Implantable Pacemakers
History of Pacing
Since the first epicardial pacing system was implanted in 1958, pacemaker technology has evolved rapidly. Sophistication of sensing circuitry led to the introduction of single-chamber demand pacing systems in 1963. Although atrial-synchronous systems and dual-chamber systems were described in the 1950s, clinical use of such devices did not occur for many years. In the 1970s, lithium batteries and programmability were introduced. Milestones of the 1980s include greater acceptance of dual-chamber pacing systems and the introduction of rate-adaptive pacing systems. The 1990s and the 2000s witnessed the introduction of advanced sensor technology, new pacemaker algorithms, and enhanced automaticity of many programmable features. The use of pacing has increased significantly from 46.7/100,000 in 1993 to 61.6/100,000 in 2009, with the percentage of dual chamber devices increasing from 62% to 82% during this period.1
Pacemaker Nomenclature
Since the first introduction of a three-letter code describing basic pacemaker functions in 1974, the code has been updated periodically by a committee comprising members of the North American Society of Pacing and Electrophysiology (NASPE) and the British Pacing and Electrophysiology Group (BPEG). The most recent code consists of five letters (Box 117-1). The first position indicates the chamber or chambers that are stimulated: A (atrium), V (ventricle), or D (dual chamber, both A and V). The second position indicates the chamber or chambers in which sensing occurs: A (atrium), V (ventricle), or D (dual chamber, both A and V). The third position indicates the function: I (inhibition), T (triggered), or D (a dual function of atrial tracking and ventricular inhibition). The fourth position of the code indicates that rate modulation is present by the letter R. Rate modulation is the use of a sensor to meet the patient’s metabolic demands, independent of intrinsic cardiac activity. The fifth position indicates whether multisite pacing is present: A (atrium), V (ventricle), or D (both A and V). Multisite pacing is defined as more than one stimulation site in any single chamber. In any of the positions, O indicates that pacing, sensing, or a function is not present.
Indications for Cardiac Pacing
Criteria established by a joint committee of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society have categorized indications for pacing as class I, generally indicated; class II, possibly indicated; and class III, not indicated.2 Class II has been divided into class IIa for recommendations for which there is general agreement and class IIb for which there is some disagreement. The evidence supporting the recommendations are ranked. The weight of evidence is ranked A if there are multiple randomized trials involving a large number of subjects and B if data were derived from a limited number of trials involving a relatively small number of subjects. The weight of evidence is ranked C if expert consensus is the primary source of the recommendation.2 Although some indications for permanent pacing are relatively certain or unambiguous, others require considerable expertise and judgment. The clinician prescribing permanent pacing systems should be aware of the indications and controversies regarding indications.
Acquired Atrioventricular Block
Acquired atrioventricular (AV) block is most commonly idiopathic and related to aging, but the potential causes are many. Class I indications for permanent pacing in patients with acquired AV block include any AV block with associated symptoms, as well as AV block after AV node ablation or persistent AV block after cardiac surgery. For some patients, heart failure is a manifestation of AV block. In addition, some patients require medications that can cause symptomatic bradycardia and might need pacing. For postoperative AV block, the guidelines do not specify a period to wait postoperatively for recovery of conduction. Class I indications also include acquired AV block below the level of the AV node or associated with marked pauses, such as longer than 3 seconds, or a ventricular escape rate less than 40 beats/min. Because the cardiovascular risk is believed to be particularly high in patients with second- or third-degree block and specific underlying conditions, permanent pacing is indicated in these patients even in the absence of symptoms. These conditions include neuromuscular diseases such as myotonic dystrophy, Kearns-Sayre syndrome, peroneal muscular atrophy, and Erb limb girdle dystrophy. Myotonic dystrophy has been associated with an increased risk of sudden death believed to be caused by progressive conduction system disease.3
Exercise-related AV block is also an indication for pacing. Advanced second-degree AV block or alternating bundle branch block in the setting of bifascicular block is considered a class I indication. Asymptomatic type II second-degree AV block with a wide QRS is a class I indication. Transient, infranodal, high-grade AV block and associated bundle branch block is a class I indication.2
Class IIa indications include asymptomatic type II second-degree AV block with a narrow QRS complex, and asymptomatic second-degree AV block at intra-His or infra-His levels based on electrophysiological testing.2 First-degree or second-degree AV block with hemodynamic compromise is also considered a class IIa indication, because some patients develop pacemaker-like syndrome as a result of conduction delay. In patients with bifascicular block, asymptomatic severe prolongation of the His-ventricle interval (>100 ms), asymptomatic pacing-induced infra-His block that is not physiological, or syncope when other causes such as ventricular tachycardia have been excluded are considered class IIa indications for pacing.
First-degree AV block in patients with neuromuscular disorders is considered a class IIb indication because the risk of progression to AV block is high. Another class IIb indication includes AV block that might have occurred because of drug use or toxicity, but there is a risk of recurrence of AV block. First-degree AV block in a patient with left ventricular dysfunction and congestive heart failure in whom hemodynamic improvement with AV interval optimization can be demonstrated is a class IIb indication.2 Asymptomatic and persistent second- or third-degree block at the AV node level is a class IIb indication.2
Congenital Complete Heart Block
Symptomatic congenital complete AV block remains a class I indication for pacing in pediatric patients.2 In addition, in pediatric patients with congenital complete AV block, the presence of a wide QRS escape rhythm, ventricular dysfunction, or complex ventricular ectopy is also a class I indication. In pediatric patients, an average heart rate less than 50 beats/min, pauses two to three times the basic cycle length, or symptoms associated with chronotropic incompetence are considered class IIa indications. In pediatric and adult patients with adequate rate, narrow QRS complex, and normal ventricular function, pacing is a class IIb indication.
