CHAPTER 13 Induction, Maintenance, and Recovery
Induction of general anesthesia
The induction of general anesthesia can be the most critical yet rewarding interaction that a pediatric anesthesiologist has with young patients and their families. Minimizing anxiety, psychological trauma, and crying during induction has many advantages, including reduced occurrence of airway complications, emergence agitation, postoperative pain, short- and long-term behavioral changes, patient and family dissatisfaction, and difficult inductions with subsequent anesthetics (Laycock and McNicol, 1988; Kotiniemi et al., 1997; Kain et al., 1999; Przybylo et al., 2005; Kain et al., 2006). With the increased focus on family-centered care, including parents’ concerns and priorities in the decision-making process being the norm, it is essential that all parties are prepared as well as possible regarding information, preferences, and expectations. If premedications or topical anesthetics are indicated, adequate time should be allotted to ensure maximal benefit. Smooth separation of child and family, be it before or after induction, should be facilitated by clearly stated instructions and participation of perioperative personnel.
Psychological Considerations
During the preoperative period, it is extremely important to identify the children and families who are likely to develop pronounced fear and anxiety before and during the induction of anesthesia. Because the level of stress and underlying temperament that predispose individuals to extreme anxiety may not be overtly apparent, it is essential that the pediatric anesthesiologist carefully evaluate each patient. Indicative behaviors, beyond the obvious crying and uncooperative child, include the absence of social interaction, vocalization, emotional expression, and age-appropriate independence from parents (Kain et al., 1997). It is also helpful to assess family members’ levels of anxiety and coping styles. Premedication with anxiolytics has been shown to be the most consistently effective intervention for facilitating induction and reducing postoperative complications in anxious patients (Bergendahl et al., 2004; Almenrader et al., 2007; Schmidt et al., 2007).
Medical Considerations
Most children and infants scheduled for elective surgical procedures are in good health, but common disorders such as upper respiratory tract infection (URI), reactive airways disease, gastroesophageal reflux, obesity, and hemodynamically stable congenital heart lesions can pose diagnostic and management challenges for the pediatric anesthesiologist. Patients with known medical problems should be carefully interviewed and examined, and if general anesthesia is to be induced, appropriate precautions and interventions should be taken. These issues are explored in detail elsewhere in this text (see Chapters 9, Preoperative Preparation, and 36, Systemic Disorders). An overview of some basic considerations is discussed below.
Upper Respiratory Tract Infections
URI is by far the most common problem the pediatric anesthesiologist encounters, especially in the ambulatory surgery setting. URI and the accompanying inflammation increase upper and lower airway irritability and secretions, and they may increase the incidence of laryngospasm, bronchospasm, and perioperative hypoxemia (see Chapter 36, Systemic Disorders) (DeSoto et al., 1988; Cohen and Cameron, 1991; Coté, 2001; Bordet et al., 2002; Elwood et al., 2003). Risk factors for associated complications include nasal congestion, copious secretions, reactive airways disease, history of prematurity, passive smoking, airway surgery, endotracheal intubation, and laryngeal mask airway insertion (Tait et al., 2001; von Ungern-Sternberg et al., 2007). Even in patients without a history of asthma, airway reactivity can develop with URI or lower respiratory tract infection and last as long as 6 to 8 weeks (Empey et al., 1976; de Kluijver et al., 2002). The decision to postpone the procedure depends on the urgency of the surgery, the severity of symptoms, and the need to instrument the airway (Tait and Malviya, 2005). In these patients, prophylactic bronchodilator treatment should be considered before the induction of anesthesia and before the emergence from anesthesia and extubation.
Reactive Airways Disease
In children with reactive airways disease (RAD), a detailed past medical history must be obtained to determine the severity of the disease and the effectiveness of current medical treatment. Recurrent emergency visits and hospital admissions, especially those to critical care units and/or involving the use of steroids, are red flags for poor control of symptoms. Mild asthma that is poorly controlled may improve with more aggressive treatment, whereas well-controlled severe RAD may require sustained optimized therapy. In a patient with active or recent bronchospasms, elective surgery should be postponed for 4 to 6 weeks. If surgery is required, preinduction treatment with a β2-agonist is recommended to minimize respiratory complications (Scalfaro et al., 2001). Recent steroid use may require perioperative stress-dose steroid coverage (see Chapter 36, Systemic Disorders).
Congenital Heart Disease
Children with congenital heart disease (CHD) may require antibiotic prophylaxis preoperatively for the prevention of bacterial endocarditis. Recommendations by the American Heart Association (AHA) were updated by Wilson et al. in 2007. The recommendations can also be downloaded from the AHA website at http://www.americanheart.org. The guidelines for the AHA subacute bacterial endocarditis (SBE) prophylaxis, which were formulated by an AHA writing group with input from national and international experts, have been significantly modified. CHD conditions associated with the highest risk for SBE are listed as follows:
Prophylaxis is only recommended for “dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa” and “invasive procedure of the respiratory tract that involves incision or biopsy of the respiratory mucosa, such as tonsillectomy and adenoidectomy” (Wilson et al., 2007). Standard antibiotic recommendations include amoxicillin PO or ampicillin IV 50 mg/kg (maximum dose 2 g) 30 to 60 minutes before the procedure. Alternative antibiotics for those patients allergic to penicillin or ampicillin include clindamycin, cefazolin, ceftriaxone, azithromycin, or clarithromycin.
Animal studies indicate that if the preoperative SBE dose is missed, the effective prophylaxis can be given within 2 hours (but not after 4 hours) after the procedure (Berney and Francioli, 1990; Dajani et al., 1997).
Preoperative Fasting
Preoperative fasting times allow for gastric emptying and reduction of aspiration risk. Evidence of rapid gastric emptying in infants and children and efforts to improve the perioperative experience of young patients and their families have resulted in liberalization of pediatric fasting guidelines. Based on clinical observation and studies of residual gastric volumes, international recommendations for NPO times before anesthetic induction in healthy children are 2 hours for clear liquids, 3 to 4 hours for breast milk, 4 hours for infant formula (in infants younger than 3 months), 6 hours for infant formula (in infants older than 6 months), 6 hours for light meals, and 8 hours for heavy meals (Schreiner et al., 1990; Litman et al., 1994; Cook-Sather et al., 2003; Søreide et al., 2005; Murat and Dubois, 2008). This growing consensus has given rise to the 2-4-6 rule. Although these fasting times do not apply to children with gastrointestinal or systemic disorders that may interfere with or slow gastric emptying, Cook-Sather and colleagues (2009) have reported that even in overweight and obese children undergoing elective surgery, the 2-hour minimum preoperative clear-liquid fasting guideline is adequate.
Allowing infants and children to have oral intake closer to the time of surgery can help reduce patient irritability, parental stress, and risk of dehydration. Conversely, the short and multiple fasting times can lead to confusion and NPO violations. It is extremely important that those giving and receiving instruction on preoperative food and fluid intake clearly understand the terminology (i.e., what constitutes a clear liquid), timing, and need for adherence to the guidelines (Schoenfelder et al., 2006). A clear liquid is a solution (as opposed to a suspension) that contains no particulate matter. Examples of clear liquids include water, Pedialyte, carbonated beverages, clear tea, plain gelatin, and fruit juices without pulp (American Society of Anesthesiologists, 1999; Ferrari et al., 1999).
Preanesthetic Preparations
Most children can be well managed in this friendly environment. An anesthesia mask may be given to the child to play with in the waiting area before induction (Fig. 13-1). Allowing children to choose a flavor (e.g., bubble gum, cherry, or grape) can provide them with a sense of control. The additional support of pacifiers, toys, and music boxes is often helpful. Children should keep the objects brought from home, particularly security blankets or other transitional objects, during the induction of anesthesia. Clowns have also been used for the prevention of preoperative anxiety in children (Vagnoli et al., 2005; Golan et al., 2009).
Premedication
As stated above, the use of premedication is the most reliably effective intervention for reducing preinduction anxiety and stress for young patients and their parents. Preoperative medications are described in detail in Chapter 9, Preoperative Preparation, and are discussed only briefly here. Historically, premedication referred to long-acting sedatives and anticholinergic agents that were often given intramuscularly (IM) or rectally (PR) to prepare children in inpatients’ units for their trip to the operating room. Contemporary administration of sedatives, typically by mouth, is administered just before induction to facilitate child-parent separation, anesthetic mask acceptance, and/or patient cooperation. IM administration is almost now solely reserved for extremely agitated, uncontrollable children.
Benzodiazepines
Midazolam, a water-soluble benzodiazepine, is the most commonly used preinduction medication (Kain et al., 1997). Given orally at 0.5 mg/kg mixed with fruit-flavored syrup, midazolam will create a calm, euphoric, or drowsy state in most children within 15 to 30 minutes. To potentiate its effect, children should be kept in a nonstimulating environment, and ambulatory children who may become unsteady should be held carefully on the parent’s lap or placed in bed. Coté et al. (2002) showed that a wider range of doses can be effective, adjusting for time of onset, and that respiratory compromise does not occur in otherwise healthy, unmedicated children. Paradoxical reactions, including restlessness, agitation, and disinhibition, occur in approximately 1% to 3% of patients (McMillan et al., 1992; Golparvar et al., 2004). The bitter taste of midazolam, which is difficult to conceal, may reduce acceptance. Midazolam can also be administered via nasal mucosal delivery at 0.2 mg/kg with a more rapid onset but has the disadvantage of an unpleasant burning sensation (Zedie et al., 1996). Prolonged recovery times are not seen with the use of midazolam premedication (Davis et al., 1995b; Bevan et al., 1997). Acetaminophen (20 mg/kg), in fruit-flavored syrup, can also be mixed with midazolam as part of premedication for postoperative analgesia, especially for short ear cases, such as myringotomies (Watcha et al., 1992).
Opioids
The use of opioids for premedication is uncommon in healthy children who are undergoing elective procedures. Even with noninvasive delivery systems such as oral transmucosal fentanyl citrate (OTFC), the advantage of relatively rapid onset is offset by the disadvantages of dysphoria, pruritus, nausea, and vomiting (Goldstein-Dressner, 1991; Ashburn et al., 1993; Epstein et al., 1996). Opioid premedication is best reserved for children experiencing pain, in which analgesia and sedation can be synergistic. The risk of respiratory depression, especially in infants younger than 6 months of age, should always be taken into account.
Ketamine
Ketamine can be given via the oral, nasal, rectal, or IM route (Stewart et al., 1990; Gutstein et al., 1992; Weksler et al., 1993; Tanaka et al., 2000). Ketamine is an effective sedative, but it can also cause increased secretions, nausea, vomiting, psychological disturbances, and prolonged recovery. IM injection of ketamine, 2 to 3 mg/kg (undiluted) may be useful in uncooperative, combative children as the last resort to avoid inhalation induction by force, which increases the risk of physical and psychological trauma to patients (Hannallah and Patel, 1989).
Alpha 2-Adrenergic Receptor Agonists
Oral (4 mcg/kg) and rectal (5 mcg/kg) clonidine can produce excellent perioperative sedation and anxiolysis while reducing the anesthetic requirement, emergence agitation, and postoperative pain, shivering, nausea, and vomiting (Bergendahl et al., 2004; Schmidt et al., 2007; Tazeroualti et al., 2007). The prolonged postoperative sedation seen with clonidine premedication may be advantageous after major surgical procedures but can delay discharge for same-day surgery patients. Similar findings have been described with transmucosal dexmedetomidine 0.5 to 1 mcg/kg (Schmidt et al., 2007; Yuen et al., 2008).
Topical Anesthesia
For children who require or prefer an intravenous (IV) induction, there are multiple approaches to achieving topical anesthesia. Local anesthetics can be delivered without needles through the skin’s protective stratum corneum into the innervated dermal layers via eutectic (EMLA) and liposomal (EL-MAX) creams or driven by mechanisms such as heat, iontophoresis, laser-assistance, or pressurized helium (Hung et al., 1997; Baron et al., 2003; Zempsky et al., 2004; 2008; Sawyer et al., 2009). These techniques, each with limitations, are capable of providing analgesia within 1 to 60 minutes of application. Reported satisfactory anesthesia varies with technique and patient age. Children younger than 6 or 7 years of age tend to report pain, secondary to fear and anticipation of needlesticks, even with apparent anesthesia (Arts et al., 1994; Kleiber et al., 2002).
Parental Presence During Induction
Parental presence during induction of anesthesia (PPIA), in the operating room or a separate area such as an induction room, has increased significantly in the United States over the last decade (Kain et al., 2004). Although the practice avoids separating children from their parents, it has not been shown to decrease patient anxiety or increase cooperation during induction (Kain et al., 1998; Arai et al., 2007). One benefit, which is important in terms of family-centered care, is an increase in parental satisfaction scores. Kain and colleagues, in 2006, confirmed the previous findings of Bevan et al. from 1990 that PPIA had a measurable benefit when a calm parent accompanied an anxious child, and a worsening effect when an anxious parent accompanied a calm child. There was no measurable benefit when both parent and child were calm. Subsequently, they have determined that parental preparation to reduce anxiety (e.g., learning distraction techniques, and avoiding reassuring behavior) can significantly improve the outcome of PPIA (Kain et al., 2007).
