16 Immunology and Infectious Disorders
Pearls
• The critically ill child may have immune compromise and is at risk for a variety of healthcare-acquired infections. The critical care nurse must be alert for evidence of inflammation and infection and signs of developing sepsis.
• It is likely that adherence to prevention measures can reduce risk of healthcare-acquired infections such as catheter-related bloodstream infections, ventilator-associated pneumonia, and urinary tract infections.
• Children with septic shock require early and aggressive fluid resuscitation, vasoactive support, early antibiotic therapy, and support of organ system function.
Anatomy and physiology: immunology
Immunology Overview
Developmental Considerations
At the time of birth, a neonate is considered to be fundamentally immunocompromised for several reasons.14 First, although infants born at term have passively acquired immunity from maternal antibodies that were transferred transplacentally before birth, the titers of these antibodies quickly wane, leaving the infant without immunity to most specific pathogens. Until exposure to common pathogens by natural infection or immunization, infants lack durable organism-specific immunity. In addition, the function of specific components of the immune system does not mature until approximately 2 years of age. Until that time, infants are unable to make a robust antibody response to pathogens that have polysaccharide molecules on their surface. This developmental defect explains why the incidence of invasive pneumococcal infection, an organism with a polysaccharide coat, is relatively high in young children.
Infectious Disease Overview
Infection is a common cause and can be a common complication of critical illness in hospitalized children.45 Common community-acquired infections such as bacterial pneumonia and viral infections can lead to life-threatening illnesses in both immunocompetent and immunocompromised children. Local and systemic complications of community-acquired infections include respiratory failure, shock, and renal insufficiency. Critically ill children are also at high risk of healthcare-acquired infections including catheter-associated bloodstream infections, ventilator-associated pneumonia, and surgical site infections. These infections can be caused by viruses, bacteria, or fungal organisms.
Colonization and Infection
At birth, a neonate is normally essentially sterile. Within hours, however, bacteria from both the environment and people who handle the infant are transferred onto and begin to grow on the baby’s skin and mucous membranes.20 These bacteria are typically referred to as colonizing flora. The predominant colonizing organisms vary with anatomic site. For example, skin organisms such as Staphylococcus epidermidis can be found on almost all keratinized skin. In contrast, anaerobic and gram-negative organisms are typically found only in the intestinal tract. Colonizing flora typically do not cause inflammation or invasive infection. Many infections, however, do arise from the patient’s colonizing flora, often when a medical device breaches the integrity of skin or mucous membranes or when skin or mucous membranes become inflamed.
Common clinical conditions
Allergic Reactions and Anaphylaxis
Etiology
Allergic or “hypersensitivity” reactions occur when the body mounts an exaggerated or inappropriate immune response to a substance perceived as foreign, resulting in local or general tissue damage. Such reactions are usually classified by severity and involvement,1 as in types I to IV (Table 16-1).
| Type | Description | Example |
| Type I (anaphylactic reaction) | Triggered in response to an exposure to an environmental antigen Mediated by IgE antibodies that bind to specific receptors on the surface of mast cells and basophils Results in the release of a host of mediators to produce a classic anaphylactic response |
Anaphylaxis Asthma Allergic rhinitis, hay fever |
| Type II (tissue specific hypersensitivity) | Triggered by the presence of an antigen found only on a cell or tissue Mediated by antibody (usually IgM, but also IgG) through two different mechanisms (complement and Fc receptors on phagocytes) Results in the destruction of the antibody-coated cell with consequences dependent on the cell or body that is destroyed (e.g., RBC, WBC, or platelet) |
ABO incompatibility Rh incompatibility Drug-induced thrombocytopenia |
| Type III (immune complex reaction) | Triggered by the formation of antigen-antibody complexes that activate the complement cascade Immune complexes are formed in the circulation and are later deposited in blood vessels or healthy tissue. Multiple forms of the response exist depending on the type and location of the antigen Results in local edema and neutrophil attraction, and thus degradative lysosomal enzymes resulting in tissue injury |
Serum sickness Glomerulonephritis |
| Type IV (delayed hypersensitivity) | Triggered by the recognition of an antigen mediated by activated T lymphocytes and release of lymphokines, which then stimulate the macrophage to phagocytize foreign invaders and some normal tissue Results in a delayed onset. Does not have an antibody component; this response is strictly a cellular reaction |
Contact sensitivities such as poison ivy and dermatitis Tuberculin reactions Graft rejection |
RBC, Red blood cell; WBC, white blood cell.
From Roberts KE, Brinker D, Murante B. Hematology and immunology. In Slota M, editor: Core curriculum for pediatric critical care nursing, ed 2. Philadelphia, 2006, Saunders Elsevier, p. 597.
Anaphylaxis is an allergic hypersensitivity reaction to a foreign protein or drug that causes a systemic response. Exposure to the antigen may be oral or intravenous, or through inhalation or via direct contact. The anaphylactic reaction can occur within seconds or minutes after exposure.42
Clinical Signs and Symptoms
Signs and symptoms of hypersensitivity or anaphylaxis can develop within seconds or minutes after exposure. Patients often initially describe a sense of impending doom, accompanied by pruritus and flushing. This can evolve rapidly into other clinical manifestations of hypersensitivity (Table 16-2).
Table 16-2 Clinical Manifestations of Hypersensitivity Reactions
| Organ System | Clinical Manifestation(s) |
| Cutaneous/ocular | Flushing, urticaria, angioedema, cutaneous and/or conjunctival pruritus, warmth, and swelling |
| Respiratory | Nasal congestion, rhinorrhea, throat tightness, wheezing, shortness of breath, cough, hoarseness |
| Cardiovascular | Dizziness, weakness, syncope, chest pain, palpitations |
| Gastrointestinal | Dysphagia, nausea, vomiting, diarrhea, bloating, cramps |
| Neurologic | Headache, dizziness, blurred vision, and seizure (very rare and often associated with hypotension) |
| Other | Metallic taste, feeling of impending doom |
Data from Linzer JF: Pediatrics, anaphylaxis. 2008. Emedicine, http://emedicine.medscape.com/article/799744-overview.
Once the clinical manifestations of the reaction become systemic, anaphylaxis is present. Mild symptoms include irritability, coughing, anxiety, disorientation, erythema, hives, and itching. Severe symptoms include dyspnea; cyanosis; difficulty speaking; swelling of the tongue, face, and airways; intense coughing; chest tightness; wheezing; stridor; laryngospasm; seizures; sense of impending doom; hypotension; and cardiorespiratory arrest.23
Management
Establish vascular access, ideally with two large-bore vascular catheters and be prepared to administer fluid boluses (to treat relative hypovolemia resulting from vasodilation and increased capillary permeability) and vasoactive support (e.g., an epinephrine infusion) to restore and maintain adequate blood pressure and systemic perfusion. (For further information, please refer to Chapter 6.)
