Chapter 52 Improving Biodegradable Interference Screw Properties by Combining Polymers
Introduction
Interference screws are widely used for graft fixation in anterior cruciate ligament (ACL) reconstruction, and good clinical results have been reported by several investigators.1–5 In addition to conventional metal screws, biodegradable interference screws are commercially available and have been shown to provide at least as strong graft fixation as metal screws.6,7 In addition, the biodegradable screws do not interfere with imaging techniques and do not need to be removed in revision cases because the implants have either degraded or can simply be drilled through. However, although biodegradable materials have been attractive for many years, they have been linked to limitations such as breakage during insertion due to brittleness of the material,8,9 tissue reactions due to poor material quality or too fast or uncontrolled degradation (e.g., polyglycolic acid),10,11 or too slow degradation offering no real advantage over metal implants (e.g., poly-L-lactic acid implants have been documented to take more than 4 years to degrade).10,12–15 It is obvious that as a result of these observations, the material properties have been identified to play a critical role, and manufacturers have thus been challenged to further develop and optimize the chemical compositions of biodegradable implants. Whether the biodegradable interference screws are actually finally replaced by bone or by some other tissue remains controversial.16–20 As a matter of fact, according to a recent study by Tecklenburg et al,21 even the recently introduced composite screws containing osteoconductive materials such as hydroxyapatite and tricalcium phosphate do not degrade in 2 years in vivo and thus cannot be replaced by bone. This clearly demonstrates the need for more optimal materials that degrade faster but are still controlled enough not to cause any clinically significant inflammatory or foreign body reactions. In addition, the material should be strong enough not to break during screw insertion and should provide adequate fixation strength during the healing period.
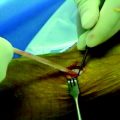
A number of biodegradable polymers have been approved for safe internal use and have been used in surgical applications for the past 30 years, initially as suture materials. Each polymer has its material-specific properties, and an implant created from a single type of polymer is naturally limited by those properties. This explains some of the problems observed with the first-generation biodegradable implants. For example, polyglycolic acid (PGA) is strong but very fast to degrade; poly-L-lactic acid (PLLA) is strong but brittle and slow to degrade; whereas trimethylene carbonate (TMC) is rather weak but elastic like rubber. Copolymer blending is a novel manufacturing method developed in an attempt to combine the desired properties of different polymers and, by doing so, to overcome the limitations of the previous biodegradable implants. By blending different copolymers it is possible to create a library of material recipes from which to select those of the appropriate strength, toughness, and degradation to meet specific clinical requirements. A biodegradable interference screw made of degradable copolymers composed of L-lactic acid, D-lactic acid, and TMC (Inion Hexalon, Inion Oy, Tampere, Finland) (Fig. 52-1) was introduced in 2002 and has since been studied both biomechanically and clinically. According to a recent preclinical sheep study, this copolymer blend fully degrades in 2 years in vivo without causing any clinically significant inflammatory, foreign body, or other tissue reactions.22
Biomechanical Results
Fixation Strength
Fixation strength of the ACL graft is commonly considered to be the weakest link of ACL reconstruction. A three-part biomechanical study was carried out to study the fixation strength of the new biodegradable copolymer interference screw (Inion Hexalon) and to evaluate its suitability for ACL reconstruction by comparing it with the previously clinically used interference screws.23 In the first part, the initial soft tissue graft fixation strength of the copolymer screw was compared with that of a conventional metal interference screw (Acufex Softsilk). In the second part of the study, the initial soft tissue graft fixation strength of the copolymer screw was compared with that of another biodegradable interference screw (Bionx SmartScrew). In the third part of the study, the initial bone–tendon–bone graft fixation strength of the copolymer screw was compared with that of another commercially available biodegradable interference screw (Linvatec Bioscrew).
