CHAPTER 21 Indications and Technology of Neurophysiologic Monitoring in Meningioma Surgery
INTRODUCTION
From the early days of neurosurgery, there has been a continuous effort to reduce operative complication rates. The use of magnification and microsurgical techniques has led to major advances contributing to a significant reduction in morbidity and mortality rates over the past 4 decades. More recently, there has been increasing emphasis in the use of neurophysiologic monitoring techniques in an attempt to detect dysfunction of the nervous system at an early stage, when it may still be reversible. Intraoperative monitoring has been used and evaluated in neurovascular surgery1 and spine surgery.2 Although its application in meningioma and skull-base surgery in general is widespread and can be extremely useful, there is a paucity of data in the literature regarding indications and outcomes. Further, definitive answers to certain basic questions regarding monitoring remain elusive: (1) Does neurophysiologic monitoring predict postoperative neurologic deficits in patients undergoing surgery for meningiomas or other skull-base tumors? (2) Which factors have a high predictive value? (3) Can intraoperative neurophysiologic monitoring prevent postoperative deficits and improve outcome?
Indeed the utility of intraoperative neurophysiologic monitoring has been questioned by some experienced neurosurgeons, who believe it adds little to a carefully planned microsurgical procedure.3 Most surgeons, however, now consider monitoring an indispensable tool of modern intracranial neurosurgery.4–8 In this chapter we review the pertinent literature on neurophysiologic monitoring of sensory and motor pathways, as well as cranial nerves, in meningioma surgery and present our experience based on monitoring of more than 2000 cases.
The ideal monitoring technique should allow recognition and localization of nervous system structures at risk during surgery and alert the neurosurgeon when that particular neural structure, whether a cranial nerve or pathway, is being stressed before irreversible damage occurs, thus allowing maximal tumor resection while preserving neurologic function. Correlation of physiologic data with surgical events can also aid in understanding mechanisms of nervous tissue injury, possibly leading to improved surgical techniques and surgical outcomes.9 Because of the numerous potential structures at risk during meningiomas surgery, especially with lesions at the skull base, we have used neurophysiologic monitoring routinely from an early stage.10
MONITORING OF THE CENTRAL MOTOR AND SOMATOSENSORY PATHWAYS
Somatosensory Evoked Potentials
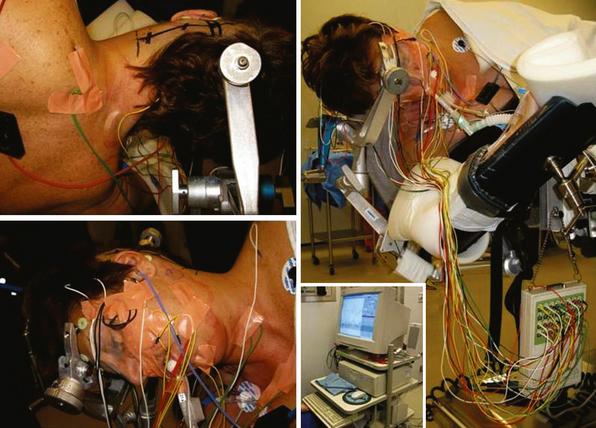
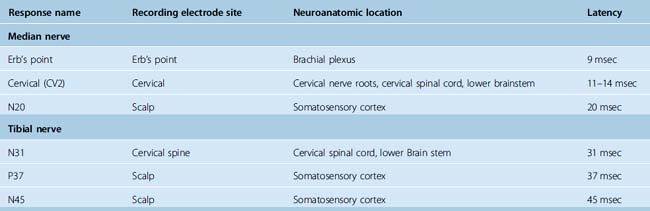
Dawson first described SSEPs in 1947.11 Initially used in the laboratory and clinical setting, further technical developments in the late 1970s and 1980s allowed for their use for intraoperative monitoring. Currently the acquisition of SSEPs is done according to international standards12,13 following stimulation of peripheral afferent nerves, generally the median or ulnar nerve for upper extremities and posterior tibial or peroneal nerve for lower extremities. Recording from the surgically exposed cortex with electrode grids or from the scalp usually with needle electrodes allows recording of a phase reversal signal between the somatosensory cortex and the motor cortex induced by the peripheral nerve stimulation. This allows the reliable identification of the central sulcus. The monitoring of somatosensory pathways throughout a procedure requires continuous monitoring by repeated stimulation. To reduce the signal-to-noise ratio induced by the significant background EEG activity of the brain, it is necessary to average about 200 to 500 stimulation trials and to filter SSEP signals with a predetermined bandwidth acquisition. The stimulation of peripheral nerves is done with needle or surface electrodes generating a bipolar signal and a single ground electrode is used for all limbs (Fig. 21-1). Stimulus intensity between 20 and 30 mA and duration between 200 and 500 µs at approximately 5 Hz is adequate to obtain satisfactory responses. This setup allows for the continuous updating of information, and any changes can be transmitted to the surgical team at less than 1-minute intervals. To differentiate SSEP changes from central or peripheral origins, cranial as well as cervical and peripheral electrodes are routinely used. The stimulation wave can be recorded at several acquisition points with a specific latency (Table 21-1), and the amplitude and morphology of the responses are compared with the baseline preoperative recording of the patient after induction of anesthesia and before positioning and with the intraoperative recordings of the contralateral side that serve as an internal control.
TABLE 21-1 SSEPs: minimal standard recording sites for upper (median nerve) and lower (tibial nerve) SSEPs and corresponding latencies
Although SSEP principally monitors the integrity of the sensory afferent pathways, it can indirectly monitor the nearby motor corticospinal tracts and motor cortex. Thus, there is a recognized utility of SSEPs for detecting disturbance not exclusively of somatosensory pathways but also of more general hemispheric changes. In this regard, there is a well established relationship between cortical cerebral blood flow and SSEP changes, which make it possible to identify vascular compromise in regions not only related to vascular supply of the somatosensory cortex. Importantly, SSEPs are also subject to change with general physiologic parameters such as blood pressure,14 body temperature, or anesthetic regimen, which have to be taken into consideration when interpreting changes seen during surgery.
