Imaging Techniques
Plain Films and Fluoroscopy
The mainstay of further assessment of suspected pathology of the hollow viscera remains fluoroscopy, although sonography, computed tomography (CT), scintigraphy, and, increasingly, magnetic resonance imaging (MRI) also are applicable and will be discussed later in this chapter. To limit the radiation dose, fluoroscopy should be intermittent; pulsed fluoroscopic techniques can decrease the radiation dose substantially without loss of clinical information.1,2 Capture and storage of the fluoroscopic images can be used liberally to document such findings as viscus distension, course of contrast, and peristaltic activity, with spot films reserved for areas in which greater anatomic detail is of diagnostic importance, such as mucosal abnormalities and potential perforation with contrast leaks.
Fluoroscopic studies typically require use of enteric contrast material for diagnosis.3,4 Barium, an inert substance that is not absorbed, remains the primary contrast medium used in fluoroscopic procedures, whether it is administered orally to evaluate the esophagus and upper GI tract or rectally in a contrast enema. Several barium preparations are available; barium sulfate powder (96% wt/wt) can be diluted with sterile water for infant upper GI examinations to the desired concentration of 40% to 60% wt/vol. Premixed suspensions (60% wt/vol) can be used in older children, adolescents, and adults. Enema kits containing 97% barium wt/wt can be mixed with water to a final concentration of 15% to 33% barium wt/vol for infants, older children, and adolescents. Although adverse reactions to barium products are rare—reportedly 2 per million or less5—they do occur and may present as a rash, loss of consciousness, and anaphylaxis, typically related to any one of several additives, such as methylparaben and carboxymethylcellulose.5–7 Aspiration of barium in small quantities is tolerated, but aspiration of a large volume of barium can be fatal.8,9
Barium is contraindicated in cases in which viscus perforation is suspected. In such cases, a low-osmolality, nonionic, water-soluble iodinated medium such as iohexol is used (Table 85-1). It is important that hypertonic media such as ionic or high-osmolality media (e.g., diatrizoate or iothalamate) not be used orally because of the risk of aspiration and consequent pulmonary edema.10–12
Table 85-1
Commonly Used Types of Contrast Media in Fluoroscopy of the Gastrointestinal Tract
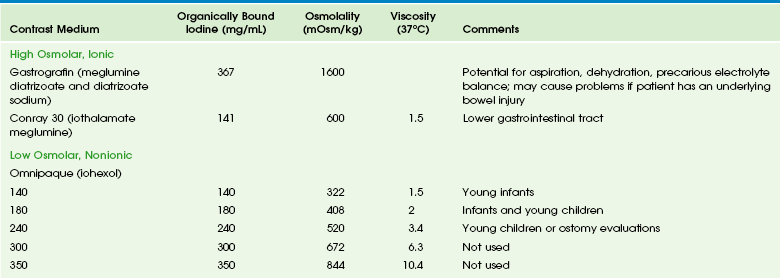
Data from package inserts.
Gastrografin (diatrizoate meglumine and diatrizoate sodium) is an ionic, markedly hypertonic iodine solution with an osmolality (mOsm/kg) of approximately 1600; a 1 : 5 dilution approximates serum osmolality (285) but also dilutes the iodine concentration. Ionic hyperosmolar media can be absorbed from the GI tract and thus pose a risk in patients with a history of hypersensitivity, particularly to iodine, and potentially in patients with thyroid disease. Hyperosmolar media cause severe pulmonary complications of edema and pneumonitis if aspirated and can cause major fluid shifts into the bowel lumen, leading to a decrease in intravascular volume, an increase in serum osmolarity, and a decrease in cardiac output. In patients with underlying bowel disease, additional injury is possible.13,14
Omnipaque (iohexol) is a nonionic water-soluble iodinated contrast medium that is available in concentrations of 140, 180, 240, 300, and 350 mg of iodine. It is poorly absorbed from the intact GI tract, with renal excretion of 0.1% to 0.5% of the administered dose. Isovue (iopamidol) also has been used in the evaluation of the pediatric GI tract, but currently only Omnipaque is officially approved for this purpose. It must be emphasized that the osmolality of both of these media is greater than that of blood and that no agent is safe in the tracheobronchial tree, and thus great care and close fluoroscopic monitoring is necessary in all patients in whom aspiration is a potential complication.15
Barium is the standard agent used in the evaluation of the colon. However, in cases of potential perforation, water-soluble agents are used and can be diluted to approximate the tonicity of serum. Higher osmolality contrast media are used rectally for therapeutic purposes in cases of uncomplicated meconium ileus after diagnosis with a low-osmolarity agent. Gastrografin (diatrizoate meglumine and diatrizoate sodium) was the original agent described for this purpose.16 However, this agent can be associated with large fluid shifts and systemic complications in severely ill infants.13 Full-strength iothalamate meglumine 30% also can be used successfully for this purpose. Close attention to water and electrolyte balance, along with surgical standby, are mandatory.
Indications and Protocols
Esophagram and Upper gastrointestinal Series
The examination is begun in the lateral projection, with the child lying on his or her left side to maintain the ingested contrast agent within the fundus of the stomach. Images of the esophagus are obtained from the nasopharynx to the esophagogastric junction, with special attention paid to nasopharyngeal aspiration, tracheal aspiration, masses, fistulas, and esophageal peristalsis and distensibility. The child is then laid supine, and the esophagus is examined in the anteroposterior projection. When the evaluation of the esophagus is completed, the barium in the fundus will be directed into the duodenum by turning the child into the prone right anterior oblique position. Gastric emptying is assessed, along with distensibility of the antrum, pylorus, duodenal bulb, and descending duodenum. Once the contrast material has reached the junction of second and third portions of the duodenum, the child is quickly placed in the supine position for assessment of the duodenojejunal junction, which is visible through the air-filled antrum. The duodenojejunal junction should lie to the left of the spine, at approximately the same level as the duodenal bulb. Once this assessment is accomplished, the child is quickly turned again, this time for a lateral projection to document the posterior course of the ascending and descending limbs of the normally rotated retroperitoneal duodenum. Evaluation for reflux can be performed after this portion of the study, if desired, or this can be done through other means such as scintigraphy or esophageal probe. A final image documents gastric emptying (Fig. 85-1).
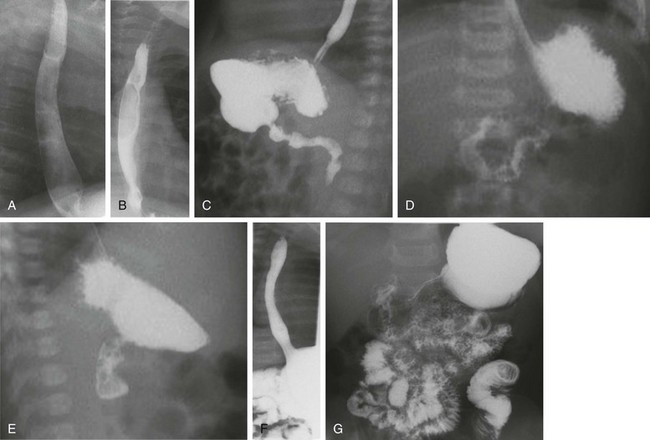
Figure 85-1 Typical upper gastrointestinal series showing reflux in otherwise healthy infant.
A and B, Lateral and anteroposterior views of the esophagus during drinking show full distensibility without intrinsic or extrinsic mass lesions. C, Oblique imaging (right anterior oblique) directs ingested contrast material to the gastric outlet and shows prompt emptying and a normal pylorus and first and second portions of the duodenum. D, An anteroposterior image immediately following C shows progress of contrast material to the normally located gastroduodenal junction at the ligament of Treitz. E, A subsequent lateral view of the duodenum shows the posterior retroperitoneal location of parallel ascending and descending limbs. F, After further drinking and gastric filling, an episode of reflux to the cervical esophagus is documented. Note the wide open gastroesophageal junction, a typical appearance during reflux. G, An image recorded at the completion of the study shows good progress of contrast material through the small bowel.