Tibial bone tunnels were created in fresh skeletally mature porcine cadaver tibiae. A porcine ACL soft tissue graft model previously described and used by Ishibashi et al24 and Harding et al25 was used in Parts I and II. Porcine patellar tendons were cut approximately 8 cm distal from their patellar insertion and left attached to the patellae. The free end of each patellar tendon was sutured using the running baseball stitch and thereafter fixed into tibial bone tunnel with an interference screw. In Part III, porcine bone–patellar tendon–bone grafts were prepared by obtaining a tibial bone block. The graft end with the tibial bone block was fixed into the tibial bone tunnel, and the maximum screw insertion torque was determined with a digital torquemeter connected to the screwdriver. The patellae were left intact to enable easy and rigid fixation to the mechanical testing machine (Lloyd LR 5K, J.J. Lloyd Instruments). The biomechanical tests were performed strictly according to the previously described single-cycle load-to-failure protocol of Kousa et al.7 The specimens were first subjected to a 50N preload for 1 minute. Thereafter, vertical tensile loading parallel to the long axis of the bone tunnel was performed at a rate of 50 mm/min until failure and the yield load, maximum failure load, and mode of failure were determined.
Torsional Strength
Screw breakage due to applied torsional forces during screw insertion rather than postoperative failure of graft fixation is the most common failure mode of biodegradable interference screws. The torsional strength of the interference screw is largely determined by the design of the screwdriver recess (socket) and the material of the screw. To test the torsional strength of the new biodegradable copolymer screw, a torsional strength study was performed according to the testing protocol of Costi et al.8,26 Six 7- × 20-mm copolymer interference screws (Inion Hexalon) were mounted in a 10-mm layer of polyurethane resin, leaving the proximal 10 mm of the screws unembedded. This mounting reproduced the failure scenario observed in vivo, in which only part of the screw length has been inserted and becomes jammed in bone. Torque was applied manually with a digital electronic torque meter (Torqueleader TSD 350, MHH Engineering) mounted on the screwdriver. The same person applied torque in all cases in an attempt to provide a constant rate of application as well as compression on the screw. Care was taken to ensure that the application of torque was performed without associated bending or excessive compression. The maximum insertion torque was recorded, and the mode of failure was visually observed. In addition, to further investigate the failure of the screw, one screw was fixed into the 7- × 20-mm screw cavity of the injection mold and torque was applied manually with a presettable torque wrench until failure.
Costi et al8 previously tested 12 different biodegradable interference screws using the same protocol. In their study, the only screws observed to continue screwing into the resin with no subsequent failure were the majority of the 7-mm PLLA Linvatec Bioscrews. In our study, all tested Inion Hexalon copolymer screws could be advanced through the resin without failure. In our additional test in which the screw was fixed into its injection mold to determine the ultimate failure point, the failure occurred first after a torque of more than 5 Nm was applied, again not by screw breakage but by bending of the metallic screwdriver shaft. Based on the previous observations made by Costi et al,8 this failure torque is above the clinically relevant insertion torques and the failure torques of most commercially available biodegradable interference screws.
Strength Retention
To investigate the effect of hydrolytic degradation on the mechanical properties of the Inion Hexalon copolymer screws over time, screw compression tests were performed after 24 hours and 4, 8, and 12 weeks of incubation of 6- × 20-mm and 7- × 20-mm screws in phosphate buffer solution at 37° C (N = 4/time point).27 In the compression test, each screw was set flat between the compression plates and loaded with a constant speed of 5 mm/min until failure (Zwick Z020, Zwick GmbH, Ulm, Germany). In the compression test, both screws retained more than 80% of their initial mechanical strength as long as 12 weeks.
Clinical Results
Clinical Experience
In our clinical work, we have used these new biodegradable interference screws made of degradable copolymers composed of L-lactic acid, D-lactic acid, and TMC (Inion Hexalon) for ACL reconstruction for more than 4 years. During this period, more than 400 of these screws have been inserted to patients, and only one screw breakage has occurred during screw insertion. In this particular case, the screwdriver broke first, which was the reason for the screw breakage. These screws can be used both with single-bundle and double-bundle technique when performing ACL reconstruction.28,29
Prospective Randomized Clinical Trial
We have done a prospective randomized clinical trial using either biodegradable screw or metallic screw in fixation of the ACL reconstruction with a hamstring autograft.29 In this study, 55 patients were randomized to either metallic interference screw (Timoni, Finland) (N = 26) or biodegradable screw fixation (Inion Hexalon) (N = 29) in ACL reconstruction with hamstring tendons. The evaluation methods were clinical examination, KT-1000 arthrometer (MEDmetric Corporation, San Diego, CA) measurements,30 radiographic evaluation, MRI, and International Knee Documentation Committee (IKDC)31 as well as Lysholm32 knee scores. There were no differences between the study groups preoperatively. For the minimum of 1-year follow-up (range 12–19 months), 23 patients of the metallic interference screw group and 26 patients of the biodegradable screw group were available (90%). The evaluation methods disclosed no statistical differences between the groups at the follow-up examinations. However, the results were significantly better at the follow-up than preoperatively, in both groups. Kaeding et al33 have reported similar results in their prospective randomized study comparing biodegradable and titanium interference screw in fixation of the bone–patellar tendon–bone autograft for the ACL reconstruction.