For adult patients with congenital complete AV block, the timing and indications for permanent pacing are more controversial and continue to evolve.4 As a result of data regarding the high incidence of unexpected syncope in adult patients with congenital complete AV block, prophylactic pacemaker implantation is often considered.2,4
Sinus Node Dysfunction
Sinus node dysfunction may be manifested by abrupt sinus pauses or gradual sinus slowing. Symptomatic chronotropic incompetence and symptomatic sinus bradycardia occurring spontaneously or as the result of drug therapy are considered class I indications for pacing. In patients with minimal or no symptoms and chronic heart rates less than 40 beats/min, pacing is a class IIb indication.2
Neurocardiogenic Syncope
Neurocardiogenic syncope typically has both cardioinhibitory and vasodepressor components. Pacing during most episodes of neurocardiogenic syncope is still associated with a significant fall in blood pressure and symptoms because of the continued vasodepressor response. Therefore, even in the presence of significant bradycardia, pacing is usually not considered first-line therapy. A number of randomized trials have failed to show a substantial benefit in increasing the freedom from recurrent syncope.5,6 As a result, neurocardiogenic syncope with documented bradycardia is considered a class IIb indication for pacing; however, there are some recent studies that indicate a selective use of pacing.7,8 In the Third International Study on Syncope of Uncertain Etiology (ISSUE-3) trial, in patients 40 years or older with at least three syncope episodes in 2 years, and with documentation of syncope with 3 seconds or more of asystole or 6 seconds or more of asystole without syncope, 77 patients were randomized to dual-chamber pacing on or off with an algorithm to increase the pacing rate with a sudden decrease in heart rate. The syncope recurrence rate was 57% in the “pacing off” group and 25% in the “pacing on” group (P = 0.039).7
When pacing is performed in this setting, dual-chamber pacing is necessary to preserve the atrial contribution to cardiac output. Modifications to the pacing strategy are being studied to make dual chamber pacing more effective in ameliorating neurocardiogenic syncope. The heart rate can be adjusted according to measured changes in impedance to estimate myocardial contractility, using so-called closed loop stimulation. Although retrospective data suggest that this approach may reduce the frequency of recurrence of syncope, additional prospective studies are needed.8
In patients with syncope of unknown origin, a randomized, multicenter study examined patients exhibiting a pause of 10 seconds or more when given an intravenous bolus of 20 mg adenosine triphosphate. These patients were randomized to atrial inhibited (AAI; 30 beats/min) or DDD pacing (70 beats/min). In the DDD pacing group, 21% of patients had recurrence of syncope compared to 66% of patients in the AAI group. Of note, the mean age in these studies was 76 years of age.9
Carotid Sinus Hypersensitivity
Carotid sinus hypersensitivity has been shown to be a cause of syncope, particularly in the elderly. However, because an abnormal response can occur even in asymptomatic persons, caution must be used when spontaneous bradycardia has not been demonstrated. In cases in which syncope has occurred during carotid sinus stimulation and carotid sinus pressure has resulted in pauses of 3 seconds or more, a class I indication is present. If such an abnormal carotid sinus pressure response is obtained but syncope did not occur in circumstances suggesting carotid sinus stimulation, a class IIa indication is present. In addition, there may be a role for pacing in patients with unexplained falls and evidence of carotid sinus hypersensitivity. Recent studies do not show a difference in the recurrence of events in patients pacing on treatment using ventricular inhibited (VVI), DDDR, or DDDR with sudden bradycardia response.10
Pacing for Atrial Fibrillation
Numerous studies have examined various pacing algorithms to prevent atrial fibrillation. Most of these algorithms involve increasing the pacing rate to suppress atrial ectopy that is believed to trigger atrial fibrillation. Whereas most randomized studies have not demonstrated a beneficial effect, some studies have suggested a modest decrease in the occurrence of atrial fibrillation. In the Study of Atrial Fibrillation (SAFARI) trial, patients with AF recorded were randomized to atrial fibrillation pacing prevention strategies. Overall, there was a small reduction in the occurrence of atrial fibrillation. In patients with a high burden of atrial fibrillation, the reduction in atrial fibrillation was greater.11 Pacing for atrial fibrillation reduction is a class III indication.
Resynchronization Therapy
Numerous randomized, controlled trials have demonstrated benefit of cardiac resynchronization therapy in improving symptoms and outcomes in patients with drug-refractory heart failure. Cardiac resynchronization therapy is the pacing of the left and right ventricles to improve the hemodynamics that are impaired because of bundle branch block. These randomized studies have shown improvement in New York Heart Association classes, 6-minute walk time, oxygen consumption, brain natriuretic peptide levels, neurohormonal levels, ejection fraction, end-diastolic and end-systolic dimension, heart failure hospitalizations, and all-cause mortality.12 The current indications for resynchronization therapy (class I) include QRS duration of 120 ms or greater, left ventricular ejection fraction of 35% or less, sinus rhythm, and class III or ambulatory class IV heart failure symptoms on optimal medical therapy. Because the largest randomized trial excluded patients with atrial fibrillation, the presence of atrial fibrillation results in classification as a class IIa indication.2,12
Hypertrophic Cardiomyopathy
Early studies suggested that right ventricular apical pacing resulted in a significant reduction in outflow tract gradient and ameliorated symptoms. However, a subsequent randomized multicenter trial failed to demonstrate benefit in patients with pacing. A subset of elderly patients might have exhibited some improvement. An analysis from the Cochrane Database concluded that clinical trial data are inconclusive regarding an effect of pacing on outcome in this patient population.13 Therefore, pacing in medically refractory symptomatic patients with significant resting or provoked gradient owing to hypertrophic cardiomyopathy is a class IIb indication.2 These patients should be considered for a dual chamber ICD because of the risk of sudden death.