Sonography
Indications and Protocols
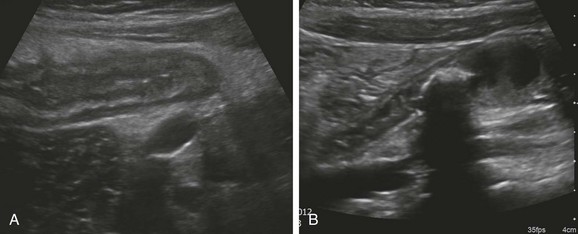
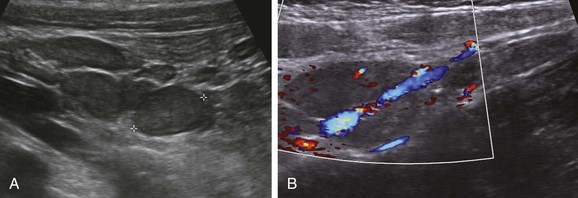
The primary role of sonography in the diagnosis of pyloric stenosis has become firmly established. Sonography is also extremely useful in the assessment of patients with clinically equivocal symptoms of appendicitis, although this setting punctuates its well-known operator dependence (e-Fig. 85-2), with published sensitivities ranging between 40% and 100%.17–19 Sonography also is extremely useful in the evaluation of mesenteric adenopathy (e-Fig. 85-3), in highly detailed assessment of the bowel wall (Fig. 85-4),20 in evaluation of small and large bowel intussusception (e-Fig. 85-5),21 and coupled with Doppler, in the effective estimation of disease activity in patients with Crohn disease.22
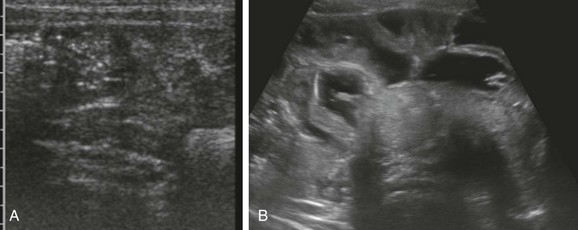
Figure 85-4 Ultrasound appearance of normal and abnormal loops of bowel.
A, A transverse image through the right lower quadrant demonstrates normal, collapsed loops of small bowel. B, A transverse image through the mid abdomen in a 14-month-old boy with vomiting and diarrhea consistent with gastroenteritis reveals multiple distended, fluid-filled loops of bowel.
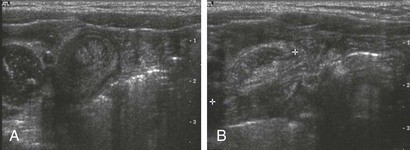
e-Figure 85-5 Small bowel intussusception.
Transverse (A) and longitudinal (B) images through incidental small bowel intussusception in a 7-month-old infant with gastroenteritis. The length between calipers in B was 2 cm. A follow-up study several hours later showed resolution of the intussusception, and the child remained asymptomatic.
Although vascular structures are seen easily with contrast-enhanced CT, the direction and velocity of flow can be evaluated with Doppler sonography. Analysis of waveform pattern can identify hepatofugal flow in collateral vessels in patients with portal hypertension, along with vascular stenosis or thrombus. In patients with heterotaxy, abdominal sonography is helpful in assessing a splenic mass (located along the greater curvature of the stomach) and the associated vascular anomalies, such as interruption of the inferior vena cava, a preduodenal portal vein, and infradiaphragmatic total anomalous pulmonary venous connection23,24 (e-Fig. 85-6).
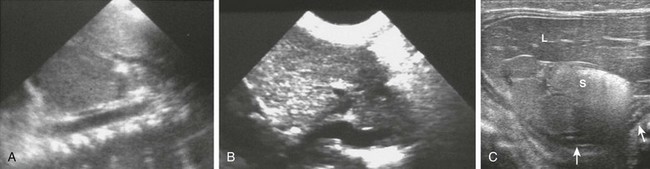
e-Figure 85-6 Ultrasound findings in patients with heterotaxy.
A, A preduodenal portal vein. A longitudinal image through the upper abdomen in a child with polysplenia shows the course of the superior mesenteric vein into the liver anterior to the gas-filled duodenal bulb. B, Infradiaphragmatic total anomalous pulmonary venous connection. A longitudinal image through the upper abdomen in an infant with asplenia shows the anomalous vessel entering the abdomen from the chest at the esophageal hiatus. C, Multiple splenules in 1-day-old girl with abnormal results of a prenatal ultrasound. Transverse ultrasound of the right upper quadrant reveals multiple splenules between the stomach (S) and the ipsilateral adrenal gland (arrows). Azygous continuation of the inferior vena cava was documented elsewhere. She did not have congenital heart disease. L, Lateral extension of liver, the bulk of which was in the left upper quadrant.
Computed Tomography
CT is a particularly useful modality in pediatric abdominal imaging. The introduction of scanners with multichannel technology and volumetric acquisition permits very rapid examinations with isotropic reconstructions in multiple planes, with decreasing need for sedation.25,26 These new capabilities require development of newer protocols to accommodate more complex and sophisticated diagnostic demands. The timing and rate of contrast administration, with the ability to scan during a specific phase of intravascular contrast distribution, demand particular attention to technical details and new approaches to image interpretation.27,28 The pediatric radiologist is further challenged by the need to balance image detail with radiation dose and implementation of the ALARA, or “as low as reasonably achievable” concept, with the increasing recognition of the potential risks of radiation exposure for pediatric patients.29,30 Improvements in equipment aimed at reducing radiation exposure include innovations such as improved collimators and iterative reconstructive algorithms. Although significant challenges persist, much progress has been made through educational and awareness-raising social marketing campaigns such as the Image Gently Campaign of The Alliance for Radiation Safety in Pediatric Imaging (www.imagegently.org).31
Indications and Protocols
Unlike sonography, CT images are sequential, standardized, and much less operator dependent, and therefore CT is particularly useful in patients with complex disease affecting multiple organ systems, because it provides reliable monitoring of change in the extent of the disease during therapy and follow-up. CT also helps solve problems in patients with unusual multi-organ abnormalities. Evaluation of both intraabdominal and extraabdominal multiorgan pathology can be accomplished with great anatomic detail (e-Figs. 85-7 and 85-8) because evaluation of solid organ, hollow viscera, and peritoneal cavity pathology is rapidly accomplished with great anatomic detail and physiologic information. The relative lack of operator dependence and high sensitivity and specificity of CT in the imaging diagnosis of appendicitis has led to its increasing use when this diagnosis is clinically equivocal, with a documented reduction in negative appendectomies.32 However, this success has led to overuse in patients with abdominal pain; therefore physical examination, followed by ultrasound when the diagnosis is clinically uncertain, is recommended by most pediatric radiologists, with CT reserved for more difficult cases.17,18 MRI is receiving increasing attention as a viable substitute for CT scanning in many indications, such as inflammatory bowel disease.33
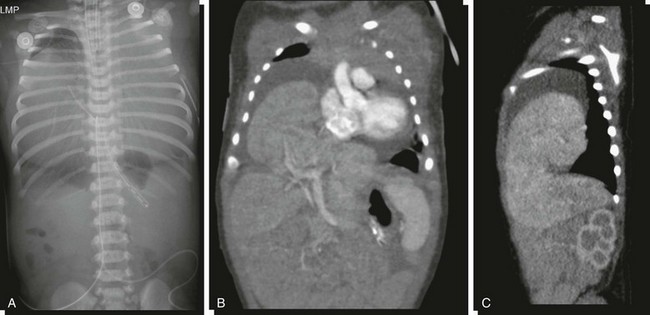
e-Figure 85-7 Pericardial hernia.
A, An abdominopelvic radiograph of a newborn infant with a prenatal diagnosis of hydrothorax. The radiograph suggests absence of most of the liver shadow from the right upper quadrant. The posterior costophrenic sulci are well outlined, indicating that chest density is not related to bilateral hydrothorax. Coronal (B) and sagittal (C) reformats of contrasted computed tomography scans outline herniation of the liver into the pericardial cavity with displacement of the heart and associated pericardial effusion.