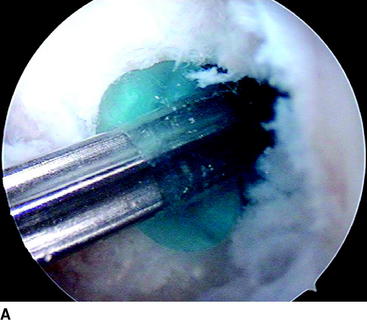
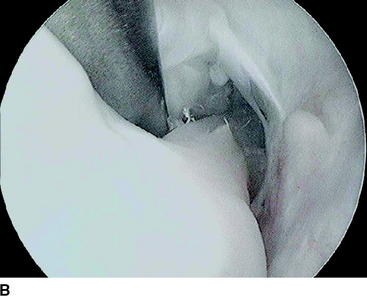
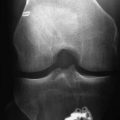
During the follow-up of our study, three revision ACL reconstructions had to be performed (two in the biodegradable screw group and one in the metallic screw group) because of new knee trauma. No other complications were found with these patients. The revision in the biodegradable screw group 8 months after the primary operation showed that the biodegradable screw was already soft. The other revision performed 18 months after primary surgery showed that the biodegradable screw was almost totally absorbed. The revision ACL reconstructions with these patients were easy to perform because we did not have to remove the screws at all. In the case in which the screw was soft but not totally absorbed yet, we simply drilled through it and created a 1 mm wider tunnel for the new graft. In addition, with another patient, the second-look arthroscopy showed that the biodegradable screw was totally absorbed 2 years after the primary operation (Fig. 52-2).
Magnetic Resonance Imaging
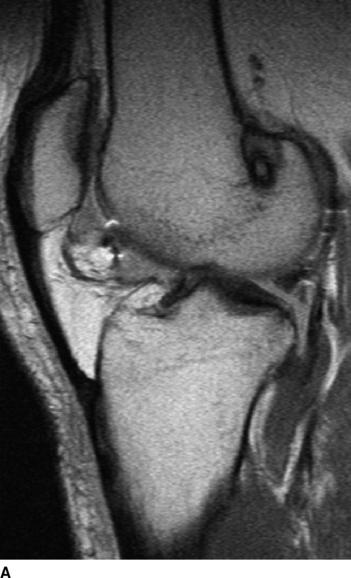
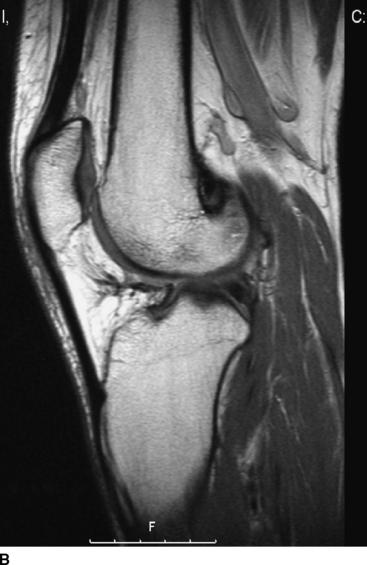
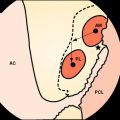
Sixteen patients (10 patients in the biodegradable screw group and six in the metallic screw group) of our prospective randomized study have been evaluated by MRI examination at a mean follow-up of 27 months (range 24–31 months). According to this evaluation, we have found that all the biodegradable screws (Inion Hexalon) were absorbed totally at the follow-up (Fig. 52-3). The MRI images appear to show that the bone tunnels are filled with fibrous tissue with signal intensity similar to that of the intraarticular ACL graft. However, because no histological analysis could be carried out in these human patients, no final conclusions regarding the tissue type that finally replaces the screw can be drawn at this point. The follow-up of these patients has been planned to continue for a minimum of 5 years postoperatively.