Preparation for Induction
Monitoring During Induction
At a minimum, monitoring during induction should include pulse oximetry and capnography. The precordial stethoscope, once the sine qua non of pediatric anesthesia, has apparently been displaced by more accurate and adaptable monitors (Watson and Visram, 2001). However, the precordial stethoscope is still an essential and more sensitive and continuous monitor for changes in breath sounds and the quality of heart beat, especially in infants and young children. Vital signs can vary markedly during the induction of anesthesia and should be observed continuously with a precordial stethoscope and in accordance with the ASA standards for patient safety (1986). If the child is anxious, it is probably best to place additional monitors like ECG pads and a blood pressure cuff after induction instead of losing the opportunity for a calm induction. However, medical condition and early infancy may necessitate full monitoring before and during induction. If this is the case, the baseline measurements should be obtained before the patient is exposed to any anesthetic agent.
Methods of Induction
Inhalation Induction
Once in the operating or induction room, the child may be given the choice to lie down or sit up. If sitting is elected and parents are present, the child can be offered the option to sit in a parent’s lap or next to them (Fig. 13-2). The anesthetic mask, either one introduced in the waiting area or one detached from the anesthesia circuit, should be shown (again) to the child. Putting artificial fruit or candy flavors in the mask may help disguise the odor of the anesthetic. One should never place the mask on the child’s face without warning. Even with preparation, some children, especially those with previous experience with inhalation inductions, may strongly reject the anesthesia mask (Przybylo et al., 2005). The mask may become more acceptable if a family or staff member initially tries it on, or if the anesthesiologist applies it to one of the child’s toys or random body parts before placing it near his or her face. Other methods to introduce the mask include having the child watch the insufflation of the anesthetic bag or hold the mask him or herself as he or she begins to breathe into the circuit. Making a game out of this activity is far better than getting into battle of wills. Distraction techniques such as storytelling, singing, counting, or just talking nonsense are strongly encouraged. If the child still emphatically objects to the mask, other approaches such as supplemental sedation or an IV induction should be considered.
DuBois et al. (1999) compared three induction techniques of conventional tidal-volume breathing of sevoflurane: incremental increasing concentrations (2% to 6% to 8%) in 100% oxygen, 8% in 100% oxygen, and 8% in a 1:1 mixture of nitrous oxide and oxygen. There were minimal differences among the three approaches. Lejus and colleagues (2006) compared the two different breathing techniques of conventional tidal volume and single-breath vital capacity of 7% sevoflurane in children older than 5 years of age. Eyelash-reflex loss was more rapid in the vital-capacity group compared with the tidal-volume group, but the time to deep anesthesia, bispectral index values 60 and 40, and the incidences of side effects were similar in both groups. Of note, the vital-capacity technique was preferred over the tidal-volume technique by the children. The authors propose that the decreased exposure to the smell of sevoflurane and decreased awareness of losing consciousness explained the higher preference scores in the vital-capacity group.
If a child has fallen asleep or is well sedated in a parent’s arms or on a stretcher, anesthesia can be induced by the “steal technique,” as originally described by Guedel in 1921 (Calverley, 1986). While avoiding moving or awakening the child, 70% nitrous oxide at high flows via an anesthesia mask is held closely over the child’s face. At first the mask should not touch the skin, but as sedation deepens it is placed gently on the face while incrementally increasing the concentration of sevoflurane. Monitoring devices should be attached as soon as possible. Once adequately anesthetized, the child can be transferred to a stretcher or operating-room bed if needed.
Maintenance of the Upper-Airway Patency
Upper airway obstruction can arise during induction of anesthesia (even in healthy infants and children) for several reasons, including tongue displacement, excess soft tissue, velopharyngeal collapse, and laryngospasm. Applying pressure to the soft tissue between the rami of the mandible when holding a mask on a patient’s face can push the tongue against the hard and soft palate or displace it posteriorly, leading to occlusion of the oral pharynx or its outlet, respectively. Even minimal relaxation of the pharyngeal and laryngeal muscles during induction can significantly reduce oral and nasal passages already compromised by enlarged or increased amounts of lymphoid tissue. Relaxation of the pharyngeal and laryngeal muscles, accompanied by a marked increase in respiratory effort and excessive generation of negative pressure, which can occur in response to pain and other stimulation, may result in collapse of the velopharynx. To prevent upper airway obstruction from these causes, the pediatric anesthesiologist must learn to hold the anesthesia mask snugly to the patient’s face and perform the triple airway maneuver—neck extension, jaw thrust, and mouth opening—without applying pressure to the soft tissue or causing pain (Fig. 13-3). In addition, a moderate amount of continuous positive airway pressure (CPAP; 10 to 15 cm H2O) can counteract the collapsing force on the relaxed upper airway (Motoyama, 1997; Hammer et al., 2001). Careful and continuous monitoring of breath sounds via a precordical stethoscope is essential to guide and maximize the effectiveness of upper-airway maintenance by hand.
When the patient is sufficiently anesthetized, an oropharyngeal airway may be inserted to further aid in maintaining a patent airway. Insertion of an oral airway in an inadequately anesthetized patient risks triggering laryngospasm. The proper length of an oral airway, with the tip behind the base of the tongue, can be estimated by holding the airway over the side of the child’s face extending from the ear to the angle of the mandible. A preferred method for inserting an oral airway is to slide it gently forward and downward over the tongue while the tongue is pulled outward by a tongue depressor. The technique of inserting the airway upside down and then correcting the orientation in the posterior pharynx should be avoided in children. It tends to push the tongue posteriorly and obstruct the oral pharynx, particularly in infants (Smith, 1980). A nasopharyngeal airway is better tolerated when the anesthesia is too light for insertion of an oropharyngeal airway. This type of airway should be well lubricated and inserted very gently to prevent mucosal injury and bleeding.
If the obstruction is not relieved by airway maneuvers, the patient may have laryngospasm, which can result from laryngeal mucosal irritation and is often initiated by the aspiration of saliva. Vigorous positive pressure ventilation may push the secretions down into the larynx, which intensifies the spasm and further inflates the stomach, compromising pulmonary gas exchange. The risk of regurgitation and aspiration of gastric contents also increases. A healthy child can tolerate a few moments of laryngospasm. A more successful approach is to maintain moderate continuous positive pressure and synchronous ventilation with the “expiratory” phase of laryngospasm, which is when the vocal cords momentarily relax. By using 100% oxygen and intermittent positive pressure, one can ventilate enough gas through the glottis to avoid serious hypoxemia. If rapid oxygen desaturation and bradycardia ensue, IV succinylcholine (2 mg/kg) and atropine (0.02 mg/kg) or 5 mg/kg of IM succinylcholine should be administered without delay (Liu et al., 1981; Hannallah et al., 1986). Airway patency needs to be reestablished with or without tracheal intubation.
Intravenous Induction
Propofol
An induction dose of propofol is 2.5 to 3.0 mg/kg in healthy unpremedicated children between the ages of 3 and 12 years (Manschot et al., 1992). In induction studies, children younger than 2 years of age required a significantly larger dose (2.6 to 3.4 mg/kg), whereas older children needed less (Aun et al., 1992; Manschot et al., 1992). Induction with propofol in children can cause significant decreases in blood pressure and inotropy, similar to those observed after thiopental (Mirakhur, 1988; Hannallah et al., 1991; Manschot et al., 1992).
A major drawback to propofol is the pain it causes on injection. In a quantitative systematic review, Picard and Tramèr (2000) determined that the only approach that had a reliably low number of needed-to-be-treated (NNT) patients was the lidocaine-tourniquet technique (similar to a Bier block; see Chapter 16, Regional Anesthesia) and that preinjection of local lidocaine and analgesics, temperature of medication, speed of infusion, and vein size had no significant effect. Yew et al. (2005) and Rochette et al. (2008) have shown that the addition of medium-chain triglyceride to propofol preparation also decreases pain on injection. Propofol offers the advantage of having antiemetic properties (Borgeat et al., 1990).
Thiopental
The induction dose of thiopental in healthy children is 5 to 6 mg/kg (Coté et al., 1981). In infants between 1 and 6 months of age, the median effective dose (ED50) is reported to be 6.8 mg/kg; in infants younger than 2 weeks of age, it is 3.4 mg/kg (Jonmarker et al., 1987). The dose for induction in infants younger than 5 months was almost twice that for older children (see Chapter 7, Pharmacology of Pediatric Anesthesia, Fig. 7-18). Like propofol, thiopental is a cardiac depressant and vasodilator, and it should be used with care in patients suspected to have hypovolemia or decreased cardiac function.
Etomidate
The induction dose of etomidate in healthy children is 0.3 mg/kg, and it is often recommended in patients who have limited hemodynamic reserve. Sarkar and colleagues (2005) confirmed that in children there are no significant changes in right atrial, aortic, or pulmonary artery pressure, or systemic or pulmonary vascular resistance after bolus dosing. Side effects include pain on injection and myoclonic movements.
Maintenance of anesthesia
Pharmacologic Agents
Inhaled Anesthetics
Inhaled anesthetics are the most commonly used agents in the administration of general anesthesia in pediatric patients. Although halothane was a staple in pediatric anesthesia for years, the development of newer agents with better safety profiles has made halothane nearly obsolete (Lerman, 2004). With discontinuation of the production of halothane in the United States, sevoflurane, desflurane, and isoflurane are now the most commonly used inhalation agents. Nitrous oxide is also commonly used as an adjunct, although there remains some controversy regarding its use.
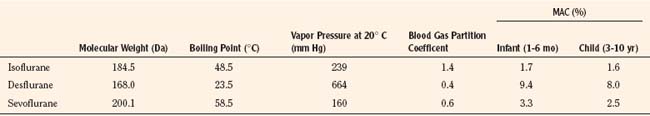
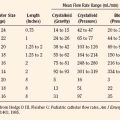
Introduced in 1995, sevoflurane became the agent of choice for inhalation inductions. In addition to its rapid and smooth induction of anesthesia, sevoflurane causes few arrhythmias, less cardiovascular depression, and minimal renal or hepatic toxicities, all of which are improvements over halothane (Lerman, 2004). Isoflurane and desflurane also have more stable cardiovascular profiles and less associated toxicity compared with halothane. However, each agent has limitations that warrant consideration in certain patients and clinical situations. Table 13-1 summarizes the characteristics of the commonly used volatile anesthetics.
Nitrous Oxide
Nitrous oxide is the oldest and has been the most widely used anesthetic in both adults and children. It has an inoffensive odor and a very low solubility (blood-gas partition coefficient of 0.47) that results in rapid uptake and distribution. Because it has a minimum alveolar concentration of 104% for general anesthesia, nitrous oxide cannot be used as a sole anesthetic at normal atmospheric pressures. Therefore, it is generally used as an adjunct to reduce the minimum alveolar concentration of a primary inhalation agent, increasing the rate of induction of general anesthesia, as well as providing or augmenting the analgesic aspect of general anesthesia (Emmanouil and Quock, 2007).
Nitrous oxide has mechanisms of action that continue to be discovered. It appears that the anesthetic effect occurs through inhibition of N-methyl-d-aspartate (NMDA) glutamate receptors in the central nervous system. Analgesia occurs through release of endogenous opioids that then stimulate opioid receptors and spinal level γ-aminobutyric acid (GABA) receptors. Anxiolysis occurs through activation of GABAA, although this pathway is still being investigated (Emmanouil and Quock, 2007).
Although nitrous oxide is widely used, there are certain patients and clinical situations in which its use is not recommended and even contraindicated. In adults with pulmonary hypertension, nitrous oxide increases pulmonary artery and pulmonary wedge pressures (Schulte-Sasse et al., 1982). However, in healthy infants, Hickey and colleagues (1986) observed mild decreases in heart rate, blood pressure, and cardiac index with no increase in pulmonary artery pressure or vascular resistance.
Nitrous oxide accumulates in closed, gas-containing spaces and should be avoided in patients at risk of toxicity caused by this expansion. This includes patients with obstructed loops of bowel, pneumothorax, pneumocephalus, and middle-ear surgery. Nitrous oxide also has been shown to increase middle cerebral-artery blood-flow velocity and may increase intracranial pressure (ICP), making its use in children with increased ICP contraindicated (Wilson-Smith et al., 2003). Furthermore, in situations in which maximal oxygen delivery is needed, such as shock, massive blood loss, severe anemia, and compromised cerebrospinal blood flow, nitrous oxide should be avoided. Children with abnormal vitamin B12 and B12-related metabolism may also be at risk of neurologic injury after exposure to nitrous oxide during a routine anesthetic procedure (Sanders et al., 2008).
The role of nitrous oxide in postoperative nausea and vomiting (PONV) has been examined extensively. Although the incidence was increased when used with propofol, no difference was seen in the incidence of PONV with or without nitrous oxide when used with sevoflurane or desflurane in children (Watcha et al., 1991; Kuhn et al., 1999; Bortone et al., 2002). Nitrous oxide supports combustion. Thus, its use in patients undergoing oral or facial procedures should be reevaluated with regard to the possibility of an airway fire.