Medications typically used to treat anaphylactic reactions include oxygen, IM epinephrine (an infusion may be needed for refractory hypotension), diphenhydramine (and possibly an H2-blocker antihistamine), albuterol nebulizer, and methylprednisolone.10,23 Antihistamines are administered to antagonize the effects of histamine. Bronchodilators relax bronchial smooth muscles. Corticosteroids are antiinflammatory agents to enhance the effects of bronchodilators. (For further information, please refer to Chapter 6.)
Skin testing may help identify patients who may experience a hypersensitivity reaction with a known high-risk agent. Patients are given a small intradermal test dose of the agent and are monitored for at least 20 minutes.17 Emergency equipment and medications should be readily available. Patients receiving medications or agents with a higher risk of producing anaphylactic reaction and those with a history of anaphylaxis should be identified and monitored appropriately.
When a patient has a known allergy or hypersensitivity reaction to a drug, premedications may be prescribed before the agent is administered. Medications commonly used for pretreatment are corticosteroids, antihistamines, and antipyretics.17 Patients with known hypersensitivity responses should wear medical alert jewelry and should have an anaphylaxis kit (epinephrine autoinjector pen) readily available.
Systemic Inflammatory Response Syndrome (SIRS)
Etiology
In 1992, the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) introduced definitions3 for systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, septic shock, and multiple organ dysfunction syndrome (MODS).
SIRS is a state of inflammatory/immune activation. SIRS is present when the adult patient demonstrates two or more of the following variables3,8a:
• Altered temperature: fever of more than 38° C rectal or less than 36° C rectal
• Tachypnea (age related) or a PaCO2 less than 32 mm Hg
• Abnormal white blood cell count: greater than 12,000/mm3 or less than 4000/mm3 or greater than 10% bands
SIRS is nonspecific and can be caused by a number of diverse clinical conditions (Table 16-3), including ischemia, inflammation, trauma, infection, or a combination of several insults. SIRS does not always occur as a result of infection. A number of underlying conditions may predispose patients to infections with specific pathogens and the development of SIRS (Table 16-4).
| Infectious Causes | Noninfectious Causes |
| Bacterial sepsis Burn wound infections Candidiasis Cellulitis Cholecystitis Community-acquired pneumonia Infective endocarditis Influenza Intraabdominal infections Meningitis Healthcare-acquired pneumonia Pyelonephritis Toxic shock syndrome Urinary tract infections |
Autoimmune disorders Burns Chemical aspiration Dehydration Erythema multiforme (Stevens-Johnson syndrome) Hemorrhagic shock Intestinal perforation Pancreatitis Surgical procedures Transfusion reactions Upper gastrointestinal bleeding Vasculitis |
From Burdette SD, et al: Systemic inflammatory response syndrome. Emedicine, http://emedicine.medscape.com/article/168943-overview. Updated July 20, 2010. Accessed April 27, 2011.
Table 16-4 Predisposing Conditions/Risk Factors for Development of SIRS
| Acquired immunodeficiency syndrome (AIDS) | Predisposes to SIRS from both typical and unusual pathogens, particularly pneumococcus |
| Hemoglobin SS (Sickle Cell) disease | 400-Fold increased risk of sepsis caused by pneumococcus and Salmonella, among other pathogens |
| Congenital heart disease (with few exceptions) | Risk for endocarditis (see Endocarditis in Chapter 8) and SIRS |
| Genitourinary anomalies | May increase the risk of urosepsis |
| Significant burns | Risk of SIRS, caused by skin flora and nosocomial gram-negative pathogens in particular |
| Splenic dysfunction or absence, as well as complement, immunoglobulin, and properdin deficiency | Predispose to infection from encapsulated organisms and resulting sepsis |
| Hematologic and solid-organ malignancies (before or during treatment) | Increased risk for SIRS from many organisms |
| Hospitalization (particularly if prolonged, in the critical care unit, or with invasive devices) | Increased risk of SIRS; prolonged stay and invasive devices increase risk of infection |
| Indwelling devices or prosthetic material and other breaches in barrier protective function | Increased risk of SIRS |
Modified from Burdette SD, et al: Systemic inflammatory response syndrome. Emedicine, http://emedicine.medscape.com/article/168943-overview. Updated July 20, 2010. Accessed April 27, 2011.
Pathophysiology
More than 15 years ago, Bone4 described the relationship between these complex inflammatory interactions. He described SIRS and MOSF as a five-stage process (Table 16-5). His definitions remain very helpful today.
Table 16-5 Bone’s Five Stages of SIRS and Multiple Organ System Failure (MOSF)
• Occurs if local defense mechanisms are insufficient to correct the local injury or eliminate the local infection
• Proinflammatory mediators are released into the systemic circulation and recruit additional cells to the local area of injury
• Systemic release of antiinflammatory mediators follows under normal circumstances, these mediators ameliorate the proinflammatory reaction and restore homeostasis
• Occurs if the systemic release of proinflammatory mediators is massive or if the antiinflammatory reaction is insufficient to permit downregulation
• Most patients have symptoms of the systemic inflammatory response syndrome (SIRS) and evidence of the multiple organ system failure (MOSF)
• Excessive systemic levels of antiinflammatory mediators develop as a response to a massive proinflammatory response
From Bone RC: Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med 125(8):680-687, 1998.