The fact that the screws used in our study had been absorbed in 2 years is contradictory to the finding in the previous studies of Ma et al14 and Radford et al.15 They found that the biodegradable screws they used did not absorb in even 2 to 4 years. The explanation for this difference seems logical: the materials of these screws are different. In our study, we used biodegradable screws made of copolymers composed of L-lactic acid, D-lactic acid, and TMC, whereas in the studies of Ma et al14 and Radford et al,15 PLLA interference screws were used.
However, two of our patients in the biodegradable screw group had some tunnel enlargement or cyst of the tunnel at the 2-year follow-up. One was in the tibial side, and another was in the femoral side (see Fig. 52-3, B). In these cases, the enlargement was only 2 to 3 mm when the width of the normal tunnel was compared. With all patients, the mean widths of the femoral and tibial tunnels were 10 mm (range 7–12 mm) and 10 mm (range 8–14 mm), respectively. No difference was found between the biodegradable and metallic screw groups. Previously in the literature, tunnel enlargement has been reported after using biodegradable fixation methods, as well as after using other fixation methods such as metallic screws and especially Endobutton fixation.14,33–35 However, the clinical importance of the tunnel enlargement still remains controversial. Theoretically, if the tunnel enlargement were large, it could be a problem when performing the revision ACL reconstruction. However, with our patients, the tunnel enlargement was so minimal that no problem would be expected later in the event that revision ACL surgery is needed. In fact, there were no difficulties in performing the revision ACL reconstruction with the two patients who underwent a revision ACL surgery in the biodegradable screw group of our study.
1 Colombet P, Allard M, Bousquet V, et al. Anterior cruciate ligament reconstruction using four-strand semitendinosus and gracilis tendon grafts and metal interference screw fixation. Arthroscopy. 2002;18:232-237.
2 Ejerhed L, Kartus J, Sernert N, et al. Patellar tendon or semitendinosus tendon autografts for anterior cruciate ligament reconstruction? A prospective randomized study with a two-year follow-up. Am J Sports Med. 2003;31:19-25.
3 Pinczewski LA, Deehan DJ, Salmon LJ, et al. A five-year comparison of patellar tendon versus four-strand hamstring tendon autograft for arthroscopic reconstruction of the anterior cruciate ligament. Am J Sports Med. 2002;30:523-536.
4 Scranton PE, Bagenstose JE, Lantz BA, et al. Quadruple hamstring anterior cruciate ligament reconstruction: a multicenter study. Arthroscopy. 2002;18:715-724.
5 Shaieb MD, Kan DM, Chang SK, et al. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:214-220.
6 Kousa P, Järvinen TLN, Vihavainen M, et al. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31:174-181.
7 Kousa P, Järvinen TLN, Vihavainen M, et al. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31:182-188.
8 Costi JJ, Kelly AJ, Hearn TC, et al. Comparison of torsional strengths of bioabsorbable screws for anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29:575-580.
9 Smith CA, Tennent TD, Pearson SE, et al. Fracture of Bilok interference screws on insertion during anterior cruciate ligament reconstruction. Case report. Arthroscopy. 2003;19:E74.
10 Andriano KP, Pohjonen T, Tormala P. Processing and characterization of absorbable polylactide polymers for use in surgical implants. J Appl Biomater. 1994;5:133-140.
11 Böstman OM, Pihlajamäki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop. 2000;371:216-227.
12 Bergsma JE, de Bruijn WC, Rozema FR, et al. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials. 1995;16:25-31.
13 Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21:2615-2621.
14 Ma CB, Francis K, Towers J, et al. Hamstring anterior cruciate ligament reconstruction: a comparison of bioabsorbable interference screw and Endobutton-post fixation. Arthroscopy. 2004;20:122-128.
15 Radford MJ, Noakes J, Read J, et al. The natural history of a bioabsorbable interference screw used for anterior cruciate ligament reconstruction with a 4-strand hamstring technique. Arthroscopy. 2005;21:707-710.