Sevoflurane
Since its introduction in the mid 1990s, sevoflurane has replaced halothane as the agent of choice for inhalation inductions in pediatric patients. It is a fluorinated methyl isopropyl ether with a blood-gas partition coefficient of 0.68, allowing for rapid induction and recovery. With a nonpungent odor and minimal airway irritation, up to 8% sevoflurane can be delivered without significant breath holding, coughing, or laryngospasm. The minimum alveolar concentration (MAC) of sevoflurane decreases from 3.3% in neonates and infants younger than 6 months old to 2.5% in children between 6 months and 5 years old and to 2.0% in adults (Hatch, 1999). Lerman and colleagues (1994) found that the MAC-sparing effect of nitrous oxide was significantly less for sevoflurane.
Sevoflurane causes minimal cardiovascular side effects in children. Compared with desflurane, sevoflurane produces less hypotension. There is also less tachycardia than isoflurane and less myocardial depression than halothane (Frink et al., 1992b; Holzman et al., 1996). Using echocardiography, Wodey et al. (1997) found that sevoflurane did not affect heart rate, cardiac index, or myocardial contractility. Furthermore, it does not sensitize the myocardium to epinephrine (Hayashi et al., 1987). Arrhythmias are uncommon with sevoflurane compared with other agents (Hatch, 1999).
Sevoflurane affects respiratory function, with some studies suggesting it does so to a greater extent than other agents (Doi et al., 1994; Yamakage et al., 1994). Brown and colleagues (1998) found that compared with halothane, minute ventilation and respiratory frequency were lower in infants on 1 MAC of sevoflurane, but that there were only moderately increased end-tidal carbon dioxide (CO2) levels. Sevoflurane is, however, an effective bronchodilator (May et al., 1996). Neurologically, sevoflurane has been associated with cortical epileptiform electroencephalograms, although no lasting clinical sequelae (such as seizures) have been attributed to sevoflurane alone. Further studies are necessary to determine which patients are at risk and what can be done to decrease the incidence of these EEG changes (Constant et al., 2005).
As much as 5% of sevoflurane is metabolized, with defluorination producing an inorganic fluoride that is then excreted in the urine. Peak fluoride concentrations, although higher in sevoflurane than in isoflurane, are still well below the accepted nephrotoxic level of 50 mmol/L. There is rapid elimination, and these levels remain below toxic levels even with prolonged anesthetics (Levine et al., 1996; Hatch, 1999). In addition, the metabolism of sevoflurane by the P450E1 system is mostly in the liver and not the kidneys. Thus, the concern of nephrogenic diabetes insipidus (DI) by elevated fluoride levels does not appear to be an issue.
Sevoflurane reacts with soda lime in the anesthesia circuit, resulting in the production of compound A. Compound A increases more when Baralyme brand (Allied Healthcare Products Inc., St. Louis, Mo) is used, when dry rather than wet absorbents are used, and when lower fresh gas flows (0.5 to 1 L) are used, resulting in increased canister temperatures (Frink et al., 1992a). Ebert and colleagues did not find evidence of renal injury after sevoflurane was administered in high concentrations (3% end-tidal) for 8 hours (1998). Kharasch and colleagues (1997) also found no significant renal dysfunction in patients with low-flow sevoflurane (1 L/min) when compound A was detected. Other studies have also documented no worsening of renal function after sevoflurane, even in patients with preexisting renal impairment (Conzen et al., 1995). New generation CO2 absorbers, including DragerSorb Free and Amsorb Plus, have been shown to provide adequate CO2 absorption while reducing the production of compound A, even at fresh gas flows as low as 500 mL/min (Marini et al., 2007).
Spontaneous ignition, fire, and explosion resulting from an exothermic reaction of sevoflurane with desiccated CO2 absorbers have also been reported (Castro et al., 2004; Wu et al., 2004). With high fresh gas flow, the increasing dryness of the absorbent increases degradation of sevoflurane. Dunning et al. (2007) demonstrated that up to 3 mol of hydrogen were produced in the reaction of sevoflurane with heated, desiccated absorbent. The authors postulate that this hydrogen is the most likely fuel in anesthesia machine fires. Special attention should be paid to situations in which absorbent desiccation may be greater, such as in seldom-used anesthesia machines and in operating rooms where high fresh-gas flows relative to body size are used (Wu et al., 2004). The use of newer absorbents or Mapleson D circuits can also help avoid this problem (Woehlck, 2004).
While the question of sevoflurane-related hepatitis has been raised in case reports, metabolism of sevoflurane does not result in the trifluoroacetylated liver proteins that trigger the immune-mediated hepatitis seen with halothane, and immune-based hepatitis after sevoflurane has not been reported (Kharasch, 1995; 2008). Malignant hyperthermia has been reported with sevoflurane use (Otsuka et al., 1991).
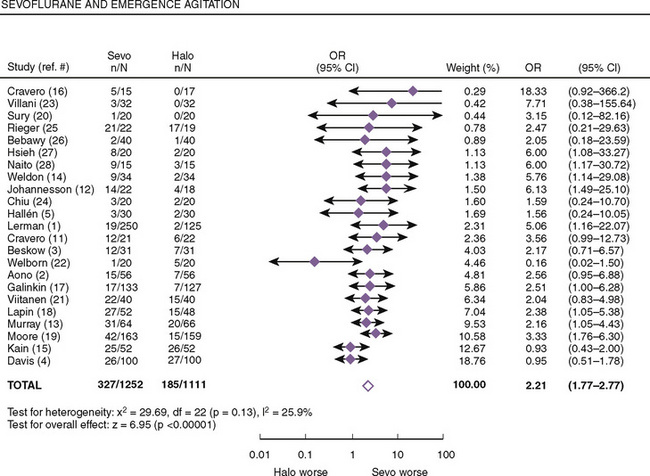
Both postanesthetic emergence time and the time when discharge criteria are met are significantly lower in patients given sevoflurane compared with those receiving halothane; however, patients receiving sevoflurane had significantly higher pain scores that required analgesics to be administered earlier (Naito et al., 1991; Sarner et al., 1995). In a meta-analysis of randomized controlled trials, Kuratani and Oi (2008) demonstrated that sevoflurane had a higher probability of emergence agitation compared with halothane (Fig. 13-4). Treatment or pretreatment with either dexmedetomidine, fentanyl, or propofol can mitigate emergence agitation after sevoflurane anesthesia.
Desflurane
Desflurane is a fluorinated methyl ethyl ether with a blood:gas solubility coefficient of 0.42, which is close to nitrous oxide and lower than any other agent. With lower potency, the MAC in newborns is 9.2%, in infants it is 8% to 9.9%, and in older children and adults it is 6% (Hatch, 1999). Because of its lower boiling point (22.8° C), desflurane requires a heated pressurized vaporizer. It is also resistant to degradation and biotransformation.
Desflurane is highly irritating to the upper airway. Zwass and colleagues (1992) found that it caused moderate to severe laryngospasm in 49% of unpremedicated patients and moderate to severe coughing in 58%. These parameters did not improve with premedication. The authors concluded that desflurane, although safe for maintenance of anesthesia, was limited in its use for inhalation inductions. Recent changes in the package insert suggest that desflurane is not recommended for use in patients undergoing bag-and-mask ventilation or in patients with an LMA in place.
Studies in both adults and children have demonstrated a dose-related effect of desflurane on ventilation, with a decrease in tidal volume, minute ventilation, and the ventilatory response to CO2. Respiratory rate was shown to increase (Lockhart et al., 1991; Behforouz et al., 1998). At 1 MAC, desflurane exhibits bronchodilator effects. However, with increasing concentration to 2 MAC, desflurane increased airway resistance as opposed to other inhalation agents that continued to produce bronchodilation (Dikmen et al., 2003). In children with known airway susceptibility, desflurane caused significant elevations in airway resistance and increased airway narrowing (von Ungern-Sternberg et al., 2008).
Desflurane has been shown to provide cardiovascular stability. Systemic vascular resistance decreases, which results in a decrease in systemic blood pressure without changes in cardiac output. Taylor and Lerman (1992) showed a mean percentage decrease in systolic arterial pressure of 29 + 13% from preanesthetic values in children, with the incidence of hypotension greatest in infants and least in children 5 to 12 years of age. These pressure differences, however, did not require intervention. Heart rate increases in a dose-dependent manner, and arrhythmias are uncommon. Sympathetic tone increases, but there does not appear to be increased sensitization of the myocardium to epinephrine as is seen with halothane (Hatch, 1999).
Because of its low blood:gas partition coefficient, desflurane allows for rapid emergence and recovery, which has been demonstrated in several studies of children (Taylor and Lerman, 1992; Welborn et al., 1996; Wolf et al., 1996). However, Welborn et al. (1996) found that recovery from desflurane was associated with a significantly greater incidence of agitation and excitement, and that there was no difference in the time to meet discharge criteria as compared with other inhaled anesthetics. Wolf and colleagues (1996) found that desflurane, unlike isoflurane, was not associated with postoperative apnea and suggested that it may be useful in ex-premature infants. More recently, however, Sale et al. (2006) showed that although there was a faster time to awakening with desflurane, apnea did occur with desflurane and there was no difference between desflurane and sevoflurane in the incidence of postoperative apnea in the ex-premature infant undergoing hernia repair (Kuratani et al., 2008).
Isoflurane
Isoflurane is a stable liquid that has a blood:gas partition coefficient of 1.4, which is much higher than both sevoflurane and desflurane. Like the other inhalation agents, the MAC of isoflurane is age-dependent, ranging from 1.3% in preterm infants to 1.7% in infants 6 to 12 months of age and decreasing to 1.6% in children 1 to 5 years old, compared with 1.2% in adults (Cameron et al., 1984; LeDez and Lerman, 1987). Because of its pungent odor, isoflurane causes coughing and laryngospasm, which precludes its use for inhalation inductions. Isoflurane is resistant to biodegradation, making the incidence of hepatotoxicity and nephrotoxicity minimal (Eger, 1984). The emergence profile is similar to that of halothane.
Like the other inhalation agents, isoflurane produces a dose-dependent respiratory depression, with an increase in respiratory rate and smaller tidal volumes that produce an increase in arterial CO2 pressure. Isoflurane depresses ventilation to a greater extent than halothane, and isoflurane anesthesia requires mechanical ventilation (Eger, 1984). It is also a bronchodilator, with continued effects at higher concentrations (Dikmen et al., 2003).
Isoflurane, in a dose-dependent manner, decreases systemic vascular resistance, resulting in a subsequent drop in the systemic arterial pressure and increase in heart rate, all while maintaining cardiac output (Wolf et al., 1986). Tachycardia is particularly seen in younger patients (Eger, 1984). However, there is no reported increase in the incidence of arrhythmia, and it does not appear to sensitize the myocardium to epinephrine.
Intravenous Agents
Propofol
Propofol is a short-acting intravenous agent that is used for both induction and maintenance of anesthesia. With its fast onset, smooth maintenance, clear headed emergence, and decreased incidence of nausea and vomiting, it has many properties that make it ideal for TIVA. It is commonly used for procedures and sedation outside of the operating room, often as the sole agent in locations such as magnetic resonance imaging (MRI) and the endoscopy suite. Although propofol has no direct analgesic properties, patients receiving it seem to have lower analgesic requirements. At lower doses, it may not produce amnesia (Marik, 2004).
Propofol is highly lipid-soluble and rapidly cleared and redistributed into the peripheral tissues, with a short, context-sensitive half time (see Chapter 7, Pharmacology of Pediatric Anesthesia, Fig. 7-16). Relative to adults, children have a larger central compartment, and they have a higher clearance that can be twice that of adults, resulting in higher dosing requirements. Hannallah and colleagues (1994) showed that with a background of 60% nitrous oxide, pediatric patients required a dose of 250 to 300 mcg/kg per minute to prevent movement with surgical stimulation. A dose of 150 to 200 mcg/kg per minute stabilized the hemodynamic indices of those who received muscle relaxants. Moderate hypotension and bradycardia are associated with propofol administration, with a decrease in mean arterial pressure (MAP) of approximately 10% on induction and another 10% during the early maintenance phase. The arterial pressure subsequently returns to baseline (Passenbacher et al., 2002). The use of adjuncts such as opioids can accentuate these responses, while coadministration of ketamine has been shown to preserve hemodynamic stability, albeit with more postoperative agitation (Aouad et al., 2008).
Pain during injection is an issue with propofol. However, preadministering or mixing it with lidocaine appears to attenuate this somewhat. Often, an inhalation induction followed by the administration of propofol for maintenance also avoids this issue in children. Propofol is associated with a shorter recovery time and a shorter time until discharge. It is also associated with significantly decreased incidence of postoperative nausea and vomiting when compared with halothane (Marsh et al., 1991; Watcha et al., 1991; Hannallah et al., 1994). Cohen et al. (2004) found in patients aged 2 to 36 months that propofol at 200 mcg/kg with analgesic supplementation had the same hemodynamic, recovery, and antiemetic profile as sevoflurane. Emergence delirium does not appear to be an issue with propofol.