The utility of SSEP monitoring has been repeatedly reported for a variety8,15,16 of spinal17–19 and cranial vascular20 as well as tumoral21–24 procedures. Based on our own experience with SSEP monitoring in more than several thousand cases of cranial procedures, we have found that reductions in amplitude of the cortical wave exceeding 50% are significant in predicting neuronal compromise and warrant alerting and reaction from the surgical team. Minor changes in amplitude and latency, which are not uncommon, are more likely related to changes in anesthesia or general physiologic parameters such as blood pressure, blood gases, and temperature. Changes in the morphology of the SSEP waveforms alone are more difficult to interpret and have no specific value in common clinical practice.
The utility of SSEP in tumor surgery including meningiomas has been assessed by several studies. Romstöck and colleagues reported a successful identification of the central sulcus with phase reversal in more than 90% out of 230 cases of central or paracentral tumors including meningiomas, but this was dependent of tumor location.24 In large and centrally located tumors causing significant displacement and deforming the central cortex it was more difficult to obtain a reliable phase reversal response. Wiedermayer and colleagues have specifically assessed the false-negative findings during SSEP intraoperative monitoring and reported 4% of 658 vascular and tumor cases in which patients with uneventful monitoring developed neurologic deficits.15 They found that the overall sensitivity was 79% and negative predictive value 96%. Not surprisingly, in their experience SSEPs were less likely to predict deficits in infratentorial lesions compressing the brain stem, small cortical lesions of the motor cortex, and small vessel injury during aneurysm surgery, situations in which motor pathways could selectively be injured without disturbing sensory pathway or causing wider hemispheric damage. In summary, these and other studies confirm the usefulness of SSEP as a routine monitoring modality in intracranial procedures, but also prompt awareness of its limitations, especially for lesion in which selective impairment of motor pathways is the major surgical risk.
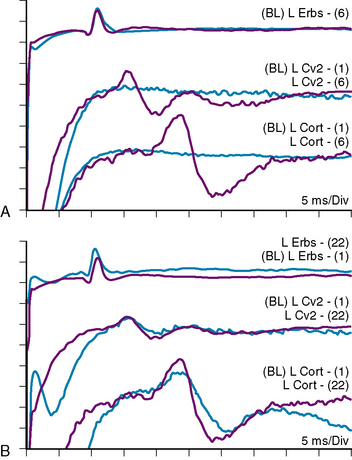
The value of SSEP in meningioma surgery in general has been assessed along with other tumors,24 and cranial base meningiomas were included in several series describing the use of SSEPs in skull-base lesions.5,10,25 Bejjani and colleagues have described several types of SSEP changes in the setting of skull-base tumor surgery.25 The proposed classification, which we have also adopted, is as follows: type I, no significant waveform change from baseline; type II, waveform changes that returned to baseline (>10% increase in latency from baseline and/or >50% decrease of amplitude from baseline); type III, waveform changes that recovered partially but incompletely; type IV, complete flattening of the SSEP waveform without improvement; type V, flat waveform from the beginning of surgery. In a series of 244 patients, these authors reported a very good correlation between SSEP type and postoperative deficits with a positive predictive value of 100%, and a negative predictive value of 90%. In our own unpublished series of 122 procedures for skull-base tumors (excluding vestibular schwannomas) during a 4-year period, we found similar results with a positive predicting value of 100% and a negative predicting value of 89%. Another important aspect in skull-base lesions compressing the brain stem, especially near the craniovertebral junction and in mass lesions of the cervical spine, is the critical value of SSEP monitoring during the positioning of the patient: passive mobilization of the spine and craniocervical junction in anesthetized and relaxed patients carries a real risk of neurologic damage.26 Further benefits to SSEP monitoring include detection of peripheral nerve injury by traction and/or compression (especially in the ulnar SSEP) attributed to improper positioning of the patient: in this situation early detection can prevent permanent nerve damage during a long procedure with complex positioning, such as in the park bench position (Fig. 21-2).
