Chapter 165 Immunization Practices
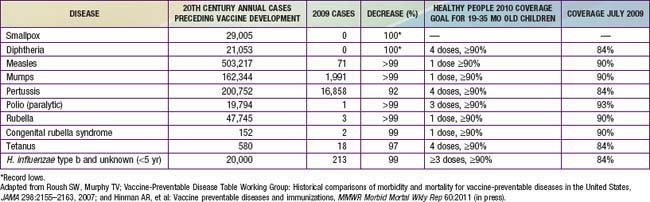
Immunization is one of the most beneficial and cost-effective disease-prevention measures. As a result of effective and safe vaccines, smallpox has been eradicated, polio is close to worldwide eradication, and measles and rubella are no longer endemic in the USA. The incidence of most other vaccine-preventable diseases of childhood has been reduced by ≥99% from the annual morbidity prior to development of the corresponding vaccine (Table 165-1). An analysis of effective prevention measures recommended for widespread use by the U.S. Preventive Services Task Force reported that childhood immunization received a perfect score, based on clinically preventable disease burden and cost-effectiveness.
Passive Immunity
The major indications for passive immunity are to provide protection to immunodeficient children with B-lymphocyte defects who have difficulties making antibodies, persons exposed to infectious diseases or who are at imminent risk of exposure where there is not adequate time for them to develop an active immune response to a vaccine, and persons with an infectious disease as part of specific therapy for that disease (Table 165-2).
Table 165-2 IMMUNE GLOBULIN AND ANIMAL ANTISERA PREPARATIONS
| PRODUCT | MAJOR INDICATIONS |
|---|---|
| Immune globulin for intramuscular injection | Replacement therapy in primary immunodeficiency disorders |
| Hepatitis A prophylaxis | |
| Measles prophylaxis | |
| Intravenous mmunoglobulin (IVIG) | Replacement therapy in primary immune-deficiency disorders |
| Kawasaki disease | |
| Immune-mediated thrombocytopenia | |
| Pediatric HIV infection | |
| Hypogammaglobulinemia in chronic B-cell lymphocytic leukemia | |
| Hematopoietic cell transplantation in adults to prevent graft-versus host disease and infection | |
| May be useful in a variety of other conditions | |
| Hepatitis B immune globulin (IM) | Postexposure prophylaxis |
| Prevention of perinatal infection in infants born to HBsAg+ mothers | |
| Rabies immune globulin (IM) | Postexposure prophylaxis |
| Tetanus immune globulin (IM) | Wound prophylaxis |
| Treatment of tetanus | |
| Varicella-zoster immune globulin (VZIG) (IM) or IVIG | Postexposure prophylaxis of susceptible people at high risk for complications from varicella |
| Cytomegalovirus IVIG | Prophylaxis of disease in seronegative transplant recipients |
| Palivizumab (monoclonal antibody) (IM) | Prophylaxis for infants against respiratory syncytial virus (RSV) (Chapter 252) |
| Vaccinia immune globulin (IV) | Prevent or modify serious adverse events following smallpox vaccination due to vaccinia replication |
| Botulism IVIG human | Treatment of infant botulism |
| Diphtheria antitoxin, equine | Treatment of diphtheria |
| Trivalent botulinum (A,B,E) and bivalent (A,B) botulinum antitoxin, equine | Treatment of food and wound botulism |
From Passive immunization. In Pickering LK, Baker CJ, Kimberlin DW, et al, editors: Red book 2006: report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics.
Intramuscular Immunoglobulin
Specific Immune Globulin Preparations
Hyperimmune globulin preparations are derived from donors with high titers of antibodies to specific agents and designed to provide protection against those agents (see Table 165-2).
Monoclonal Antibodies
Monoclonal antibodies are antibody preparations produced against a single antigen. They are mass-produced from a hybridoma, created by fusing an antibody-producing B cell with a fast-growing immortal cell such as a cancer cell. A major monoclonal antibody used in infectious diseases is palivizumab, which can prevent severe disease from respiratory syncytial virus (RSV) among children ≤24 mo of age with chronic lung disease (CLD, also called bronchopulmonary dysplasia), with a history of premature birth or with congenital heart lesions or with neuromuscular diseases. The American Academy of Pediatrics (AAP) has developed specific recommendations for use of palivizumab (Chapter 252). RSV-IVIG, a hyperimmune globulin formulated for intravenous administration, is no longer produced in the USA. Monoclonal antibodies also are used to prevent transplant rejection and to treat some types of cancer and autoimmune diseases. Monoclonal antibodies against interleukin 2 (IL-2) and tumor necrosis factor-α (TNF-α) are being used as part of the therapeutic approach to patients with a variety of malignant and autoimmune diseases.