Basic Pacemaker Function and Modes
Ventricular Inhibited Pacing
In the ventricular inhibited (VVI) pacing mode, pacemaker output is inhibited by a sensed ventricular event (Figure 117-1). The lower rate interval determines the longest interval between any sensed or paced ventricular events. The interval from the previous sensed or paced ventricular event to the subsequent paced ventricular event is the lower rate interval. If the time interval from the last sensed ventricular event to the first paced ventricular beat is longer than the time interval between ventricular paced beats, ventricular hysteresis is present. Any ventricular event occurring after a paced or sensed ventricular event within the ventricular refractory period is not sensed and does not reset the timing cycle.
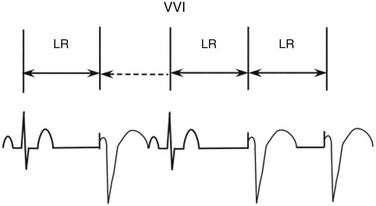
Figure 117-1 In the ventricular inhibited (VVI) pacing, the lower rate interval (LR) is the time from the last intrinsic or paced ventricular event to a ventricular paced event. When the LR interval has been completed, a ventricular paced event will occur. If an intrinsic beat occurs before the LR is completed, the ventricular output will be inhibited. The first QRS complex is intrinsic, and after the LR interval is completed a second intrinsic beat has not occurred; therefore, the second QRS complex is a paced ventricular event. The third QRS complex is again intrinsic. Following the third QRS complex, intrinsic QRS complexes do not occur within the LR interval. Thus, the fourth and fifth QRS complexes are paced. The double-headed arrows indicate a full LR interval has transpired. The dotted single-headed arrow indicates that the full LR interval has not been completed and an intrinsic QRS complex inhibits ventricular pacing.
Atrial Inhibited Pacing
Atrial inhibited (AAI) pacing incorporates the same timing cycles as VVI pacing, with the obvious difference that pacing and sensing occur in the atrium, and pacemaker output is inhibited by a sensed atrial event (Figure 117-2). An atrial paced or sensed event initiates a refractory period during which atrial events do not reset the timing cycle. Confusion can arise when multiple ventricular events occur during atrial pacing. For example, when the atrial timing cycle ends, an atrial pacing stimulus is delivered regardless of ventricular events, because an AAI pacemaker does not sense in the ventricle. If the ventricular signal is inappropriately sensed by the atrial lead (far-field sensing), the atrial timing cycle is reset. This abnormality can sometimes be corrected by making the atrial channel less sensitive or by lengthening the atrial refractory period so that a conducted ventricular complex is not sensed.
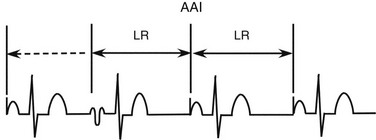
Figure 117-2 In atrial inhibited (AAI) pacing, the lower rate interval (LR) is the time from the last intrinsic or paced atrial event to an atrial paced event. When the LR interval has been completed, an atrial paced event will occur. If an intrinsic beat occurs before the LR interval is completed, the atrial output will be inhibited. The first atrial complex is paced. The second atrial complex occurs before the LR is completed. A third atrial complex does not occur spontaneously and instead, after the LR interval has been completed, an atrial paced beat occurs. The fourth atrial complex is also paced and occurs after another LR interval. The double-headed arrows indicate that a full LR interval has transpired. The dotted single-headed arrow indicates that the full LR interval has not been completed, and an intrinsic QRS complex inhibits ventricular pacing.
DDD Pacing
Four different rhythms can be seen as a result of normal DDD function: normal sinus rhythm, atrial pacing, AV sequential pacing, and P-synchronous pacing. In the DDD mode, the lower rate limit determines the lowest rate at which the atrial and ventricular events will occur (Figure 117-3). If the intrinsic atrial rate is too slow, an atrial paced event will occur. After a paced or intrinsic atrial event, if there is no ventricular event, a paced ventricular event occurs, at the programmed interval called the AV delay or interval. If an intrinsic atrial event occurs before the programmed lower rate, the atrial event will be followed by a ventricular paced event, a function called atrial tracking. This function permits the ventricular pacing to follow the atrial rate as the metabolic demands change. There is a maximal tracking rate to prevent the tracking of atrial arrhythmias and extraneous signals at excessively rapid rates. In addition, if the P wave occurs too early after the previous ventricular event, it will occur in the postventricular atrial refractory period (PVARP) and will not be tracked. The atrial rate at which two atrial sensed events will be followed by one ventricular paced event is equal to the total atrial refractory period, which is the sum of the programmed AV delay and the PVARP.
