Chapter 1 Importance of plants in modern pharmacy and medicine
Types of drugs derived from plants
Herbal drugs derived from specific parts of a medicinal plant
Botanical drugs which form the basis for herbal remedies or phytomedicines include, for example:
• the herb of St John’s wort (Hypericum perforatum), used in the treatment of mild to moderate depression
• the leaves of Ginkgo biloba, used for cognitive deficiencies (often in the elderly), including impairment of memory and affective symptoms such as anxiety
• the flower heads of chamomile (Chamomilla recutita), used for mild gastrointestinal complaints and as an anti-inflammatory agent
• the leaves and pods of senna (Cassia spp.), used for constipation.
Natural products or compounds isolated from nature
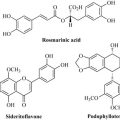
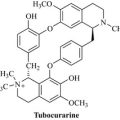
• morphine, from opium poppy (Papaver somniferum), used as an analgesic
• digoxin and other digitalis glycosides, from foxglove (Digitalis spp.), used to treat heart failure
• taxol, from the Pacific yew (Taxus brevifolia), used as an anticancer treatment
• quinine, from Cinchona bark (Cinchona spp.), used in the treatment of malaria
• galanthamine from Galanthus and Leucojum species, used in the management of cognitive disorders.
Use of herbal medicines
The use of these remedies is extensive, increasing and complex. In several surveys 20–33% of the UK population claimed to regularly use CAM alone or in addition to orthodox or conventional medicine and treatments. In the UK, usage is particularly frequent amongst those who are over-the-counter medicines-users. There is not, on the whole, a wide understanding of what herbal medicines are (or are not) (IPSOS-MORI 2008). Healthcare professionals and students also commonly use such products. Forty-three percent of students at a University School of Pharmacy reported using at least one type of CAM during the last 12 months (Freymann et al 2006).
In the United States, approximately 38% of adults and approximately 12% of children are using some form of CAM (NIH/NCCAM). Kennedy et al (2008) showed that in the preceding 12 months about 38 million adults in the US (18.9% of the population) used herbal medicines or supplements, but that only one-third revealed this use to their physician. Data for other regions are even more limited, but the usage of herbal medicines is widespread in countries like India, Indonesia, Australia and China, to name just a few.
In developed countries, most purchases of HMPs are made on a self-selection basis from pharmacies and health-food stores, as well as from supermarkets, by mail order and via the Internet. Normally, with the exception of pharmacists, there is no requirement for a trained healthcare professional to be available on the premises to provide information and advice. In any case, most HMPs can be sold or supplied without the involvement of a healthcare professional and several studies have confirmed that many individuals do not seek professional advice before purchasing or using such products, even when purchased from a pharmacy (Barnes et al 1998, Gulian et al 2002). Rather, consumers of HMPs tend to rely on their own (usually limited) knowledge, or are guided by advice from friends and relatives or the popular media. Consumers who do seek professional advice (e.g. from their pharmacist or general practitioner) may find that he or she is not able to answer their question(s) fully. In some cases this may be because the information simply is not available, but it is also recognized that, at present, many healthcare professionals are not adequately informed about herbal medicines, particularly with regard to their quality, safety and efficacy. This book attempts to redress that omission.
HMPs are used for general health maintenance, as well as for treating diseases, including serious conditions such as cancer, HIV/AIDS, multiple sclerosis, asthma, rheumatoid arthritis and osteoarthritis. Older patients, pregnant and breastfeeding women, and children also take HMPs, and this raises concerns because, as with conventional medicines, precautions need to be taken. For example, few medicines (whether conventional or herbal) have been established as safe for use during pregnancy and it is generally accepted that no medicine should be taken during pregnancy unless the benefit to the mother or fetus outweighs any possible risk to the fetus. Similarly, HMPs should be used with caution in children and the elderly, who, as with conventional medicines, differ from adults in their response to, and metabolism and clearance of drugs. The use of herbal medicines by patients who are already taking prescribed medicines is of particular concern as there is the potential for drug–herb interactions to occur. For example, important pharmacokinetic and pharmacodynamic interactions between St John’s wort (Hypericum perforatum) and certain conventional medicines have been documented (Williamson et al 2009) and mechanisms for such interactions have been identified. Generally, information on interactions between HMPs and conventional medicines is limited, although potential drug–herb interactions can sometimes be identified based on the known phytochemistry and pharmacological properties of the herbs involved.