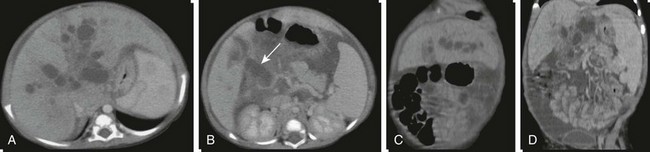
e-Figure 85-8 A choledochal cyst with intrahepatic ductal dilatation and absence of the portal vein.
A and B, Axial contrasted computed tomography images through the liver and porta hepatis outline intrahepatic cystic dilatation of the biliary tree (Caroli disease) and cystic dilatation of the common bile duct at the porta hepatis (arrow). C and D, Coronal reconstructions better define the cystic intrahepatic ductal dilatation, absence of the portal vein, and splenomegaly.
CT protocols vary and undergo change with the ongoing introduction of new applications and advances in equipment capability; generalizable protocols applicable to pediatric patients can be downloaded at http://www.imagegently.org. However, some underlying principles underscore most successful pediatric examinations. Administration of intravenous contrast material is extremely important, particularly in pediatric patients in whom a paucity of intraabdominal fat decreases intrinsic intraabdominal contrast.27,34 CT angiography requires a rapid contrast bolus injection, which in pediatrics can be challenging because of the caliber of IV access. Lowering kVp is important in patients in whom high-contrast structures are of interest, such as those undergoing angiography or bone examinations; in neonates, the kVp can be decreased to as low as 80, with some adjustment of the milliamperes-second (mAs) to produce acceptable image quality.29 Precontrast images are seldom necessary and serve to increase the radiation exposure without adding diagnostic information. If necessary (e.g., to identify the presence of calcifications in an abdominal mass), the mAs of the precontrast scans can be decreased significantly and the scan should be limited to the appropriate specific area (e.g., scan only the mass, not the entire abdomen). The use of oral contrast material is usually important when outlining some types of intraperitoneal pathology, such as abscess or masses, but in other cases, its use is more controversial.35 Positive oral contrast material will mask mucosal enhancement; use of water-density contrast material may be more appropriate in such cases.
Despite radiation concerns, CT remains an important life-saving modality in pediatric diagnosis. As with any other tool, it needs to be used judiciously, according to the principles of appropriateness, justification, optimization, and training.36
Magnetic Resonance Imaging
Patient Preparation and Equipment Requirements
Use of an oral contrast agent is essential for enterography examinations. A number of choices are acceptable, although most regimens consist of a biphasic agent, that is, one that gives the bowel lumen a long T2 and T1 relaxation time. Agents include VoLumen (E-Z-Em, New York, NY), mannitol, polyethylene glycol l, and locust bean gum (a type of galactomannan) solutions. Little difference is seen in efficacy of these choices, although patient tolerance may vary.37 Most important is rapid consumption of a large volume of the contrast agent; 25 mL per kilogram of body weight over an hour is adequate. Placing the patient in the right decubitus position for the final 15 minutes before the start of imaging aids in emptying of the stomach.
Equipment specifications are important because of children’s smaller sizes, with the consequent need for improved signal to noise and faster acquisition times to decrease the need for and length of sedation. Although the literature to date is still sparse on pediatric abdominal imaging at 3 T,38,39 increasing experience suggests that most children will benefit from the higher signal. Phased array surface coils are now standard, typically with eight to 32 channels. In the following situations, 1.5 T often provides improved image quality compared with 3 T: when the patient is very large, when ascites is present, when enterography examinations are being performed (1.5 T results in fewer banding artifacts in steady-state imaging), and during hepatic iron quantification.
Indications and Protocols
Hepatic tumors are well evaluated by MRI relative to CT,40 with the goal of imaging being tumor characterization, staging, and assessment of resectability. For lesion characterization, determination of the T2-weighted signal and enhancement characteristics is essential.41 Staging and resectability of tumors (such as hepatoblastomas) require delineation of anatomic boundaries, lymph node involvement, vascular invasion, and delineation of the biliary tree, according to accepted staging systems such as PRETEXT (PRETreatment tumor EXTension) outlined by the International Childhood Liver Tumor Strategy Group.42,43 Biliary and pancreatic diseases also are well assessed by MRI.44,45 Common indications include cholelithiasis, pancreatitis, sclerosing cholangitis,46,47 ductal plate malformations and choledochal cysts,48–50 and biliary complications of liver transplantation. In the case of liver transplantation, assessment of vascular complications often is essential. Diffuse liver disease, such as fibrosis, steatosis,51–53 and iron deposition, can be quantified by MRI. Fibrosis has been quantitatively assessed by elastography,54 as well as qualitatively by T2-weighted imaging and delayed contrast enhancement.55 Although steatosis can be assessed by spectroscopic methods,56 more commonly steatosis, as well as iron deposition, are assessed by multi-echo gradient echo imaging.53,57 MR enterography is most commonly performed for evaluation of inflammatory bowel disease.58–60 The goals of MR include detection of bowel inflammation, distinction of active inflammation from chronic fibrosing disease, and fistula/abscess detection and characterization. Fistulography, particularly for fistula in ano, is performed well by MRI.61 The goals of the examination include detection of fistulae, classification (i.e., intersphincteric, transsphincteric, suprasphincteric, or extrasphincteric), and abscess detection.
Suggested protocols are provided in Table 85-2, with examples in Figures 85-9 and 85-10 and e-Figures 85-11 and 85-12 and details provided in the following sections. In general, matrix, field of view, and slice thickness should be adjusted to the patient size and thus are not emphasized in the following sections.
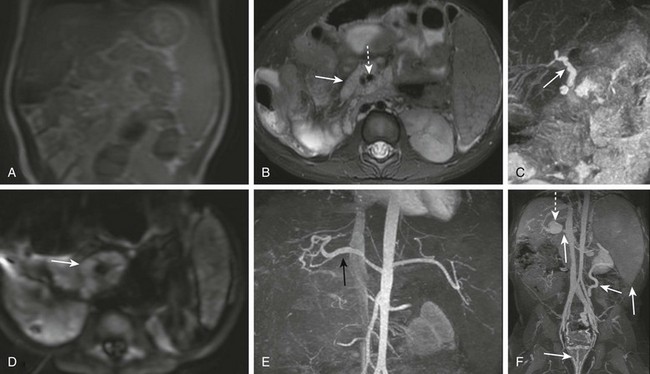
Figure 85-9 Volumetric, diffusion, and angiographic imaging of a 1-year-old girl with a left lobe lateral segment liver transplant.
A, A localizer is performed quickly. B, Axial fat-suppressed T2-weighted imaging shows lymph nodes (solid arrow) and surrounding mesenteric vessels (dashed arrow), suggesting posttransplant lymphoproliferative disorder. C, Maximum intensity projection of a volumetric T2-weighted image showing patent bile ducts (arrow). D, Axial diffusion again showing lymph nodes (arrow). E, A contrast-enhanced angiogram (spoiled gradient with fat suppression) in the arterial phase showing a patent hepatic artery (black arrow). F, Venous phase imaging shows the proximal portal vein (small arrow), poststenotic dilation of the intrahepatic portal vein (dashed arrow), hemorrhoidal varices (medium arrows), and splenomegaly (large arrow).
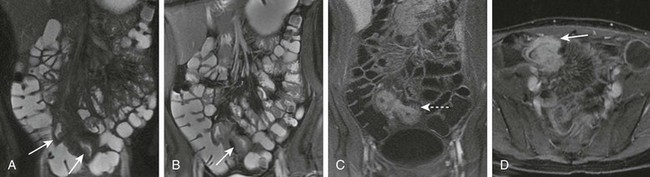
Figure 85-10 Magnetic resonance enterography. A 20-year-old girl with typical findings of Crohn disease.
A, Single shot imaging shows a thickened terminal ileum with submucosal edema (arrows). B, Balanced steady state imaging shows wall thickening (arrow). C, Hyperemia and early transmural enhancement is seen after contrast administration, as well as a “comb sign” (dashed arrow). D, Delayed imaging shows persistent enhancement of the terminal ileum (arrow).
