79-91.
113 Naftolin F, MacLusky NJ, Leranth CZ, et al. The cellular effects of estrogens on neuroendocrine tissues. J Steroid Biochem. 1988;30:195-207.
114 Naftolin F. Brain aromatization of androgens. J Reprod Med. 1994;39:257-261.
115 Sioufi A, Gauducheau N, Pineau V, et al. Absolute bioavailability of letrozole in healthy post-menopausal women. Biopharm Drug Dispos. 1997;18:779-789.
116 Sioufi A, Sandrenan N, Godbillon J, et al. Comparative bioavailability of letrozole under fed and fasting conditions in 12 healthy subjects after a 25 mg single oral administration. Biopharm Drug Dispos. 1997;186:489-497.
117 DePaolo LV. Inhibins, activins, and follistatins: The saga continues. Proc Soc Exp Biol Med. 1997;214:328-339.
118 Nett TM, Turzillo AM, Baratta M, Rispoli LA. Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest Anim Endocrinol. 2002;23:33-42.
119 Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: From endocrinology to signaling. A paradigm for the new millennium. Biol Med (Maywood). 2002;2279:724-752.
120 McNeilly AS, Crawford JL, Taragnat C, et al. The differential secretion of FSH and LH: Regulation through genes, feedback and packaging. Reprod Suppl. 2003;61:463-476.
121 Roberts V, Meunier H, Vaughan J, et al. Production and regulation of inhibin subunits in pituitary gonadotropes. Endocrinology. 1989;124:552-554.
122 Mason AJ, Berkemeier LM, Schmelzer CH, Schwall RH. Activin B: Precursor sequences, genomic structure and in vitro activities. Mol Endocrinol. 1989;3:1352-1358.
123 Mather JP, Roberts PE, Krummen LA. Follistatin modulates activin activity in a cell- and tissue-specific manner. Endocrinology. 1993;132:2732-2734.
124 Baratta M, West LA, Turzillo AM, Nett TM. Activin modulates differential effects of estradiol on synthesis and secretion of follicle-stimulating hormone in ovine pituitary cells. Biol Reprod. 2001;64:714-719.
125 Shupnik MA, Rosenzweig BA. Identification of an estrogen-responsive element in the rat LH β gene. DNA–estrogen receptor interactions and functional analysis. J Biol Chem. 1991;66:17084-17091.
126 DiGregorio GB, Nett TM. Estradiol and progesterone influence the synthesis of gonadotropins in the absence of GnRH in the ewe. Biol Reprod. 1995;35:166-172.
127 Roberts VJ, Barth S, El-Roeiy A, Yen SSC. Expression of inhibin/activin system messenger ribonucleic acids and proteins in ovarian follicles from women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79:1434-1439.
128 Yamoto M, Minami S, Nakano R. Immunohistochemical localization of inhibin subunits in polycystic ovary. J Clin Endocrinol Metab. 1993;77:859-862.
129 Anderson RA, Groome NP, Baird DT. Inhibin A and inhibin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono-ovulation. Clin Endocrinol Oxf. 1998;48:577-584.
130 Lockwood GM, Muttukrishna S, Groome NP, et al. Mid-follicular phase pulses of inhibin B are absent in polycystic ovarian syndrome and are initiated by successful laparoscopic ovarian diathermy: A possible mechanism regulating emergence of the dominant follicle. J Clin Endocrinol Metab. 1998;83:1730-1735.
131 Weil SJ, Vendola K, Zhou J, et al. Androgen receptor gene expression in the primate ovary: Cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;837:2479-2485.
132 Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;848:2951-2956.
133 Vendola KA, Zhou J, Adesanya OO, et al. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;10112:2622-2629.
134 Vendola K, Zhou J, Wang J, et al. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;612:353-357.
135 Adashi E. Intraovarian regulation: The proposed role of insulin-like growth factors. Ann NY Acad Sci. 1993;687:10-12.
136 Giudice LC. Insulin-like growth factors and ovarian follicular development. Endocr Rev. 1992;13:641-669.
137 Yen SSC, Laughlin GA, Morales AJ. Interface between extra-and intra-ovarian factors in polycystic ovary syndrome (PCOS). Ann NY Acad Sci. 1993;687:98-111.
138 Agarwal SK, Judd HL, Magoffin DA. A mechanism for the suppression of estrogen production in polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;8110:3686-3691.
139 Jakimiuk AJ, Weitsman SR, Brzechffa PR, Magoffin DA. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod. 1998;41:1-8.
140 Nirmala PB, Thampan RV. Ubiquitination of the rat uterine estrogen receptor: Dependence on estradiol. Biochem Biophys Res Commun. 1995;2131:24-31.