Motor Evoked Potentials
Because SSEPs offer a valuable but only indirect mean of monitoring motor pathways within the limits discussed in the preceding text, many efforts have been made to develop direct surveillance of motor pathway integrity during intracranial and spinal surgery. The identification of the motor cortex is possible through direct bipolar stimulation and observation of muscle contraction or muscle activity recording with EMG in either awake or anesthetized patients. However, as anesthesia has an impact on the stimulation threshold required to induce recognizable muscle contraction, and as awake surgery is not generally practical in most of brain and spine tumors including meningiomas, more refined monitoring modalities were developed for continuous intraoperative motor monitoring. The first attempts to explore the human cerebral cortex by evoking a motor response from a cortical electrical stimulation during surgery were described by Sir Victor Horsley and further refined by Penfield27 and others. However, the scientific groundwork for motor evoked potentials (MEPs) stems from the work on monkeys of Patton and Amassian in the mid-1950s.28,29 By applying direct cortical stimulation they were able to elicit two distinct recordable discharges from of the corticospinal tracts. The first one is a nonsynaptic early response named the D-wave translating a direct stimulation of the corticospinal tract and the second is a late, synaptic response called the I wave resulting from the action potential firing of stimulated cortical motoneurons. MEP was subsequently applied in humans either by direct cortical stimulation30 or by transcranial electrical stimulation (TES) in awake patients.31 Recording of MEPs is possible either from electrodes placed directly in the spinal cord epidural space or currently more often by recording muscular EMG. Volatile anesthetic agents suppress the I response, and intraoperative MEPs in the 1980s relied on the more robust D wave recorded from the spinal epidural space. Transcranial magnetic stimulation29 alleviating scalp electrodes but mainly inducing I waves is valuable in awake patients but does not offer a significant advantage in anesthetized patients because of the suppression of I waves induced by anesthetic agents. The major breakthrough for the reliable use of MEPs in anesthetized patients was the demonstration that the summation of short-train impulses at high frequency by direct cortical stimulation allowed to record MEPs (both the I and D waves) in the presence of most anesthetics.32 This was followed by further work establishing the feasibility of pulse-train TES.33 Moreover, the wider adoption of intravenous anesthetic agents such as propofol further facilitated the intraoperative use of MEPs that are less suppressed with intravenous substances such as remifentanyl and propofol than with volatile anesthetics.34–36
The value of current modern MEP’s techniques has been demonstrated by several authors for intra-axial and extra-axial lesions in the perirolandic area,32,37–41 for intracranial vascular lesions,39,42–45 and for spinal deformity and spine tumor surgery.39,46–48 More recently, its use with transcranial stimulation has been suggested to monitor cranial nerves,38,39,49 as discussed later. In clinical practice it has been established that more than a 50% amplitude reduction of the D wave during intramedullary spinal surgery is associated with injury of the corticospinal tract and long-term motor deficit.50,51 In perirolandic surgery, more than a 30% to 50% reduction in the D waves is predictive of permanent deficit.37 Preservation of the D waves is a good predictor of the absence of motor deficit although probably not with 100% predictive value. Fujiki and colleagues compared the value of corticospinal D versus I wave in brain tumors. They report that I waves recorded from the muscles are more sensitive than corticospinal MEPs recorded from the spinal epidural space that depend more on D waves.37
Although the use of MEPs for intracranial meningiomas have been reported by some authors,37,39 there is no convincing evidence of a benefit for the use of MEPs in surgery for these lesions. In our own practice we would recommend the use of MEPs in selective cases of meningiomas in or adjacent to the motor cortex, in parasagittal meningiomas with encasement of the anterior cerebral arteries or invasion of the superior sagittal sinus in its posterior two thirds, in spinal meningiomas, as well as in skull-base meningiomas with suspected encasement of major arteries of the circle of Willis (Fig. 21-3).
MONITORING OF CRANIAL NERVES
Cranial nerve monitoring in skull-base operations has been shown to reduce the risk of permanent postoperative neurologic deficits.4,7
Techniques for monitoring the facial nerve were the first to be described,52,53 followed by intraoperative monitoring of neural conduction in the auditory nerve.54,55 These techniques came into common use for operations in the cerebellopontine angle during the 1980s.56,57 With the development of skull-base surgery methods for intraoperative monitoring of several other cranial nerves often at risk were also introduced.58 Sensory nerves are typically monitored with evoked potentials and motor nerves with EMG recordings.
Initially, the presence of a visible muscle twitch following electrical stimulation was used as a gross measure of peripheral nerve integrity. In the 1960s and 1970s, nerve conduction studies (NCSs) were adapted to the intraoperative monitoring of peripheral nerves, and the recording of evoked potentials to the study of visual and auditory, as well as spinal sensory pathways during surgery.9 In 1986, Harner and colleagues59 first described the use of continuous EMG recordings as an intraoperative monitoring tool for peripheral nerve function. The past two decades have seen refinement of these techniques as well as their wider application.
The aim of cranial nerve monitoring in meningioma surgery can be simplified and summarized in three points:7
Optic Nerve Monitoring
The search for an efficacious and reliable monitoring method of the optic nerves (ONs) in neurosurgery has been ongoing for several decades,60,61 as it would have an immediate and obvious utility. The rate of new visual deficits after the surgical treatment of tuberculum sellae meningiomas is approximately 12% to 20%.62,63 Various techniques that might be able to diminish trauma to the optic nerve during surgery have been described. An optimal monitoring technique would be able to show the advantages of one technique versus another and allow as safe a removal of the meningioma as possible.64
Postoperative visual decline from manipulation and/or devascularization of the ON and chiasm remains one of the most devastating consequences of modern central skull-base neurosurgery.65 While the use of visual evoked potential (VEP) has been in common use in the diagnostic laboratory, unfortunately the ability of flash-induced visual evoked potentials (fVEPs) to predict intraoperative or postoperative visual pathway disturbances has proven unreliable because of high variability and a lack of specificity66–69 of this technique.
An attempt to use a checkerboard stimulus intraoperatively, a more reliable stimulus that better correlates with acuity than the flash stimulus, has to date not proved feasible. Recently a feasibility study on the epidural stimulation of the optic nerve has been reported,65 but overall there remains little data on results and outcomes with electrical stimulation of the optic nerve.70–72 Unfortunately, to date there is no accurate technique to monitor the function of the optic nerve intraoperatively.