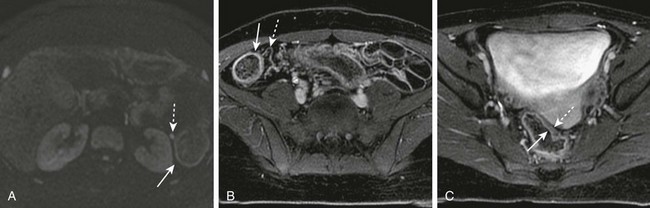
e-Figure 85-11 Diffusion and contrast enhancement in a 16-year-old girl with ulcerative colitis.
A, Diffusion highlights colonic wall (arrow) as well as a small pericolonic lymph node (dashed arrow). B, Contrast-enhanced spoiled gradient shows cecal mucosal enhancement (arrow) and prominent adjacent vessels (dashed arrow). C, Enhancement is pancolonic; mucosal enhancement is denoted by the signal intensity difference between the luminal (arrow) and serosal (dashed arrow) portions of the bowel.
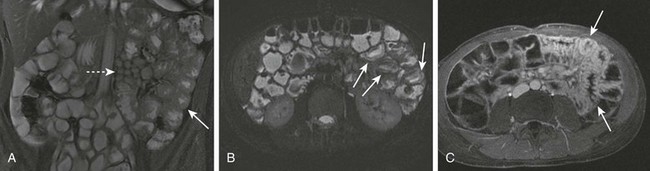
e-Figure 85-12 Balanced steady state and small bowel imaging in a 14-year-old boy with jejunitis as an atypical manifestation of Crohn disease.
A, Jejunal wall thickening (arrow) and prominent mesenteric lymph nodes (dashed arrow) on balanced steady-state imaging. B, Submucosal edema (arrows) on single shot imaging. C, Contrast-enhanced spoiled gradient echo with fat suppression immediately after intravenous administration of glucagon shows hyperemic bowel and again demonstrates thickened folds (arrows).
Localizers
At 1.5 T, three-plane single shot fast spin echo with low bandwidth (20 kHz), matrix, and repetition time are performed as localizers. If diagnostic single shot images are required, adjustments can be made to the matrix (320 × 256), slice thickness (4 to 5 mm), and echo time (TE) (200 ms) and images acquired with respiratory triggering.62 At 3 T, localizers may be more efficiently achieved with balanced steady-state imaging to decrease specific absorption rate, especially for small patients. All localizer sequences should employ parallel imaging.
Conventional T2-Weighted Imaging
Conventional T2-weighted imaging can be performed with fast spin echo. Here parallel imaging is not optimal, because the primary emphasis is on tissue characterization with high signal/noise ratio (SNR). Sequences should be performed at least in the axial plane, and respiratory triggering or navigation is significantly helpful in improving image quality.63,64 Typical TEs are 80 to 90 ms at 1.5 T and 70 to 80 ms at 3 T. Fast recovery may be used to improve SNR. Current literature is mixed on the ability of T2-weighted single shot images to provide an equivalent alternative to longer conventional sequences,65 and thus protocols almost always include conventional T2-weighted imaging.
Volumetric T2-Weighted Imaging
Volumetric T2-weighted sequences have been described mostly for musculoskeletal imaging and neuroimaging.66–68 These pulse sequences are similar to fast spin echo in that a 90° excitation is required, which excites a slab rather than a slice. These sequences permit thin slices (1 to 2 mm) and reformatting in arbitrary planes. Parallel imaging is essential to maintain a reasonable scan time of 4 to 5 minutes. Navigation or respiratory triggering is important in optimizing image quality, along with a high bandwidth (e.g., 62 kHz). For dedicated MR with cholangiopancreatography examinations, a higher TE (>500 ms) can be used to permit excellent maximum intensity reformation of the biliary tree and pancreatic duct. For other examinations, a TE of 70 to 90 ms permits delineation of relevant anatomy.
Single Shot Imaging
Although volumetric imaging usually displays the bile ducts well,69 image quality likely will be suboptimal in patients with voluntary or involuntary motion, such as irregular breathing and peristalsis. Thus single shot imaging is complementary70 for biliary imaging and essential for bowel imaging. In patients with irregular breathing, the user can hand trigger acquisition of each slice by observing the respiratory belt tracing, enabling reasonable assessment of bile ducts in even the most challenging patients.
T1-Weighted Imaging: Dual echo imaging (spoiled gradient with both in-phase and opposed-phase echoes) offers volumetric images at high spatial resolution, as well as with fat-water separation. These sequences can be performed while the patient hold his or her breath, and data can be processed to yield four image sets from one acquisition: water, in-phase, opposed-phase, and fat. Typical parameters are high bandwidth (100 kHz), 4-mm slice thickness, and 12° to 15° flip angle.
Dynamic Gadolinium
Several contrast agents are now available. Consideration should be given to macrocyclic agents, such as gadobutrol (Gadavist), because they may provide enhanced safety in the setting of renal insufficiency. If the primary clinical concern is evaluation of the vascular structures, gadobenate dimeglumine (MultiHance) is a reasonable option, given its higher relaxivity and longer intravascular residence time. The agent has approximately 5% hepatobiliary elimination. Gadofoveset trisodium (Ablavar) recently has been approved by the Food and Drug Administration for assessment of aortoiliac disease in adults, and it may be an option if vascular evaluation is the sole clinical question. Finally, gadoxetate disodium (Eovist) may be a good option if the primary clinical concern is biliary.71–77 This agent has 50% hepatobiliary excretion in the setting of normal hepatic and renal function and thus provides a functional as well as an anatomic MR with cholangiopancreatography examination. This agent also is preferred in the setting of evaluation of suspected focal nodular hyperplasia. Although the literature is still limited, gadoxetate may prove useful for characterization of other liver tumors. Even though the gadoxetate dose of 0.025 mmol/kg contains only a quarter of the gadolinium of other agents, its higher T1 relaxivity still allows adequate first-pass hepatic imaging. For all agents, the rate of single dose administration at 1 mL/sec followed by a saline solution flush is acceptable.
For tumor characterization, the minimum full echo time should be chosen to maximize SNR.
Noncontrast Magnetic Resonance Angiography
Although time-of-flight–based approaches have been used, these sequences produce limited image quality in the abdomen. Noncontrast-enhanced techniques based on balanced steady-state approaches have been gaining favor.78 A variation of this method is based on a respiratory-triggered inversion pulse covering the imaged volume, as well as a region inferior to it,79 followed by a balanced steady-state echo train. Thus blood flowing from superior to the inverted region is bright, producing MRA. These sequences have pitfalls similar to time-of-flight–based techniques, including flow-related dephasing, slow-flow–related signal dropout, and intrinsic high T1-weighted signal, but on the whole they provide a nice complement to contrast-enhanced MRA.
Iron/Fat Quantification
For assessment of steatosis, qualitative evaluation may be performed by dual echo imaging (in/opposed phase gradient echo). For quantitative assessment, low flip angle multi-echo imaging may be used, or alternatively spectroscopy can be performed.80–82 In general, the gradient echo methods are faster and easier, although considerable care must be taken for quantitative accuracy to be maintained.