141 Rosenfeld CR, Roy T, Cox BE. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul Pharmacol. 2002;382:115-125.
142 Krasnopolskaya K, Kaluina A: Application of aromatase inhibitors Anastrosol in IVF program for the treatment of infertility associated with severe endometriosis. In Program and abstracts of the 18th Annual Meeting of the European Society for Human Reproduction and Embryology (ESHRE). Vienna, 2002, 17:75, P-438.
143 Tredway DR, Buraglio M, Hemsey G, Denton G. A phase I study of the pharmacokinetics, pharmacodynamics, and safety of single- and multiple-dose anastrozole in healthy, premenopausal female volunteers. Fertil Steril. 2004;82:1587-1593.
144 Ligibel JA, Winer EP. Clinical differences among the aromatase inhibitors. Clin Cancer Res. 2003;91(Pt 2):473S-479S.
145 Hu Y, Cortvrindt R, Smitz J. Effects of aromatase inhibition on in vitro follicle and oocyte development analyzed by early preantral mouse follicle culture. Mol Reprod Dev. 2002;614:549-559.
146 Guo Y, Guo KJ, Huang L, et al. Effect of estrogen deprivation on follicle/oocyte maturation and embryo development in mice. Chin Med J Engl. 2004;1174:498-502.
147 Mitwally MFM, Casper RF. Single dose administration of an aromatase inhibitor for ovarian response. Fertil Steril. 2005;83:229-231.
148 Letrozole (Femara) package insert. East Hanover, N.J.: Novartis, July 1997.
149 Anastrozole (Arimidex) package insert. Wilmington, Del.: Astra-Zeneca, September 2005.
150 Mitwally MFM, Casper RF: Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol 192:381–386.
151 Bulun SE, Zeitoun KM, Takayama K, Sasano H. Estrogen biosynthesis in endometriosis: Molecular basis and clinical relevance. J Mol Endocrinol. 2000;1:35-42.
152 Vignali M, Infantino M, Matrone R, et al. Endometriosis: Novel etiopathogenetic concepts and clinical perspectives. Fertil Steril. 2002;78:665-678.
153 Hamilton A, Piccart M. The third-generation nonsteroidal aromatase inhibitors: A review of their clinical benefits in the second-line hormonal treatment of advanced breast cancer. Ann Oncol. 1999;10:377-384.
154 Goss PE. Risks versus benefits in the clinical application of aromatase inhibitors. Endocr Relat Cancer. 1999;6:325-332.
155 Palter SF, Tavares AB, Hourvitz A, et al. Are estrogens of import to primate/human ovarian folliculogenesis? Endocr Rev. 2001;223:389-424.
156 Yanase T, Simpson ER, Waterman M. 17-hydroxylase/17,20-lyase deficiency: From clinical investigation to molecular definition. Endocr Rev. 1991;12:91-108.
157 Rabinovici J, Blankstein J, Goldman B, et al. IVF and primary embryonic cleavage are possible in 17-hydroxylase deficiency despite extremely low intrafollicular 17β E2. J Clin Encrinol Metab. 1989;68:693-697.
158 Schoot DCJM, Herjan JT, Bennink C, et al. Human recombinant FSH induced growth of preovulatory follicles without concomitant increase in androgen and estrogen biosynthesis in a woman with isolated GT deficiency. J Clin Endocrinol Metab. 1992;74:1471-1473.
159 Shoham Z, Mannaerts B, Insler V, Coelingh HB. Induction of follicular growth using recombinant human follicle-stimulating hormone in two volunteer women with hypogonadotropic hypogonadism. Fertil Steril. 1993;59:738-742.
160 Shoham Z, Balen A, Patel A, Jacobs HS. Results of ovulation induction using human menopausal gonadotropins or purified follicle-stimulating hormone in hypogonadotropic hypogonadism patients. Fertil Steril. 1991;56:1048-1053.
161 Dawood MY. In vitro fertilization, gamete intrafallopian transfer and superovulation with intra-uterine insemination: Efficacy and potential health hazards on babies delivered. Am J Obstet Gynecol. 1996;174:1208-1217.
162 Births. Final data for 2001. Natl Vital Stat Rep. 51, 2003.
163 Collins MS, Bleyl JA. Seventy-one quadruplet pregnancies: Management and outcome. Am J Obstet Gynecol. 1990;162:1384-1392.
164 Kaufman E, Malone FD, Harvey-Wilkes KB, et al. Neonatal morbidity and mortality associated with triplet pregnancy. Obstet Gynecol. 1998;91:342-348.
165 Botting BJ, Davies IM, Macfarlane AJ. Recent trends in the incidence of multiple births and associated mortality. Arch Dis Child. 1987;62:941-950.