Although many properties of propofol make it ideal for sedation, it has been associated with several adverse effects in children and adults undergoing prolonged infusions (greater than 48 hours) at high doses of greater that 4 mg/kg per hour (Vasile et al., 2003). This propofol infusion syndrome (PRIS) is characterized by bradycardia, hypotension, lipemic plasma, fatty liver enlargement, significant lactic acidosis, rhabdomyolysis, and myoglobinuria. Usually, PRIS leads to fatal renal, cardiac, and circulatory failure (Fudickar and Bein, 2009).
PRIS is caused by propofol’s impairment of oxygen utilization and inhibition of mitochondrial electron transport, leading to cytolysis of both skeletal and cardiac muscle cells (Kam and Cardone, 2007). Children appear to be more prone to PRIS because of low glycogen storage and high dependence on fat metabolism (Fudickar and Bein, 2009). Current therapy involves immediate stoppage of the propofol infusion, cardiorespiratory support, and hemodialysis or hemofiltration to eliminate the propofol and its toxic metabolites. Carbohydrate supplementation is also recommended (Fudickar and Bein, 2009).
Dexmedetomidine
Dexmedetomidine is a highly selective α2-adrenoreceptor agonist with both sedative and analgesic properties. Its elimination half-life is 2 hours, with a rapid distribution half-life of only 6 minutes (Dyck and Shafer, 1993), making it ideal for IV infusion. U.S. Food and Drug Administration (USFDA)-approved for sedation in the intensive care unit in adult patients who are mechanically ventilated, dexmedetomidine has now been used in the pediatric ICU setting, as well as for sedation in noninvasive and invasive procedures (Chrysostomou et al., 2006; 2009). It has minimal effects on respiration, which may be beneficial in pediatric patients with upper airway obstruction, and it allows for agitation-free emergence (Mahmoud et al., 2010).
Several studies have reported efficacy of dexmedetomidine for sedation in noninvasive radiologic imaging. Compared with midazolam, dexmedetomidine provided better quality of sedation and less need for rescue sedation (Koroglu et al., 2005). In another study by Koroglu and colleagues (2006), patients randomly received either dexmedetomidine or propofol. Both agents demonstrated equally effective sedation, but propofol had shorter induction, recovery, and discharge times; however, hypotension and oxygen desaturation were more common with propofol. Mason and colleagues (2006) found that the effective mean loading dose of dexmedetomidine for children during radiologic imaging was 2.2 mcg/kg, followed by a continuous infusion of 1 mcg/kg per hour. There was no change in end-tidal CO2 and no oxygen desaturation while these children were breathing room air.
For invasive procedures, use of dexmedetomidine as the sole agent for sedation has produced mixed results. For cardiac catheterization, Munro et al. (2007) used an average maintenance infusion rate of 1.15 + 0.29 mcg/kg per hour with a range of 0.6 to 2 mcg/kg per hour. Hammer and colleagues (2008) noted that dexmedetomidine significantly depressed sinus and AV nodal function with sinus cycle length and sinus node recovery time increasing. Sixty percent of patients needed a propofol bolus for movement, for an increasing bispectral index (BIS) number, or in anticipation of stimulus. Because of dexmedetomidine’s limited analgesic effects, use of ketamine or inhalational agents as an adjunct to dexmedetomidine has been shown to be effective in several studies (Ard et al., 2003; Tosun et al., 2006).
In adults, hypotension and bradycardia are reported, especially when propofol is given as a large and rapid bolus dose or with other agents that are negative chronotropes. There appears to be a biphasic response, with an initial increase in systolic blood pressure and a reflex decrease in heart rate, followed by stabilization of both parameters below baseline values (Bloor et al., 1992). In pediatric patients, Hammer and colleagues (2008) demonstrated an increase in MAP and a decrease in heart rate from baseline values after a 10-minute loading dose of dexmedetomidine 1 mcg/kg. These effects did not continue once an infusion of 0.7 mcg/kg per hour was administered. Respiratory rate and end-tidal CO2 did not change. It also appears that concurrent use of medications that affect cardiac conduction, such as digoxin, enhance the risk of bradycardia. Mukhtar et al. (2006) demonstrated a sympatholytic effect of dexmedetomidine in pediatric patients undergoing cardiopulmonary bypass. Dexmedetomidine, through its centrally acting α2-adrenergic agonist effect, also can reduce the shivering threshold in children and be used to reduce postoperative shivering (Blaine et al., 2007).
Adjuvant Agents
Opioids
Opioids and their synthetic analogs are widely used in infants and children as adjuncts during maintenance of anesthesia, as well as to provide for better postoperative pain management. Whereas morphine and fentanyl have historically been the opioids of choice, newer agents such as remifentanil have become an important part of pediatric anesthesia. The agent chosen, as well as the dosage used, depends on the overall goal and the plan for postoperative management (spontaneous vs. mechanical ventilation). Opioids and other analgesics are discussed in greater detail in Chapter 7, Pharmacology of Pediatric Anesthesia. The intravenous dosages of opioids for children are listed in Table 13-2.
Morphine is a long-acting hydrophilic analgesic that is commonly used for management of both intraoperative and postoperative pain. There is a prolonged duration of action in very young infants because of its lower clearance and longer elimination half-life, which requires lower doses and a longer dosing interval in the newborn period (Lynn and Slattery, 1987). However, total body morphine clearance is 80% that of adults by 6 months of age (Bouwmeester et al., 2004). There does not appear to be an age-related difference in the respiratory depression that occurs (Lynn et al., 1993). Morphine can cause hypotension as a result of histamine release, and urticaria and bronchospasm can be seen. Postoperative nausea and vomiting can also occur.
Fentanyl is a lipophilic synthetic opioid that is approximately 100 times as potent as morphine but has a relatively short duration of action of 1 to 2 hours. Fentanyl is safely used as a primary anesthetic agent and as a supplemental analgesic during an inhalational anesthetic. Pharmacokinetic studies in neonates, infants, and children have demonstrated a highly variable volume of distribution, rate of clearance, and half-life (Koehntop et al. 1986; Singleton et al. 1987). Because the effect is less predictable in neonates and premature infants, postoperative ventilatory support should be considered.
Fentanyl is ideal for patients who need short-term anesthesia and analgesia in which a quick return to baseline respiratory function is desired. It is widely used in outpatient procedures as well as in neurosurgical cases. In cardiac surgery patients, higher doses (upward of 50 mcg/kg) have been found to provide patients with hemodynamic stability. For longer procedures, it can also be administered as a continuous infusion. However, its context sensitive half-time profile changes dramatically after 2 hours of the infusion (see Chapter 7, Pharmacology of Pediatric Anesthesia, Fig. 7-15).
Sufentanil is approximately 1000 times as potent as morphine, with an elimination half-life about half that of fentanyl but a similar neonatal pharmacokinetic profile (Davis et al., 1987a, 1987b). It is often administered as a continuous infusion.
Alfentanil is approximately 25 to 100 times as potent as morphine. It has a small volume of distribution and an elimination half-life one third that of fentanyl. In neonates, it has a prolonged elimination time and increased volume of distribution (Davis et al. 1989). It also has great patient-to-patient variability, making its effects more difficult to predict. Like other opioids, alfentanil has a high incidence of PONV.
Remifentanil is an ultrashort-acting opioid with potency similar to fentanyl. It undergoes esterase cleavage to inactive metabolites, making its clearance independent of hepatic or renal clearance. Ross et al. (2001) found a consistent pharmacokinetic profile, with neonates and infants younger than 2 years of age having the largest volume of distribution and most rapid clearance. It has a constant context-sensitive half-life of 3.4 to 5.7 minutes in all age groups despite the duration of administration and the dose infused. With a mean rate of 0.55 mcg/kg per minute, Davis et al. (2001) demonstrated that remifentanil was safe and provided stable intraoperative and postoperative conditions in premature and full-term infants undergoing pyloromyotomy. It allowed for rapid emergence without postoperative apnea. For tonsillectomy and adenoidectomy, remifentanil provided faster extubation times compared with fentanyl. However, these patients had higher pain discomfort scores postoperatively, indicating that supplemental analgesia for the postoperative period is necessary (Davis et al., 2000).
Meperidine is one tenth as potent as morphine and does not have the increased narcotic effect in the newborn. In older infants and children, it offers the same advantages as morphine. Histamine release is associated with its use. Doses of meperidine that are less than 0.5 mg/kg reliably treat postanesthetic shivering (Macintyre et al., 1987).
Nonsteroidal Antiinflammatory Drugs
Nonsteroidal antiinflammatory drugs (NSAIDs) are often used intraoperatively to help decrease postoperative pain. Ketorolac has been shown to improve postoperative analgesia with a decrease in nausea and vomiting (Watcha et al., 1992; Cohen et al., 1993). There is continued controversy, however, as to whether the effects on platelet function should limit its use in the perioperative setting. Splinter and colleagues (1996) found that ketorolac increased bleeding in tonsillectomy patients. However, other studies have shown that ketorolac improved postoperative analgesia without increasing the incidence of bleeding after tonsillectomy and cardiac surgery (Romsing et al., 1998; Moffett et al., 2006). It has also been shown to be effective and safe in neonates and premature infants, and its kinetics have been described in children 6 to 18 months of age (Papacci et al., 2004; Lynn et al., 2007). It should be avoided in patients with compromised renal and hepatic function.
Acetaminophen is often given per rectum intraoperatively to supplement opioid analgesia. Korpela et al. (1999) found that there was a dose-related reduction in the number of children requiring a postoperative rescue opioid when given acetaminophen 40 or 60 mg/kg rectally after induction of anesthesia. There was also a decrease in PONV. Doses higher than 40 mg/kg, however, are at or near toxic levels, and higher dosing warrants further safety studies. Subsequent doses should be modified.
Intravenous acetaminophen provides effective analgesia, with 100% bioavailability, a rapid onset of action within 5 minutes of administration, and peak analgesic efficacy at 1 hour after administration (Wilson-Smith and Morton, 2009). Prins and colleagues (2008) compared IV with rectal acetaminophen in children younger than 2 years old who were undergoing craniofacial surgery. They found that the IV form was more effective, producing analgesia more rapidly and with less interpatient variability compared with the rectal administration. In the United States, ongoing studies are looking at the efficacy and safety of IV acetaminophen in the pediatric population for future approval by the Food and Drug Administration.
Hypnotics and Sedatives
Benzodiazepines, although most commonly used for premedication, can be used intraoperatively to ensure amnesia as part of a balanced anesthetic and to prevent emergence delirium. The shorter half-life of midazolam makes it well-suited for intraoperative use. In a meta-analysis, midazolam premedication was not found to cause a significant delay in either time to emergence or time to discharge from the PACU (Cox et al., 2006).
Ketamine
Ketamine, an NMDA-receptor antagonist, has long been used in pediatric anesthesia because of its analgesic properties and its ability to produce a dissociative state. Blood pressure is well maintained with spontaneous ventilation and preservation of laryngeal reflexes. However, it can produce psychodysmorphic symptoms that require supplementation with a benzodiazepine. To produce satisfactory conditions for diagnostic or therapeutic procedures, ketamine can be administered as intermittent bolus doses of 0.2 to 1 mg/kg along with glycopyrrolate to reduce oral secretions, or as an infusion of 1 to 2 mg/kg per hour after a loading dose (Morton, 1998). Ketamine at subanesthetic infusion doses of 0.1 to 0.25 mg/kg per hour has been shown to improve pain control and have an opioid-sparing effect (Finkel et al., 2007). Further studies to determine ketamine’s antihyperalgesic properties in the pediatric population are needed.
The question of whether ketamine affects central nervous system development in newborns has been raised. In humans, organogenesis of the central nervous system begins in utero and continues for several years after birth. Ketamine is associated with neuronal apoptosis in animal models during organogenesis. However, no long-term learning or behavioral disturbance has ever been noted in children after ketamine administration for anesthesia (Lois and De Kock, 2008).
Antiemetics
Prevention of PONV is an important aspect of any anesthetic. Prophylactic use of serotonin receptor (5-HT3) antagonists such as ondansetron and granisetron has decreased the incidence of PONV with minimal side effects and a decreased length of stay when compared with droperidol (Davis et al., 1995a; Fujii et al., 1996; Patel et al., 1997; Khalil et al., 2005). The half-life of ondansetron in infants and children younger than 2 years of age is 50% greater than in adults, making repeat dosing in the PACU unnecessary.
Metoclopramide (0.15 mg/kg) has antiemetic effects via antagonism of central dopaminergic receptors, as well as prokinetic effects caused by increased gastric emptying. Other agents with demonstrated effectiveness include dimenhydrinate at 0.5 mg/kg and perphenazine at 70 mcg/kg (Splinter and Rhine, 1998; Kranke et al., 2003).
Dexamethasone has been shown to be effective in reducing the incidence of PONV in children after tonsillectomy and after strabismus surgery (Splinter and Roberts, 1996; Madan et al., 2005). Kim et al. (2007) have shown no dose escalation response to dexamethasone in children having tonsillectomy or adenoidectomy. A dose of 0.0625 mcg/kg was as effective as 1.0 mcg/kg. There is also improved pain control in tonsillectomy patients (Elhakim et al., 2003). Several studies have shown that the best available treatment for patients at increased risk of PONV was a combination of a 5-HT3 antagonist and dexamethasone (Henzi et al., 2000; Gombar et al., 2007).