Nuclear Medicine
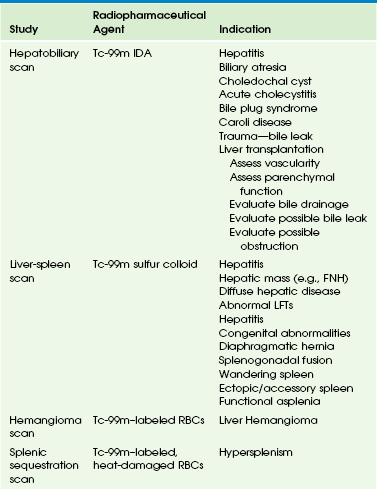
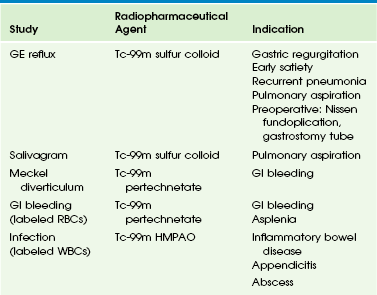
Nuclear scintigraphy plays an important role in the evaluation of hepatobiliary dysfunction and disorders of the gastrointestinal tract in infants and children. Although some of the studies offer unique diagnostic information, others provide functional information complementary to that obtained with sonography, CT, MRI, and fluoroscopy. Radionuclide imaging of the GI system can be divided into two major categories: imaging of the hepatobiliary system and spleen (Table 85-3) and imaging of the GI tract (Table 85-4).
Patient Preparation and Equipment Requirements
Some of the studies discussed in this section require a period of fasting before imaging. Hepatobiliary imaging typically is performed after premedication with phenobarbital, and Meckel diverticulum imaging can be enhanced with pentagastrin (not currently available in the United States), histamine H2 blockers, or glucagon.83
Radiopharmaceutical Agents
The dose of a radiopharmaceutical agent used in a child should be determined by the minimal dose required to yield a high-quality diagnostic examination. Administered activity typically is calculated on the basis of either body surface area or weight. Recent work by Gelfand and colleagues84 determined that weight-based formulas result in lower radiation exposures, especially in young infants. The group has worked with The Alliance for Radiation Safety in Pediatric Imaging (www.imagegently.org) and published weight-based consensus guidelines for recommended doses of radiopharmaceutical agents in children84 and is supported by the Society for Pediatric Radiology, the Society of Nuclear Medicine, and the American College of Radiology.
Indications and Protocols
Hepatobiliary scintigraphy is a useful adjunct to sonography in the evaluation of infants with jaundice and cholestasis, as well as in older children with hyperbilirubinemia. Its value in jaundiced neonates is the timely differentiation of neonatal hepatitis and biliary atresia, because surgical intervention in patients with biliary atresia is most successful when it is performed early in life. Postoperatively, hepatobiliary imaging may be requested to confirm patency of the bilioenteric anastomosis. Imaging with sonography, CT, and MRI frequently is diagnostic for choledochal cysts, but hepatobiliary imaging is a useful adjunct. Similarly, the evaluation of Caroli disease often is facilitated with hepatobiliary imaging. Cystic duct patency can be assessed in older children with suspected acute cholecystitis. Hepatobiliary imaging is highly sensitive and specific for the detection of spontaneous, postoperative, or posttraumatic biliary leaks.85 Other clinical indications are listed in Table 85-3.
Currently, 99mTc mebrofenin is used widely for hepatobiliary imaging because it has a high hepatic extraction; it is transported into the hepatocytes and then excreted with bile into the bile ducts. The radiopharmaceutical agent is administered intravenously, and static anterior images of the abdomen are acquired in the supine position every 5 minutes for 30 minutes. A simultaneous dynamic acquisition is suggested during the first 30 minutes. Delayed images may be obtained at 45 minutes, 60 minutes, and up to 24 hours, as necessary, to visualize the gallbladder and biliary tree and excretion into the duodenum. A normal scan typically demonstrates radiopharmaceutical uptake in the liver by 5 minutes, in the biliary tree by 15 minutes, and in the small bowel by 15 to 45 minutes (Fig. 85-13). In patients with neonatal hepatitis, delayed and diminished uptake by the liver occurs, but the radiopharmaceutical agent eventually reaches the small bowel. In patients with biliary atresia, radiopharmaceutical uptake by the liver is usually adequate, but the radiotracer never reaches the bowel, even on delayed 24-hour images (e-Fig. 85-14).
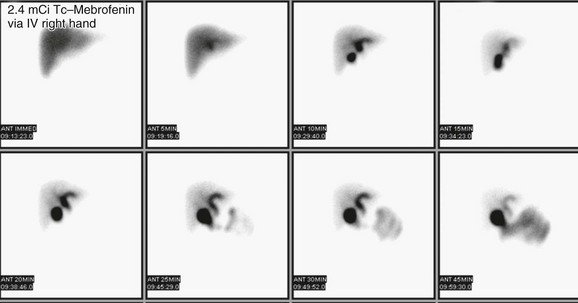
Figure 85-13 Normal results of a hepatobiliary study in a 5-year-old with right upper abdominal pain.
Anterior static images of the abdomen were obtained every 5 minutes for 45 minutes after the intravenous (IV) administration of technetium (Tc)-99m mebrofenin. Homogeneous radiotracer accumulation is seen throughout the liver, with prompt visualization of the intrahepatic ducts by 5 minutes, the common bile duct and gallbladder by 10 minutes, and the small bowel by 25 minutes.
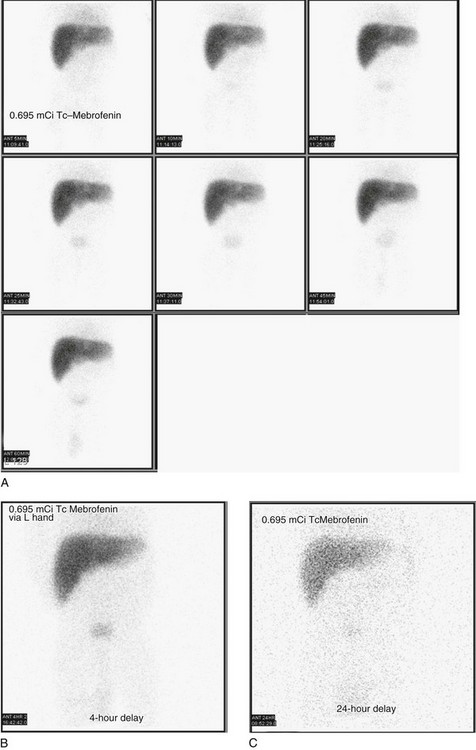
e-Figure 85-14 Biliary atresia in an 8-week-old infant with jaundice and conjugated hyperbilirubinemia.
Technetium (Tc)-99m mebrofenin biliary scintigraphy demonstrates relatively good hepatic extraction of the radiotracer, nonvisualization of the biliary system, and no evidence of radiotracer passage into the bowel. 0 to 60-minute (A), 4-hour (B), and 24-hour (C) anterior projection images are shown. All images reveal normal, faint radiopharmaceutical agent accumulation in the urinary bladder.
When differentiation between biliary atresia and neonatal hepatis is required, premedication with phenobarbital (5 mg/kg per day for 5 days) improves hepatic extraction of tracer,86 and 3 to 5 days of premedication is recommended in the setting of elevated conjugated hyperbilirubinemia. A child should fast for 2 to 4 hours; 2 hours is sufficient for infants. Prolonged fasting is not recommended.
Liver-Spleen Scintigraphy
The traditional liver-spleen scan is seldom used because of advances in sonography, CT, and MRI, which provide far greater spatial and contrast resolution and more precise anatomic details. However, several important indications for the liver-spleen scan still exist, including the evaluation of congenital anomalies, such as the location of accessory or ectopic spleens, the evaluation of functional asplenia, the functional evaluation of hepatitis and cirrhosis, and the diagnostic differentiation of certain hepatic masses, such as focal nodular hyperplasia. Some authors have reported its utility in the assessment of children with heterotaxy syndrome; however, differentiating a normal abdominal situs from asplenia with a midline, transverse liver can be difficult when the splenic fossa is occupied by liver tissue. CT or MRI makes this distinction more clearly.24 The physiologic basis of the liver-spleen scan is the phagocytosis of radioactive colloid particles by the reticuloendothelial cells of the liver, spleen, and bone marrow. Normal images reveal homogeneous distribution of the radiopharmaceutical agent in the liver and spleen, which can be evaluated for size, position, configuration, and any focal areas of radiotracer deficit. Functional asplenia is better documented with 99mTc-labeled, heat-damaged red blood cell imaging.