Active Immunization
Vaccines are defined as whole or parts of microorganisms administered to prevent an infectious disease. Vaccines can consist of whole inactivated microorganisms (e.g., polio and HepA), parts of the organism (e.g., acellular pertussis, HPV, and HepB), polysaccharide capsules (e.g., pneumococcal and meningococcal polysaccharide vaccines), polysaccharide capsules conjugated to protein carriers (e.g., Hib, pneumococcal, and meningococcal conjugate vaccines), live-attenuated microorganisms (measles, mumps, rubella, varicella, rotavirus, and live-attenuated influenza vaccines), and toxoids (tetanus and diphtheria) (Table 165-3). A toxoid is a modified bacterial toxin that is made nontoxic but is still able to induce an active immune response against the toxin.
| PRODUCT | TYPE |
|---|---|
| Anthrax vaccine adsorbed | Cell free filtrate of components including protective antigen |
| Bacille Calmette-Guérin (BCG) vaccine | Live-attenuated mycobacterial strain used prevent tuberculosis in very limited circumstances |
| Diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine | Toxoids of diphtheria and tetanus and purified and detoxified components from Bordetella pertussis |
| DTaP with Haemophilus influenzae type b (DTaP/Hib) | DTaP and Hib polysaccharide conjugated to tetanus toxoid |
| DTaP–hepatitis B–inactivated polio vaccine (DTaP-HepB-IPV) | DTaP with hepatitis B surface antigen produced through recombinant techniques in yeast with inactivated whole polioviruses |
| DTaP with IPV and Hib (DTaP-IPV/Hib) | DTaP with inactivated whole polio viruses and Hib polysaccharide conjugated to tetanus toxoid |
| DTaP and inactivated polio vaccine (DTaP-IPV) | DTaP with inactivated whole polio viruses |
| Hib conjugate vaccine (Hib) | Polysaccharide conjugated to either tetanus toxoid or meningococcal group B outer membrane protein |
| Hepatitis A vaccine (HepA) | Inactivated whole virus |
| Hepatitis A-hepatitis B vaccine (HepA-HepB) | Combined hepatitis A and B vaccine |
| Hepatitis B vaccine (HepB) | HBsAg produced through recombinant techniques in yeast |
| Hepatitis B-Hib vaccine (Hib-HepB) | Combined hepatitis B–Hib vaccine; the Hib component is polysaccharide conjugated to meningococcal group B outer membrane protein |
| Human papillomavirus vaccine (bivalent) (HPV2) and (quadrivalent) (HPV4) | The L1 capsid proteins of HPV types 6, 11, 16, 18 and to prevent cervical cancer and genital warts (HPV4) and types 16 and 18 to prevent cervical cancer (HPV2) |
| Influenza virus vaccine inactivated (TIV) | Trivalent (A/H3N2, A/H1N1, and B) split and purified inactivated vaccine containing the hemagglutinin (H) and neuraminidase (N) of each type and other components |
| Influenza virus vaccine live, intranasal (LAIV) | Live-attenuated, temperature-sensitive, cold-adapted trivalent vaccine containing the H and N genes from the wild strains reassorted to have the 6 other genes from the cold-adapted parent |
| Japanese encephalitis vaccine | Inactivated whole virus that is purified |
| Measles, mumps, rubella (MMR) vaccine | Live-attenuated viruses |
| Measles, mumps, rubella, varicella (MMRV) vaccine | Live-attenuated viruses |
| Meningococcal conjugate vaccine against serogroups A, C, W135, and Y (MCV4) | Polysaccharide from each serogroup conjugated to diphtheria toxoid or CRM 197 |
| Meningococcal polysaccharide vaccine against serogroups A, C, W135, and Y (MPSV4) | Polysaccharides from each of the serogroups |
| Pneumococcal conjugate vaccine (13 valent) (PCV13) | Pneumococcal polysaccharides conjugated to a nontoxic form of diphtheria toxin CRM197 |
| Contains 13 serotypes that accounted for >80% of invasive disease in young children prior to vaccine licensure. | |
| Pneumococcal polysaccharide vaccine (23 valent) (PPSV23) | Pneumococcal polysaccharides of 23 serotypes responsible for 85-90% of bacteremic disease in the USA |
| Poliomyelitis (inactivated, enhanced potency) (IPV) | Inactivated whole virus |
| Rabies vaccines (human diploid and purified chick embryo cell) | Inactivated whole virus |
| Rotavirus vaccines (RV5 and RV1) | Bovine rotavirus pentavalent vaccine (RV-5) live reassortment attenuated virus, and human live-attenuated virus (RV1) |
| Smallpox vaccine | Vaccinia virus, an attenuated pox virus that provides cross-protection against smallpox |
| Tetanus and diphtheria toxoids, adsorbed (Td, adult use) | Tetanus toxoid plus a reduced quantity of diphtheria toxoid compared to diphtheria toxoid used for children <7 yr of age |
| Tetanus and diphtheria toxoids adsorbed plus acellular pertussis (Tdap) vaccine | Tetanus toxoid plus a reduced quantity of diphtheria toxoid plus acellular pertussis vaccine to be used in adolescents and adults and in children 7 through 9 yr of age who have not been appropriately immunized with DTaP |
| Typhoid vaccine (polysaccharide) | Vi capsular polysaccharide of Salmonella typhi |
| Typhoid vaccine (oral) | Live-attenuated Ty21a strain of Salmonella typhi |
| Varicella vaccine | Live-attenuated Oka strain |
| Yellow fever vaccine | Live-attenuated 17D strain |
Data from Centers for Disease Control and Prevention: U.S. vaccine names (website). www.cdc.gov/vaccines/about/terms/USvaccines.html. Accessed March 4, 2011.