Muscle Relaxants
Muscle relaxants are discussed in greater detail in Chapter 7, Pharmacology. The following overview briefly discusses the advantages and disadvantages of the different agents currently available.
Succinylcholine is a depolarizing muscle relaxant with a fast onset (able to produce intubating conditions within 1 minute) and short duration of action. It can be given IV (2 mg/kg in infants and small children or 1 mg/kg in older children) or IM (3 to 4 mg/kg with an onset of 3 to 4 minutes) (Liu et al., 1981). Because of the incidence of bradycardia and possible asystole, atropine (0.02 mg/kg) is administered before succinylcholine. Although its use in children is warranted in emergency situations, caution should be used because of the serious complications that can be associated with it. Succinylcholine can cause significant hyperkalemia in patients with neuromuscular disorders and burns, dysrhythmias, muscle rigidity, masseter spasm, and postoperative myalgias. In susceptible individuals, it can also trigger malignant hyperthermia.
Vecuronium is an intermediate-acting steroidal nondepolarizing muscle relaxant. Vecuronium does not produce the vagolytic response that one sees with pancuronium. In children, it has a higher ED95 than in infants and adults (Meretoja et al., 1988). The duration of its effect is more prolonged in infants (73 minutes) compared with children (35 minutes) and adults (53 minutes) (Meretoja, 1988).
Rocuronium is an intermediate-acting steroidal nondepolarizing neuromuscular blocker that is similar in structure to vecuronium but one tenth as potent. It produces the most rapid onset of paralysis of the nondepolarizing agents and is used for rapid sequence inductions as an alternative to succinylcholine. At a dose of 0.6 mg/kg, the onset of maximal block is 1.3 + 2 minutes in children between 1 and 5 years old, and the time to recovery is 26.7 + 1.9 minutes (Woelfel et al., 1992). It can produce tachycardia and causes pain on injection in lightly anesthetized patients. Interaction of rocuronium with volatile anesthetic agents augments the intensity of neuromuscular blockade without effects on duration of or recovery from the block (Wulf et al., 1998).
Cisatracurium is an intermediate nondepolarizing agent that is a 1R-isomer of atracurium. At a dose of 0.15 mg/kg, the onset of maximal block is 2 + 0.8 minutes in infants and 3 + 1.2 minutes in children. Time to recovery was longer in infants (43.3 + 6.2 minutes) than in older children (36 + 5.4 minutes) (Taivainen et al., 2000). Cisatracurium undergoes hydrolysis at body temperature and physiologic pH (Hoffman elimination). It maintains hemodynamic stability and does not cause the release of histamine.
Regional Anesthesia
Regional anesthesia has become an important part of the anesthetic management of pediatric patients in the perioperative period. Regional techniques can decrease the intraoperative requirement of inhaled and intravenous agents and allow for a more rapid emergence with effective postoperative analgesia and minimal sedation (Markakis, 2000). Although caudal anesthesia remains the most popular regional technique in this population, epidural anesthesia, field blocks, ultrasound-guided peripheral nerve blocks, and neuroaxial opioids have also been used to a greater extent. The various regional analgesia techniques, as well as the choices and dosages of local anesthetics and opioids for regional analgesia are detailed in Chapter 16, Regional Anesthesia.
Caudal Anesthesia
Caudal blocks continue to be the most commonly used regional technique in infants and children. As an adjunct to general anesthesia, caudal blockade provides both intraoperative and postoperative analgesia for procedures in the lower abdomen, pelvis, or lower extremities. This block is easily performed with few associated complications, such as dural puncture and intravascular injection (Dalens and Hasnaoui, 1989). Anatomic features that contribute to these occurrences are the caudal position of the dural sac in infants at S3, the increased vascularity of the area, and the development of the sacral fat pad in school-aged children. Awareness of these factors should decrease the risk of complications.
Because infants and children do not cooperate and remain still while they are awake, caudal blocks are typically performed under general anesthesia. Once the airway is secured, the patient is placed in the lateral decubitus position and the sacral hiatus is identified and cleaned. Although several needle types and sizes have been investigated, short, beveled (22-gauge) needles have a lower incidence of intravascular injection compared with standard needles (Dalens and Hasnaoui, 1989). The needle is inserted through the sacrococcygeal ligament and advanced into the caudal space. Aspiration to confirm that there is no blood or cerebrospinal fluid (CSF), which would indicate inadvertent intravascular or intrathecal puncture, is performed before injection of the drug. Because the patient is under general anesthesia, the response to a standard test dose of epinephrine may be masked with minimal heart rate or blood pressure change seen. However, an increase of 25% or more in the T-wave amplitude appears to be a more reliable positive predictor of intravascular injection and was seen consistently with an epinephrine dose of 0.25 to 0.5 mcg/kg (Tanaka and Nishikawa, 1999; Kozek-Langenecker et al., 2000).
Bupivacaine is a local anesthetic that has long been used due to its relative safety and longer duration of action. It has a maximal safe dose of 2.5 mg/kg. Yaster and Maxwell (1989) recommend caudal dosing of bupivacaine 0.25% at 1 mL/kg, which should block approximately 10 spinal segments, with a maximal volume of 20 mL. For procedures longer than 3 hours, the caudal procedure can be repeated with a more dilute concentration of 0.125% or 0.175% to minimize the risk of toxicity as well as motor blockade. Levo-bupivacaine is the (S)-(-)-enantiomer of racemic bupivacaine, with similar local-anesthetic properties and potency. However, it is less toxic to the central nervous system and is less likely to cause myocardial depression and fatal arrhythmias than racemic bupivacaine (Tsui and Berde, 2005). Levo-bupivacaine 0.25% at a dose of 0.8 mL/kg provides adequate analgesia for penile or groin surgery (Taylor et al., 2003).
Ropivacaine is an S-enantiomer of bupivacaine with less cardiovascular and central nervous system toxicity than racemic bupivacaine. It produces significantly less motor blockade and stronger vasoconstriction at low concentrations (Zink and Graf, 2004). Several studies have shown comparable analgesic efficacy between ropivacaine, levo-bupivacaine, and bupivacaine (Ivani et al., 2002; Breschan et al., 2005; Locatelli et al., 2005). With lower systemic absorption and lower toxicity, ropivacaine is the drug of choice for prolonged use in neonates or patients with abnormal metabolism (Hansen et al., 2001). Hong and colleagues have shown that a high volume–low concentration of ropivacaine provides a higher level of block and longer duration of analgesia than a high concentration–low volume of ropivacaine (Hong et al., 2009), where the total drug amount is the same.
Other medications with analgesic properties, injected into the caudal space with and without local anesthetics, have been shown to be efficacious. Opioids, clonidine, and ketamine have been used with varying success and are discussed in greater detail in Chapter 16, Regional Anesthesia.
Contraindications to caudal anesthesia include active sepsis, local infection of the skin at the injection site, coagulopathy, sacral anomalies (including previous meningomyelocele), and uncorrected hypovolemia (Markakis, 2000). After caudal blockade, urinary retention occurs in approximately 10% of patients but is usually short-lived (Yaster and Maxwell, 1989).
Epidural Anesthesia
Placement of an epidural catheter through the caudal, lumbar, or thoracic route allows for continuous epidural delivery of local anesthesia both intraoperatively and postoperatively at the appropriate segmental level. Many have found success with catheters that were introduced caudally then threaded to the lumbar or thoracic level (Bosenberg et al., 1988). However, in 2002, Valairucha and colleagues found only 67% of catheter tips were in optimal position when threaded from below, and Blanco et al. (1996) could only advance 22% of catheters threaded from between L4 and L5 to predicted thoracic levels.
Direct placement at the lumbar and thoracic levels can be technically more challenging and present an increased risk of complications. However, weighing risks and benefits, anesthesiologists with experience in their placement and use have found that they provide successful analgesia for upper abdominal and thoracic procedures when specifically indicated. Rapp et al. (2005) demonstrated that ultrasound guidance allows for identification of the epidural space, ligamentum flavum, and dural structures, as well as the depth at which loss of resistance occurs. Willschke et al. (2006c) had a faster catheter placement, less bone contact, and direct visualization of epidural local anesthetic spread when ultrasound guidance was used instead of the standard loss-of-resistance technique. Further studies to evaluate whether there is a reduction in the complication rate in pediatric epidural anesthesia when using ultrasound for placement are ongoing.
Epidural opioids can also provide effective analgesia without sympathetic, sensory, or motor blockade (Shapiro et al., 1984; Rosen and Rosen, 1989). Respiratory depression is a major complication that requires careful dosing and observation. Pruritus, nausea, vomiting, and urinary retention can also occur but can be minimized with smaller doses of opioids or a small dose of naloxone (0.5 to 1 mcg/kg). Preservative-free morphine (30 to 70 mcg/kg caudally or 50 mcg/kg epidurally) is hydrophilic, which allows for a delayed onset, decreased systemic uptake, and prolonged duration of action (15 to 20 hours) (Attia et al., 1986; Rash et al., 1990). Fentanyl and hydromorphone are lipophilic, with a more rapid onset, greater systemic absorption, and shorter duration of action, making them more appropriate as adjuncts for continuous infusions (Dalens et al., 1986).
Subarachnoid Opioids
Subarachnoid injection of morphine has been found to have a similar efficacy and side effect profile to epidurally placed morphine. The recommended dose is 10 to 20 mcg/kg (Krechel and Helikson, 1993). Delayed respiratory depression may occur and is typically more severe than when given in the epidural space (Nichols et al., 1993). Careful monitoring and delayed further dosing of opioids are necessary.
Peripheral Nerve Blocks and Field Blocks
With advances in technology, peripheral nerve blocks are increasingly being used in the pediatric population as adjuncts to general anesthesia and to manage postoperative pain. These blocks can supply analgesia for the upper and lower extremities and allow for one-sided blockade as opposed to the central regional techniques. Axillary, interscalene, sciatic, and femoral nerve blocks have all been described. The more recent use of ultrasound allows for real-time imaging of the anatomy and direct visualization of the needle in anesthetized patients who cannot indicate discomfort or pain caused by direct nerve injection. As a result, ultrasonography is gaining in popularity over landmark-based techniques and neurostimulation (Marhofer and Frickey, 2006; Roberts, 2006). It also decreases the volume of local anesthetic per block by 30% to 50%, which allows the anesthesiologist to stay within maximum dosing guidelines and still achieve success (Ecoffey, 2007). Toxicity can further be avoided by using diluted solutions and solutions that contain epinephrine (Berde, 1989). Disposable pumps that can deliver continuous peripheral nerve block infusions of local anesthesia can be used for continued postoperative pain control at the patient’s home (Ganesh et al., 2007). Ludot and colleagues (2008) demonstrated successful analgesia and overall satisfaction with this technique in children who had a suitable family environment and proper parental instruction on the catheter’s management.
Field blocks are also an important aspect of pediatric anesthesia and allow for improved postoperative pain control while decreasing the incidence of side effects associated with opioids. Compared with caudal anesthesia, ilioinguinal-iliohypogastric nerve block, wound infiltration, local anesthesia “splash,” dorsal-penile nerve block, and subcutaneous ring block have all been shown to be effective for the chosen surgical procedure (Cross and Barrett, 1987; Hannallah et al., 1987; Fell et al., 1988; Casey et al., 1990). The use of ultrasound has also caused a revision in the technique of these blocks. The unpredictable depth of the posterior rectus sheath in children is more defined with ultrasound, allowing for improved analgesia. Furthermore, use of ultrasound for ilioinguinal-iliohypogastric nerve blocks reduced the volume of local anesthetic used to 0.075 mL/kg (Willschke et al., 2006a).
Monitoring
In addition to observation, standard monitoring devices include a pulse oximeter, capnography, ECG, an automated blood pressure measuring device, a temperature probe, and a precordial stethoscope (Fig. 13-5). For longer surgical procedures or when there are anticipated fluid shifts, a Foley catheter to monitor urinary output as an indication of IV volume is also recommended. Invasive monitoring of arterial blood pressure or central venous pressure is also indicated in certain clinical situations.
Standards of Intraoperative Monitoring
In the tradition of safety that has been a hallmark of anesthesia care and practice, standards for basic anesthetic monitoring were initially proposed in 1986 by the Harvard University teaching hospitals (Eichhorn et al., 1986). These were then adopted and amended by the American Society of Anesthesiologists (ASA), with the last iteration approved in 2005. As stated in the document, these standards apply to all general anesthetics, regional anesthetics, and monitored anesthesia care. Although some rare clinical situations may preclude use of all of the recommended monitors, it is expected that these standards are technologically attainable and should be used by all practitioners.
Standard I states that “qualified anesthesia personnel shall be present in the room throughout the conduct of all general anesthetics, regional anesthetics, and monitored anesthesia care” (ASA, 2005). It stipulates that this continuous care is “without any interruption at any time” because of the rapidly changing status of patients under anesthesia. Standard II stipulates that oxygenation, ventilation, circulation, and temperature be continually evaluated. “Continually” is defined as “repeated regularly and frequently in steady rapid succession” ASA, 2005. For each component, specific objectives and methods are outlined. A combination of clinical observation and technical methods is recommended. Whereas there are no mandates for specific instrumentation, quantitative measurements are “strongly encouraged” over qualitative measures alone. Standard monitoring and supplemental measures recommended in pediatric anesthesia are shown in Box 13-1.