Some fundamental aspects of the regulation of herbal medicines
United kingdom
Herbal products are available on the UK market as:
• traditional herbal medical products registered under the European ‘Traditional Herbal Medicinal Product Directive’ (THMPD)
• herbal medicines exempt from licensing
• unlicensed herbal products, sold as food or dietary supplements
• prescription-only medicines (POM); these potentially hazardous plants may only be dispensed by order of a prescription by a registered doctor
• pharmacy-only medicines (P); certain others may only be supplied by a registered pharmacist, or may be subject to dose (but not duration of treatment) and route of administration restrictions.
Traditional herbal medical products
The THMPD 2004/24/EC (see http://www.mhra.gov.uk) is a regulatory process established to provide a mechanism whereby manufacturers of good-quality herbal medicines can register their products as medicinal products and make (restricted) medicinal claims on the packaging and the patient information leaflet (PIL). Examples of the types of information that may be displayed are given here:
• Evidence that a corresponding herbal product (i.e. one derived from the same botanical drug and prepared in a similar way) has been used traditionally for at least 30 years (15 years’ non-EU and 15 years in the EU, or more than 30 years in the EU).
• Bibliographic data on safety with an expert report.
• A quality dossier specifying how the company complies with the quality guidance requirements of the regulators.
• Details on the patient information leaflet (PIL), packaging, naming and labelling.
Australia
In Australia, Western herbal medicine is one of the most popular forms of CAM and a range of ethnic medicines, especially TCM, are increasingly becoming popular. All medicines, including herbal and other complementary medicines, are covered by the Therapeutic Goods Act (1989) and regulated by a federal agency, the Therapeutic Goods Administration (TGA, http://www.tga.gov.au/). A statutory expert committee, the Advisory Committee on Complementary Medicines (formerly the Complementary Medicines Evaluation Committee) provides the TGA with advice on the regulation of complementary medicines. The Australian regulatory guidelines for complementary medicines ( http://www.tga.gov.au/docs/html/argcm.htm) provide information to help producers and distributors of complementary medicines to meet their obligations under therapeutic goods legislation.
Canada
Similar to the UK, an Adverse Reaction Reporting System for natural health products is in place and is used to warn the public (http://www.hc-sc.gc.ca/dhp-mps/prodnatur/about-apropos/index-eng.php).
India
India has an ancient heritage of traditional medicine (see Chapter 12) with a well-recorded and widely practised knowledge of herbal medicine. The Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy (AYUSH) within the Ministry of Health & Family Welfare focuses on its regulation and on the improvement of standards in the areas of quality control and standardization of drugs, the availability of raw material, research and development, education/training of professionals, and a wider outreach with regard to these traditional medical systems.
The Traditional Herbal Medicines Act, 2006 regulates the sale of the traditional herbal medicines which are marketed without any licence and control on the basis of being from ancient texts. According to the Act every retailer or seller of traditional herbal medicines needs to have a licence to sell traditional herbal medicines from the Authority. Every manufacturer of traditional herbal medicine needs to work under the principles of GMP and has to list the ingredients of each medicine on the packing of the medicine along with their accurate quantity. Side effects and warning of contraindications need to be stated on the package. Pharmacopoeia Committees have been established to develop quality standards for the main groups of therapeutically relevant drugs of Ayurveda, Unani, Siddha and homoeopathy (Mukherjee et al 2007).
The Indian Government also established an independent body – the ‘National Medicinal Plants Board’ under the Ministry of Health and Family Welfare. It is responsible for co-ordinating all matters relating to the development of medicinal plants, including policies and strategies for conservation, proper harvesting, cost-effective cultivation and marketing of raw material in order to protect, sustain and develop this sector. Uniquely, the Indian government has established programmes for the documentation of traditional Indian knowledge, which is already available in public domain. The political goal is to safeguard the sovereignty of this traditional knowledge and to protect it from being misused in patenting on non-patentable inventions. The Traditional Knowledge Digital Library (TKDL) is an original proprietary database, which is fully protected under national and international laws of Intellectual Property Rights and is maintained and developed by the government. TKDL also allows automatic conversion of information from Sanskrit into various languages. The information includes names of plants, Ayurvedic description of diseases under their modern names and therapeutic formulations (Mukherjee et al 2007).