Vaccination System in the USA
Vaccine Development
Basic scientific knowledge about an organism, its pathogenesis, and the immune responses thought to be associated with protection are financed primarily through government sponsorship of academic research, although private industry plays a major role (Fig. 165-1). Private industry usually assumes the lead role for guiding potential vaccine candidates through preclinical testing in humans into human clinical trials. There are three phases of prelicensure clinical trials: phase I, involving generally <100 participants to gauge safety and dosing; phase II, involving several hundred or more participants to refine safety and dosing; and phase III or pivotal trials that can involve thousands or tens of thousands of participants. Phase III trials are the major basis for licensure. Following successful clinical development, the sponsor applies to the FDA for vaccine licensure. Estimates for the cost of development for each vaccine range to $800 million or more. Following licensure by the FDA, postlicensure monitoring is performed on hundreds of thousands to millions of people to monitor safety and effectiveness.
Vaccine Policy
There are 2 major committees that make vaccine policy recommendations for children: the Committee on Infectious Diseases (COID) of the AAP (the Red Book Committee) and the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC). At least annually, the AAP, the ACIP, and the American Academy of Family Physicians (AAFP) issue a harmonized childhood and adolescent immunization schedule (www.cdc.gov/vaccines/recs/schedules/default.htm). The COID consists primarily of academic pediatric infectious disease specialists with liaisons from practicing pediatricians, professional organizations, and government agencies including the FDA, CDC, and National Institutes of Health (NIH). Recommendations of the COID must be approved by the AAP Board of Directors. The ACIP consists of 15 members who are academic infectious disease experts (for both children and adults), family physicians, state and local public health officials, nurses, and consumers. The ACIP also has extensive liaison representation from major medical societies, government agencies, managed care, and others. The AAP recommendations are published in the Red Book and in issues of Pediatrics. The ACIP recommendations, available at www.cdc.gov/vaccines/pubs/ACIP-list.htm, are official only after approval by the CDC director, which leads to publication in the Morbidity and Mortality Weekly Report (MMWR).
Vaccine Financing
The greatest portion comes from the Vaccines for Children (VFC) program, a federal entitlement program. The VFC program covers children on Medicaid, children without any insurance (uninsured), and Native Americans and Alaska Natives. In addition, children who have insurance but whose insurance does not cover immunization (underinsured) can be covered through VFC, but only if they go to a federally qualified health center (FQHC) (http://www.cms.gov/center/fqhc.asp). In contrast to other public funding sources that require approval of discretionary funding by legislative bodies, VFC funds are immediately available for new recommendations provided the ACIP votes the vaccine and the recommendation for its use into the VFC program, the federal government negotiates a contract, and the Office of Management and Budget (OMB) apportions funds. The VFC program can provide free vaccines to participating private providers.
Vaccine Safety Monitoring
Monitoring vaccine safety is the responsibility of the FDA, CDC, and vaccine manufacturers. A critical part of that monitoring depends on reports to the Vaccine Adverse Event Reporting System (VAERS). Adverse events following immunization can be reported by calling 1-800-822-7967 or completing a VAERS form that can be obtained from www.vaers.hhs.gov. Individual VAERS case reports may be helpful in generating hypotheses about whether vaccines are causing certain clinical syndromes, but in general they are not helpful in evaluating the causal role of vaccines in the adverse event. This is because most clinical syndromes that follow vaccination are similar to syndromes that occur in the absence of vaccination. For causality assessment, epidemiologic studies are often necessary, comparing the incidence rate of the adverse event after vaccination with the rate in the unvaccinated. A statistically significant higher rate in the vaccinated would be consistent with causation.
The Vaccine Safety Datalink (VSD) consists of inpatient and outpatient records of some of the largest managed-care organizations in the USA and facilitates causality evaluation. In addition, the clinical immunization safety assessment network (CISA) has been established to advise primary care physicians on evaluation and management of adverse events (www.cdc.gov/vaccinesafety/Activities/CISA.html). The Institute of Medicine (IOM) has reviewed independently a variety of vaccine safety concerns (available at www.iom.edu/imsafety and Table 165-4). In no instance did the IOM find that evidence favored acceptance of a causal link between the postulated adverse event and vaccines.