The cumulative effect of this inflammatory cascade is an unbalanced state with inflammation and coagulation. To counteract the acute inflammatory response, the body is equipped to reverse this process via a counter-inflammatory response syndrome (CARS). Co-morbidities and other factors can influence a patient’s ability to respond appropriately. The balance of SIRS and CARS determines a patient’s prognosis after an insult. Some researchers believe that, because of CARS, many of the new medications meant to inhibit the proinflammatory mediators may lead to deleterious immunosuppression.4
If SIRS continues to progress, cardiac output may fall, peripheral vascular resistance may increase, and shunting of blood may ensue (i.e., cold shock). This results in development of tissue hypoxia, end-organ dysfunction, metabolic acidosis, end-organ injury and/or failure, and can be fatal.35
Clinical Signs and Symptoms
Fever is the most common presenting symptom of children with SIRS. Fever is one component of the triad of hyperthermia (or hypothermia), tachypnea, and tachycardia that typifies the earliest, mildest manifestation of SIRS. The international consensus terminology defines SIRS in children as present when the patient demonstrates two or more of the following (see details in Box 16-1)18:
• Alteration in temperature: fever of more than 38° C rectal or temperature less than 36° C rectal
• Alteration in heart rate (age related): tachycardia or bradycardia (in infants)
• Tachypnea (age related) or a PaCO2 less than 32 mm Hg
• Abnormal white blood cell count (greater than 12,000/mm3 or less than 4000/mm3 or greater than 10% bands)
Box 16-1 Pediatric Signs of Sepsis/Systemic Inflammatory Response Syndrome
Manifested by two or more of the following four criteria:
Mean heart rate more than 2 standard deviations (SD) above normal for age in absence of external stimulus, chronic drugs, or painful stimuli OR
Otherwise unexplained persistent elevation over a 0.5- to 4-h time period
Mean heart rate less than the 10th percentile for age in absence of external vagal stimulus, ß-blocker drugs, or congenital heart disease
Leukocyte count elevated or depressed for age (not secondary to chemotherapy-induced leukopenia) or more than 10% immature neutrophils
Modified from Goldstein B, et al: International pediatric sepsis consensus conference. Pediatr Crit Care Med 6(1):5, 2005.
Management
Treatment of SIRS is focused on treating the inciting cause. Empiric antibiotics are not administered routinely to all patients. Indications for empiric antimicrobial therapy include suspected or diagnosed infectious etiology, hemodynamic instability, neutropenia, and asplenia.8a Broad spectrum antibiotics are initiated when there is concern for an infectious cause but no definitive infection has been diagnosed.
Drotrecogin alpha, a recombinant form of human recombinant activated protein C (APC), reduces microvascular dysfunction by reducing inflammation and coagulation and increasing fibrinolysis. It has been hypothesized that APC may be beneficial in the management of SIRS. However, the supporting evidence to date is limited. In the prospective, randomized multicenter controlled PROWESS trial,2 mortality was reduced by 28% in adult patients with severe sepsis who received APC. Patients who received APC also demonstrated significantly more bleeding than control patients. However, a Cochrane meta-analysis of adult trials25 involving over 4000 patients (including some children who were not randomized) did not find overall evidence of improved survival when APC was administered; a multicenter pediatric study was halted because excessive bleeding occurred when children received APC.
Fluid resuscitation should be initiated in those patients who exhibit signs of hypovolemia and hypovolemic shock (see section, Sepsis and Septic Shock and Chapter 6). All patients require establishment of adequate intravenous access. Administer isotonic fluids boluses (typically 20 mL/kg boluses; smaller volumes may be used in children with poor myocardial function) as needed to treat shock and monitor hemodynamic status closely.22,33 If signs of shock are present, antibiotics, aggressive fluid resuscitation and vasoactive support should be provided within the first hour after the onset of symptoms (see section, Sepsis and Septic Shock and Chapter 6).5
Assess and support adequate oxygenation and ventilation. The oxyhemoglobin saturation in the superior vena cava (SCVO2) allows tracking of the balance between oxygen delivery and oxygen use; therapy should be titrated to maintain this SCVO2 70% or higher.5 Patients with increased oxygen requirement should receive supplementary oxygen. Intubation and mechanical ventilation may be required in some patients.
Sepsis and Septic Shock
Etiology
The term sepsis is often used to refer to a broad spectrum of pathophysiologic and clinical derangements ranging from initial infection and bacteremia to SIRS, generalized sepsis, severe sepsis, septic shock, and refractory septic shock. Sepsis is the systemic response to infection and is defined as the presence of SIRS in addition to a documented or presumed infection.15 Severe sepsis is defined as sepsis plus sepsis-induced MOSF or tissue hypoperfusion or insufficient end-organ perfusion.15,43
Septic shock is defined as sepsis criteria plus persistent hypotension despite the administration of 20 mL/kg of crystalloid or colloid plus an inotrope/vasopressor requirement, a Glasgow coma score less than 15 in the absence of CNS disease, arterial lactate greater than 1.6 mmol/L (verify laboratory normal), or urine output less than 1 m/kg per hour for two consecutive hours with a urinary catheter in place.43 Septic shock is addressed in greater detail in Chapter 6.
Pathophysiology
Sepsis is a complex clinical disorder that results from three potential factors that interact with one another, leading to severe sepsis and potentially to septic shock. First, an infecting pathogen can cause direct injury to the tissues, which results in the development of MOSF. Second, sepsis may develop as a secondary response to an excessive host inflammatory response. Third, sepsis may involve a failure of counter regulatory mechanisms.43 The end result of sepsis is dysregulation of the inflammatory cascades leading to endothelial injury, coagulation and fibrinolytic abnormalities, microcirculatory disturbances, myocardial depression, organ dysfunction, and increased susceptibility to healthcare-acquired infections.39
Clinical Signs and Symptoms
Hypotension is a late sign of septic shock. Diastolic blood pressure begins to fall as sepsis produces a decrease in vascular tone. Systolic blood pressure is typically maintained for a longer period of time and only falls once a significant compromise in hemodynamic status has occurred. Clinical diagnosis is made in children with a suspected infection manifested by hypothermia or hyperthermia, and clinical signs of inadequate tissue perfusion. Hypotension is not necessary for a clinical diagnosis of septic shock. However, the presence of hypotension in a child with a clinical suspicion of infection is confirmatory for septic shock.5
Management
The management of pediatric sepsis and septic shock is targeted at maintaining cardiopulmonary stability and preventing or correcting metabolic abnormalities.21 The 2007 update to the ACCM guidelines for the hemodynamic support of pediatric and neonatal patients with septic shock5 emphasizes the importance of early antibiotics and aggressive fluid resuscitation and use of vasoactive agents among other age-specific therapies to achieve time-sensitive goals (Fig. 16-1).