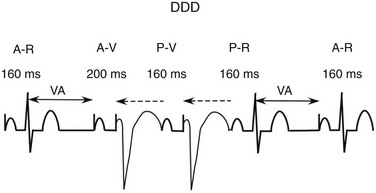
Figure 117-3 The DDD timing cycle consists of a lower rate limit interval, an atrioventricular (AV) interval, a postventricular atrial refractory period (PVARP), and an upper rate limit. In the figure, the first complex consists of an atrial paced event and an intrinsic QRS complex. Following the sensed ventricular event, a ventriculoatrial (VA) interval is created to indicate the time of the next atrial paced event if no intrinsic atrial event (after the postventricular atrial refractory period) and no intrinsic ventricular event occur. The VA interval equals the lower rate interval minus the programmed AV interval. A completed VA interval is indicated by the double-headed solid arrow. In the second complex, after the atrial paced event there is no intrinsic QRS complex within the AV interval of 200 ms; therefore, a paced ventricular event occurs. If an intrinsic atrial event occurs before the VA interval is completed but after the postventricular atrial refractory period, the atrial event will be tracked or followed by a paced ventricular event (the third QRS complex). As long as the V-V interval does not occur before the upper rate limit interval is complete, the AV interval will occur after the tracked atrial event. Following the third QRS complex, an intrinsic atrial event occurs before the VA interval is complete, but an intrinsic QRS complex occurs before the AV interval is finished. Following the fourth QRS complex, the VA interval is completed and another atrial paced event occurs. The dotted single-headed arrow indicates that the full LR interval has not been completed and an intrinsic QRS complex inhibits ventricular pacing.
Selecting the Appropriate Pacing Mode
Selection Criteria
In selecting the optimal pacing mode, the patient’s overall physical condition, associated medical problems, exercise capacity, and chronotropic response to exercise must be considered, along with the underlying rhythm disturbance.14
VVIR pacing is indicated for patients with chronic atrial fibrillation and slow ventricular response. Although VVI pacing protects the patient from lethal bradycardia, its limitations include its inability to restore or to maintain AV synchrony and to provide rate responsiveness in the chronotropically incompetent patient. In addition, some patients with VVI pacing experience symptomatic hemodynamic deterioration with ventricular pacing. Adverse hemodynamics resulting from pacing are known as pacemaker syndrome if the normal coordination of atrial filling and ventricular emptying is absent because of VA conduction or long AV conduction times. The systolic blood pressure might decline during ventricular pacing with VA conduction (Figure 117-4). The incidence of pacemaker syndrome depends on the definition being used, and its incidence has been estimated to be 7% to 10% of patients with VVI or VVIR pacing. The most common symptoms reported were shortness of breath, dizziness, fatigue, pulsations in the neck or abdomen, cough, and apprehension.
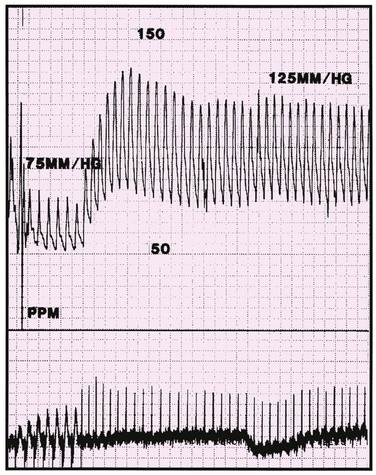
Figure 117-4 Hemodynamic tracing of a patient with pacemaker syndrome. The initial portion of the tracing shows ventricular pacing with a systolic arterial pressure of approximately 75 mm Hg. The patient’s intrinsic sinus rhythm inhibits ventricular pacing, and the arterial systolic pressure increases to approximately 125 mm Hg. (From Hayes DL, Holmes DR Jr: Hemodynamics of cardiac pacing. In Furman S, Hayes DL, Holmes DR Jr, editors: A practice of cardiac pacing, ed 3, Mount Kisco, N.Y., 1993, Futura Publishing, pp 195–218, with permission from Blackwell Publishing.)
Effect of Pacing Mode on Morbidity and Mortality
Several large, randomized, controlled trials have demonstrated benefits of physiological AAIR or DDDR versus VVIR. Andersen et al. published prospective data on pacing mode and survival with the random assignment of 225 patients with sinus node dysfunction to AAI or VVI pacing.14a The study demonstrated a lower incidence of atrial fibrillation and thromboembolism in the AAI group than in the VVI group. The Canadian Trial of Physiologic Pacing (CTOPP) randomly assigned 2568 patients to physiologic pacing (dual-chamber or AAIR) or ventricular pacing (VVI). No difference in mortality rate or quality of life was demonstrated; however, there was an 18% relative risk reduction of atrial fibrillation.