Table 165-4 INSTITUTE OF MEDICINE IMMUNIZATION SAFETY REVIEW COMMITTEE REPORTS, 2001-2004
| REPORT | DATE OF RELEASE |
|---|---|
| Measles-mumps-rubella vaccine and autism | April 2001 |
| Thimerosal-containing vaccines and neurodevelopmental disorders | October 2001 |
| Multiple immunizations and immune dysfunction | February 2002* |
| Hepatitis B vaccine and demyelinating neurologic disorders | May 2002 |
| SV40 contamination of polio vaccine and cancer | October 2002 |
| Vaccinations and sudden unexpected death in infancy | March 2003 |
| Influenza vaccines and neurologic complications | October 2003 |
| Vaccines and autism | May 2004 |
Data from http://www.iom.edu/imsafety.
* Reviews relationship of vaccines to asthma, diabetes, and heterologous infections.
From Cohn AC et al: Immunizations in the US: a rite of passage, Pediatr Clin North Am 52:669–693, 2005.
The National Vaccine Injury Compensation Program (NVICP), established in 1988, is designed to compensate persons injured by vaccines in the childhood and adolescent immunization schedule. The program is funded through an excise tax of $0.75 per disease prevented per dose. As of 2010, all of the routinely recommended vaccines that protect children against 16 diseases are covered by this program. The NVICP was established to provide a no-fault system. There is a table of related injuries and time frames. All persons alleging injury from covered vaccines must first file with the program. If the injury meets the requirements of the table, compensation is automatic. If not, the claimant has the responsibility to prove causality. If compensation is accepted, the claimant cannot sue the manufacturer or physician administering the vaccine. If the claimant rejects the judgment of the compensation system, he or she can enter the tort system, but this has only occurred rarely. Information on the NVICP is available at 1-800-338-2382 and www.hrsa.gov/vaccinecompensation/. All physicians administering a vaccine covered by the program are required by law to give the approved Vaccine Information Statement (VIS) to the child’s parent or guardian at each visit before administering vaccines. Information on the VIS can be obtained from www.cdc.gov/vaccines/pubs/vis/default.htm.
Vaccine Delivery
To ensure potency, vaccines should be stored at recommended temperatures before and after reconstitution (www.cdc.gov/vaccines/recs/storage/default.htm). Expiration dates should be noted, and expired vaccine should be discarded. Lyophilized vaccines often have long shelf lives. However, the shelf life of reconstituted vaccines generally is short, ranging from 30 min for the varicella vaccine to 8 hr for the MMR vaccine.
Recommended Immunization Schedule
All children in the USA should be vaccinated against 15 diseases (Figs. 165-2 and 165-3) (annually updated schedule available at http://www.cdc.gov/vaccines/recs/acip/default.htm). Girls 11-12 yr old should also receive HPV. Boys may receive HPV4 to prevent genital warts, but this is a permissive recommendation.

Figure 165-2 Recommended immunization schedule for persons aged 0 through 6 years—USA, 2011.
(From www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed February 14, 2011.)

Figure 165-3 Recommended immunization schedule for persons aged 7 through 18 years—USA, 2011.
(From www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed February 14, 2011.)
The recommended childhood and adolescent immunization schedule establishes a routine adolescent visit at 11-12 yr of age. MCV4 and a Tdap booster should be administered during this visit and HPV can be started. Influenza vaccine should be administered annually. In addition, the 11-12 yr old visit is also an opportune time to review all of the immunizations the child has received previously, to provide any doses that were missed, and to review other age-appropriate preventive services. The 11-12 year old visit establishes an important platform for incorporating other vaccines. Information on the current status of new vaccine licensure and recommendations for use can be obtained at aapredbook.aappublications.org/news/vaccstatus.shtml and www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM093833.
For children who are at least 1 mo behind in their immunizations, catch-up immunization schedules are available for children 4 mo to 18 yr of age (Fig. 165-4); also available for children <6 yr of age at www.cdc.gov/vaccines/recs/schedules/child-schedule.htm#catchup) is an interactive immunization scheduler.