Splenic Sequestration Scintigraphy
Splenic sequestration scintigraphy is used in children with hypersplenism, which results in acceleration of the normal sequestration and phagocytosis of abnormal erythrocytes, neutrophils, and platelets. Spleen scintigraphy also can be used to identify accessory spleens, posttraumatic “splenosis,” and to evaluate splenic uptake in a “wandering” spleen (see Chapter 95).
Gastroesophageal Reflux Scintigraphy
A study with normal findings shows the radiopharmaceutical agent in the stomach but no activity in the esophagus or lungs. If reflux is detected, the number of episodes is counted over the entire imaging period, and the proximal extent is noted (Fig. 85-15).
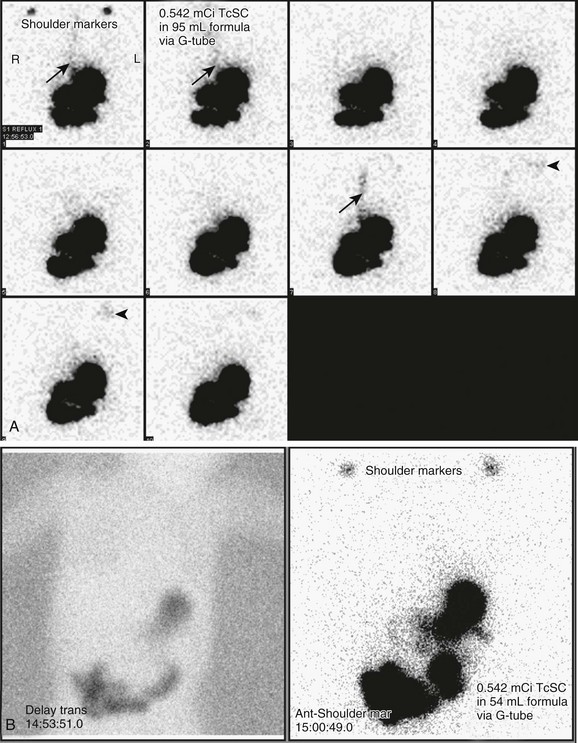
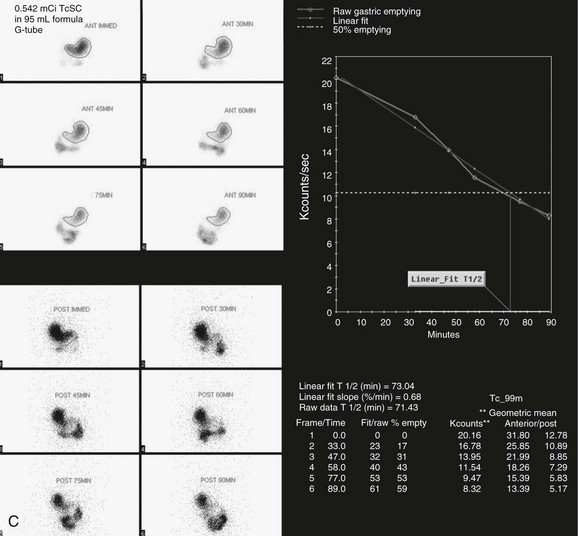
Figure 85-15 A combined gastroesophageal reflux and gastric emptying imaging study in a 1-year-old child with persistent emesis.
A, Initial images of the chest and abdomen reveal three episodes of reflux (arrows in the first, second, and seventh images), with subsequent visualization of the radiopharmaceutical agent in the child’s mouth (arrowheads in the eighth and ninth images). B, Three-hour delayed transmission and routine supine image of the chest and upper abdomen reveal no pulmonary aspiration. C, Progressive gastric emptying is occurring. A time-activity curve constructed from regions of interest drawn around the stomach reveals a half time for emptying of 73 minutes, which is within normal limits. TcSC, Technetium-99m sulfur colloid.
Gastric Emptying Scintigraphy
A study with normal findings reveals radiotracer in the stomach on the initial images, followed by progressive emptying of the stomach (see Fig. 85-15). Computer processing is performed by drawing a region of interest around the stomach at selected time points to calculate fractional emptying. A time-activity curve is then generated and plotted on a linear scale using the geometric mean of the anterior and posterior counts. A half-time for emptying (T½) is calculated, that is, the length of time required for the initial number of counts to decrease by 50%. In normal studies the time-activity curve should exhibit a continuous decline in activity over time.
Reported normal values are variable. Seibert and colleagues87 showed 60-minute gastric emptying values of 48% ± 16% in infants and 51% ± 7% in children who were fed a radiolabeled milk formula (roughly, a gastric emptying T½ of 60 minutes in each age group). A different study performed with healthy infants who were fed radiolabeled milk demonstrated a T½ of 87 ± 29 minutes.88 Singh et al.89 developed a standard solid meal (99mTc-labeled “Technecrispy cake”), determined a standard meal volume (30 g), and prospectively established normal gastric emptying values in healthy children, which typically are normalized by each individual nuclear medicine laboratory or imaging department.
Colonic Transit Scintigraphy
Patient preparation requires discontinuance of laxatives for 5 days before the transit study, and 4 hours of patient fasting is recommended. A 99mTc sulfur colloid radiolabeled liquid or solid meal is administered orally. Anterior and posterior images are obtained in the supine position at 0 to 2, 6, 24, 30, and 48 hours. Radioactivity is measured in six regions (precolonic, ascending, transverse, descending, rectosigmoid colon, and evacuated feces). An examination with normal results shows radioactivity in the cecum by 6 hours and evacuation by 30 to 58 hours. Retention in the proximal colon at 48 hours indicates “slow colonic transit,” whereas retention in the rectum at 48 hours indicates “functional fecal retention.”90,91 Quantitative assessment of transit also can be performed with geometric center analysis.92
Salivagram
Fasting is not required. The child is positioned supine, and a small dose of 99mTc sulfur colloid mixed in 0.1 to 0.5 mL of water or saline solution is placed on the anterior tongue and allowed to mix with oral secretions. Rapid dynamic images of the neck, chest, and upper abdomen are acquired from the posterior projection every 60 seconds for 1 hour. Static images are then obtained at 1 hour and 3 hours. Detection of any radiopharmaceutical agent in the tracheobronchial tree is abnormal (Fig. 85-16).
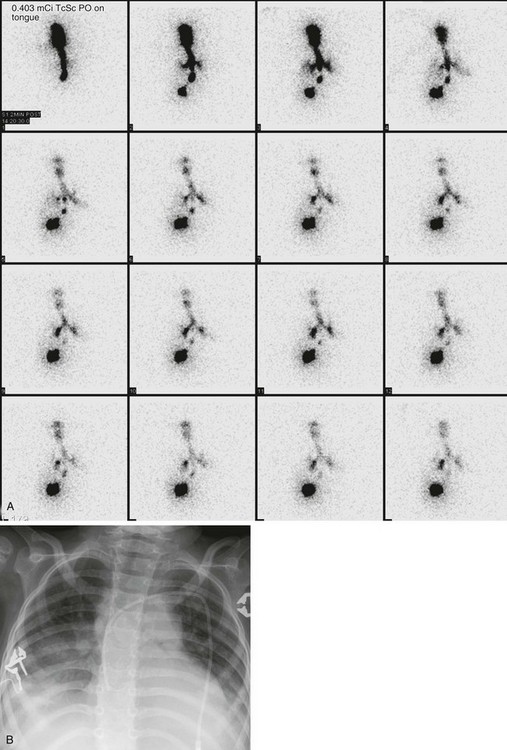
Figure 85-16 A salivagram.
A, A radionuclide salivagram (posterior images) obtained in a child with recurrent pneumonia and neuromuscular incoordination demonstrates technetium-99m sulfur colloid (TcSC) outlining the tracheobronchial tree. Some of the swallowed radiopharmaceutical agent is seen in the stomach. B, A chest radiograph obtained the same day reveals bibasilar lung opacity, consistent with aspiration pneumonitis.