Box 13-1 Standard Monitors and Supplementary Measurements in Pediatric Anesthesia
Pulse oximetry, introduced in the 1980s, has become a mandatory instrument in the perioperative care of pediatric patients. The pulse oximeter accurately reflects saturation in all age groups with various hematocrit values, including premature infants with fetal hemoglobin over the range of 60% to 100% Sao2 (Deckardt and Steward, 1984). Recent advances in pulse oximetry include newer designs that claim to improve performance during low-perfusion states and patient motion, often a consideration in newborns and infants. Signal extraction technology (SET) uses algorithms that filter out interference, resulting in a 90% lower false-alarm rate (Miyasaka, 2002). Other devices provide continuous noninvasive measurement of total hemoglobin concentration and allow for differentiation between different hemoglobin types (Noiri et al., 2005; McMorrow and Mythen, 2006). The reliability of these new technologies continues to be investigated. Further information on monitoring devices, their mechanisms of action and clinical uses is presented in Chapter 11, Monitoring.
Invasive Monitoring
Indications for direct arterial pressure monitoring are beat-to-beat monitoring of blood pressure when hemodynamic fluctuations are expected or pharmacologic manipulation can occur; an inability to measure blood pressure noninvasively; the need to frequently measure arterial blood gas samples; or other laboratory analyses to aid in management of the patient (Barbeito and Mark, 2006). Arterial catheters are typically placed percutaneously in the wrist (radial or ulnar), groin (femoral), or foot (posterior tibial or pedal). They should not be placed anywhere where there is evidence of vascular compromise or lack of collateral circulation. A noninvasive blood pressure monitor should always be available to verify the readings of the direct monitor.
The risks of central catheter placement include vascular injury, unintentional arterial puncture, and pneumothorax, just to name a few. However, Verghese and colleagues (1999) showed that real-time ultrasound guidance for placement of an internal jugular catheter was superior to the landmark technique, with a high success rate and fewer carotid punctures. Another study demonstrated advantages of this technique in children older than 1 year of age or heavier than 10 kg (Leyvi et al., 2005).
Bispectral Index
The bispectral algorithm uses a statistical process to analyze EEG signals and compute a number between 0 and 100 that represents the degree of awareness. The variables analyzed include the frequency and power spectrum of the EEG, the amount of burst suppression, and the degree of synchronization of the EEG (Bowdle, 2006). In infants and children, studies have validated the bispectral index (BIS) and demonstrated that more accurate titration of general anesthesia is achieved with its use, resulting in shorter recovery times (Denman et al., 2000; Bannister et al., 2001; McCann et al., 2002; Powers et al., 2005). However, several studies have demonstrated low BIS values in young infants (younger than 1 year old) before awakening and poor correlations between anesthetic concentration and BIS (Davidson et al., 2005; Klockars et al., 2006; Malviya et al., 2007). Newer indices to measure anesthesia depth continue to be developed, including the Narcotrend index, the A-line ARX index, and the cerebral-state index. All of these are currently being studied for validation in children and with regard to BIS.
Anesthesia Record
With advances in technology, automated anesthetic record systems have become more widely used (Egger Halbeis et al., 2008). Sanborn et al. (1996) found that electronic scanning recorded a higher incidence of intraoperative incidents than those reported voluntarily. Others have reported improvements in patient safety and care with automated systems (Junger et al., 2001; Merry et al., 2001). As automated records become more user friendly, affordable, and integrated with the care process, they eventually could be the standard method of recording information in the operating room.
Fluids and Electrolytes
Fluid therapy in the perioperative period needs to account for several different fluid requirements: fluid deficits, maintenance fluid, and the volume of fluid necessary to maintain adequate tissue perfusion. Fluid deficits come from numerous sources, including preoperative fasting, gastrointestinal and renal losses, hemorrhage and third space losses (see Chapter 5, Regulation of Fluids and Electrolytes).
Historically, maintenance fluid requirements were based on the recommendations of Holliday and Segar (1957), which state that the basal caloric needs of infants and children dictate their fluid requirements. They proposed that an infant needs 100 mL of water per 100 kcal of caloric expenditure. On the basis of 1 mL of fluid per 1 kcal of caloric requirement, the fluid requirements in infants and children were approximated by the following formulas:
For every 100 calories of energy expenditure or 100 mL of water, a child needs 3 mmol of sodium, 2 mmol of potassium, 5 mmol of chloride, and 5 g of dextrose. While 5% dextrose in 0.2% or 0.45% NaCl meets these requirements for maintenance fluids, several recent articles describing clinically significant hyponatremia caused by perioperative fluids have raised some concerns (Arieff, 1998; Halberthal et al., 2001). Practitioners are tending to reduce the average fluid maintenance volume to half or two thirds of the classic recommendations proposed by Holliday and Segar (1957) based on these findings or are using isotonic saline solutions (Paut and Lacroix, 2006; Bailey et al., 2010). Further studies are necessary to evaluate what the most appropriate maintenance fluids are; however, one must individualize fluid therapy and monitor blood sodium levels in patients who are receiving prolonged infusions.
Pediatric patients are typically kept fasting for 2 hours from clear fluids and 4 to 8 hours (sometimes longer) from breast milk and solid food before induction for elective surgery. To determine the fasting deficit, one multiplies the hourly maintenance fluid requirement by the number of hours of restriction. Half of the fluid deficit plus the hourly maintenance fluid requirement should be given during the first hour of anesthesia. One quarter of the fluid deficit plus the hourly maintenance fluid is infused during the second and third hours, along with the replacement of any third-space fluid loss (Furman et al., 1975). These deficits should be replaced with a balanced salt solution such as normal saline or Lactated Ringer’s solution that does not contain glucose.
Glucose requirements in pediatric patients need to account for the hazards of both hyperglycemia and hypoglycemia. Hyperglycemia produces an osmotic diuresis that leads to dehydration and electrolyte abnormalities. It also appears to worsen neurologic outcome in cerebral ischemia and should be avoided (Lanier et al., 1987). On the other hand, hypoglycemia is known to cause cerebral ischemia and brain damage, especially in newborns. Infants and some children who have fasted for a prolonged period without glucose supplementation can become hypoglycemic during anesthesia yet show no overt clinical symptoms (Welborn et al., 1986). Intraoperative monitoring of blood glucose levels in these patients is recommended. However, preoperative hypoglycemia occurs rarely in normal, healthy infants and children (1% to 2%), making it less likely that the majority of patients need glucose administered in the perioperative period (Murat and Dubois, 2008).
Recommendations on the appropriate glucose-containing solution to administer, if IV glucose is to be given, have changed over the years (Bailey et al., 2010). Whereas a 5% dextrose solution consistently causes hyperglycemia, 2.5% dextrose in Lactated Ringer’s solution maintains normal blood glucose levels (Welborn et al., 1987). Dubois et al. (1992) also investigated the use of lower dextrose solutions (1 or 0.9%) and found normal blood glucose concentrations without hypoglycemia.
Ventilation
To maintain alveolar ventilation in infants and small children under general anesthesia, partial rebreathing or nonrebreathing Mapleson D- or F-type circuits (such as the Bain or Jackson-Reese) are commonly used. With their light weight and low flow resistance, they have desirable qualities for the pediatric population, although the lack of scavenging is a drawback. Today, adult circle systems with smaller pediatric circuits are safely used in children. However, in small infants, one must account for the potential for increased airway resistance and increased circuit compliance that diminishes the accuracy of measured tidal volume. For a more detailed discussion of pediatric anesthesia circuits and ventilators, refer to Chapter 10, Equipment.
For controlled ventilation in infants and children without major respiratory dysfunction, the ventilator can be set initially at a tidal volume of 10 mL/kg or peak pressure of 16 to 18 cm H2O. Respiratory rates between 20/min for infants and 12/min for older children are sufficient, although higher respiratory rates are recommended for young infants (Peters et al., 1998). A positive end-expiratory pressure (PEEP) of at least 5 cm H2O prevents airway closure and atelectasis in anesthetized infants and young children (Motoyama, 1996). Once the steady state of ventilation is established, tidal volume and respiratory rate can be adjusted to maintain an end-tidal CO2 between 35 and 40 mm Hg (see Chapter 3, Respiratory Physiology in Infants and Children).
Temperature Maintenance
Infants and small children are at particular risk of hypothermia because of their relative lack of adipose tissue and their increased body surface area relative to their body weight, which increases the difference between heat loss and heat production. Under general anesthesia, core temperature control is impaired and regulatory defenses, including nonshivering thermogenesis, are not triggered (Sessler, 2008). Furthermore, vasodilation, exposure to the cold operating room environment, insensible heat loss, and infusion of cold intravenous fluids all contribute to the development of hypothermia. The mechanisms of thermoregulation are further discussed in Chapter 6, Thermoregulation: Physiology and Perioperative Disturbances.
Accurate monitoring of body temperature is imperative to maintaining normothermia as well as detecting malignant hyperthermia. Core temperature monitoring is the best indicator of thermal status and can be easily measured with an esophageal probe (Sessler, 2008). Because this is not always a possibility, near-core sites including the mouth, axillae, bladder, rectum, and skin surface are other possibilities, although they each have limitations. The safe range for a child’s core temperature is between 35.5° and 37.5° C. To prevent complications caused by hypothermia, anesthesiologists need to be proactive and begin warming the patient as soon as or before the temperature deviates from this range.
There are several means by which body temperature can be maintained. Forced-air warmers preserve intraoperative normothermia better than circulating-water mattresses (Kurz et al., 1993). Humidifiers in the anesthesia circuit also help prevent evaporative heat loss, damage to the ciliated airway epithelia, and postoperative pulmonary complications (Chalon et al., 1979). Additionally, increasing room temperature, using radiant warming lamps, wrapping the child’s head and other exposed areas with plastic, and using warmed intravenous fluids help to conserve heat. Careful monitoring, however, is necessary to prevent hyperthermia that can sometimes occur when all of these measures are combined.
Blood Loss and Blood Component Therapy
Accurate and continuous monitoring of blood loss and its timely replacement are critical in pediatric patients. Relatively small losses in infants can produce significant changes in hemoglobin concentration and hemodynamic stability, making careful determination of acceptable blood loss mandatory. Estimated blood loss needs to account for the contents of suction bottles as well as a visual estimate of blood lost on the surgical drapes and the weight of blood-soaked sponges. Allowable blood loss is determined by the patient’s estimated blood volume, preoperative and intraoperative hematocrit values, cardiopulmonary and general medical conditions to provide adequate oxygen transport, and risk versus benefit of the transfusion. Estimated blood volumes in pediatric patients are age dependent and are listed in Table 13-3.
| Age of Patient | Blood Volume (mL/kg) |
| Premature newborn | 90-100 |
| Full-term newborn | 80-90 |
| 3 mo-1 yr | 75-80 |
| 3-6 yr | 70-75 |
| >6 yr | 65-70 |
Once the estimated blood volume (EBV) is known, one can determine the maximum allowable blood loss (MABL). This should be done for each patient before the induction of anesthesia and is achieved with the use of a simple mathematical calculation as follows (Barcelona et al., 2005):
The use of a trigger value alone to determine the need for transfusion is no longer recommended in most guidelines, because hemoglobin and hematocrit values may not accurately reflect blood volume or oxygen carrying capacity (Weldon, 2005). In actively bleeding patients without volume replacement, hematocrit values alone can be misleading (Hume and Limoges, 2002). However, the hematocrit value along with good clinical judgment can serve as a guideline for when to consider transfusion (National Institutes of Health Consensus Conference, 1988).
Debate continues as to whether a crystalloid or colloid solution should be used to replace blood volume as the hematocrit is allowed to decrease (Bailey et al., 2010). The SAFE Study (2004) randomized adult ICU patients to receive either 4% albumin or normal saline for fluid resuscitation with the primary outcome being death from any cause. In 6997 patients, they found no difference in death rate or in number of days in either the ICU or the hospital between the groups. This was in contrast to a meta-analysis by the Cochrane Injuries Group Albumin Reviewers (1998), which suggested that the administration of albumin resulted in a 6% increase in the absolute risk of death when compared with the administration of crystalloid. If a colloid solution (e.g., albumen, hetastarch) is used, blood loss is replaced milliliter for milliliter. If a crystalloid solution is used, 2 to 3 times the volume of the blood loss is replaced. Although colloid may be more physiologic, it is more expensive and has not been shown definitively to be superior to crystalloid replacement. In otherwise healthy patients, the use of crystalloid is safe and effective to replace the MABL.