Vaccines Recommended in Special Circumstances
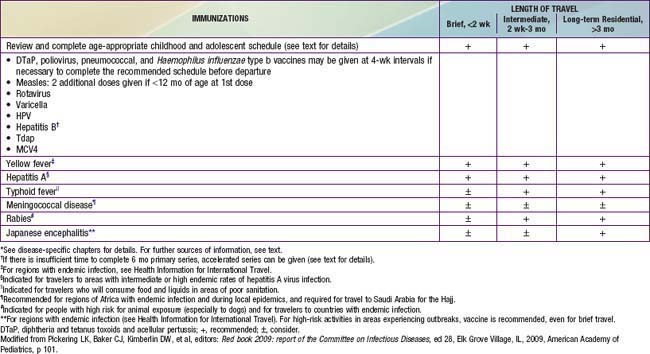
A variety of vaccines are available for children who will be traveling to areas of the world where certain infectious diseases are common in addition to vaccines in the recommended childhood and adolescent schedule (Table 165-5). Vaccines for travelers include typhoid fever, HepA, HepB, Japanese encephalitis, MCV4 or MPS4, rabies, and yellow fever, depending on the location and circumstances of travel. Measles is endemic in many parts of the world. Children 6-11 mo of age may receive a dose of MMR before travel. However, doses of measles vaccine received before the 1st birthday should not be counted in determining compliance with the recommended 2-dose MMR schedule. Additional information on vaccines for international travel can be found at www.cdc.gov/travel/content/vaccinations.aspx.
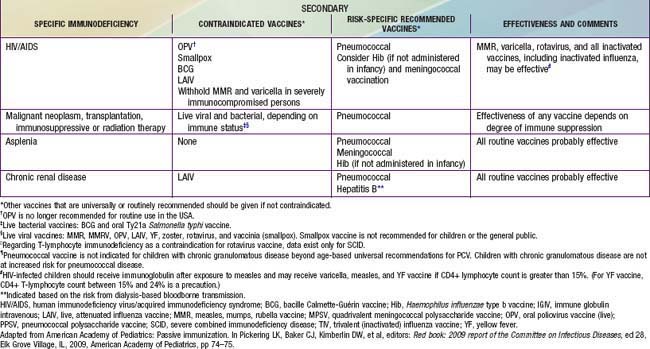
Vaccine recommendations for children with immunocompromise, either primary (inherited) or secondary (acquired), vary according to the underlying condition, the degree of immune deficit, the risk for exposure to disease, and the vaccine (Table 165-6). Immunization of children with immunocompromise poses the following potential concerns: the incidence or severity of some vaccine-preventable diseases is higher, and therefore certain vaccines are recommended specifically for certain conditions; vaccines may be less effective during the period of altered immunocompetence and may need to be repeated when immune competence is restored; and because of altered immunocompetence, some children and adolescents may be at increased risk for an adverse event following receipt of a live viral vaccine. Live-attenuated vaccines generally are contraindicated in immunocompromised people. The exceptions include MMR, which may be given to a child with HIV infection provided the child is asymptomatic or symptomatic without evidence of severe immunosuppression, and varicella vaccine, which may be given to HIV-infected children if the CD4+ lymphocyte count is at least 15%. MMRV is not recommended in these situations.
Preterm infants generally can be vaccinated at the same chronologic age as full-term infants according to the recommended childhood immunization schedule. An exception is the birth dose of HepB vaccine. Infants weighing ≥2 kg and who are stable may receive a birth dose. However, HepB vaccination should be deferred in infants weighing <2 kg at birth until 30 days of age, if born to an HBsAg-negative mother. All preterm, low birth weight infants born to HBsAg-positive mothers should receive HepB IG and HepB vaccine within 12 hr of birth. However, such infants should receive an additional 3 doses of vaccine starting at 30 days of age (see Fig. 165-2).
Many agents have been considered for potential use as weapons of bioterrorism. For most of these agents, licensed vaccines are not available in the USA, although vaccines are being developed for some organisms, including botulinum toxoid, Ebola virus, plague, and others. Anthrax vaccine and smallpox (vaccinia) vaccine are available, but they are not recommended for children. Both are indicated in a pre-exposure setting only for selected adults with potential occupational risks of exposure (www.bt.cdc.gov/ provides details on which groups are recommended for vaccination).
Precautions and Contraindications
Vaccines usually should be deferred in children with moderate to severe acute illnesses, regardless of the presence of fever, until the child recovers. However, children with mild illnesses may be vaccinated. Studies of undervaccinated children have documented opportunities that were missed because mild illness was used as an invalid contraindication. Complete tables of contraindications and contraindication misperceptions can be found at www.cdc.gov/vaccines/recs/vac-admin/contraindications.htm.
Improving Immunization Coverage
Standards for child and adolescent immunization practices have been developed to support achievement of high levels of immunization coverage while providing vaccines in a safe and effective manner and educating parents about risks and benefits of vaccines (Table 165-7).
Table 165-7 STANDARDS FOR CHILD AND ADOLESCENT IMMUNIZATION PRACTICES
AVAILABILITY OF VACCINES
ASSESSMENT OF VACCINATION STATUS
EFFECTIVE COMMUNICATION ABOUT VACCINE BENEFITS AND RISKS
PROPER STORAGE AND ADMINISTRATION OF VACCINES AND DOCUMENTATION OF VACCINATIONS
IMPLEMENTATION OF STRATEGIES TO IMPROVE VACCINATION COVERAGE
From the National Vaccine Advisory Committee: Standards for child and adolescent immunization practices. Pediatrics 112:958–963, 2003.