Two additional trials have examined the outcomes of physiological pacing: Pacing Mode Selection in the Elderly and the Mode Selection Trial. The Pacing Mode Selection in the Elderly study failed to demonstrate any statistically significant benefit of physiological pacing over ventricular pacing. When the patients were separated by underlying rhythm disturbance—that is, sinus node dysfunction versus AV block—the sinus node dysfunction group had some quality-of-life improvements. In addition, there was a crossover of 26% of patients assigned to ventricular pacing because they were unable to tolerate ventricular pacing. The Mode Selection Trial, like the CTOPP, did not demonstrate any difference in mortality rates, but it did demonstrate a lower incidence of atrial fibrillation with physiological pacing, reduced signs and symptoms of heart failure, and a slightly improved quality of life. The study concluded that overall dual-chamber pacing offered significant improvement compared with ventricular pacing. Most recently, the DANPACE trial randomized 1415 patients to AAIR or DDR pacing. There was no significant difference in stroke, heart failure, death, and chronic atrial fibrillation, but there was a lower incidence of paroxysmal atrial fibrillation—28.4% in the AAIR group compared to 23.0% in the DDDR group.15
Selecting the Appropriate Sensor for Rate-Adaptive Pacing
A variety of sensors appropriate for rate-adaptive pacing have been developed and achieve excellent responses in heart rate to exertion and changes in metabolic demand.16
Right Ventricular Impedance-Based Sensor
Closed-loop stimulation measures impedance from the right ventricular unipolar pacing. The impedance measurements correlate with dP/dtmax, which is a surrogate for ventricular contractility and in turn is a reflection of autonomic activity. As a reflection of autonomic activity, this sensor has the potential to respond to nonexertional stimuli such as mental stress.17
Dual-Sensor Combinations
The perfect sensor would be resistant to all nonphysiological stimuli. A multisensor, rate-adaptive pacing system could improve specificity by having one sensor verify or crosscheck the other. The most widely used sensor combination is the accelerometer–minute ventilation combination or blended sensor. Using this combination, the accelerometer provides a rapid response to exertion, and the minute ventilation provides an excellent physiologic response, achieving a more normal physiological heart rate response.18 A dual sensor using a closed-loop sensor and an accelerometer could have the advantage of being able to result in increased heart rates with mental stress.19
Troubleshooting Electrocardiographic Abnormalities
Electrocardiographic abnormalities in patients with pacemakers can be categorized broadly into failure to capture, failure to output, oversensing, undersensing, and inappropriate rate change.20
Failure to capture indicates that a pacing artifact is present without subsequent cardiac depolarization (Figure 117-5, Box 117-3).
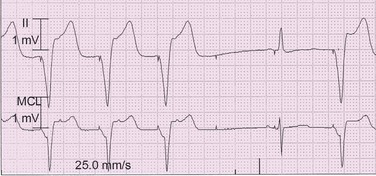
Figure 117-5 Electrocardiographic tracing demonstrating intermittent ventricular failure to capture. The first three ventricular pacing stimuli demonstrate ventricular capture. The fourth and the fifth ventricular pacing stimuli demonstrate failure to capture. The sixth ventricular stimulus results in ventricular capture. An intrinsic sinus beat occurs after the fifth ventricular pacing stimulus.
True failure to output is caused when the electrical signal cannot reach the heart from the pacemaker so that the electrical signal cannot reach the heart (see Box 117-3).
Oversensing is caused by the sensing of signals interpreted as the atria or ventricles deflections in their respective channels. Sensing of signals originating in the opposite chamber is called crosstalk. For example, the ventricular deflection can be sensed on the atrial channel (Figure 117-6). More seriously, the atrial stimulus may be detected on the ventricular channel, resulting in ventricular inhibition and asystole. Such an occurrence can be prevented by programming on safety pacing so that a ventricular stimulus is given if a ventricular event is sensed shortly after an atrial stimulus is delivered. The electrocardiographic hallmark of safety pacing is the presence of pacing with an shortened AV interval, ranging from 80 to 130 ms. Ventricular oversensing results in a prolonged ventricular interval, and atrial oversensing in the DDD mode results in tracking of atrial signals and rapid ventricular pacing (Figure 117-7).
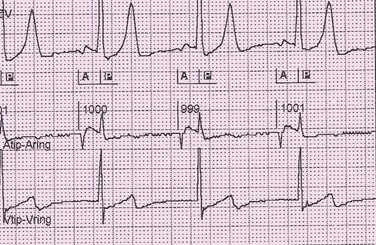
Figure 117-6 Electrocardiographic tracing demonstrating oversensing of the ventricular deflection on the atrial channel. Top channel, Surface electrocardiogram. Middle channel, Bipolar atrial electrogram (tip-ring). Bottom channel, Bipolar ventricular electrogram. Atrial pacing at a cycle length of 1000 ms occurs with intact AV conduction. There is sensing of the intrinsic ventricular deflection on the atrial channel, indicated by the P.
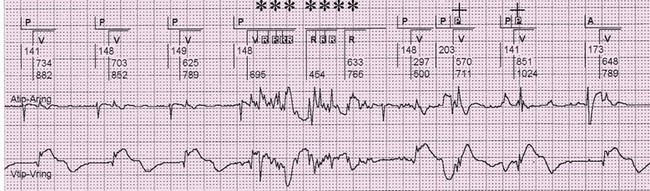
Figure 117-7 Electrograms demonstrating oversensing on the atrial and ventricular channels. Top channel is the marker channel. A indicates atrial paced event, P indicates intrinsic atrial activity, V indicates ventricular paced event, R indicates ventricular sensed event. Middle channel is the bipolar atrial electrogram. Bottom channel is the bipolar ventricular channel. In the first three, AV complexes represent intrinsic atrial activity (P) followed by ventricular paced beats (V). Artifact is seen on both the atrial and ventricular channels. The artifact is sensed on the ventricular channel, indicated by the R markers and the asterisks. The artifact is sensed on the atrial channel, indicated by the P and the plus sign.
Sensing abnormalities can be divided into true abnormalities and functional sensing abnormalities (see Box 117-3). True abnormalities include undersensing, which is a failure to recognize normal intrinsic cardiac activity, and oversensing, which is unexpected sensing of an intrinsic or extrinsic electrical signal (Figure 117-8). Artifact can also create the appearance of oversensing. True undersensing is most commonly caused by lead dislodgment or inadequate intrinsic amplitude. Sensing abnormalities are commonly seen secondary to insulation defects and lead fracture.