Ocular Motor Nerve Monitoring
Preserving the integrity of the ocular motor nerves (oculomotor, trochlear, and abducens) during operations in the skull base is critical in preventing the postoperative complications of functional blindness.73
One monitoring technique based on EMG monitoring of the extraocular muscles requires extraocular but intraorbital insertion of EMG needle electrodes. The procedure is not trivial, and different techniques have been described: electrodes can be inserted free-hand on the basis of anatomical landmarks,74 under ultrasound control75 or with neuronavigation with a slightly modified pointer;76 special ring electrodes have also been described for this use.73,77 Although some reports on “bursts” and “train” EMG patterns after mechanical stimulation of the nerves have been reported,74,76,78 the exact classification and attribution of the EMG responses and eyeball movement remain a challenge in clinical practice.79 Monopolar recording electrodes are used rather than bipolar electrodes because of insufficient space to place bipolar electrodes. The reference electrodes are placed on the opposite side of the head or bridge of the nose to avoid recording EMG activity from facial muscles on the operated side of the head, where they may be activated by surgical manipulation of the facial nerve.7
A less invasive monitoring technique for cranial nerve (CN) III and VI is electrooculography (EOG)80: from an electrophysiologic standpoint, the eye can be observed as a dipole, the two poles being the cornea and the retina, connected by the optic axis. Skin electrodes, arranged symmetrically to the cornea–retina axis at the outer and inner canthus, can register eye movements recorded by changes in the voltage differences between the anterior and posterior eye pole. As compared with EMG monitoring of extraocular muscles, EOG recording is less invasive and yet provides similar information concerning which part of the oculomotor nerve and abducent nerve is being stimulated, with a real-time response. However, EOG cannot monitor the trochlear nerve. As the nerve imparts a rotational movement to the eyeball around the cornea–retina axis, with no major change in the axis, EOG monitoring is unable to detect the effect of the trochlear nerve.80,81
Trigeminal Nerve Monitoring
The motor portion (portio minor) of the trigeminal nerve may be stimulated when probing large acoustic tumors to find the facial branch eliciting contractions of the muscles of mastication (masseter and temporalis muscles). Because of the spread of EMG activity, the activity from these muscles may be picked up by the recording electrodes that are placed in facial muscles and become mistaken for CN VII activation, causing misidentification of the facial nerve. This type of activity and misinterpretation is referred to as “cross talk.” The peak latency of the response to stimulation of the trigeminal nerve is less than 6 msec (masseter muscle), and the peak latency of the response from the facial muscles (orbicularis oris/oculi) to intracranial stimulation of the facial nerve is longer than 8 msec. The latency of the beginning of the masseter muscle’s response to intracranial stimulation is 1.5 to 2.0 msec for the trigeminal nerve and 5 to 6 msec for the facial nerve. If a second recording channel is available, it is best to use it to record directly from the mastication muscles, such as the masseter muscle.7
Even when not monitoring the trigeminal nerve the neurosurgeon should be aware of the trigemino–cardiac reflex and inform the anesthesiologist when working near the trigeminal nerve.82,83
Facial Nerve Monitoring
Facial nerve monitoring was prompted by the high incidence of postoperative facial nerve deficits after surgery in the cerebellopontine angle. The principle of probing the surgical field with a hand-held stimulator has not changed since such monitoring was introduced in the 1960s although the way that muscle contractions are monitored has been modified7: initially muscle contractions were checked visibly, then with mechanical transducers and, more recently, EMG recording has become standard practice. Monopolar or bipolar stimulation can be used to exclude tumor areas that do not involve the nerve and to track the nerve along its course; it is also useful to ascertain the nerve integrity at the end of tumor removal. To monitor the nerve function throughout the surgical procedure continuous EMG recording was introduced.84 Various types of electrical activity can be registered and only some have been proven to be signs of nerve injury. Recently Romstock and colleagues85 have stressed the importance of the so-called A waves, a series of high-frequency discharges with abrupt onset and termination. Spikes, bursts, B trains (sequence of single components with maximum intervals of 500 msec), and C trains (continuous irregular EMG activity) did not exhibit any correlation with pre- and postoperative paresis; the A train was observed in 19 patients, with one false-positive and three false-negative, leading to a sensitivity of 86% and a specificity of 89%.85 Waveform pattern definition was therefore crucial, whereas amplitude, duration, and frequency of EMG potentials proved irrelevant.85
Wedeking and Klug86 recently reported a high correlation of the loss of nasal muscle F-waves to postoperative facial nerve deficit. F-wave recordings are a standard electrophysiologic means to reveal lesions in the proximal segments of peripheral nerves and facial F-waves can be obtained by stimulating the zygomatic branch of the nerve.87 The diagnostic sensitivity of a permanent F-wave loss for development of a severe-to-total facial paresis was 91%, based on the study of 33 patients.86 The specificity was calculated to be 100% as was the positive predictive value of this finding; the negative predictive value of a permanent F-wave loss was 96%.
More recently different groups have reported the use of MEPs for continuous monitoring of the facial nerve (FNMEPs): stimulating electrodes are placed at positions C3, C4, and Cz to cortically activate FNMEPs; standard needle electrodes in the orbicularis oculi and orbicularis oris muscles can be used to monitor the response. A good correlation with facial postoperative function has been documented using a 50% final-to-baseline MEP amplitude ratio,38,49,88 although a correlation with the postoperative deficit grade was not evident.88 It is important to exclude stimulation artifacts during FNMEP monitoring achieved by transcranial electrical stimulation. Because the distance between the stimulation and recording sites is short, the stimulation artifact is usually large and sometimes overrides the FNMEP responses themselves.88 In patients with severe preoperative facial nerve dysfunction, FNMEPs could not be used for intraoperative monitoring.88
Cochlear Nerve Monitoring
Meningiomas are the second most common tumor of the cerebellopontine angle. Although most of them will not involve the cochlear nerve, meningiomas with an extension in the internal auditory meatus have a intimate relationship with the CN VII–VIII complex and therefore surgical implications that are very similar to vestibular schwannomas.85,89–91
In a recently published study on meningiomas involving the internal auditory meatus, Nakamura and colleagues89 reported hearing preservation in 31 of 37 patients (83.8%). Although they noticed a good predictive value of normal brain stem auditory evoked potential (BAEP) during surgery, one patient experienced postoperative deafness even though the monitoring was apparently normal; in two cases of postoperative deafness the authors noticed a temporary deterioration of the BAEP. The negative predictive value of BAEP (i.e., normal hearing after normal BAEP) was therefore 95.8%.
The presence or absence of BAEPs cannot signify preservation or loss of hearing with complete reliability and this might be explained by certain atypical combinations: under the assumption that electrical activity still works along the auditory pathway in these patients, it is hypothesized that some impulse asynchrony along the auditory brain stem pathway prevents the formation of typical BAEPs; more frequently, complete wave absence is encountered in high-frequency hearing losses.92
The authors89 also evaluated the positive predictive value of BAEP: an overall risk of deafness of 40% was seen in patients with a transient BAEP deterioration. The duration of the BAEP deterioration seems to be a relevant factor: 75% of patients, in whom the duration of BAEP deterioration to class B4 was longer than 5 minutes, experienced hearing deterioration as compared to 16.7% of patients who experienced a shorter deterioration of BAEP. Although with these limitations, ABR are very useful for hearing preservation and have contributed to improved patient outcomes in modern series. As in acoustic neuroma surgery, combining BAEP monitoring with an electrocochleogram (EcochG) and possibly direct CN VIII nerve action potential (NAP) recording may improve the sensitivity and specificity of the monitoring of the cochlear nerve.