The minimum recommended hemoglobin concentration allowable has changed over the years. With the exception of patients who are in the early neonatal period (with high oxygen affinity of fetal hemoglobin and decreased oxygen unloading at tissue levels) and those with cyanotic cardiopulmonary disease, the previously recommended 10 g/dL is no longer a prerequisite hemoglobin concentration. Several studies have demonstrated cardiovascular stability with significant anemia (hemoglobin of 6 to 7 g/dL) in both animals and children who underwent acute normovolemic hemodilution (Stehling and Zauder, 1991; Fontana et al., 1995). Lacroix and colleagues (2007) found no difference in outcomes in children randomized to either a restrictive-strategy group (transfusion threshold of 7 g/dL) or a liberal-strategy group (transfusion threshold of 9.5 g/dL). However, a randomized, controlled trial comparing two hemodilution protocols (to a hematocrit value of 21.5% versus 27.8%) in children during hypothermic cardiopulmonary bypass found that a lower hematocrit value was associated with a poorer performance on psychomotor development tests at 1 year of age (Jonas et al., 2003).
Once the blood loss exceeds one blood volume (massive blood loss), a coagulopathy can develop because of both a reduction in platelets (dilutional thrombocytopenia) and a reduction in labile clotting factors. In patients with clinical signs of nonsurgical bleeding, the administration of other blood components, including platelets, fresh frozen plasma, and cryoprecipitate becomes necessary. The management of blood component therapy and massive blood loss is detailed in Chapter 14, Blood Conservation.
Emergence and extubation
Emergence
If neuromuscular blockers were used, recovery should be monitored using peripheral nerve stimulation and clinical assessment. Kopman et al. (1997) observed that recovery of a train-of-four (TOF) ratio over 0.90 is needed to assure adequate neuromuscular function, especially in the ambulatory setting. Neuromuscular blockade is reversed with intravenous atropine (0.02 mg/kg) followed by neostigmine (0.06 mg/kg). In infants, a higher dose of atropine (0.03 mg/kg) is recommended. Glycopyrrolate (0.01 mg/kg) is as effective as atropine and may produce a more stable heart rate (Warran et al., 1981).
Sugammadex, a new rapid-acting reversal agent, which has been approved for use in Europe, Australia, and New Zealand, encapsulates rocuronium and vecuronium, preventing binding to neuromuscular junction receptors. Studies in adults have shown recovery from weak blockade to a train-of-four ratio of 0.9 occurs in approximately 2 minutes with doses of 2 mg/kg or higher (Sorgenfrei et al., 2006; Suy et al., 2007; Vanacker et al. 2007). Plaud and colleagues (2009) demonstrated that in recovery from neuromuscular blockade from 0.6 mg/kg rocuronium, a train-of-four of 0.9 was attained in 0.6, 1.2, and 1.1 minutes in infants, children, and adolescents, respectively, with 2 mg/kg of sugammadex.
Extubation of the Trachea
The endotracheal tube can be removed from a patient who is well anesthetized (deep extubation) or from one who has successfully emerged from general anesthesia (awake extubation). Deep extubations should be performed in patients whose airway was well maintained by mask ventilation during induction of anesthesia and who are still receiving intravenous or inhalation agents at levels that will prevent coughing and laryngospasm. Before extubation, an oropharyngeal airway should be placed to avoid upper airway obstruction. Depth of anesthesia should be confirmed by the absence of any response to suctioning of the oral pharynx and deflation of the endotracheal tube cuff, if one is present. If airway patency was satisfactory before intubation, the return to spontaneous breathing is not a prerequisite for extubation. Deep extubation can be performed safely when using sevoflurane or desflurane to allow expedient awakening after extubation (Valley et al., 2003). This technique is especially useful for patients with a history of reactive airways disease.
Awake extubations, with protective laryngeal reflexes intact, should be performed in the child with difficult airways and full stomach risks. Before an awake extubation, infants and young children may respond to laryngeal stimulation by breath holding, bronchospasm, chest-wall rigidity, marked cyanosis, and oxygen desaturation. This phenomenon is impressive and may resemble the cyanotic spells seen in infants with tetralogy of Fallot. Fortunately, these episodes are self-limited, provided that alveolar ventilation is maintained. Older children show less of a tendency toward sudden desaturation, but they are at a greater risk of forceful coughing against the endotracheal tube (bucking) when extubation is delayed. This may lead to a greater incidence of postintubation croup (Koka et al., 1977). Thus, older children should be extubated as soon as protective reflexes return.
Until recently, it was a widely accepted practice to ventilate the patient with high flows of 100% oxygen for several minutes before extubation in order to wash out residual anesthetics and replace them with pure oxygen. Recent studies, however, have demonstrated that this practice of “preoxygenation” should be modified and that patients should be ventilated with a mixture of oxygen and air (1:1 to 2:1 ratios of oxygen and air provide about 60% to 70% oxygen), instead of 100% oxygen, to minimize airway closure and atelectasis, which almost always develop during general anesthesia, especially in infants and children (see Chapter 3, Respiratory Physiology). To eliminate atelectasis, before extubation the lungs must be reinflated with several “vital capacity maneuvers” (sustained positive airway pressure of 35 to 40 cm H2O for several seconds) with air-oxygen mixtures and then maintain PEEP/CPAP (5 to 7 cm H2O) (Benoit et al., 2002; Lindahl and Mure, 2002; Tusman et al., 2003).
Technique of Extubation
The removal of the endotracheal tube should be performed with as much attention to detail as when it was inserted. Placement of an oral airway or a bite block before awakening can greatly reduce the risk of complete occlusion by the teeth on the endotracheal tube and compromise of the airways. Severe or complete occlusion of the tube or the upper airways associated with marked inspiratory effort, and intrathoracic negative pressure can result in postobstructive pulmonary edema (Galvis et al., 1980; Sofer et al., 1984). Oropharyngeal airways are preferred in patients whose airway patency was less than satisfactory during anesthetic induction. Soft bite blocks made of rolled gauze and tape can reduce the incidence of soft-tissue damage to the gums, palate, and lips associated with plastic airways. After reinflation of the lungs with high pressures to eliminate atelectasis, the lungs are inflated again with oxygen-air mixture synchronously with the child’s inspiration by gently squeezing the anesthesia bag. The bag is then held momentarily at end-inspiration with a positive pressure of 15 to 20 cm H2O to maintain a high lung volume as the endotracheal tube is gently pulled out. This last maneuver serves the following three functions:
Transport to the Postanesthetic Care Unit
When breathing is satisfactory, the patient is transported to the PACU in the lateral position with supplemental oxygen via a mask, keeping the airway clear of the tongue and secretions and protecting against aspiration (Smith, 1959; Isono et al., 2002). By holding the chin up and extending the neck, the anesthesiologist can further ensure a patent airway and feel the warm breaths, indicating gas exchange. During transport to the PACU, the guardrails should be up and the safety straps should be securely fastened to prevent injury. Patients should be covered with warmed blankets to reduce heat loss during transport. Monitoring during transport should include clinical observation of chest movement, color, and gas exchange in awake and active patients and heart and breath sounds with a precordial stethoscope in sleeping patients.
Postanesthetic recovery
The postanesthetic recovery period is a time of high risk for pediatric patients. A large percentage of otherwise healthy infants and children (20% to 40%) develop oxygen desaturation (SpO2 = 94%) during transport and on arrival at the PACU (Patel et al., 1988). Oxygen desaturation occurs sooner, is more pronounced, and has a longer duration in infants than in children and a longer duration in children than in adults (Xue et al., 1996). Postoperative hypoxemia is most likely caused by atelectasis, but upper airway problems such as obstruction, croup, and laryngospasm, are more likely in children (4% to 5%) than adults (Cohen et al., 1990). All children, therefore, should be administered oxygen supplementation during their transport from the operating room and on arrival at the PACU, until they can maintain satisfactory oxygen saturation in room air or at their baseline Fio2. Nausea, vomiting, temperature instability, and postoperative pain also require prompt and effective treatment to ensure patient comfort and efficient discharge timing.
Recovery in the Postanesthetic Care Unit
Awakening Responses
Measures other than the use of pharmacologic agents can often be effective in calming the awakening pediatric patient. Some patients simply need reassurance or the comfort of being touched or held to alleviate anxiety (Fig. 13-6). Parental presence has been shown to reduce the incidence of fear, crying, anger, and clinging during hospitalization (Fiorentini, 1993; Fina et al., 1997; Kamerling et al., 2008). Both parents and PACU staff have reported beneficial results from parental involvement. Once the infant or child has documented stable vital signs, a parent should be present.
Common Problems in the Postanesthetic Care Unit
Apnea of Prematurity
Apnea may be central (no respiratory effort), obstructive (respiratory effort without gas flow), or mixed (both central and obstructive). Apnea is defined as cessation of breathing for longer than 15 seconds or for less than 15 seconds associated with bradycardia, cyanosis, or pallor (Nelson et al., 1978; Thatch, 1985). Repetitive pauses of breathing, lasting 5 to 10 seconds and not associated with other changes in infants, are termed periodic breathing. These abnormal respiratory patterns, which are observed commonly in neonates and preterm infants, can appear or worsen in preterm infants after exposure to anesthetic agents (Rigatto, 1986). This is particularly true for prematurely born infants with a previous history of apnea (Liu et al., 1983).
In a combined analysis of eight studies from the halothane era, Coté et al. (1995) found that postoperative apnea was:
They concluded that the risk of apnea in infants without a history of apnea or anemia is greater than 5% (95% CI) until PCA of 48 weeks with a gestational age of 35 weeks, and the risk remains greater than 1% until PCA of 54 to 56 weeks with gestational age of 32 weeks. They further concluded that older infants with apnea in the PACU and those with anemia should be admitted and monitored overnight. Because of these findings, it is generally recommended that preterm infants of fewer than 44 to 46 weeks PCA be admitted for monitoring after general anesthesia (Gregory et al., 1983; Coté et al., 1995). Regional anesthesia and subarachnoid blocks without sedation have been shown to decrease the risk of postoperative apnea (Williams et al., 2001). Sale et al. (2006) in a prospective comparison study, found no increase in respiratory events with the use of sevoflurane and desflurane. Welborn et al. (1998) found caffeine (10 mg/kg) to be effective in treating apnea in premature infants undergoing elective surgery. These patients, however, are still routinely admitted for observation and treatment.
Airway Obstruction
Although patients should be able to maintain airway patency before leaving the operating room, it is not uncommon for an infant or a child to have an obstruction after the stimulation of extubation and transportation has subsided. The anesthesiologist must be acutely aware of any changes in the breathing pattern at this time, because hypoventilation can lead to a reaccumulation of volatile agents in the alveoli that can further blunt the respiratory drive. Hypercarbia may result in dysrhythmias and hypertension, and hypoxemia in infants may lead to further suppression of breathing (Knill and Clement, 1984; Motoyama and Glazener, 1986). Neck extension, mouth opening, and jaw thrust alone or together may be enough to correct the problem. Nasopharyngeal airways, if necessary, are better tolerated than oropharyngeal airways in this setting. If obstruction continues, reassessment of anesthetic and neuromuscular blockade reversal should be conducted and possible reintubation may be considered.
Obstructive Sleep Apnea
Patients with obstructive sleep apnea syndrome (OSA) are predisposed to postoperative apnea (see Chapter 24, Anesthesia for Pediatric Otorhinolaryngologic Surgery). OSA is characterized by prolonged partial and/or intermittent complete upper airway obstruction that disrupts normal breathing and sleeping patterns (American Thoracic Society, 1996). Although OSA in adults is commonly associated with obesity, in children it more often arises from enlarged tonsils and adenoids (Young et al., 1993). Surgical removal of enlarged adenoids and tonsils often markedly improves upper airway patency (Schechter et al., 2002). OSA also occurs in infants and young children with a narrowing of upper airways secondary to craniofacial abnormalities, neuromuscular disorders, and Trisomy 21, which may worsen during the postoperative period (Marcus, 2001; Au and Li, 2009). Children with OSA have an increased sensitivity to opioids (Brown et al., 2004) (Fig. 13-7).
Postobstructive Pulmonary Edema
Pulmonary edema developing shortly after the relief of upper airway obstruction is known as postobstructive pulmonary edema (POPE) and negative pressure pulmonary edema (NPPE). It was first noted after difficult intubation in children and subsequently described after relief of laryngospasm both in infants and children (Travis et al., 1977; Galvis et al., 1980; Sofer et al., 1984). The first signs, which include rales, wheezing, desaturation, and copious production of pink, frothy respiratory secretions, may occur immediately after the relief of the obstruction. The cause of this type of pulmonary edema is attributed to the marked negative intrathoracic pressure generated by forced inspiration against a closed glottis (Mueller’s maneuver), which leads to increased interstitial negative pressure, increased right-side cardiac preload, increased pulmonary vascular resistance, acute hypoxia, and increased capillary permeability (Thiagarajan and Laussen, 2007).
Postintubation Croup
The incidence of postintubation croup was reported to be about 1% (Koka et al., 1977). The most common cause is a tight-fitting endotracheal tube without an air leak at 30 to 40 cm H2O with positive airway pressure (Koka et al., 1977). Other factors associated with postintubation croup may include traumatic or repeated intubation, “bucking” or coughing with the ET tube in place, changing the head position, the duration of surgery, and neck surgery. The incidence of postintubation croup seems to have decreased since this initial report. This may be secondary to the better quality of implant-tested sterile endotracheal tubes and the increased use of smaller-sized cuffed endotracheal tubes and corticosteroids.