Some parents refuse immunization for their child. Pediatricians should try to open a dialogue with such parents to understand the reasons for refusal and try to work with them to overcome their concerns over time during the course of visits. Discussion should be based on the reason for refusal and the knowledge of the parent. Pediatricians should refer patients to reputable sources for vaccine information (Table 165-8) and discuss risks and benefits of vaccines. Physician concerns about liability should be addressed by appropriate documentation of discussions in the chart. The Committee on Bioethics of the AAP has published guidelines for dealing with parents’ refusal of immunization. Physicians also might wish to consider having parents sign a refusal waiver. A sample of a refusal to vaccinate waiver can be found at www.aap.org/immunization/pediatricians/pdf/ReducingVaccineLiability.pdf.
| ORGANIZATION | WEBSITE |
|---|---|
| HEALTH PROFESSIONAL ASSOCIATIONS | |
| American Academy of Family Physicians (AAFP) | www.familydoctor.org/online/famdocen/home.html |
| American Academy of Pediatrics (AAP) | www.aap.org/ |
| AAP Childhood Immunization Support Program | www.aap.org/immunization/ |
| American Medical Association (AMA) | www.ama-assn.org/ |
| American Nurses Association (ANA) | www.nursingworld.org/ |
| Association of State and Territorial Health Officials (ASTHO) | www.astho.org/ |
| Association of Teachers of Preventive Medicine (ATPM) | www.atpm.org/ |
| National Medical Association (NMA) | www.nmanet.org/ |
| NONPROFIT GROUPS AND UNIVERSITIES | |
| Albert B. Sabin Vaccine Institute | www.sabin.org/ |
| Allied Vaccine Group (AVG) | www.vaccine.org/ |
| Children’s Vaccine Program | www.path.org/vaccineresources/ |
| Every Child By Two (ECBT) | www.ecbt.org/ |
| Global Alliance for Vaccines and Immunization (GAVI) | www.gavialliance.org/ |
| Health on the Net Foundation (HON) | www.hon.ch/ |
| National Healthy Mothers, Healthy Babies Coalition (HMHB) | www.hmhb.org/ |
| Immunization Action Coalition (IAC) | www.immunize.org/ |
| Institute for Vaccine Safety (IVS), Johns Hopkins University | www.vaccinesafety.edu/ |
| Institute of Medicine | www.iom.edu/Activities/PublicHealth/ImmunizationSafety.aspx |
| National Alliance for Hispanic Health | www.hispanichealth.org/ |
| National Network for Immunization Information (NNii) | www.immunizationinfo.org/ |
| Parents of Kids with Infectious Diseases (PKIDS) | www.pkids.org/ |
| The Vaccine Education Center at the Children’s Hospital of Philadelphia | www.chop.edu/service/vaccine-education-center/home.html |
| The Vaccine Place | www.vaccineplace.com/?fa=home |
| GOVERNMENT ORGANIZATIONS | |
| Centers for Disease Control and Prevention (CDC) | |
| Public Health Image Library | www.phil.cdc.gov/phil/home.asp |
| Travelers’ Health | www.cdc.gov/travel/ |
| Vaccines and Immunizations | www.cdc.gov/vaccines/ |
| Vaccine Safety | www.cdc.gov/vaccinesafety/index.html |
| Department of Health and Human Services (HHS) | |
| National Vaccine Program Office (NVPO) | www.hhs.gov/nvpo/ |
| Health Resources and Services Administration | |
| National Vaccine Injury Compensation Program | www.hrsa.gov/vaccinecompensation/ |
| National Institute of Allergy and Infectious Diseases (NIAID) | |
| Vaccines | www.niaid.nih.gov/topics/vaccines/Pages/Default.aspx |
| World Health Organization (WHO) | |
| Immunization, Vaccines, and Biologicals | www.who.int/immunization/en/ |
American Academy of Pediatrics. Policy statement: recommended childhood and adolescent immunization schedules—United States, 2011. Pediatrics. 2011;127(20):387-388.
American Academy of Pediatrics Committee on Practice and Ambulatory Medicine and Council on Community Pediatrics. Policy statement: increasing immunization coverage. Pediatrics. 2010;125:1295-1304.
Atkinson W, Wolfe C, Hamborsky J, McIntyre L, editors. Epidemiology and prevention of vaccine-preventable diseases, ed 11, Washington, DC: Public Health Foundation, 2009.
Berberich FR, Landman Z. Reducing immunization discomfort in 4- to 6-year-old children: a randomized clinical trial. Pediatrics. 2009;124:e203-e209.