Lower Cranial Nerve Monitoring
The literature regarding IOM of lower cranial nerves (LCN, i.e., CN IX–XII) is scant.93–96 The majority of experience in monitoring these nerves has been limited to the extracranial segments of the vagal nerve such as the superior and recurrent laryngeal nerves.96 Although a considerable number of studies has been published on different applications of facial nerve monitoring, only very few papers have focused on technical modalities for intraoperative monitoring of the LCN during neurosurgical operations.95,97
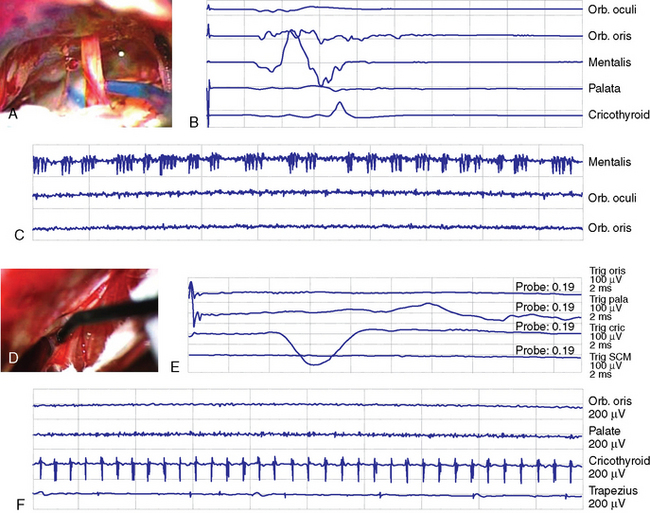
The incidence of high false-positive and -negative CMAP and the variability in CMAP amplitude and threshold can vary depending on individual and technical factors.93,96 Nonetheless monitoring of lower cranial nerves has the important function of allowing recognition of the nerve and definition of its location (Fig. 21-4). Our own experience with lower cranial nerve monitoring indicates that quantification of the nerve deficit may be possible. Threshold current for stimulation can be a potential predictor for the quantitative function (e.g., correlation of threshold current for stimulation and functional grade) and the possibility and extent of recovery. A prospective study is needed to confirm this impression.
The cranial nerve and the corresponding muscle used for monitoring at Toronto Western Hospital are shown in Table 21-2. The neurophysiologic monitoring technique used at Toronto Western Hospital is shown in Figure 21-1.
TABLE 21-2 Cranial nerves and corresponding muscle/s used for their monitoring
| Cranial nerve | Muscle |
|---|---|
| III | Inferior rectus |
| IV | Superior oblique |
| V | Temporalis, masseter |
| VI | Lateral rectus |
| VII | Orbicularis oculi, orbicularis oris, mentalis |
| VIII | (Sensory only) BSAEP |
| IX | Stylopharyngeus |
| X | Cricothyroid |
| XI | Trapezius, sternocleidomastoid (SCM) |
| XII | Intrinsic tongue muscles |
MULTIMODALITY ELECTROPHYSIOLOGIC MONITORING
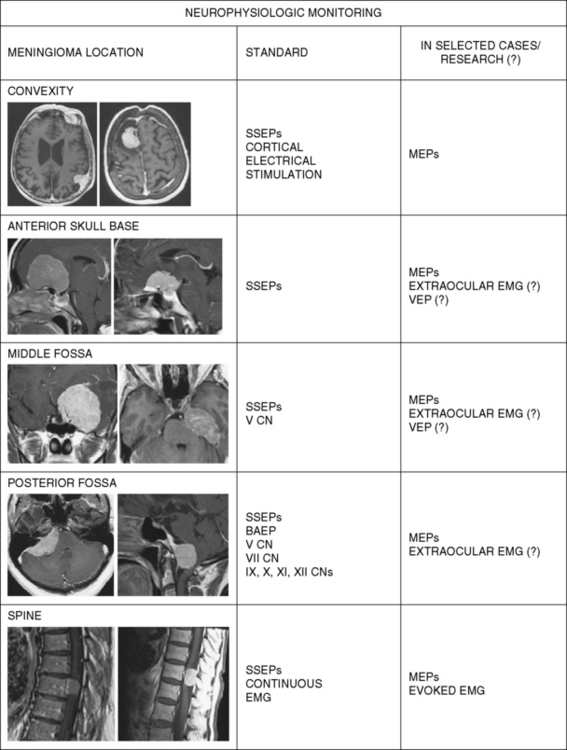
While electrophysiological monitoring offers promise as an noninvasive means of detecting damage to the nervous system, surgeons must be aware of potential difficulties with these techniques. This may include quality control, confounding variables, and interpretation of results. Further, each monitoring modality whether it be SSSP, BAEP, MEP, and individual cranial nerve monitoring will assess only the transmission in that particular pathway or nerve. Thus one has to decide preoperatively what particular neural structure(s) is/are at most risk during surgery and customize the monitoring to best assess potential damage to those structures. For most skull base and posterior fossa meningiomas, the use of multimodality monitoring of multiple pathways and cranials nerve is recommended (Fig. 21-5).
[1] Schramm J., Koht A., Schmidt G., et al. Surgical and electrophysiological observations during clipping of 134 aneurysms with evoked potential monitoring. Neurosurgery. 1990;26:61.
[2] Fehlings M.G., Kelleher M.O. Intraoperative monitoring during spinal surgery for neuromuscular scoliosis. Nat Clin Pract Neurol. 2007;3:318.