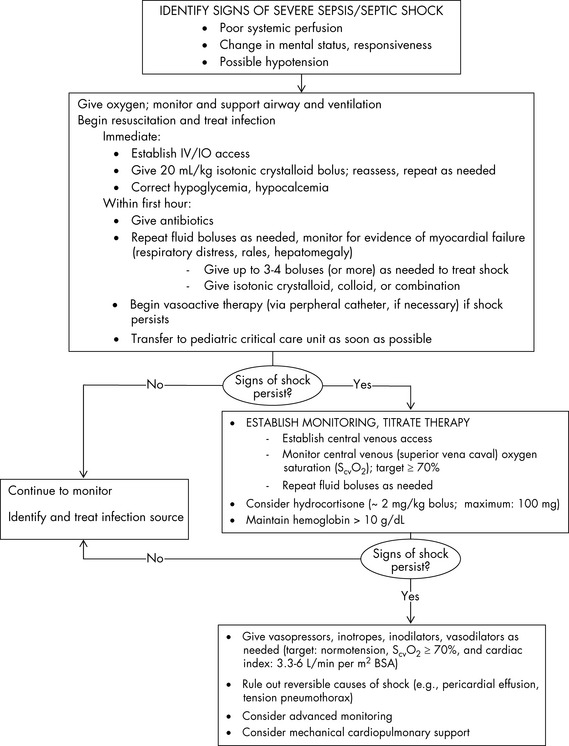
Fig. 16-1 Suggestions for hemodynamic support in pediatric septic shock.
BP, Blood pressure; CI, cardiac index; SVRI, systemic vascular resistance index.
Based on recommendations of Brierley J, Carcillo JA, Choong K, Cornell T, et al: Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 37:666–688, 2009.
Nursing care measures to reduce risk of infection
Infection Control: Basic Principles
Hospitals and healthcare systems now recognize the importance of protecting the critically ill child from developing healthcare-acquired infections (HAIs). HAIs are a serious problem for hospitalized children; they contribute to increased morbidity, mortality, and cost of care.11 For example, investigators have demonstrated that catheter-associated bloodstream infections have an attributable mortality of 10% to 35%, can prolong hospitalization by an average of 7 days, and increase hospital costs up to $39,000 per episode.45 New technologies are under investigation to attempt to reduce the incidence of these infections. Approaches include use of antiseptic- or antibiotic-impregnated catheters, use of maximal sterile barrier during placement of catheters, and consistent use of effective skin disinfectants (i.e., chlorhexidine gluconate/70% isopropyl alcohol). Risk factors for HAI in critically ill children are highlighted in Table 16-6.
| Type of Infection | Risk Factors |
| Catheter-related bloodstream infection | Arterial catheter Multiple venous catheters Transport out of the critical care unit (e.g., to operating room or radiology) Genetic syndrome |
| Ventilator-associated pneumonia | Invasive ventilation Reintubation Genetic syndrome Immunodeficiency Aspiration of oropharyngeal or gastric secretions |
| Surgical site infection | Malnutrition Steroid use Inappropriate perioperative antibiotic prophylaxis Placement of prosthetic material or device |
| Multidrug resistant organism (MDRO) infection | History of transplantation or preexisting lung disease Presence of an indwelling medical device Duration and spectrum of antibiotic exposure |
| Viral infection | Young age Multi-bed room Poor adherence to standard and transmission-based precautions |
Using Standard and Expanded Precautions to Prevent Disease Transmission (SC)
One of the most important aspects of infection prevention and control is the prevention of microorganism transmission between patients and between healthcare workers and patients. Standard precautions should be used for every patient encounter,37 regardless of the setting in which care is being delivered.
Hand hygiene is one of the most basic, yet most important strategies in infection prevention and control by healthcare providers. And yet, healthcare providers continue to struggle to comply with the Recommendations of the 2002 Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force.9 See Table 16-7 for a summary of these recommendations.
• When decontaminating hands with an alcohol-based hand rub, apply product to palm of one hand and rub hands together, covering all surfaces of hands and fingers, until hands are dry.
• When washing hands with soap and water, wet hands first with water, apply an amount of product recommended by the manufacturer to hands, and rub hands together vigorously for at least 15 seconds, covering all surfaces of the hands and fingers. Rinse hands with water and dry thoroughly with a disposable towel. Use towel to turn off the faucet.
• Avoid using hot water, because repeated exposure to hot water may increase the risk of dermatitis.
• Liquid, bar, leaflet, or powdered forms of plain soap are acceptable when washing hands with a non-antimicrobial soap and water. When bar soap is used, soap racks that facilitate drainage and small bars of soap are recommended.
• Multiple-use cloth towels of the hanging or roll type are not recommended for use in healthcare settings.
• Remove rings, watches, and bracelets before beginning the surgical hand scrub.
• Remove debris from underneath fingernails using a nail cleaner under running water.
• Surgical hand antisepsis using either an antimicrobial soap or an alcohol-based hand rub with persistent activity is recommended before donning sterile gloves when performing surgical procedures.
• When performing surgical hand antisepsis using an antimicrobial soap, scrub hands and forearms for the length of time recommended by the manufacturer, usually 2-6 min. Long scrub times (e.g., 10 min) are not necessary.
• When using an alcohol-based surgical hand-scrub product with persistent activity, follow the manufacturer’s instructions.
• Before applying the alcohol solution, prewash hands and forearms with a non-antimicrobial soap and dry hands and forearms completely.
• After application of the alcohol-based product as recommended, allow hands and forearms to dry thoroughly before donning sterile gloves.
• Provide personnel with efficacious hand-hygiene products that have low irritancy potential, particularly when these products are used multiple times per shift.
• This recommendation applies to products used for hand antisepsis before and after patient care in clinical areas and to products used for surgical hand antisepsis by surgical personnel.
• To maximize acceptance of hand-hygiene products by healthcare workers, solicit input from these employees regarding the feel, fragrance, and skin tolerance of any products under consideration.
• The cost of hand hygiene products should not be the primary factor influencing product selection.
• When selecting non-antimicrobial soaps, antimicrobial soaps, or alcohol-based hand rubs, solicit information from manufacturers regarding any known interactions between products used to clean hands, skin care products, and the types of gloves used in the institution.
• Before making purchasing decisions, evaluate the dispenser systems of various product manufacturers or distributors to ensure that dispensers function adequately and deliver an appropriate volume of product.
• Do not add soap to a partially empty soap dispenser. This practice of “topping off” dispensers can lead to bacterial contamination of soap.
• Provide healthcare workers with hand lotions or creams to minimize the occurrence of irritant contact dermatitis associated with hand antisepsis or handwashing.
• Solicit information from manufacturers regarding any effects that hand lotions, creams, or alcohol-based hand antiseptics may have on the persistent effects of antimicrobial soaps used in the institution.
• Do not wear artificial fingernails or extenders when having direct contact with patients at high risk (e.g., those in critical care units or operating rooms).
• Keep tips of natural nails less than 1/4-inch long.
• Wear gloves when contact with blood or other potentially infectious materials, mucous membranes, and nonintact skin could occur.