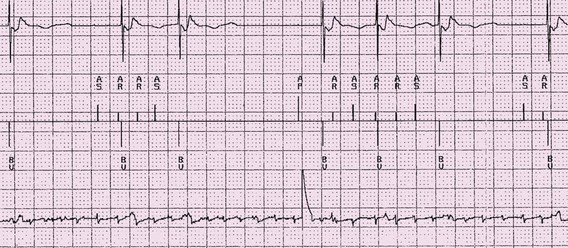
Figure 117-8 Electrogram indicating intermittent undersensing on the atrial channel of atrial fibrillation. Top channel is the ventricular electrogram. Middle channel is the marker channel. AS indicates an intrinsic atrial event that is sensed, AR indicates an intrinsic atrial event that falls within a refractory period. BV indicates a biventricular paced event. Bottom channel is the bipolar atrial electrogram. The atrial activity indicates the presence of atrial fibrillation.
Every pacing mode has a defined lower rate limit, and dual-chamber and rate-adaptive pacemakers require a defined upper rate limit. One must be familiar with the timing cycle of a particular pacing mode, as well as any idiosyncrasies of the specific pacemaker, to determine whether the paced rate is appropriate. The causes of an altered pacing rate are numerous (see Box 117-3).
Drugs can affect sensing and pacing thresholds and can result in electrocardiographic abnormalities.31,32 Although many drugs have been reported to affect pacing thresholds, only the class IC agents commonly result in a clinical problem. If these drugs are administered to a patient with a pacemaker, especially a pacemaker-dependent patient, he or she should be monitored for an increase in pacing threshold. Class IC agents can also cause sensing abnormalities.
Automatic Pacemaker Function
Atrial Arrhythmia Detection and Automatic Mode Switching
The onset of atrial arrhythmias in the DDD or DDDR mode results in tracking of the atrial activity and rapid ventricular pacing, typically at the upper rate limit. Modern algorithms detect the rapid atrial rate on the atrial channel and result in automatic switching of the mode to a nontracking mode such as DDI, DDIR, VVI, or VVIR (Figure 117-9). In any of these modes, tracking of atrial activity ceases. Once the atrial arrhythmia stops, the mode switches back to the DDD or DDDR mode. Atrial mode switching is generally considered accurate. Simulation studies have demonstrated that rate-based algorithms are most effective in detecting atrial arrhythmias.21
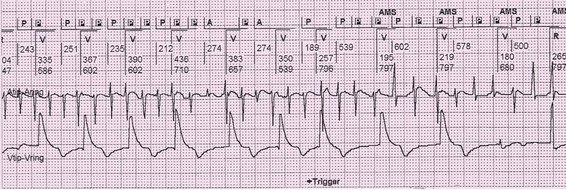
Figure 117-9 Electrogram demonstrating automatic mode switching after detection of atrial fibrillation. Top channel is the marker channel. P indicates a sensed atrial event. A indicates a paced atrial event. R indicates intrinsic ventricular event and V indicates a paced ventricular event. AMS indicates automatic mode switching from DDDR to DDIR mode. Middle channel is the bipolar atrial electrogram. Bottom channel is the bipolar ventricular electrogram. The ventricular pacing rate abruptly drops with mode switching.
There is some variability in the ability to detect atrial fibrillation by different atrial tachyarrhythmia detection algorithms. Undersensing can lead to decreased detection of episodes and in the incorrect determination that an episode has terminated. In such a situation, a greater number of shorter episodes might be recorded rather than fewer longer episodes. Longer detection times during onset can decrease inappropriate far-field oversensing. Atrial electrodes with shorter tip-to-ring distances, particularly shorter than 10 mm, can also reduce far-field R wave sensing. Postventricular atrial blanking periods can also contribute to undersensing.22
Intrinsic Conduction Preference
There has been an increasing recognition that hemodynamics are improved when intrinsic ventricular conduction is permitted in the absence of bundle branch block. New algorithms have been designed to permit the automatic restoration of intrinsic conduction. These algorithms can represent a form of AV search hysteresis or mode switching from DDD or DDDR to AAI or AAIR. When ventricular pacing occurs, AV search hysteresis periodically lengthens the AV interval so that intrinsic AV conduction occurs. If AV conduction does not occur, AV pacing resumes. In mode-switching algorithms, the pacing mode is AAI or AAIR until AV block occurs and the mode switches to DDD or DDDR (Figure 117-10). Periodically, the mode switches back to AAI or AAIR to check that AV conduction has resumed (Figure 117-11). These algorithms are extremely successful in decreasing the incidence of ventricular pacing in patients with intact AV conduction and can decrease the incidence of atrial fibrillation.
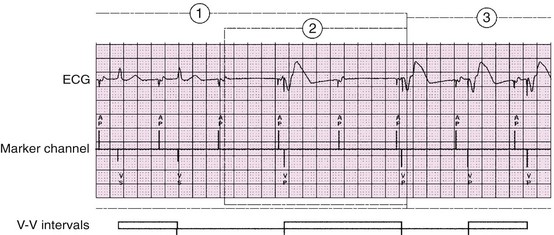
Figure 117-10 An electrocardiographic tracing reveals mode switching algorithm from AAI or AAIR to DDD or DDDR. The managed ventricular pacing algorithm significantly decreases the incidence of ventricular pacing in patients with intact AV conduction. If transient AV block occurs in the AAIR mode after an A-A interval without a ventricular sensed event, a ventricular paced event occurs 80 ms after the escape interval. If two of the four most recent A-A intervals are missing a ventricular event, the device will switch from AAIR to DDDR or from AAI to DDD. (From Flammang D, Church TR, De Roy L: Medtronic Adapta/Versa/Sensia Device Manual, Minneapolis, 2010, Medtronic, Inc.)