[3] Malis L.I. Intra-operative monitoring is not essential. Clin Neurosurg. 1995;42:203.
[4] Moller A.R. Intra-operative neurophysiologic monitoring in neurosurgery: benefits, efficacy, and cost-effectiveness. Clin Neurosurg. 1995;42:171.
[5] Sekhar L.N., Bejjani G., Nora P., et al. Neurophysiologic monitoring during cranial base surgery: is it necessary? Clin Neurosurg. 1995;42:180.
[6] Monfared A., Agrawal S., Jackler R.K. Cranial base approaches to inaccessible intracranial tumors. Curr Opin Neurol. 2007;20:726.
[7] Moller A. Monitoring and mapping the cranial nerves and the brainstem. In: Deletis V., Shils J., editors. Neurophysiology in Neurosurgery – A Modern Intraoperative Approach. Philadelphia: Elsevier; 2002:291.
[8] Wiedemayer H., Fauser B., Sandalcioglu I.E., et al. The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg. 2002;96:255.
[9] Harper C.M. Intraoperative cranial nerve monitoring. Muscle Nerve. 2004;29:339.
[10] Gentili F., Lougheed W.M., Yamashiro K., et al. Monitoring of sensory evoked potentials during surgery of skull base tumours. Can J Neurol Sci. 1985;12:336.
[11] Dawson G.D. Investigations on a patient subject to myoclonic seizures after sensory stimulation. J Neurol Neurosurg Psychiatry. 1947;10:141.
[12] Mauguiere F., Allison T., Babiloni C., et al. Somatosensory evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl.. 1999;52:79.
[13] Burke D., Nuwer M.R., Daube J., et al. Intraoperative monitoring. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl.. 1999;52:133.
[14] Yamada S., Brauer F., Knierim D., et al. Can somatosensory evoked potential monitoring predict energy dynamics during controlled hypotension? Neurol Res. 1992;14:325.
[15] Wiedemayer H., Sandalcioglu I.E., Armbruster W., et al. False negative findings in intraoperative SEP monitoring: analysis of 658 consecutive neurosurgical cases and review of published reports. J Neurol Neurosurg Psychiatry. 2004;75:280.
[16] Wiedemayer H., Sandalcioglu I.E., Regel J., et al. Enhanced stability of somatosensory evoked potentials attained in the median nerve by using temporal electrodes for intraoperative recording in patients in the semisitting position. J Neurosurg. 2003;99:986.
[17] Kelleher M.O., Tan G., Sarjeant R., et al. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: a prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8:215.
[18] Paradiso G., Lee G.Y., Sarjeant R., et al. Multimodality intraoperative neurophysiologic monitoring findings during surgery for adult tethered cord syndrome: analysis of a series of 44 patients with long-term follow-up. Spine. 2006;31:2095.
[19] Krassioukov A.V., Sarjeant R., Arkia H., et al. Multimodality intraoperative monitoring during complex lumbosacral procedures: indications, techniques, and long-term follow-up review of 61 consecutive cases. J Neurosurg Spine. 2004;1:243.
[20] Lopez J.R., Chang S.D., Steinberg G.K. The use of electrophysiological monitoring in the intraoperative management of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1999;66:189.
[21] Rowed D.W., Houlden D.A., Basavakumar D.G. Somatosensory evoked potential identification of sensorimotor cortex in removal of intracranial neoplasms. Can J Neurol Sci. 1997;24:116.
[22] Ma H.L., Yu C.L., Chang C.N. Intraoperative somatosensory evoked potentials for localization in excision of recurrent parasagittal meningioma–a case report. Acta Anaesthesiol Sin. 1995;33:237.
[23] Witzmann A., Beran H., Bohm-Jurkovic H., et al. The prognostic value of somatosensory evoked potential monitoring and tumor data in supratentorial tumor removal. J Clin Monit. 1990;6:75.
[24] Romstöck J., Fahlbusch R., Ganslandt O., et al. Localisation of the sensorimotor cortex during surgery for brain tumours: feasibility and waveform patterns of somatosensory evoked potentials. J Neurol Neurosurg Psychiatry. 2002;72:221.
[25] Bejjani G.K., Nora P.C., Vera P.L., et al. The predictive value of intraoperative somatosensory evoked potential monitoring: review of 244 procedures. Neurosurgery. 1998;43:491.
[26] Deinsberger W., Christophis P., Jodicke A., et al. Somatosensory evoked potential monitoring during positioning of the patient for posterior fossa surgery in the semisitting position. Neurosurgery. 1998;43:36.
[27] Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937:339.
[28] Patton H.D., Amassian V.E. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345.
[29] Barker A.T., Jalinous R., Freeston I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106.
[30] Horikoshi T., Omata T., Uchida M., et al. Usefulness and pitfalls of intraoperative spinal motor evoked potential recording by direct cortical electrical stimulation. Acta Neurochir (Wien). 2000;142:257.
[31] Merton P.A., Morton H.B. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227.
[32] Taniguchi M., Cedzich C., Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery. 1993;32:219.
[33] MacDonald D.B. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19:416.
[34] Scheufler K.M., Zentner J. Total intravenous anesthesia for intraoperative monitoring of the motor pathways: an integral view combining clinical and experimental data. J Neurosurg. 2002;96:571.
[35] Taniguchi M., Nadstawek J., Langenbach U., et al. Effects of four intravenous anesthetic agents on motor evoked potentials elicited by magnetic transcranial stimulation. Neurosurgery. 1993;33:407.
[36] Taniguchi M., Nadstawek J., Pechstein U., et al. Total intravenous anesthesia for improvement of intraoperative monitoring of somatosensory evoked potentials during aneurysm surgery. Neurosurgery. 1992;31:891.
[37] Fujiki M., Furukawa Y., Kamida T., et al. Intraoperative corticomuscular motor evoked potentials for evaluation of motor function: a comparison with corticospinal D and I waves. J Neurosurg. 2006;104:85.