• Remove gloves after caring for a patient. Do not wear the same pair of gloves for the care of more than one patient, and do not wash gloves between uses with different patients.
• Change gloves during patient care if moving from a contaminated body site to a clean body site
• No recommendation can be made regarding wearing rings in healthcare settings. This remains an unresolved issue.
• As part of an overall program to improve hand hygiene practices of healthcare workers, educate personnel regarding the types of patient-care activities that can result in hand contamination, and the advantages and disadvantages of various methods used to clean their hands.
• Monitor healthcare workers’ adherence with recommended hand-hygiene practices and provide personnel with information regarding their performance.
• Encourage patients and their families to remind healthcare workers to decontaminate their hands.
• Make improved hand-hygiene adherence an institutional priority, and provide appropriate administrative support and financial resources.
• Implement a multidisciplinary program designed to improve adherence of healthcare personnel to recommended hand-hygiene practices.
• As part of a multidisciplinary program to improve hand-hygiene adherence, provide healthcare workers with a readily accessible alcohol-based hand rub product.
• To improve hand-hygiene adherence among personnel who work in areas where high workloads and high intensity of patient care are anticipated, make an alcohol-based hand rub available at the entrance to the patient’s room or at the bedside, in other convenient locations, and in individual pocket-sized containers to be carried by healthcare workers.
• Store supplies of alcohol-based hand rubs in cabinets or areas approved for flammable materials.
Adapted from Centers for Disease Control and Prevention: Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR 51(RR-16):32-34, 2002.
For patients with known or suspected infection with pathogens of epidemiologic significance, expanded transmission-based precautions should also be employed, particularly during hospitalization. Table 16-8 categorizes the appropriate expanded precautions needed to prevent the spread of specific organisms, and Table 16-9 summarizes the types of expanded precautions.
Table 16-8 Modes of Organism Transmission and Isolation Precautions
| Mode of Transmission | Isolation Precaution |
|
Standard |
|
|
Contact |
|
|
Droplet |
|
|
Airborne |
Table 16-9 Isolation Precautions for Specific Infections
| Isolation Precautions | Clinical Example |
| Standard | |
| Airborne | |
| Droplet | |
| Contact |
Specific diseases
Severe Combined Immunodeficiency Disorder (SCID)
Etiology
Severe combined immunodeficiency disorder (SCID) is a rare combined B- and T-lymphocyte disorder. Estimations of incidence range from 1:70,000 to 1:1,000,000 live births, but may be as frequent as 1:35,000.27,44 The majority of cases are inherited in an X-linked or autosomal recessive pattern. SCID is fatal if untreated and is considered a pediatric emergency. In most cases survival requires expeditious stem cell transplantation.
Pathophysiology
SCID occurs as the result of mutations in 1 of 10 known genes.36 There are many variants of the disorder ranging from partial to almost complete loss of T-lymphocyte function. X-linked SCID is the most common form. Other forms of SCID usually follow an autosomal recessive inheritance pattern or are the result of spontaneous mutations. One of these other forms is linked to a deficiency of the enzyme adenosine deaminase (ADA). B and T lymphocytes produce chemicals that can accumulate to toxic levels within these cells. Normally, these cells produce an enzyme (ADA) that destroys the excess toxins. When this vital “detoxifying enzyme” is missing, these toxins accumulate, “poisoning” the B and T lymphocytes.
Clinical Signs and Symptoms
SCID is characterized by multiple severe or recurrent illnesses such as dermatitis, otitis media, and diarrhea.36 Infection is not present at birth, because the infant is protected from bacterial infections by transplacental delivery of maternal IgG antibody. However, signs and symptoms of infection develop soon after birth with the majority of cases presenting in patients less than 3 months of age.36
Management
Early immune reconstitution offers the best chance of long-term survival. Bone marrow or stem cell transplantation can be performed within the first 3 months of life, with reported survival as high as 97%.7 Donors may be human leukocyte antigen-identical siblings, cord blood units, or matched unrelated donors identified through the national registries or haploidentical parents. Bone marrow transplantation (BMT) using stem cells obtained from a related, HLA-identical donor (RID) is the optimal treatment for patients with SCID. In the absence of a RID, HLA-mismatched related donors (MMRDs) are often used. However, compared with RIDs, use of MMRDs for BMT is associated with reduced survival and inferior long-term immune reconstitution. Grunebaum and co-workers19 found that use of HLA-matched unrelated donors (MUDs) had an 80% cure rate for SCID and are associated with long-term robust immune reconstitution. They suggest that this mode of treatment may be an important therapeutic alternative for patients with SCID when a RID is not available.
Meningitis
Bacterial meningitis is a life-threatening illness that results from bacterial infection of the meninges. Following the neonatal period, the three most common organisms that cause acute bacterial meningitis are Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b (Hib). With the routine use of Hib, conjugate pneumococcal, and conjugate meningococcal vaccines in the United States, the incidence of meningitis has decreased significantly.13 See Chapter 11 for a complete discussion of the etiology, pathophysiology, clinical signs and symptoms, and management of meningitis.
Meningococcemia
Pathophysiology
N. meningitidis is transmitted from person to person via direct contact with infected droplets of respiratory secretions. Carriers are often asymptomatic at the time of transmission. Immunity to N. meningitidis is thought to be acquired through intermittent nasal carriage of meningococci and antigenic cross-reaction with enteric flora during the first two decades of life.16 Once the bacteria colonize and cross the nasal mucosa, disease symptoms often appear within 10 days.
The virulence or severity of meningococcemia is related to the release of endotoxin. The endotoxin of meningococcus is structurally different from that of other gram-negative bacteria.34 Levels of endotoxin correlate with the severity of illness and may be 50- to 100-fold that occurring with other gram-negative infections.
The clinical manifestations of meningococcemia result from capillary leak, myocardial failure, coagulopathy, and metabolic derangement. These processes work together to produce a state of multisystem organ failure that typically causes cardiorespiratory dysfunction and, frequently, renal, neurologic, and gastrointestinal dysfunction.30
In the first 2 to 4 days after onset of illness, there is a massive increase in vascular permeability with resulting capillary leak. Initially, the body compensates through normal homeostatic mechanisms, including vasoconstriction. As the capillary leak continues, hypovolemia develops. This hypovolemia leads to decreased venous return and cardiac output. Vasodilation and maldistribution of blood flow contribute to inadequate tissue perfusion, tissue hypoxia, and lactic acidosis, and may produce cell death and organ dysfunction. For additional information, see Chapter 6.