The croup scoring system by Downs and Raphaely (1975) objectively quantifies the severity of the condition and its use can be helpful in treatment decisions (Table 13-4). Cool, humidified mist administered after extubation may be helpful in mild cases of croup. Racemic epinephrine (0.5 mL of 2.25% solution), diluted in 3 to 5 mL of normal saline solution and administered by nebulizer for 5 to 10 minutes, assists patients with progressively worsening symptoms and stridor by producing mucosal vasoconstriction, resulting in a shrinking of swollen airway mucosa. The “rebound effect” and recurrence of symptoms are well described and necessitate observing the patient for up to 4 hours after treatment. Anene et al. (1996), in a prospective, double-blind, placebo-controlled study, found that dexamethasone is effective in reducing the incidence of postintubation croup in children who have been intubated for longer than 48 hours.
Cardiovascular Instability
Cardiac rhythm disturbances and blood pressure fluctuations are rare in infants and children recovering from general anesthesia in comparison with adults (Hines et al., 1992; Murat et al., 2004). Bradycardia is typically a response to medications such as neuromuscular-blockade reversing agents or fentanyl, or it can be a normal variant that should be treated only if it is associated with hypotension. Hypoxia is also an important cause of bradycardia, especially in neonates and young infants. Tachycardia may be secondary to hypovolemia, inadequately treated pain, or anticholinergic medications. Careful assessment and appropriate therapy should be instituted to correct volume deficit or the need for analgesia. Hypertension may also reflect inadequate analgesia, an anticholinergic effect, or excessive hydration, or it may be an artifact caused by the use of an inappropriately small blood-pressure cuff. Hypotension is more unusual and is most often caused by hypovolemia secondary to inadequate fluid replacement or ongoing blood loss. Appropriate fluid resuscitation should be instituted.
Nausea and Vomiting
Postoperative vomiting (POV) is the most common complication of anesthesia in infants and children and a major cause of delayed PACU discharge and unscheduled admission for ambulatory patients (Patel and Hannallah, 1988; Murat et al., 2004). Nausea, also a common postoperative complication, is rarely included in pediatric studies because of the challenges of accurate assessment in infants and young children. Factors that increase the risk of POV include surgeries such as strabismus repair, adenotonsillectomy, and orchiopexy; age; gender; history of motion sickness; and intraoperative opioids. Eberhart noted four independent predictors of pediatric POV, including a duration of surgery equal to 30 minutes, an age of 3 years, strabismus surgery, and a positive history of POV in the patient’s parent or sibling. When present, these risk factors increased the likelihood of POV (Fig. 13-8) (Gan et al., 2007). Early ambulation and clear liquids, in an attempt to meet the discharge criteria in the short-stay surgery settings, may precipitate vomiting, particularly in susceptible patients (Schreiner et al., 1992). Although rarely life threatening in the PACU, vomiting has the potential for causing aspiration, hypovolemia, and/or hypernatremia.
For prophylaxis against POV, intravenous serotonin (5-HT3) receptor antagonists, such as ondansetron (0.1 to 0.15 mg/kg) and granisetron (0.04 mg/kg) given intraoperatively, have been shown to be highly effective with rare side effects (Patel et al., 1997; Fujii et al., 2002). Dexamethasone (0.2 to 0.5 mg/kg) given intraoperatively is effective in decreasing the incidence of both nausea and vomiting (Aouad et al., 2001). In combination these medications are synergistic (Davis et al., 2008). Gan and colleagues (2003) published guidelines for preventing and treating POV and recommended prophylaxis only in patients with moderate to high risk. In pretreated patients who develop POV in the PACU, a dose of an alternative 5-HT3 blocker or an antiemetic from another family of medications (antihistamine, antidopaminergic) should be considered.
Emergence Delirium
Emergence delirium (ED) is a significant problem for children (Vlajkovic and Sindjelic, 2007). ED in children appears to be a dissociated state of consciousness characterized by inconsolability, thrashing, and incoherence (Fig. 13-9). Having all but disappeared with the discontinued use of older anesthetic agents, ED reemerged as a problem in children with the introduction of desflurane and sevoflurane (Davis et al., 1994; Aono et al., 1997; Grundmann et al., 1998). Theoretic explanations for this occurrence include rapid awakening in a strange environment, variable recovery of central nervous system function, precipitous withdrawal from GABA receptors, and a yet-to-be-defined psychomotor side effect. Inadequately treated pain has also been proposed as a major contributor to this phenomenon, but studies have demonstrated a high incidence of ED in pain-free patients (Wells et al., 1999; Uezono et al., 2000). Risk factors include an age younger than 5 years old, otolaryngologic and ophthalmologic procedures, use of isoflurane, rapid emergence, the need for intraoperative opioids, anxious parents, poor socialization, and low adaptability scores (Voepel-Lewis et al., 2003; Kain et al., 2006). In this state, children pose a danger to themselves and care providers with complications arising in the forms of increased bleeding, pain, medication use, and need for staff. Other complications include patients pulling at surgical drains, loss of intravenous catheters and tubing, and longer PACU stays.
Several instruments and therapies have been proposed and studied to evaluate and prevent ED. The Pediatric Anesthesia Emergence Delirium Scale (PAEDS), using five criteria (eye contact, purposeful movement, awareness of environment, restlessness, inconsolability), has been demonstrated to be valid and reliable (Box 13-2) (Sikich and Lerman, 2004). A single intravenous prophylactic treatment of fentanyl 2.5 mcg/kg, clonidine 2 mcg/kg, ketamine 0.25 mg/kg, nalbuphine 0.1 mg/kg, or dexmedetomidine 0.15 mcg/kg has been shown to decrease incidence of ED (Cohen et al., 2001; Kulka et al., 2002; Dalens et al., 2006; Ibacache et al., 2004). Other routes of administration such as intranasal fentanyl 1 mcg/kg and oral clonidine 4 mg/kg are also effective (Finkel et al., 2001; Tazeroualti et al., 2007). Aouad and colleagues (2007) found that 1 mg/kg propofol after discontinuation of sevoflurane at the end of surgery decreases the incidence of ED without prolonging recovery.
Pain Measurement and Assessment
Most techniques measure the intensity of pain by assigning incremental values. The most common technique, self-reporting, depends on verbal, cognitive, and developmental skills. Adapting adult patient surveying tools such as the McGill Pain Questionnaire, researchers have developed scales using verbal description and graphic rating (Melzack, 1975). Such tools are modified for age, culture, and cognitive ability (McGrath et al., 1985; Varni et al., 1987). Word lists and questioning techniques have been developed (Abu-Saad et al., 1990; Wilkie et al., 1990). Graphic or symbolic representations of pain intensity also require modification. The visual analog pain scale, initially developed for adult pain measurement, is typically a 10-cm horizontal line defined by “no pain” on the left end and “severe pain” on the right. In older children and adolescents, this instrument has been used with success (Abu-Saad, 1984; McGrath et al., 1985). In younger children, replacing the definers with different words, such as “no hurt” and “most hurt,” numbers, or happy and sad faces has also been tested (Broadman et al., 1988; Savedra and Tesler, 1989). For younger children with preoperational reasoning, less abstract quantitative measurements, including counting poker chips, selecting color scales, and marking graduated thermometers, are more easily understood and used (Eland and Anderson, 1977; Hester, 1979; Jeans and Johnston, 1985). A further variation involves a progression of happy to crying faces; the faces are either illustrated, as in McGrath’s Facial Affective Scale and the Wong-Baker FACES Pain Rating Scale, or photographed, as in Beyer’s Oucher Scale (McGrath et al., 1985; Beyer and Aradine, 1988; Wong and Baker, 1988). Numeric values are assigned in each of these methods for progressive levels of pain intensity.
In infants and nonverbal children, observational pain scales must be implemented. For the evaluation of postoperative pain, the Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) and the Objective Pain Scale grade behavioral manifestations of pain (McGrath et al., 1985; Hannallah et al., 1987). The CHEOPS assesses six categories—cry, facial expression, verbal response, torso position, leg activity, and arm movement—in relationship to the surgical wound. The Objective Pain Scale contains physiologic and behavioral changes associated with pain. These scales were designed for research purposes and are specific for age. The Facial Expression-Leg Movement-Activity-Cry-Consolability (FLACC) Scale has be shown to be valid and reliable in children who were preverbal, cognitively impaired, recovering from surgery, or undergoing painful procedures (Merkel et al., 1997; Voepel-Lewis et al., 2002; Manworren and Hynan, 2003; Nilsson et al., 2008). The Multidimensional Assessment of Pain Scale (MAP) has also shown promise of wide applicability (Ramelet et al., 2007). Neonatal and premature infant pain scales have been created and verified; these include the Neonatal Infant Pain Scale (NIPS), the Crying-Requires Oxygen-Increased Vital Signs-Expression-Sleep (CRIES) Scale, and the Premature Infant Pain Profile.
Observational pain assessment techniques are also fraught with variability. Studies have demonstrated that scoring by parents, nurses, and physicians can be unreliable when assessing pain by cry, behavior, and constructed pain scales (Wasz-Hocket et al., 1985; Beyer et al., 1990; Favaloro and Touzel, 1990; Watt-Watson et al., 1990). As with older children, previous experience, emotional and medical status, and clinician interaction can affect the response.
Pain Management
Morphine (0.025 to 0.05 mg/kg) or fentanyl (0.5 to1.0 mcg/kg), given in incremental doses, can be used to achieve an analgesic state in patients recovering from a general anesthetic. If hospital admission overnight or longer is planned, then morphine use is preferable because of its longer duration of action. For patients undergoing extensive surgical procedures with moderate to severe pain anticipated, continuous infusion of an opioid should be considered. Hendrickson et al. (1990) demonstrated better analgesia and greater patient satisfaction with continuous infusion compared with intermittent dosing. Continuous infusion can create consistent analgesic blood levels of morphine and remove the need for children to communicate their pain (Berde, 1989; Esmail et al., 1999). Patient-controlled analgesia allows the patient to self-administer small incremental doses of a local anesthetic and an opioid. Patient-controlled analgesia has been extensively studied in children, and studies support its efficacy and safety (Gaukroger et al., 1989; Lawrie et al., 1990; Tyler, 1990; Berde et al., 1991). In younger children and infants, nurse-assisted patient-controlled analgesia is a useful alternative (Monitto et al., 2000; Anghelescu et al., 2005). However, there are risks associated with its use (Voepel-Lewis et al., 2008). It is most effective if patients are selected, evaluated, and instructed before surgery. Side effects of opioid use, including nausea, vomiting, pruritus, and urinary retention, should be anticipated and treated when they occur. Placement of indwelling catheters in the epidural space, body cavities, and nerve sheaths allows for continued use of local anesthetics and opioids for several days after surgery. In older children, patient-controlled epidural anesthesia (PCEA) has been shown to be safe and effective (Birmingham et al., 2003). Careful dosing and monitoring for side effects, including oversedation, respiratory arrest, and toxicity of local anesthetics, are essential. The advantages, limitations, and possible dangers of these techniques are discussed in Chapter 15, Pain Management.
Discharge from the Postanesthetic Care Unit
With more rapid recovery from general anesthesia and a greater variety of surgical cases being scheduled on an outpatient basis, strict time criteria for discharge from the PACU are becoming less useful. The Modified Aldrete Score examines the criteria of motor activity, respiration, blood pressure, consciousness, and color (Table 13-5). The Simplified Postanesthetic Recovery Score assesses consciousness, airway, and movement (Steward, 1975). Both scores can be helpful as guidelines in determining when a patient is ready for discharge. Inclusion of oxygen saturation by pulse oximetry is indicated. Before a child can be safely discharged from the PACU, a careful examination should be conducted to ensure safety on the patient floor, with its reduced nursing care and observation (Box 13-3).
| Criteria | Characteristics | Points |
| Activity | Voluntary movement of all limbs to command Voluntary movement of two extremities to command Unable to move |
2 1 0 |
| Respiration | Breathe deeply and cough Dyspnea, hypoventilation Apneic |
2 1 0 |
| Circulation | BP +/– 20 mm Hg of preanesthesia level BP >20-50 mm Hg of preanesthesia level BP >50 mm Hg of preanesthesia level |
2 1 0 |
| Consciousness | Fully awake Arousable Unresponsive |
2 1 0 |
| Color | Pink Pale, blotches Cyanotic |
2 1 0 |
From Aldrete JA: The postanesthesia recovery score revisited, J Clin Anesth 7:89-91, 1995.
Short-Stay Recovery Unit
Patients undergoing outpatient procedures continue to recover in an ambulatory or a short-stay recovery unit (SSRU). Complications seen in the PACU can also occur here. The most common causes for unplanned hospital admission from the SSRU are vomiting, croup, fever, and family request (Patel and Hannallah, 1988). Criteria for discharge have been established (Box 13-4).
Box 13-4 Criteria for Discharge to Home
(From Patel RI, Rice LJ: Special considerations in the recovery room of children from anesthesia, Int Anesthesiol Clin 29:55, 1991.)