[38] Akagami R., Dong C.C., Westerberg B.D. Localized transcranial electrical motor evoked potentials for monitoring cranial nerves in cranial base surgery. Neurosurgery. 2005;57:78.
[39] Neuloh G., Schramm J. Intraoperative neurophysiological mapping and monitoring for supratentorial procedures. In: Neurophysiology in Neurosurgery – A Modern Intraoperative Approach. California: Academic Press; 2002:339.
[40] Sala F., Lanteri P. Brain surgery in motor areas: the invaluable assistance of intraoperative neurophysiological monitoring. J Neurosurg Sci. 2003;47:79.
[41] Yamamoto T., Katayama Y., Nagaoka T., et al. Intraoperative monitoring of the corticospinal motor evoked potential (D-wave): clinical index for postoperative motor function and functional recovery. Neurol Med Chir (Tokyo). 2004;44:170.
[42] Horiuchi K., Suzuki K., Sasaki T., et al. Intraoperative monitoring of blood flow insufficiency during surgery of middle cerebral artery aneurysms. J Neurosurg. 2005;103:275.
[43] Sakuma J., Suzuki K., Sasaki T., et al. Monitoring and preventing blood flow insufficiency due to clip rotation after the treatment of internal carotid artery aneurysms. J Neurosurg. 2004;100:960.
[44] Suzuki K., Kodama N., Sasaki T., et al. Intraoperative monitoring of blood flow insufficiency in the anterior choroidal artery during aneurysm surgery. J Neurosurg. 2003;98:507.
[45] Quinones-Hinojosa A., Lyon R., Du R., et al. Intraoperative motor mapping of the cerebral peduncle during resection of a midbrain cavernous malformation: technical case report. Neurosurgery. 2005;56:E439. discussion E439
[46] Calancie B., Harris W., Brindle G.F., et al. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001;95:161.
[47] Sala F., Palandri G., Basso E., et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129.
[48] Kothbauer K.F. Motor evoked potential monitoring for intramedullary spinal cord tumor surgery. In: Deletis V., Shils J., editors. Neurophysiology in Neurosurgery – A Modern Intraoperative Approach. California: Academic Press; 2002:73.
[49] Dong C.C., Macdonald D.B., Akagami R., et al. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol. 2005;116:588.
[50] Deletis V. Intraoperative neurophysiology and methodologies used to monitor the functional integrity of the motor system. In: Deletis V., Shils J., editors. Neurophysiology in Neurosurgery – A Modern Intraoperative Approach. California; 2002:25.
[51] Kothbauer K.F. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007;37:407.
[52] Rand R.W., Kurze T.L. Facial nerve preservation by posterior fossa transmeatal microdissection in total removal of acoustic tumours. J Neurol Neurosurg Psychiatry. 1965;28:311.
[53] Moller A.R., Jannetta P.J. Preservation of facial function during removal of acoustic neuromas. Use of monopolar constant-voltage stimulation and EMG. J Neurosurg. 1984;61:757.
[54] Grundy B.L. Monitoring of sensory evoked potentials during neurosurgical operations: methods and applications. Neurosurgery. 1982;11:556.
[55] Silverstein H., McDaniel A.B., Norrell H. Hearing preservation after acoustic neuroma surgery using intraoperative direct eighth cranial nerve monitoring. Am J Otol Suppl. 1985:99.
[56] Silverstein H., Smouha E., Jones R. Routine identification of the facial nerve using electrical stimulation during otological and neurotological surgery. Laryngoscope. 1988;98:726.
[57] Harner S.G., Daube J.R., Ebersold M.J., et al. Improved preservation of facial nerve function with use of electrical monitoring during removal of acoustic neuromas. Mayo Clin Proc. 1987;62:92.
[58] Moller A.R. Electrophysiological monitoring of cranial nerves in operations in the skull base. In: Sekhar L.N., Schramm V.L., editors. Tumors of the cranial base: Diagnosis and treatment. Mt. Kisco, NY: Futura Publishing; 1987:123.
[59] Harner S.G., Daube J.R., Ebersold M.J. Electrophysiologic monitoring of facial nerve during temporal bone surgery. Laryngoscope. 1986;96:65.
[60] Wilson W.B., Kirsch W.M., Neville H., et al. Monitoring of visual function during parasellar surgery. Surg Neurol. 1976;5:323.
[61] Feinsod M., Selhorst J.B., Hoyt W.F., et al. Monitoring optic nerve function during craniotomy. J Neurosurg. 1976;44:29.
[62] Nakamura M., Roser F., Struck M., et al. Tuberculum sellae meningiomas: clinical outcome considering different surgical approaches. Neurosurgery. 2006;59:1019.
[63] Jallo G.I., Benjamin V. Tuberculum sellae meningiomas: microsurgical anatomy and surgical technique. Neurosurgery. 2002;51:1432.
[64] Akabane A., Saito K., Suzuki Y., et al. Monitoring visual evoked potentials during retraction of the canine optic nerve: protective effect of unroofing the optic canal. J Neurosurg. 1995;82:284.
[65] Bosnjak R., Benedicic M. Direct epidural electrical stimulation of the optic nerve: a new method for intraoperative assessment of function. J Neurosurg. 2008;109:647.
[66] Cedzich C., Schramm J., Mengedoht C.F., et al. Factors that limit the use of flash visual evoked potentials for surgical monitoring. Electroencephalogr Clin Neurophysiol. 1988;71:142.
[67] Harding G.F., Bland J.D., Smith V.H. Visual evoked potential monitoring of optic nerve function during surgery. J Neurol Neurosurg Psychiatry. 1990;53:890.
[68] Wiedemayer H., Fauser B., Armbruster W., et al. Visual evoked potentials for intraoperative neurophysiologic monitoring using total intravenous anesthesia. J Neurosurg Anesthesiol. 2003;15:19.