Bryant KA, Block SL, Baker SA, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:866-875.
Centers for Disease Control and Prevention. General recommendations on immunization. MMWR. 2011;60(2):3-61.
Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine. MMWR. 2011;59(No. RR-11):1-19.
Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2011. MMWR. 2011;60(4):1-4.
Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the advisory committee on immunization practices, 2010. MMWR. 2011;60(1):13-16.
Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—advisory committee on immunization practices (ACIP), 2010. MMWR. 2011;60(3):72-76.
Centers for Disease Control and Prevention. ACIP recommendations (Advisory Committee on Immunization Practices). (website) www.cdc.gov/vaccines/pubs/ACIP-list.htm Accessed March 4, 2011
Centers for Disease Control and Prevention. Use of combination measles, mumps, rubella, and varicella vaccine. MMWR Recomm Rep. 2010;59(RR-3):1-12.
Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58:51-52.
Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2006;55(RR 15):1-48.
Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2009. MMWR. 2009;57(51&52):Q1-Q4.
Centers for Disease Control and Prevention. Vaccine preventable deaths and the global immunization vision and strategy, 2006–2015. JAMA. 2006;295:2840-2842.
Cohn AC, Broder KR, Pickering LK. Immunizations in the US: a rite of passage. Pediatr Clin North Am. 2005;52:669-693.
Diekema DS. American Academy of Pediatrics Committee on Bioethics: responding to refusals of immunization of children. Pediatrics. 2005;115:1428-1431.
Kelso JM, Li JT. Adverse reactions to vaccines. Ann Allerg Asthma Immunol. 2009;103:S1-S14.
The Medical Letter. A new conjugate meningococcal vaccine (Menveo). Med Lett. 2010;52(1343):59-60.
Omer SB, Salmon DA, Orenstein WA, et al. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360:1981-1988.
O’Brien KL, Levine OS. Effectiveness of pneumococcal conjugate vaccine. Lancet. 2006;368:1469-1470.
Orenstein WA, Douglas RG, Rodewald LE, Hinman AR. Immunizations in the US: success, structure and stress. Health Affairs. 2005;24:559-610.
O’Ryan M, Matson DO. New rotavirus vaccines: renewed optimism. J Pediatr. 2006;149:448-451.
Pickering LK, Baker CJ, Kimberlin DW, Long SS, editors. Red book: 2009 report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL: American Academy of Pediatrics, 2009.
Pickering LK, Baker CS, Freed GL, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Disease Society of America. Clin Infect Dis. 2009;49:817-840.
Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines, ed 5, Philadelphia: Elsevier, 2008.
Prymula R, Siegrist CA, Chilbek R, et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomized controlled trials. Lancet. 2009;374:1339-1350.
Tan LKK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitides. N Engl J Med. 2010;362(16):1511-1520.
Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287-294.
Zimmerman RK. Size of the needle for infant vaccination: longer needles reduce incidence of local reactions. BMJ. 2006;333:563-564.
165.1 International Immunization Practices
Vaccines are used to prevent infectious diseases around the world. However, the types of vaccines in use, the indications and contraindications, and the immunization schedules vary substantially. Most developing countries follow the immunization schedules promulgated by the World Health Organization’s Immunization Programme; the latest update is available at www.who.int/immunization/policy/Immunization_routine_table2.pdf.
Immunization schedules in the industrialized world are substantially more variable than in the developing world. Immunization recommendations for Canada are developed by the Canadian National Advisory Committee on Immunization (NACI) but are implemented somewhat differently by each province. The Canadian schedule is similar to the U.S. immunization schedule (http://www.phac-aspc.gc.ca/im/is-cv/index-eng.php). Conjugate meningococcal serogroup C vaccine (MCV-C) is recommended in a 3-dose series at 2, 4, and 6 mo of age. A single dose is recommended after 12 mo of age if the child has never been immunized or has received <3 doses in infancy. The province of Ontario, Canada, has a recommendation for annual vaccination of all persons 6 mo and older with trivalent influenza vaccine.
There is tremendous variation in vaccines used and the immunization schedules recommended in Europe. European immunization schedules can be reviewed at www.who.int/vaccines/globalsummary/immunization/ScheduleSelect.cfm. As an example, the UK developed an immunization schedule during the late 1980s that includes visits at 2, 3, and 4 mo of age where a combination DTaP-Hib-IPV vaccine is administered. Following evidence that a three-dose series of Hib vaccine at these ages was insufficient to ensure long-term, high-grade protection, a booster dose was added at 12 mo of age. MMR is recommended in a 2-dose schedule at 13 mo and 3-5 yr of age. During the 2nd MMR visit, a booster of DTaP and IPV is provided. A Td/IPV booster is recommended between 13 and 18 yr of age. PCV7 is recommended at 2, 4, and 13 mo of age. The UK was the first country to use MCV-C vaccine during a massive catch-up campaign for children, adolescents, and young adults. The effectiveness of the vaccine in the 1st year was 88% or greater, and herd immunity was induced with an approximate two-thirds reduction in the incidence among unvaccinated children. MCV-C is administered at 3, 4, and 12 mo of age. In September 2008, HPV vaccine was recommended for girls 12-13 yr old. As of July 2009, the UK schedule did not include HepB vaccine, varicella vaccine, or influenza vaccine for universal childhood immunization (see www.immunisation.nhs.uk/).