Patients with meningococcemia often present with disseminated intravascular coagulation (DIC). The combination of microvascular thrombosis and bleeding (caused by initiation of the clotting cascade) is particularly challenging to manage. DIC, in combination with the described hypoxia and hypovolemia, can cause significant end-organ damage. Waterhouse-Friderichsen syndrome (massive adrenal hemorrhage) can complicate meningococcemia and may further exacerbate cardiovascular collapse. See Chapter 15 for additional information about DIC.
Clinical Signs and Symptoms
The clinical manifestations of meningococcal disease can be extremely variable, ranging from the typical signs of meningitis to full-blown septic shock (Table 16-10). In cases of severe meningococcemia, the disease may progress extremely quickly, with death occurring within a few hours of the onset of symptoms. The classic petechial rash is considered to be pathognomic for meningococcemia. However, up to 20% of children with this disorder have no rash or petechiae at presentation.26
| System | Clinical Manifestations |
| Neurologic/neuromuscular | Headache Photophobia Lethargy Neck stiffness ±Brudzinski sign ±Kernig sign Seizures Myalgia |
| Cardiovascular | Tachycardia Bradycardia Hypotension Cool extremities Diminished peripheral pulses Delayed capillary refill time |
| Respiratory | Tachypnea |
| GI | Vomiting Abdominal pain |
| Skin | Rash (may be erythematous initially and then progress to petechiae and purpura) |
Management
Antibiotic therapy is the only definitive treatment for meningococcemia and should be initiated as soon as the diagnosis is made. The sequelae of this disease may be devastating, but N. meningitidis itself is relatively easy to eradicate. A blood culture should be obtained before initiation of antibiotic therapy but should not delay treatment. In a stable patient, a lumbar puncture may be performed. However, it may be deferred in those who are considered unstable or are exhibiting signs of increased intracranial pressure. Recommended antibiotic therapy may include cefotaxime, ceftriaxone, or penicillin G.28,32,41 Doses vary based on the patient’s clinical condition (i.e., presence or absence of associated meningitis).
Further management is aimed at restoring adequate oxygen and substrate delivery to the tissues. This is achieved through aggressive fluid resuscitation to replete intravascular volume, correction of DIC, and optimization of oxygenation and ventilation. Patients with meningococcemia may require as much as 80 mL/kg of crystalloid during the first hour of therapy and 240 mL/kg or more during the first 8 hours of therapy. This fluid is administered in boluses of 20 mL/kg per Pediatric Advanced Life Support (PALS) guidelines.10,22,33 The child’s hemodynamic and cardiovascular status should be reassessed after each intervention. If shock persists after initial volume therapy, inotropic support is recommended, and should be instituted within the first hour of therapy, even if this requires administration via peripheral venous access. Epinephrine and dopamine may be used to improve myocardial contractility and enhance vasoconstriction.
After initial resuscitation and stabilization, afterload reducing agents such as milrinone are often needed. Arterial and central venous catheters should be placed to allow for close monitoring of hemodynamic status and response to interventions. Insertion of a central venous catheter should not delay administration of fluid boluses and vasoactive support; both can be initiated through large-bore peripheral venous catheters until a central venous catheter is placed. For additional information about management of septic shock, see Chapter 6.
Administration of corticosteroids in the pediatric patient with meningococcemia is controversial. In those patients who are at risk for adrenal insufficiency (those with chronic steroid use, recent steroid use, purpura fulminans, or hypothalamic, pituitary, or adrenal disease) or those with persistent hypotension after adequate volume resuscitation and initiation of inotropic support, it may be prudent to administer hydrocortisone.28 However, the safety and efficacy of stress-dose steroids as adjunctive therapy for pediatric septic shock have not been established.46 Glucocorticoid administration does add potential risks, including anti-anabolic effects, attenuated immunity, depressed wound healing, calcium mobilization, impaired insulin action, and associated hyperglycemia and possible alteration in brain development.46
Correction of DIC is essential to prevent complications such as intracranial bleeding, GI hemorrhage, and bleeding into skin lesions. Fresh-frozen plasma, platelets, cryoprecipitate, and factor VII are administered as indicated to correct specific deficiencies. Packed red blood cells are administered to restore hemoglobin concentration and improve oxygen carrying capacity. For additional information about management of DIC, see Chapter 15.
Optimizing oxygenation and ventilation typically requires the use of supplementary oxygen and mechanical ventilation. The patient with depressed level of consciousness may require endotracheal intubation to establish a patent airway and reduce risk of aspiration. If increased capillary permeability produces pulmonary edema, noninvasive positive pressure ventilation (e.g., bi-level positive airway pressure) or ventilation with positive end-expiratory pressure will be needed. For additional information, see Chapter 9.
Treatment of septic shock is presented in more detail in Chapter 6.
Colonization with Multidrug Resistant Organisms
Over the past decade, antibiotic resistant organisms have emerged as a growing problem for patients with community- or healthcare-acquired infections.40 Some organisms carry genetic elements that confer resistance to multiple classes of antibiotics. These multidrug-resistant organisms (MDRO) can easily colonize hospitalized patients and are a common cause of invasive infection in critically ill children.
Infections Associated with Medical Devices or the Gastrointestinal Tract
Medical devices are among the greatest risk factors for healthcare-acquired infection (see Table 16-6).45 Central venous catheters, urinary catheters, and endotracheal tubes all provide portals of entry that permit organisms to migrate from the skin and mucous membranes to sterile body sites (Box 16-2). Implantable devices can also disrupt host defenses and provide a site sequestered from the surveillance of the immune system where bacteria can flourish. Box 16-3 lists common organisms related to medical devices.
The risk of device-related infections can be reduced by several important preventive measures.38 First, devices should only be used when they are essential to patient care. Strict aseptic technique must be observed during insertion or manipulation of a device. In addition, limiting the frequency of device manipulation reduces the likelihood of contamination. Finally, temporary devices should be removed as soon as they are no longer needed. Although the use of antimicrobial prophylaxis at the time of insertion is commonplace, the efficacy of such measures for many devices has not been established.
Central Venous Catheter Infections
Evidence-based practices that are associated with a reduced risk of catheter-associated infections include: (1) use of maximal sterile barrier precautions (e.g., cap, mask, sterile gown, sterile gloves, and large sterile drape) during catheter placement; (2) use of 2-3% chlorhexidine gluconate/70% isopropyl alcohol or other appropriate antiseptic agents to prepare the skin before placement or during routine care of the catheter; (3) prompt removal of catheters as soon as they are no longer required; and (4) strict adherence to appropriate hand hygiene practices.29 For additional information, see Box 22-6.