Meckel Scintigraphy
Meckel diverticulum is a congenital anomaly resulting from incomplete closure of the omphalomesenteric duct; it is present in approximately 2% of the population.93 Most of these diverticula are asymptomatic and are lined by ileal mucosa. However, those that contain ectopic gastric mucosa are capable of producing hydrochloric acid and pepsin, thereby inducing mucosal ulceration. Peptic ulceration causes acute GI hemorrhage in children, usually in the first 2 years of life. A Meckel diverticulum can be quite small and difficult to differentiate from bowel loops by anatomic imaging. The Meckel scan is the study of choice for unexplained GI bleeding in children. Those diverticula with ectopic gastric mucosa can be detected with scintigraphy because the radiopharmaceutical agent accumulates within the ectopic gastric mucosa. Bleeding enteric duplication cysts containing ectopic gastric mucosa also can be detected.
Pharmacologic pretreatment is not considered necessary to produce a high-quality Meckel scan. However, if a false-negative study is suspected, a follow-up examination with pentagastrin or an H2 blocker can increase diagnostic sensitivity. Pentagastrin stimulates gastric secretions and increases gastric mucosa uptake of the pertechnetate. It also stimulates secretion of pertechnetate and GI motility. Histamine H2 blockers (e.g., cimetidine, ranitidine, and famotidine) block secretions from the cells and increase gastric mucosa uptake. Glucagon may be given to decrease intestinal peristalsis.94
A study with normal findings reveals radiotracer accumulation in the stomach and the urinary bladder, sometimes with faint uptake by the kidneys. An abnormal study demonstrates accumulation of radiotracer in ectopic tissue simultaneously with the appearance of gastric mucosa, usually between 6 and 10 minutes after injection. The abnormality is usually a small, rounded focus of radiopharmaceutical agent uptake in the right lower abdomen (e-Fig. 85-17). Single photon emission tomography (SPECT) imaging co-registered with simultaneously acquired low-dose CT (SPECT/CT) on a hybrid scanner has proved useful in discriminating between a Meckel diverticulum and possible artifact from urinary or other visualized activity.95,96
Gastrointestinal Inflammation and Infection Scintigraphy
The radionuclide labeling of white blood cells can be used for the localization of sites of abdominal infection, including appendicitis,97 and for the localization and evaluation of the intensity and extent of inflammatory bowel disease (Crohn disease and ulcerative colitis).
Data suggest that 99mTc hexamethylpropyleneamine oxime–labeled leukocyte imaging may be superior to CT98 for assessing the extent and activity of inflammatory bowel disease, and a review of these techniques has been published more recently.99 Labeled leukocyte imaging may be used as a screening study to determine if a child should undergo more invasive testing. The study also is useful for patients who refuse endoscopy or contrast radiography or for those who have luminal narrowing that precludes endoscopic evaluation.
Positron Emission Tomography
PET using fluorine-18 (18F) fluorodeoxyglucose has been reported as a noninvasive, sensitive alternative to conventional studies in the identification and localization of intestinal inflammatory and infectious processes in children. Specific inflammatory conditions investigated using 18F-fluorodeoxyglucose PET imaging include inflammatory bowel disease,100–102 chronic granulomatous disease,31 appendicitis,103 and fever of unknown origin.104
PET imaging using 18F-dihydroxyphenylalanine has been used to distinguish focal from diffuse pancreatic disease in infants with hyperinsulism. Patients with focal disease may undergo resection of the offending adenoma, whereas those with diffuse disease may be treated with octreotide or subtotal pancreatectomy.105,106 The imaging techniques for these PET studies are beyond the scope of this chapter.
Molecular Imaging
Molecular imaging techniques that use tiny concentrations of radiotracers have been used as probes in animal models and human tissue samples to characterize, measure, and treat disease processes at the cellular or molecular level. Such techniques show promise in preclinical studies of patients with inflammatory bowel disease, but translation into routine clinical use in children awaits further evaluation.107
References
Full references for this chapter can be found on www.expertconsult.com.
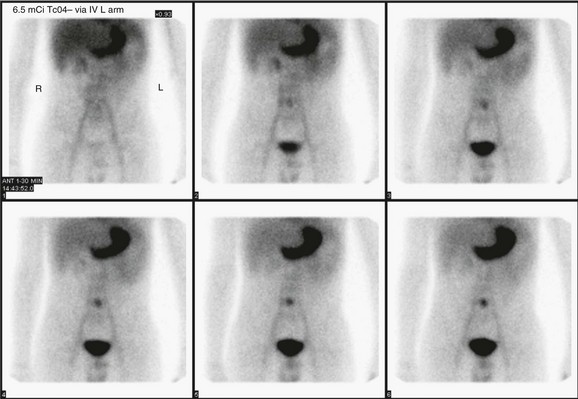
e-Figure 85-17 A Meckel scan in an 11-year-old with lower gastrointestinal bleeding reveals ectopic gastric mucosa in the midline of the lower abdomen.
The timing coincides with the appearance of the stomach, with increasing conspicuity over time. Lateral views (not shown) revealed abnormal uptake anteriorly in the lower abdomen.
References
1. Hernanz-Schulman, M, et al. Pause and pulse: ten steps that help manage radiation dose during pediatric fluoroscopy. AJR Am J Roentgenol. 2011;197(2):475–481.
2. Newman, B, et al. Pause and pulse: radiation dose in pediatric fluoroscopy. Pediatr Rev. 2011;32(9):e83–e90.
3. Cohen, MD. Choosing contrast media for the evaluation of the gastrointestinal tract of neonates and infants. Radiology. 1987;162(2):447–456.
4. Cohen, MD. Choosing contrast media for pediatric gastrointestinal examinations. Crit Rev Diagn Imaging. 1990;30(4):317–340.
5. Muroi, N, et al. Anaphylaxis from the carboxymethylcellulose component of barium sulfate suspension. N Engl J Med. 1997;337(18):1275–1277.
6. Janower, ML. Hypersensitivity reactions after barium studies of the upper and lower gastrointestinal tract. Radiology. 1986;161(1):139–140.
7. Skucas, J. Anaphylactoid reactions with gastrointestinal contrast media. AJR Am J Roentgenol. 1997;168(4):962–964.
8. Katsanoulas, C, et al. Severe barium sulphage aspiration: a report of two cases and review of the literature. Signa Vitae. 2007;2(1):25–28.
9. Tamm, I, Kortsik, C. Severe barium sulfate aspiration into the lung: clinical presentation, prognosis and therapy. Respiration. 1999;66(1):81–84.
10. Trulzsch, DV, et al. Gastrografin-induced aspiration pneumonia: a lethal complication of computed tomography. South Med J. 1992;85(12):1255–1256.
11. Reich, SB. Production of pulmonary edema by aspiration of water-soluble nonabsorbable contrast media. Radiology. 1969;92(2):367–370.
12. Tuladhar, R, Patole, S, Whitehall, J. Gastrografin aspiration in a neonate with tracheoesophageal fistula. J Paediatr Child Health. 2000;36(1):94–95.
13. Rowe, MI, et al. The neonatal response to gastrografin enema. Pediatrics. 1971;48(1):29–35.
14. Tuladhar, R, et al. Oral gastrografin in neonates: a note of caution. Int J Clin Pract. 1999;53(7):565.
15. Moore, DE, et al. Comparison of nonionic and ionic contrast agents in the rabbit lung. Invest Radiol. 1991;26(2):134–142.
16. Noblett, HR. Treatment of uncomplicated meconium ileus by Gastrografin enema: a preliminary report. J Pediatr Surg. 1969;4(2):190–197.
17. Hernanz-Schulman, M. CT and US in the diagnosis of appendicitis: an argument for CT. Radiology. 2010;255(1):3–7.
18. Strouse, PJ. Pediatric appendicitis: an argument for US. Radiology. 2010;255(1):8–13.
19. Trout, AT, Sanchez, R, Ladino-Torres, MF, et al. A critical evaluation of US for the diagnosis of pediatric acute appendicitis in a real-life setting: how can we improve the diagnostic value of sonography. Pediatr Radiol. 2012;42(7):813–823.
