Chapter 38
Implantable Cardioverter-Defibrillators
1. What are the components of an implantable cardioverter-defibrillator (ICD) system?
ICDs are composed of a pulse generator (typically implanted in the left pectoral region) and one or more intracardiac leads. Typically the right ventricular (RV) lead contains two distal electrodes (tip and ring) that are used to sense local electrical ventricular signals and to pace if necessary. This lead also contains one or two defibrillation coils (one sits in the RV and the other in the superior vena cava) specifically designed to deliver high voltages. Additional leads may include an atrial lead (for pacing, sensing, and arrhythmia discrimination) or a left ventricular (LV) lead for cardiac resynchronization (Fig. 38-1). All transvenous defibrillators are also capable of pacemaker functions.
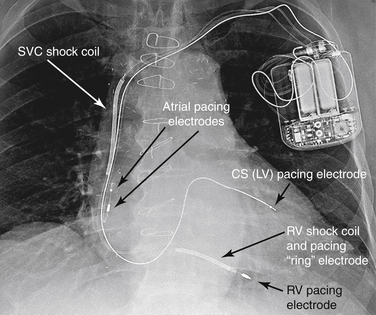
Figure 38-1 A radiograph of a patient with an implantable cardioverter-defibrillator (ICD). With this ICD, shock coils are present in both the right ventricle (RV) and the superior vena cava (SVC). There is also a coronary sinus (CS) lead present for biventricular pacing. (From Miller R, Eriksson L, Fleisher L, et al: Miller’s anesthesia, ed 7, Philadelphia, 2009, Churchill Livingstone.) LV, left ventricle.
2. How does an ICD deliver shocking energy?
ICDs use lithium-vanadium batteries that are reliable energy sources with predictable discharge curves. An ICD is able to deliver a charge larger than its battery voltage because of a system of internal capacitors. These hold a charge and then release it all at once when it is needed. Shocking energy travels around a circular circuit, usually formed between the defibrillator lead coils and the device titanium-housed can.
3. How does the ICD detect and define a rhythm?
4. How does ICD therapy improve survival?
5. What are the key clinical trials that evaluated the benefit of ICD for primary prevention?
Clinical trials have evaluated the risks and benefits of ICDs in prevention of sudden death and have improved survival in multiple patient populations, including those with prior myocardial infarction (MI) and heart failure caused by either coronary artery disease or nonischemic dilated cardiomyopathy (DCM). Table 38-1 summarizes the important trials that evaluated the mortality benefit of ICDs for primary prevention.
TABLE 38-1
TRIALS THAT EVALUATED THE BENEFIT OF IMPLANTABLE CARDIOVERTER-DEFIBRILLATORS FOR PRIMARY PREVENTION OF SUDDEN CARDIAC DEATH
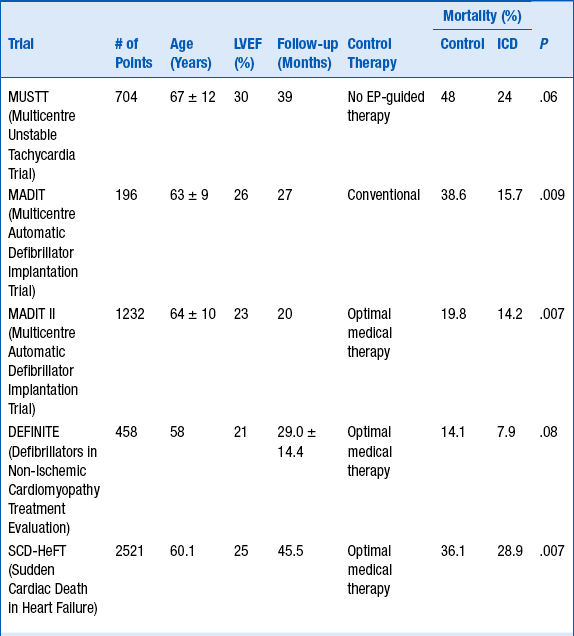
EP, Electrophysiologic; ICD, implantable cardioverter-defibrillator.
6. What are the current class I indications for ICD implantation for primary prevention of SCD?
 Patients with left ventricular ejection fraction (LVEF) less than 35% as a result of prior MI, who are at least 40 days post-MI, and are in New York Heart Association (NYHA) functional class II or III.
Patients with left ventricular ejection fraction (LVEF) less than 35% as a result of prior MI, who are at least 40 days post-MI, and are in New York Heart Association (NYHA) functional class II or III.
 Patients with LV dysfunction as a result of prior MI who are at least 40 days post-MI, have an LVEF less than 30%, and are in NYHA functional class I.
Patients with LV dysfunction as a result of prior MI who are at least 40 days post-MI, have an LVEF less than 30%, and are in NYHA functional class I.
 Patients with nonischemic DCM who have an LVEF 35% or less and who are in NYHA functional class II or III.