In studies performed in adult critical care units, antiseptic impregnated catheters have been associated with reduced rates of catheter associated bloodstream infection8b,10b (Box 16-4). Studies performed in children have described delayed time to infection but not reduced infection rate associated with use of antibiotic-impregnated catheters.10a
Box 16-4 Prevention of Vascular Catheter-Associated Infections
Catheter Selection
Select a catheter with the fewest lumens needed for management of the patient
Consider use of antiseptic- or antimicrobial-coated central catheters and silver-impregnated, collagen-cuffed catheters when infection rates remain high despite other measures (e.g., maximal barrier precautions)
For long-term (>30 days) access in children older than 4 years, consider peripherally inserted central catheters or tunneled or totally implantable devices
Urinary Tract Infections
Experts estimate that catheter-associated urinary tract infections are the most common device-associated infection among hospitalized patients, although the burden of disease is likely greater in adult, as compared with pediatric patients. Inappropriate and prolonged use of urinary catheters has been found in as many as 50% of patients who develop a catheter-associated urinary tract infection. Important strategies to prevent urinary catheter infections are: (1) limiting catheterization to those for whom it is medically necessary; (2) attention to strict aseptic technique during catheter insertion; and (3) removal of the catheter as soon as possible.24 Catheters are commonly left in place for too long; one study of adult medical inpatients revealed that clinicians were unaware that their patients had a catheter 28% of the time.34a
Ventilator-Associated Pneumonia (VAP)
An endotracheal tube provides an ideal portal of entry for the many organisms colonizing the oropharynx to migrate into the lower respiratory tract. The tube itself is an ideal substrate for the formation of biofilm. Pediatric patients appear to have lower risk of ventilator-associated pneumonia than adults, likely because they have fewer co-morbid conditions such as chronic heart or lung disease or immunosuppressing conditions. However, pediatric critical care clinicians have embraced strategies to reduce the risk of VAP, including whenever possible: (1) the use of noninvasive ventilation; (2) the avoidance of nasotracheal intubation; (3) the use of in-line suctioning to prevent the aspiration of pooled tracheal sections; and (4) the elevation of the head of the bed at 45 degrees from horizontal, especially for patients receiving enteral nutrition.12 Additional information is included in Box 22-7.
Clostridium difficile Colitis
In both children and adults, the most important cause of healthcare-acquired gastrointestinal disease is C. difficile. The epidemiology of C. difficile colitis is complex and somewhat confusing in the pediatric population.6 Up to 50% of healthy neonates may be colonized with toxigenic forms of C. difficile. After the age of 2 years, colonization rates decrease to adult levels of less than 5%, although higher levels are seen in hospitalized patients with or without exposure to antimicrobial agents.
Diagnostic tests
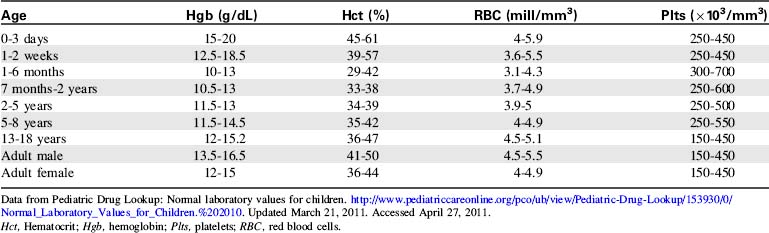
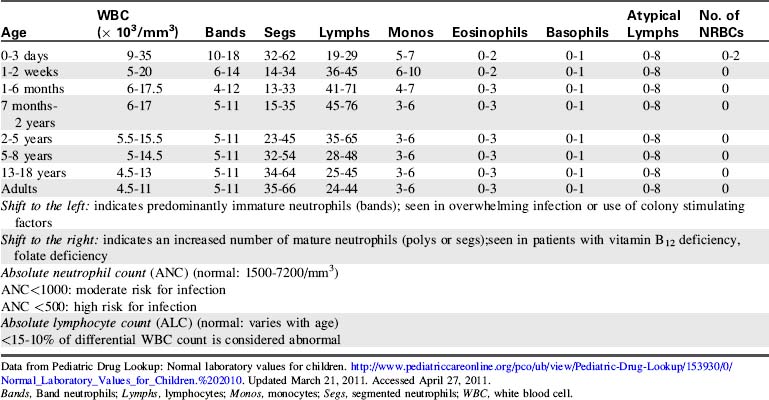
The most common diagnostic tests performed to evaluate critically ill children with a potential immune disorder or infectious process are hematology studies (including evaluation of hemoglobin and hematocrit) and evaluation of the white blood cell (WBC) count and differential. For quick reference, normal results of these and other common studies are listed in Tables 16-11 to 16-13.
| Diagnostic Test | Normal Values | Comments |
| Erythrocyte sedimentation rate (ESR) (Westergren) | Child: 0-20 mm/hour Adult male: 0-15 mm/hour Adult female: 0-20 mm/hour |
Nonspecific indicator of acute inflammatory response Measures the amount of RBCs that settle in 1 hour |
| Reticulocyte count | Newborns: 2-6% Children: 0-2.8% Adults: 0.5-1.5% |
|
| C-reactive protein (CRP) | <6 mg/dL | Increase within 6-8 h of onset of infection or injury CRP is produced by the liver during periods of inflammation |
Data from Pediatric Drug Lookup: Normal laboratory values for children. http://www.pediatriccareonline.org/pco/ub/view/Pediatric-Drug-Lookup/153930/0/Normal_Laboratory_Values_for_Children.%202010. Updated March 21, 2011. Accessed April 27, 2011.
1 Baines P.B., Hart C.A. Severe meningococcal disease in childhood. Br J Anesthes. 2003;90(1):72-83.
2 Bernard G.R., et al. Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group, Efficacy and safety of recombinant human activated protein C for severe sepsis. NEJM. 2001;344(10):699-709.
3 Bone R.C., et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis, the ACCP/SCCM Consensus Conference Committee. Chest. 1992;101:1644-1655.
4 Bone R.C. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med. 1996;125:680-777.
5 Brierley J., et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37(2):666-681.
6 Bryant K., McDonald L.C. Clostridium difficile infections in children. Pediatr Infect Dis J. 2009;28:145-146.
7 Buckley R.H. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;55:625-656.
8 . Burdette S.D., et al. Systemic inflammatory response syndrome, Emedicine, http://emedicine.medscape.com/article/168943-overview, 2011 Page updated July 20, 2010. Accessed April 26 Casey A.L., Elliott T.S. Prevention of central venous catheter-related infection: update. Br J Nurs. 2010;19(2):78. 80, 82
9 Centers for Disease Control and Prevention. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR. 2002;51(No. RR-16):32-34.