166 Lipitz S, Frenkel Y, Watts C, et al. High-order multifetal gestation—management and outcome. Obstet Gynecol. 1990;76:215-218.
167 Callahan L, Hall JE, Ettner SL, et al. The economic impact of multiple-gestation pregnancies and the contribution of assisted-reproduction techniques to their incidence. NEJM. 1994;331:244-249.
168 Thurin A, Hausken J, Hillensjo T, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. NEJM. 2004;351:2392-2402.
169 Adashi EY, Barri PN, Berkowitz R, et al. Infertility therapy-associated multiple pregnancies births: An ongoing epidemic. Reprod Biomed Online. 2003;75:515-542.
170 Martin KA, Hall JE, Adams JM, Crowley WFJr. Comparison of exogenous gonadotropins and pulsatile gonadotropin-releasing hormone for induction of ovulation in hypogonadotropic amenorrhea. J Clin Endocrinol Metab. 1993;77:125-129.
171 Schenker JG, Jarkoni S, Granat M. Multiple pregnancies following induction of ovulation. Fertil Steril. 1981;35:105-123.
172 Jain T, Missmer SA, Hornstein MD. Trends in embryo-transfer practice and in outcomes of the use of assisted reproductive technology in the United States. NEJM. 2004;35016:1639-1645.
173 Wapner RJ, Davis GH, Johnson A, et al. Selective reduction of multifetal pregnancies. Lancet. 1990;335:90-93.
174 Berkowitz RL, Lynch L, Lapinski R, Bergh P. First-trimester transabdominal multifetal pregnancy reduction: A report of two hundred completed cases. Am J Obstet Gynecol. 1993;169:17-21.
175 Gilstrap LCIII, Brown CE. Prevention and treatment of preterm labor in twins. Clin Perinatol. 1988;15:71-77.
176 Lipitz S, Reichman B, Paret G, et al. The improving outcome of triplet pregnancies. Am J Obstet Gynecol. 1989;161:1279-1284.
177 Corson SL, Dickey RP, Gocial B, et al. Outcome in 242 in vitro fertilization–embryo replacement or gamete intrafallopian transfer-induced pregnancies. Fertil Steril. 1989;51:644-650.
178 De Geyter C, De Geyter M, Nieschlag E. Low multiple pregnancy rates and reduced frequency of cancellation after ovulation induction with gonadotropins, if eventual supernumerary follicles are aspirated to prevent polyovulation. J Assist Reprod Genet. 1998;15:111-116.
179 Pasqualotto E, Falcone T, Goldberg J, et al. Risk factors for multiple gestation in women undergoing intrauterine insemination with ovarian stimulation. Fertil Steril. 1999;72:613-618.
180 Richmond JR, Deshpande N, Lyall H, et al. Follicular diameters in conception cycles with and without multiple pregnancy after stimulated ovulation induction. Hum Reprod. 2005;20:756-760.
181 Antman AM, Politch JA, Ginsburg ES. Conversion of high-response gonadotropin intrauterine insemination cycles to in vitro fertilization results in excellent ongoing pregnancy rates. Fertil Steril. 2002;77:715-720.
182 Whittemore AS, Harris R, Itnyre J, et al. Characteristics relating to ovarian risk: Collaborative analysis of 12 US case-control studies. Am J Epidemiol. 1992;136:1184-1203.
183 Rossing MA, Daling JR, Weiss NS, et al. Ovarian tumors in a cohort of infertile women. NEJM. 1994;331:771-776.
184 Venn A, Watson L, Lumley J, et al. Breast and ovarian cancer incidence after infertility and in vitro fertilization. Lancet. 1995;346:995-1000.
185 Modan B, Ron E, Lerner-Geva L, et al. Cancer incidence in a cohort of infertile women. Am J Epidemiol. 1998;147:1038-1042.
186 Mosgaard BJ, Lidegaard O, Kjaer SK, et al. Infertility, fertility drugs, and invasive ovarian cancer: A case-control study. Fertil Steril. 1997;67:1005-1012.
187 Potashnik G, Lerner-Geva L, Genkin L, et al. Fertility drugs and the risk of breast and ovarian cancers: Results of a long-term follow-up study. Fertil Steril. 1999;71:853-859.
188 Pike MC, Pearce CL, Wu AH. Prevention of cancers of the breast, endometrium and ovary. Oncogene. 2004;23:6379-6391.
189 Khoo SK. Cancer risks and the contraceptive pill. What is the evidence after nearly 25 years of use? Med J Aust. 1986;17:185-190.
190 Fathalla MF. Factors in the causation and incidence of ovarian cancer. Obstet Gynecol Surv. 1972;2711:751-768.