[69] Bergholz R., Lehmann T.N., Fritz G., et al. Fourier transformed steady-state flash evoked potentials for continuous monitoring of visual pathway function. Doc Ophthalmol. 2008;116:217.
[70] Kikuchi Y., Sasaki T., Matsumoto M., et al. Optic nerve evoked potentials elicited by electrical stimulation. Neurol Med Chir (Tokyo). 2005;45:349.
[71] Wang K., Li X.X., Jiang Y.R., et al. Influential factors of thresholds for electrically evoked potentials elicited by intraorbital electrical stimulation of the optic nerve in rabbit eyes. Vision Res. 2007;47:3012.
[72] Moller A.R., Burgess J.E., Sekhar L.N. Recording compound action potentials from the optic nerve in man and monkeys. Electroencephalogr Clin Neurophysiol. 1987;67:549.
[73] Sekiya T., Hatayama T., Iwabuchi T., et al. Intraoperative recordings of evoked extraocular muscle activities to monitor ocular motor nerve function. Neurosurgery. 1993;32:227.
[74] Eisner W., Schmid U.D., Reulen H.J., et al. The mapping and continuous monitoring of the intrinsic motor nuclei during brain stem surgery. Neurosurgery. 1995;37:255.
[75] Schlake H.P., Goldbrunner R., Siebert M., et al. Intra-operative electromyographic monitoring of extra-ocular motor nerves (Nn. III, VI) in skull base surgery. Acta Neurochir (Wien). 2001;143:251.
[76] Alberti O., Sure U., Riegel T., et al. Image-guided placement of eye muscle electrodes for intraoperative cranial nerve monitoring. Neurosurgery. 2001;49:660.
[77] Sekiya T., Hatayama T., Iwabuchi T., et al. A ring electrode to record extraocular muscle activities during skull base surgery. Acta Neurochir (Wien). 1992;117:66.
[78] Schlake H.P., Goldbrunner R., Milewski C., et al. Technical developments in intra-operative monitoring for the preservation of cranial motor nerves and hearing in skull base surgery. Neurol Res. 1999;21:11.
[79] Samii M., Rosahl S. Comment on: Alberti O, Sure U, Riegel T, Bertalanffy H. Image-guided placement of eye muscle electrodes for intraoperative cranial nerve monitoring. Neurosurgery. 2001;3(49):660-663. Neurosurgery. 2001;49:2.
[80] Fukaya C., Katayama Y., Kasai M., et al. Intraoperative electrooculographic monitoring of oculomotor nerve function during skull base surgery. Technical note. J Neurosurg. 1999;91:157.
[81] Fukaya C., Katayama Y., Kasai M., et al. Intraoperative electro-oculographic monitoring for skull base surgery. Skull Base Surg. 2000;10:11.
[82] Koerbel A., Gharabaghi A., Samii A., et al. Trigeminocardiac reflex during skull base surgery: mechanism and management. Acta Neurochir (Wien). 2005;147:727.
[83] Meng Q., Yang Y., Zhou M., et al. Trigemino-cardiac reflex: the trigeminal depressor responses during skull base surgery. Clin Neurol Neurosurg. 2008;110:662.
[84] Prass R.L., Luders H. Acoustic (loudspeaker) facial electromyographic monitoring: Part 1. Evoked electromyographic activity during acoustic neuroma resection. Neurosurgery. 1986;19:392.
[85] Romstöck J., Strauss C., Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000;93:586.
[86] Wedekind C., Klug N. Recording nasal muscle F waves and electromyographic activity of the facial muscles: a comparison of two methods used for intraoperative monitoring of facial nerve function. J Neurosurg. 2001;95:974.
[87] Wedekind C., Klug N. Nasal muscle F-wave for peri- and intraoperative diagnosis of facial nerve function. Electromyogr Clin Neurophysiol. 1998;38:481.
[88] Fukuda M., Oishi M., Takao T., et al. Facial nerve motor-evoked potential monitoring during skull base surgery predicts facial nerve outcome. J Neurol Neurosurg Psychiatry. 2008;79:1066.
[89] Nakamura M., Roser F., Dormiani M., et al. Intraoperative auditory brainstem responses in patients with cerebellopontine angle meningiomas involving the inner auditory canal: analysis of the predictive value of the responses. J Neurosurg. 2005;102:637.
[90] Schaller B., Heilbronner R., Pfaltz C.R., et al. Preoperative and postoperative auditory and facial nerve function in cerebellopontine angle meningiomas. Otolaryngol Head Neck Surg. 1995;112:228.
[91] Nakamura M., Roser F., Mirzai S., et al. Meningiomas of the internal auditory canal. Neurosurgery. 2004;55:119.
[92] Matthies C., Samii M. Management of vestibular schwannomas (acoustic neuromas): the value of neurophysiology for evaluation and prediction of auditory function in 420 cases. Neurosurgery. 1997;40:919.
[93] Schlake H.P., Goldbrunner R.H., Milewski C., et al. Intra-operative electromyographic monitoring of the lower cranial motor nerves (LCN IX-XII) in skull base surgery. Clin Neurol Neurosurg. 2001;103:72.
[94] Cheek J.C. Posterior fossa intraoperative monitoring. J Clin Neurophysiol. 1993;10:412.
[95] Jackson L.E., Roberson J.B.Jr. Vagal nerve monitoring in surgery of the skull base: a comparison of efficacy of three techniques. Am J Otol. 1999;20:649.
[96] Topsakal C., Al-Mefty O., Bulsara K.R., et al. Intraoperative monitoring of lower cranial nerves in skull base surgery: technical report and review of 123 monitored cases. Neurosurg Rev. 2008;31:45.
[97] Lanser M., Jackler R., Yingling C. Regional monitoring of the lower [ninth through twelfth] cranial nerves. In: Kartush J., Bouchard K., editors. Neuromonitoring in otology and head and neck surgery. New York: Raven Press; 1992:131.