16 Fink C, Benedetto KP, Hackl W, et al. Bioabsorbable polyglyconate interference screw fixation in anterior cruciate ligament reconstruction: a prospective computed tomography-controlled study. Arthroscopy. 2000;16:491-498.
17 Lajtai G, Noszian I, Humer K, et al. Serial magnetic resonance imaging evaluation of operative site after fixation of patellar tendon graft with bioabsorbable interference screws in anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:709-718.
18 Lajtai G, Schmiedhuber G, Unger F, et al. Bone tunnel remodeling at the site of biodegradable interference screws used for anterior cruciate ligament reconstruction: 5-year follow-up. Arthroscopy. 2001;17:597-602.
19 Morgan CD, Gehrmann RM, Jayo MJ, et al. Histologic findings with a bioabsorbable anterior cruciate ligament interference screw explant after 2.5 years in vivo. Case report. Arthroscopy. 2002;18:E47.
20 Weiler A, Hoffmann RFG, Bail HJ, et al. Tendon healing in a bone tunnel. Part II: histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:124-135.
21 Tecklenburg K, Burkart P, Hoser C, et al. Prospective evaluation of patellar tendon graft fixation in anterior cruciate ligament reconstruction comparing composite bioabsorbable and allograft interference screws. Arthroscopy. 2006;22:993-999.
22 Nieminen T, Rantala I, Hiidenheimo I, et al. Degradative and mechanical properties of a novel resorbable plating system during a 3-year follow-up in vivo and in vitro. J Mater Sci Mater Med. 18, 2007.
23 Nurmi JT, Suuriniemi N. The fixation strength of the biodegradable Hexalon interference screw. The 12th Congress of European Society of Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA), Innsbruck, Austria. 2006;234 Book of Abstracts [poster]
24 Ishibashi Y, Rudy TW, Livesay GA, et al. The effect of anterior cruciate ligament graft fixation site at the tibia on knee stability: evaluation using a robotic testing system. Arthroscopy. 1997;13:177-182.
25 Harding N, Barber FA, Herbert MA. The effect of the EndoPearl on soft-tissue graft fixation. J Knee Surg. 2002;15:150-154.
26 Nurmi JT, Ahvenjärvi P, Suuriniemi N. Torsional strength of a new biodegradable interference screw. The 12th Congress of European Society of Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA), Innsbruck, Austria. 2006;233. Book of Abstracts: [poster]
27 Väänänen P, Nurmi JT. The biomechanical properties of a biodegradable 6-mm interference screw. Podium presentation at the European Conference on Biomaterials (ESB 2006), Nantes, France. 2006.
28 Järvelä T. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective, randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2007;15:500-507.
29 Järvelä T, Järvinen M. Anterior cruciate ligament reconstruction with a hamstring graft: prospective, randomized clinical study using metallic or bioabsorbable screw in fixation. Podium presentation at the 12th Congress of European Society of Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA), Innsbruck, Austria. 2006;55. Book of Abstracts
30 Daniel DM, Malcom LL, Losse G, et al. Instrumented measurement of anterior laxity of the knee. J Bone Joint Surg. 1985;67A:720-726.
31 Hefti F, Drobny T, Hackenbusch W, et al. Evaluation of knee ligament injuries: the OAK and IKDC forms. In: Jakob RP, Staubli H-U, editors. The knee and the cruciate ligament. Berlin: Springer; 1990:134-139.
32 Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of scoring scale. Am J Sports Med. 1982;10:150-154.
33 Kaeding C, Farr J, Kavanaugh T, et al. A prospective randomized comparison of bioabsorbable and titanium anterior cruciate ligament interference screw. Arthroscopy. 2005;21:147-151.
34 Buelow JU, Siebold R, Ellermann A. A new biocortical tibial fixation technique in anterior cruciate ligament reconstruction with quadruple hamstring graft. Knee Surg Sports Traumatol Arthrosc. 2000;8:218-225.
35 Jansson KA, Harilainen A, Sandelin J, et al. Bone tunnel enlargement after anterior cruciate ligament reconstruction with the hamstring autograft and Endobutton fixation technique. A clinical, radiographic and magnetic resonance imaging study with 2 years follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7:290-295.