Patients with nonischemic DCM who have an LVEF 35% or less and who are in NYHA functional class II or III.
 Patients with nonsustained VT as a result of prior MI, LVEF less than 40%, and inducible VF or sustained VT at electrophysiologic study.
Patients with nonsustained VT as a result of prior MI, LVEF less than 40%, and inducible VF or sustained VT at electrophysiologic study.
 Patients with syncope of undetermined origin with clinically relevant, hemodynamically significant sustained VT or VF induced at electrophysiologic study.
Patients with syncope of undetermined origin with clinically relevant, hemodynamically significant sustained VT or VF induced at electrophysiologic study.
 Patients with structural heart disease and spontaneous sustained VT, whether hemodynamically stable or unstable.
Patients with structural heart disease and spontaneous sustained VT, whether hemodynamically stable or unstable.
7. What are the current class I indications for ICD implantation for secondary prevention of SCD?
Secondary prevention refers to prevention of SCD in those patients who have survived a prior sudden cardiac arrest or sustained VT. Evidence from multiple randomized controlled trials supports the use of ICDs for secondary prevention of SCD regardless of the type of underlying structural heart disease. In patients resuscitated from cardiac arrest, the ICD is associated with clinically and statistically significant reductions in SCD total mortality compared with antiarrhythmic drug therapy in prospective randomized controlled trials. Hence, ICDs are indicated for secondary prevention of SCD in patients who survived VF or hemodynamically unstable VT or experienced VT with syncope and have an LVEF 40% or less, after evaluation to define the cause of the event and to exclude any completely reversible causes.
8. What other special populations may also benefit from ICD therapy?
 Patients with congenital heart disease who are survivors of cardiac arrest, after evaluation to define the cause of the event and exclude any reversible causes
Patients with congenital heart disease who are survivors of cardiac arrest, after evaluation to define the cause of the event and exclude any reversible causes
 Hypertrophic cardiomyopathy (HCM) with one or more risk factor for SCD (VF, VT, family history of SCD, unexplained syncope, LV thickness 30 mm or more, abnormal blood pressure response to exercise)
Hypertrophic cardiomyopathy (HCM) with one or more risk factor for SCD (VF, VT, family history of SCD, unexplained syncope, LV thickness 30 mm or more, abnormal blood pressure response to exercise)
 Brugada syndrome with a history of previous cardiac arrest, documented sustained VT, or classic electrocardiogram (ECG) phenotype with unexplained syncope
Brugada syndrome with a history of previous cardiac arrest, documented sustained VT, or classic electrocardiogram (ECG) phenotype with unexplained syncope
 Long- and short-QT syndromes with previous cardiac arrest or unexplained syncope
Long- and short-QT syndromes with previous cardiac arrest or unexplained syncope
 Catecholaminergic polymorphic VT
Catecholaminergic polymorphic VT
 Arrhythmogenic RV dysplasia and/or cardiomyopathy
Arrhythmogenic RV dysplasia and/or cardiomyopathy
 Infiltrative cardiomyopathies such as sarcoidosis, amyloidosis, Fabry disease, hemochromatosis, giant cell myocarditis, or Chagas disease
Infiltrative cardiomyopathies such as sarcoidosis, amyloidosis, Fabry disease, hemochromatosis, giant cell myocarditis, or Chagas disease
9. What is defibrillator threshold testing?
10. What is antitachycardia pacing?
 ATP is not painful (sometimes barely noticed by some patients).
ATP is not painful (sometimes barely noticed by some patients).
 ATP may reduce battery drainage by terminating the arrhythmia without the need for shocks.
ATP may reduce battery drainage by terminating the arrhythmia without the need for shocks.
For this reason, it is mostly used in patients that remain stable and asymptomatic during episodes of slow VT, generally below 200 beats/min. However, more aggressive ATP programming has been shown to decrease ICD shocks, and newer ICDs incorporate ATP while charging for a shock.
11. How common are inappropriate shocks in patients with ICDs?
12. What is the totally subcutaneous defibrillator?
Bibliography, Suggested Readings, and Websites
1. Bardy, G.H., Lee, K.L., Mark, D.B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237.
2. Bardy, G.H., Smith, W.M., Hood, M.A., et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44.
3. Daubert, J.P., Zareba, W., Cannom, D.S., et al. Inappropriate implantable cardioverter-defibrillators shocks in the MADIT II study: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol 51 1357–1365. J Am Coll Cardiol. 2008;51:1357–1365.
4. DiMarco, J.P. Implantable cardioverter-defibrillators. N Engl J Med. 2003;349:1836–1847.
5. Epstein, A.E., DiMarco, J.P., Ellenbogen, K.A., et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). J Am Coll Cardiol. 2008;51:2085–2105.
6. Hohnloser, S.H., Kuck, K.H., Dorian, P., et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488.
7. Kadish, A., Dyer, A., Daubert, J.P., et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158.
8. Moss, A.J., Zareba, W., Hall, W.J., et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883.