United states of america (USA)
In the USA, herbal medicines are generally regulated as ‘dietary supplements’. The US Food and Drug Administration (FDA, www.fda.gov/Food/DietarySupplements/) is in charge of these comparatively loose regulations (http://nccam.nih.gov/health/supplements/wiseuse.htm). Some key characteristics stand out:
• Prior to marketing, dietary supplements do not have to be assessed for safety and effectiveness. Limited therapeutic claims may be made, e.g. that a dietary supplement addresses a nutrient deficiency, supports health, or is linked to a particular body function (e.g. immunity). However, this requires supportive prior research. Such a claim must be followed by the words ‘This statement has not been evaluated by the U.S. Food and Drug Administration (FDA). This product is not intended to diagnose, treat, cure, or prevent any disease.’
• Since 2008 ‘Good manufacturing practices’ (GMPs) is expected in order to ensure that dietary supplements are processed consistently and meet quality standards.
• Once a dietary supplement is on the market, the FDA monitors the claims made and the product’s safety. If inappropriate claims are made the manufacturer receives a warning letter or is required to remove the product from the marketplace. If a product is found to be unsafe, the FDA takes similar action against the manufacturer and/or distributor.
Barnes J., Mills S.Y., Abbot N.C., et al. Different standards for reporting ADRs to herbal remedies and conventional OTC medicines: face-to-face interviews with 515 users of herbal remedies. Br. J. Clin. Pharmacol.. 1998;45:496-500.
Freymann H., Rennie T., Bates I., Nebel S., Heinrich M. Knowledge and use of herbal medical products and other CAM among British undergraduate pharmacy students. Pharm. World Sci.. 2006;27:13-18.
Gulian C., Barnes J., Francis S.A., et al. Types and preferred sources of information concerning herbal medicinal products: face-to-face interviews with users of herbal medicinal products. International Journal of Pharmaceutical Practice. 2002;10(Suppl.):R33.
IPSOS – Mori. Public perceptions of herbal medicines general public qualitative & quantitative research. London, UK: IPSOS-Mori; 2008.
Kennedy J., Wang C.C., Wu C.H. Patient disclosure about herb and supplement use among adults in the US. Evid. Based Complement. Alternat. Med.. 2008;5:451-456.
Williamson E.M., Driver S., Baxter K., editors. Stockley’s herbal medicines. London: Interactions Pharmaceutical Press, 2009.
Barnes J., Phillipson J.D., Anderson L.A. Herbal medicines. A guide for healthcare professionals, third ed. London: Pharmaceutical Press; 2009.
Bruneton J. Pharmacognosy, phytochemistry, medicinal plants. Berlin: Springer-Verlag; 1995.
Cavaliere C., Rea P., Lynch M.E., Blumenthal M. Herbal supplement sales rise in all channels in 2009. HerbalGram. 2010;86:82-85.
Evans W.C. Trease and Evans’s pharmacognosy, sixteenth ed. London: WB Saunders; 2009.
Hänsel R., Sticher O., editors. Pharmakognosie – phytopharmazie. Berlin: Springer, 2010.
McEwen J. What does TGA approval of medicines mean? Australian Prescriber. 2004;27:156-158.
Mills S., Bone K. Phytotherapy. Principles and practice. London: Churchill Livingstone; 2000.
Mukherjee P.K., Venkatesh M., Kumar V. An overview on the development in regulation and control of medicinal and aromatic plants in the Indian system of medicine. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas (BLACPMA). 2007;6:129-136.
Robbers J.E., Speedie M.K., Tyler V.E. Pharmacognosy and pharmacobiotechnology. Baltimore: Williams & Wilkins; 1996.
Ross I. Medicinal plants of the world. Totawa, NJ: Humana Press, 1999;vol. I.
Ross I. Medicinal plants of the world. Totawa, NJ: Humana Press, 2000;vol. II.
Schulz V., Haensel R., Tyler V. Rational phytotherapy. Berlin: Springer-Verlag; 1998.
Thomas K.J., Nicholl J.P., Coleman P. Use and expenditure on complementary medicine in England: a population based survey. Complement Ther. Med.. 2001;9:2-11.
Williamson E. Potter’s herbal cyclopedia. Saffron Walden: CW Daniels; 2003.