10 Chameides L., Samson R., Schexnayder S., Hazinski M.F.: Pediatric Advanced Life Support Provider Manual, Dallas. . American Heart Association 2011. editors: Part 10: management of shock
10a Chelliah A., Heydon K.H., Zaoutis T.E., et al. Observational trial of antibiotic-coated central venous catheters in critically ill pediatric patients. Pediatr Infect Dis J. 2007;26:816-820.
10b Cicalini S., Palmieri F., Petrosillo N. Clinical review: New technologies for prevention of intravascular catheter-related infections. Crit Care. 2007;8(3):157-162. Published online 2003 September 29. doi: 10.1186/cc2380
11 Coffin S.E., Zaoutis T.E. Healthcare-associated infections. In Long S., Pickering L., Prober C., editors: Principles and practice of pediatric infectious diseases, ed 3, Philadelphia: Churchill Livingstone, 2008.
12 Coffin S.E., et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Cont Hosp Epidemiol. 2008;29:S31-S40.
13 Cohn A.C. Immunizations in the United States: a rite of passage. Pediatr Clin North Am. 2005;52:669-693.
14 de la Morena M. Immunologic development and susceptibility to infection. In Long S., Pickering L., Prober C., editors: Principles and practice of pediatric infectious diseases, ed 3, Philadelphia: Churchill Livingstone, 2008.
15 Dellinger R.P., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. CCM. 2008;36(1):296-327.
16 Faust M.A., Cathie K., Levin M. Meningococcal infections. Emedicine http://emedicine.medscape.com/article/966333-overview, 2011. Updated July 20, 2010. Accessed April 27
17 Gobel B. Chemotherapy induced hypersensitivity reactions. Oncol Nursing Forum. 2005;32(5):1027-1055.
18 Goldstein B., et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2-8.
19 Grunebaum E., et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295(5):508-518. 2006
20 Hunstad D.A., St. Geme J.W. Mechanisms of pediatric bacterial disease. In Bergelson J.A., editor: Pediatric infectious disease requisites, ed 1, Philadelphia: Mosby, 2008.
21 Khilnani P., Deopujari S., Carcillo J. Recent advances in sepsis and septic shock. Ind J Pediatr. 2008;75:821-830.
22 Kleinman M., et al. Part 14, pediatric advanced life support in 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S876-S908.
23 Linzer J.F. Pediatrics, anaphylaxis. Emedicine http://emedicine.medscape.com/article/799744-overview, 2008. Accessed January 15, 2012
24 Lo E., et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Cont Hosp Epidemiol. 2008;29:901-994.
25 Marti-Carvajal A., Salanti G., Cardona A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Sys Rev. (1):2008. CD004388
26 Marzouk O., et al. Features and outcomes in meningococcal disease presenting with maculopapular rash. Arch Dis Child. 1991;66:485-487.
27 McGhee SA, Stiehm ER, McCabe ERB: Potential costs and benefits of newborn screening for severe combined immunodeficiency. J Pediatr 147:603-608.
28 Milonovich L. Meningococcemia: epidemiology, pathophysiology and management. J Pediatr Healthcare. 2006;21:75-80.
29 O’Grady N.P., et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2009;35:1281-1307.
30 Pathan N., Faust S.N., Levin M. Pathophysiology of meningococcal meningitis and septicaemia. Arch Dis Child. 2003;88(7):601-607.
31 Pediatric Drug Lookup. Normal laboratory values for children. http://www.pediatriccareonline.org/pco/ub/view/Pediatric-Drug-Lookup/153930/0/Normal_Laboratory_Values_for_Children.%202010, 2011. Updated March 21, 2011. Accessed April 27
32 Pollard A.J., et al. Emergency management of meningococcal disease: eight years on. Arch Dis Child. 2007;92:283-286.
33 Ralston M., et al, editors. Pediatric advanced life support provider manual. Dallas: American Heart Association, 2006.
34 Rosenstein N.E., et al. Medical progress: meningococcal disease. NEJM. 2001;344:1378-1388.
34a Saint S., Wiese J., Amory J.K., et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476-480.
35 Santhanam S., Tolan R.W. Sepsis. Emedicine http://emedicine.medscape.com/article/972559-overview, 2011. Updated April 25, 2011. Accessed April 27
36 Schwartz R.A., Sinha S. Severe combined immunodeficiency. Emedicine http://emedicine.medscape.com/article/888072, 2010. Updated September 29, 2010. Accessed April 27
37 Siegel J.D., Grossman L. Pediatric infection prevention and control. In Long S., Pickering L., Prober C., editors: Principles and practice of pediatric infectious diseases, ed 3, Philadelphia: Churchill Livingstone, 2008.
38 Siegel J.D., et al. Preventing transmission of infectious agents in healthcare settings. http://www.cdc.gov/hicpac/2007IP/2007isolationPrecautions.html.
39 Skippen P., et al. Sepsis and septic shock: progress and future considerations. Ind J Pediatr. 2008;75:599-607.
40 Stockwell J.A. Nosocomial infections in pediatric intensive care unit. Ped Crit Care Med. 2007;8:S21-S37.
41 Thielen U., et al. Management of invasive meningococcal disease in children and young people: summary of SIGN guidelines. BMJ. 2008;336:1367-1370.
42 Venes D., Taber C.W., editors. Taber’s cyclopedic medical dictionary, ed 20, Philadelphia: Davis, 2005.
43 Wong H. Sepsis/inflammatory response syndrome. In: Conway E., editor. Pediatric multiprofessional critical care review. Des Plaines, IL: Society of Critical Care Medicine, 2006.
44 Yee A., et al. Severe combined immunodeficiency: a national surveillance study. Pediatr Allergy Immunol. 2008;19:298-302.
45 Zaoutis T.E., Coffin S.E. Clinical syndromes of device-associated infections. In Long S., Pickering L., Prober C., editors: Principles and practice of pediatric infectious diseases, ed 3, Philadelphia: Churchill Livingstone, 2008.
46 Zimmerman J.J. A history of adjunctive glucocorticoid treatment for pediatric sepsis: moving beyond steroid pulp fiction toward evidence-based medicine. Pediatr Crit Care Med. 2007;8(6):530-539.




 Before donning sterile gloves
Before donning sterile gloves