American Academy of Pediatrics Committee on Infectious Diseases. Recommended childhood and adolescent immunization schedule—United States, 2007. Pediatrics. 2007;119:207-208.
Atkinson W, Hamborsky J, McIntyre L, et al, editors. Epidemiology and prevention of vaccine-preventable diseases, ed 9, Washington, DC: Public Health Foundation, 2006.
Centers for Disease Control and Prevention. General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2007;55:Q1-Q3.
Centers for Disease Control and Prevention. Recommended adult immunization schedule, United States, October 2006–September 2007. MMWR Morb Mortal Wkly Rep. 2006;55:Q1-Q4.
Centers for Disease Control and Prevention. Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1120-1124.
Centers for Disease Control and Prevention. Progress in global measles control and mortality reduction, 2000–2007. MMWR Morb Mortal Wkly Rep. 2008;57:1303-1306.
Centers for Disease Control and Prevention. vaccine preventable deaths and the global immunization vision and strategy, 2006–2015. JAMA. 2006;295:2840-2842.
Clark HF, Offit PA, Plotkin SA, et al. The new pentavalent rotavirus vaccine composed of bovine (strain WC3) human rotavirus reassortments. Pediatr Infect Dis J. 2006;25:577-582.
Cohn AC, Broder KR, Pickering LK. Immunizations in the US: a rite of passage. Pediatr Clin North Am. 2005;52:669-693.
Diekema DS. American Academy of Pediatrics Committee on Bioethics: Responding to refusals of immunization of children. Pediatrics. 2005;115:1428-1431.
O’Brien KL, Levine OS. Effectiveness of pneumococcal conjugate vaccine. Lancet. 2006;368:1469-1470.
Omer SB, Pan WKY, Halsey NA, et al. Nonmedical exemptions to school immunization requirements: secular trends and association of state policies with pertussis incidence. JAMA. 2006;296:1757-1763.
Orenstein WA, Douglas RG, Rodewald LE, et al. Immunizations in the US: success, structure and stress. Health Affairs. 2005;24:559-610.
O’Ryan M, Matson DO. New rotavirus vaccines: renewed optimism. J Pediatr. 2006;149:448-451.
Pickering LK, Baker CJ, Long SS, et al, editors. Red book: 2009 report of the Committee on Infectious Diseases, ed 28. American Academy of Pediatrics, Elk Grove Village, IL, 2009;1-104.
Plotkin SA, Orenstein WA, editors. Vaccines, ed 5, Philadelphia: Elsevier, 2007.
Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287-294.
World Health Organization. Progress in global measles control and mortality, 2000–2007. Wkly Epidemiol Rec. 2008;83:441-448.
Zimmerman RK. Size of the needle for infant vaccination: longer needles reduce incidence of local reactions. BMJ. 2006;333:563-564.

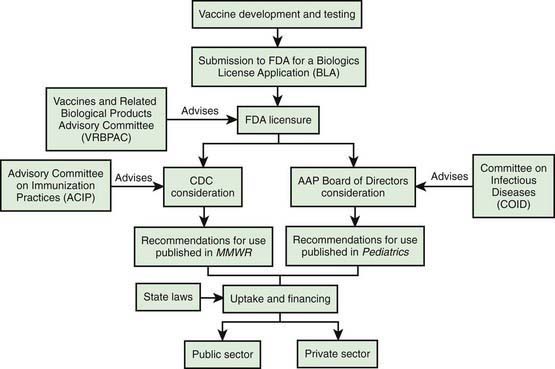
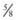 inch for newborn infants, 1 inch for infants 2-12 mo of age, and 1-
inch for newborn infants, 1 inch for infants 2-12 mo of age, and 1-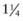 inches for older children. For adolescents and adults, the deltoid muscle of the arm is the preferred site for IM administration with needle lengths of 1-
inches for older children. For adolescents and adults, the deltoid muscle of the arm is the preferred site for IM administration with needle lengths of 1-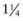 inches depending on the size of the patient. Most IM injections can be made with 23-25 gauge needles. For SC injections, needle lengths generally range from
inches depending on the size of the patient. Most IM injections can be made with 23-25 gauge needles. For SC injections, needle lengths generally range from 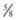 to
to 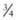 inches with 23-25 gauge needles.
inches with 23-25 gauge needles.