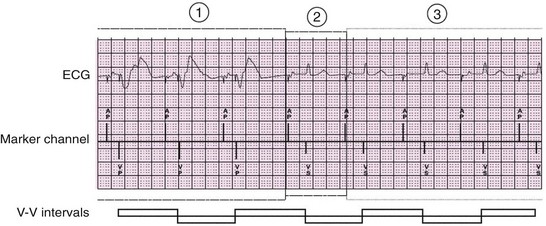
Figure 117-11 An electrocardiographic tracing reveals mode switching algorithm from DDD or DDDR to AAI or AAIR. The algorithm checks for AV conduction at progressively longer intervals, initially 1 minute in duration but increasing up to 16 hours. If atrioventricular conduction is present, the mode changes to AAIR. (From the Medtronic Adapta/Versa/Sensia Device Manual.)
Automatic Testing and Data Storage
Many pacemakers can perform reliable automatic threshold testing of the leads. These data can be used to adjust the pacing output automatically. Automatic capture verification appears to be reliable in long-term follow-up and provides the benefit of maintaining an adequate safety margin, even when the threshold has changed. In addition, the use of automatic capture verification can prolong pacemaker battery life.23 Such automatic features can be effectively programmed with a need for reprogramming in 6% of patients in 12 months.24 Pediatric patients with constant intrinsic high heart rates might not be able to have effective automatic threshold testing.25 In addition, many pacemakers keep long-term data regarding lead thresholds, lead impedances, and R and P wave amplitudes. Such trends are extremely helpful in detecting early abnormalities in lead function and in identifying pacing abnormalities. Many pacemakers can store events that are classified as pacemaker-mediated tachycardia, atrial tachyarrhythmia, or rapid ventricular rate.
Electromagnetic Interference
Electromagnetic interference (EMI) can be defined as any signal, either biologic or nonbiologic, that falls within a frequency spectrum that can be detected by the sensing circuitry of the pacemaker. EMI can result in rate alteration, sensing abnormalities, magnet mode response, initiation of pacing algorithms, or reprogramming.26,27
Certain antitheft devices have the potential for pacemaker interference, causing pacemaker inhibition, sensing abnormalities, and induction of extrasystoles.33,34 From a practical standpoint, if the patient walks through antitheft gates at a regular pace, clinically significant abnormalities are unlikely.
Consumer products can also interfere with pacemaker function. In one report, iPods placed within 2 inches of implanted pacemakers resulted in telemetry interference, but no interference on pacemaker function or programmed parameters.28
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) has been shown to pose serious risks, including life-threatening arrhythmias and death. A pacemaker system has been designed for safe use in MRIs. Wilkoff et al.29 examined 464 patients randomized to undergo MRI scan between 9 and 12 weeks after implantation or not to undergo an MRI scan. During 1.5-T brain and lumbar MRI scans, there were no clinically significant ventricular arrhythmias, pacemaker inhibition, or changes in threshold.30 MRI imaging can pose serious risks if preexisting leads are in place, even if a pacemaker system designed for MRI is in place.
References
1. Greenspon, AJ, Patel, J, Lau, E, et al. Trends in permanent pacemaker implantation in the United States 1993-2009: Increasing complexity of patients and procedures. J Am Coll Cardiol. 2012; 59:1016–1097.
2. Epstein, AE, Dimarco, JP, Ellenbogen, KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Heart Rhythm. 2008; 5:1–22.
3. Nazarian, S, Wagner, KT, Caffo, BS, et al. Clinical predictors of conduction disease progression in type I myotonic muscular dystrophy. Pacing Clin Electrophysiol. 2011; 34:171–176.
4. Serwer, GA, Shetty, I. Pediatric pacing and defibrillator use. In: Ellenbogen KA, Kay GN, Lau CP, et al, eds. Clinical Cardiac Pacing and Defibrillation, and Resynchronization Therapy. ed 4. Philadelphia: WB Saunders; 2011:393–427.
5. Romme, JJ, Reitsma, JB, Black, CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev. (5):2011.
6. Sud, S, Massel, D, Klein, GJ, et al. The expectation effect and cardiac pacing for refractory vasovagal syncope. Am J Med. 2007; 120:54–62.
7. Brignole, M, Menozzi, C, Moya, A, et al. Pacemaker therapy in patients with neutrally-mediated syncope and documented asystole: Third International Study on Syncope of Uncertain Etiology (ISSUE-3): A randomized trial. Circulation. 2012; 125:2566–2571.
8. Palmisano, P, Zaccaria, M, Luzzi, G, et al. Closed-loop cardiac pacing vs. conventional dual-chamber pacing with specialized sensing and pacing algorithms for syncope prevention in patients with refractory vasovagal syncope: results of a long-term follow up. Europace. 2012; 14:1038–1043.
9. Flammang, D, Church, TR, De Roy, L, et al. Treatment of unexplained syncope: a multicenter, randomized trial of cardiac pacing quided by adenosine 5’-triphosphate testing. Circulation. 2012; 125:31–36.
10. McLeod, CJ, Trusty, JM, Jenkins, SM, et al. Method of pacing does not affect the recurrence of syncope in carotid sinus syndrome. Pacing Clin Electrophysiol. 2012; 35(7):827–833.
11. Gold, MR, Adler, S, Fauchier, L, et al. Impact of atrial prevention pacing on atrial fibrillation burden: primary results of the Study of Atrial fibrillation (SAFARI) trial. Heart Rhythm. 2009; 6:295–301.
12. Talwar, S, Saxon, LA. Clinical trials of cardiac resynchronization therapy: Pacemakers and defibrillators. In: Ellenbogen KA, Kay GN, Lau CP, et al, eds. Clinical Cardiac Pacing and Defibrillation, and Resynchronization Therapy. ed 4. Philadelphia: WB Saunders; 2011:279–299.
13. Qintar, M, Morad, A, Alhawasli, H, et al. Pacing for drug-refractory or drug-intolerant hypertrophic cardiomyopathy. Cochrane Database Syst Rev. 5, 2012.
14. Prinzen, F, Strik, M, Regoli, F, et al. Basic physiology and hemodynamics of cardiac pacing. In: Ellenbogen KA, Kay GN, Lau CP, et al, eds. Clinical Cardiac Pacing and Defibrillation, and Resynchronization Therapy. ed 4. Philadelphia: Elsevier Saunders; 2011:203–233.
14a. Andersen, HR, Nielson, JC, Thomsen, PE, et al. Long-term folow-up of patients from a randomized trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997; 350:1200–1216.
15. Nielsen, JC, Thomsen, PE, Hojberg, S, et al. A comparison of single-lead atrial pacing with dual chamber pacing in sick sinus syndrome. Eur Heart J. 2011; 32:686–696.
16. Lau, CP, Siu, CW, Tse, HF. Implantable sensors for rate adaption and hemodynamic monitoring. In: Ellenbogen KA, Kay GN, Lau CP, et al, eds. Clinical Cardiac Pacing and Defibrillation, and Resynchronization Therapy. ed 4. Philadelphia: Elseveir Saunders; 2011:144–174.
17. Proietti, R, Manzoni, G, DiBiase, L. Closed loop stimulation is effective in improving heart rate and blood pressure response to mental stress: Report of a single-chamber pacemaker study in patients with chronotropic incompetent atrial fibrillation. PACE. 2012. [Early online: 9 JUN].
18. Coman, J, Freedman, R, Koplan, BA, et al. A blended sensor restores chronotropic response more favorably than an accelometer alone in pacemaker patients: the LIFE study results. Pacing Clin Electrophysiol. 2008; 31:1433–1442.
19. Coenen, M, Malinowski, K, Spitzer, W, et al. Closed loop stimulation and accelerometer-based rate adaptation: results of the PROVIDE study. Europace. 2008; 10:327–333.
20. Love, CJ. Pacemaker troubleshooting and follow-up. In: Ellenbogen KA, Kay GN, Lau CP, et al, eds. Clinical Cardiac Pacing and Defibrillation, and Resynchronization Therapy. ed 3. Philadelphia: WB Saunders; 2007:1005–1062.
21. Santamauro, M, Ottaviano, L, Borreli, A, et al. Efficacy of automatic mode switching in DDDR mode parameters: the most 2 study. J Interv Card Electrophysiol. 2008; 21:13–17.
22. Silberbauer, J, Arya, A, Veasey, R, et al. The effect of bipole tip-to-ring distance in atrial electrodes upon atrial tachyarrhythmia sensing capability in modern dual-chamber pacemakers. Pacing Clin Electrophysiol. 2010; 33:85–93.
23. Biffi, M, Bertini, M, Mazzotti, A, et al. Long-term RV threshold behavior by automated measurements: safety is the standpoint of pacemaker longevity. Pacing Clin Electrophysiol. 2011; 34:89–95.
24. Ailings, M, Vireca, E, Bastian, D, et al. Clinical use of automatic pacemaker algorithms: results of AUTOMATICITY registry. Europace. 2011; 13:976–983.
25. Hiippala, A, Serwer, G, Clausson, E, et al. Automatic atrial threshold measurement and adjustment in pediatric patients. Pacing Clin Electrophysiol. 2010; 33:309–313.
26. Wang, PJ, Chen, H, Okamura, H, et al. Timing cycles of implantable devices. In: Ellenbogen KA, Kay GN, Lau CP, et al, eds. Clinical Cardiac Pacing and Defibrillation, and Resynchronization Therapy. ed 3. Philadelphia: WB Saunders; 2007:969–1004.
27. Misiri, J. Kusomoto F and Goldshlager N: Electromagnetic interference and implanted devices: the non-medical environment (Part I). Clin Cardiol. 2012; 35:276–280.
28. Misiri, J. Kusomoto F, Goldshlager N: Electromagnetic interference and implanted devices: the medical environment (Part II). Clin Cardiol. 2012; 35:321–328.
29. Wilkoff, B, Bellow, D, Taborsky, M, et al. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm. 2011; 8:65–73.
30. Thaker, JP, Patel, MP, Shah, AJ. Do media players cause interference with pacemakers? Clin Cardiol. 2009; 32:653–657.
31. Hellestrand, KJ, Burnett, PJ, Milne, JR, et al. Effect of the antiarrhythmic agent flecainide acetate on acute and chronic pacing thresholds. Pacing Clin Electrophysiol. 1983; 6(5 Pt 1):892–899.
32. Mohan, JC, Kaul, U, Bhatia, ML. Acute effects of antiarrhythmic drugs on cardiac pacing threshold. Acta Cardiol. 1984; 39(3):191–201.
33. Irnich, W. Electronic security systems and active implantable medical devices. Pacing Clin Electrophysiol. 2002; 25:1235–1258.
34. Kainz, W, Neubauer, G, Alesch, F, et al. Electromagnetic compatibility of electronic implants—review of the literature. Wien Klin Wochenschr. 2001; 113:903–914.