20. Ledermann, HP, et al. Bowel wall thickening on transabdominal sonography. AJR Am J Roentgenol. 2000;174(1):107–117.
21. Verschelden, P, et al. Intussusception in children: reliability of US in diagnosis—a prospective study. Radiology. 1992;184(3):741–744.
22. Spalinger, J, et al. Doppler US in patients with Crohn disease: vessel density in the diseased bowel reflects disease activity. Radiology. 2000;217(3):787–791.
23. Applegate, KE, et al. Situs revisited: imaging of the heterotaxy syndrome. Radiographics. 1999;19(4):837–852. [discussion 853-854].
24. Hernanz-Schulman, M, et al. Pictorial essay. Current evaluation of the patient with abnormal visceroatrial situs. AJR Am J Roentgenol. 1990;154(4):797–802.
25. Pappas, JN, Donnelly, LF, Frush, DP. Reduced frequency of sedation of young children with multisection helical CT. Radiology. 2000;215(3):897–899.
26. Lemos, AA, et al. Single-versus multidetector-row CT: comparison of sedation rates, conventional angiograms and motion artefacts in young children following liver transplantation. Radiol Med. 2006;111(7):911–920.
27. Frush, DP. Pediatric abdominal CT angiography. Pediatr Radiol. 2008;38(suppl 2):S259–S266.
28. Siegel, MJ. Computed tomography of pediatric cardiovascular disease. J Thorac Imaging. 2010;25(3):256–266.
29. Strauss, KJ, et al. Image gently: ten steps you can take to optimize image quality and lower CT dose for pediatric patients. AJR Am J Roentgenol. 2010;194(4):868–873.
30. Frush, DP, Yoshizumi, T. Conventional and CT angiography in children: dosimetry and dose comparisons. Pediatr Radiol. 2006;36(suppl 2):154–158.
31. Goske, MJ, et al. Image gently: progress and challenges in CT education and advocacy. Pediatr Radiol. 2011;41(suppl 2):461–466.
32. Applegate, KE, et al. Effect of cross-sectional imaging on negative appendectomy and perforation rates in children. Radiology. 2001;220(1):103–107.
33. Johnson, AK, et al. Ultrafast 3-T MRI in the evaluation of children with acute lower abdominal pain for the detection of appendicitis. AJR Am J Roentgenol. 2012;198(6):1424–1430.
34. Frush, DP. Technique of pediatric thoracic CT angiography. Radiol Clin North Am. 2005;43(2):419–433.
35. Donnelly, LF. Commentary: oral contrast medium administration for abdominal CT—reevaluating the benefits and disadvantages in the pediatric patient. Pediatr Radiol. 1997;27(9):770–772.
36. Brody, AS, et al. Radiation risk to children from computed tomography. Pediatrics. 2007;120(3):677–682.
37. Kuehle, CA, et al. Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol. 2006;187(4):W375–W385.
38. Dagia, C, Ditchfield, M. 3T MRI in paediatrics: challenges and clinical applications. Eur J Radiol. 2008;68(2):309–319.
39. Schindera, ST, et al. Abdominal magnetic resonance imaging at 3.0 T: what is the ultimate gain in signal-to-noise ratio. Acad Radiol. 2006;13(10):1236–1243.
40. Weinreb, JC, et al. Imaging the pediatric liver: MRI and CT. AJR Am J Roentgenol. 1986;147(4):785–790.
41. Finn, JP, et al. Primary malignant liver tumors in childhood: assessment of resectability with high-field MR and comparison with CT. Pediatr Radiol. 1990;21(1):34–38.
42. Roebuck, DJ, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37(2):123–132. [quiz 249-250].
43. Roebuck, DJ, Olsen, O, Pariente, D. Radiological staging in children with hepatoblastoma. Pediatr Radiol. 2006;36(3):176–182.
44. Arcement, CM, et al. MRCP in the evaluation of pancreaticobiliary disease in children. Pediatr Radiol. 2001;31(2):92–97.
45. Delaney, L, et al. MR cholangiopancreatography in children: feasibility, safety, and initial experience. Pediatr Radiol. 2008;38(1):64–75.
46. Ferrara, C, et al. Magnetic resonance cholangiopancreatography in primary sclerosing cholangitis in children. Pediatr Radiol. 2002;32(6):413–417.
47. Chavhan, GB, et al. Primary sclerosing cholangitis in children: utility of magnetic resonance cholangiopancreatography. Pediatr Radiol. 2008;38(8):868–873.
48. Hamada, Y, et al. Magnetic resonance cholangiopancreatography on postoperative work-up in children with choledochal cysts. Pediatr Surg Int. 2004;20(1):43–46.
49. Irie, H, et al. Value of MR cholangiopancreatography in evaluating choledochal cysts. AJR Am J Roentgenol. 1998;171(5):1381–1385.
50. Kim, MJ, et al. Using MR cholangiopancreatography to reveal anomalous pancreaticobiliary ductal union in infants and children with choledochal cysts. AJR Am J Roentgenol. 2002;179(1):209–214.
51. Fishbein, M, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39(7):619–625.
52. Fishbein, MH, et al. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15(3):287–293.
53. Qayyum, A, et al. Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques—initial experience. Radiology. 2005;237(2):507–511.
54. Dietrich, O, et al. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26(2):375–385.
55. Fujita, T, et al. Hepatic parenchymal enhancement in the cirrhotic liver: evaluation by triple-phase dynamic MRI. Abdom Imaging. 2002;27(1):29–33.
56. Lee, JK, et al. Fatty infiltration of the liver: demonstration by proton spectroscopic imaging. Preliminary observations. Radiology. 1984;153(1):195–201.
57. Wood, JC, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–1465.
58. Chalian, M, et al. MR enterography findings of inflammatory bowel disease in pediatric patients. AJR Am J Roentgenol. 2011;196(6):W810–W816.
59. Darge, K, Anupindi, SA, Jaramillo, D. MR imaging of the bowel: pediatric applications. Magn Reson Imaging Clin North Am. 2008;16(3):467–478. [vi].
60. Gee, MS, et al. Prospective evaluation of MR enterography as the primary imaging modality for pediatric Crohn disease assessment. AJR Am J Roentgenol. 2011;197(1):224–231.
61. Essary, B, et al. Pelvic MRI in children with Crohn disease and suspected perianal involvement. Pediatr Radiol. 2007;37(2):201–208.
62. Runge, VM, et al. Respiratory gating in magnetic resonance imaging at 0.5 Tesla. Radiology. 1984;151(2):521–523.
63. Kim, YK, et al. Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2006;186(1):149–157.
64. Klessen, C, et al. Magnetic resonance imaging of the upper abdomen using a free-breathing T2-weighted turbo spin echo sequence with navigator triggered prospective acquisition correction. J Magn Reson Imaging. 2005;21(5):576–582.
65. Lee, SS, et al. Image quality and focal lesion detection on T2-weighted MR imaging of the liver: comparison of two high-resolution free-breathing imaging techniques with two breath-hold imaging techniques. J Magn Reson Imaging. 2007;26(2):323–330.
66. Busse, RF, et al. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med. 2006;55(5):1030–1037.
67. Hennig, J, Weigel, M, Scheffler, K. Multiecho sequences with variable refocusing flip angles: optimization of signal behavior using smooth transitions between pseudo steady states (TRAPS). Magn Reson Med. 2003;49(3):527–535.
68. Kijowski, R, et al. Knee joint: comprehensive assessment with 3D isotropic resolution fast spin-echo MR imaging—diagnostic performance compared with that of conventional MR imaging at 3.0 T. Radiology. 2009;252(2):486–495.
69. Nandalur, KR, et al. Possible biliary disease: diagnostic performance of high-spatial-resolution isotropic 3D T2-weighted MRCP. Radiology. 2008;249(3):883–890.
70. Miyazaki, T, et al. Single-shot MR cholangiopancreatography of neonates, infants, and young children. AJR Am J Roentgenol. 1998;170(1):33–37.
71. Carlos, RC, et al. Biliary imaging with Gd-EOB-DTPA: is a 20-minute delay sufficient? Acad Radiol. 2002;9(11):1322–1325.
72. Koelblinger, C, et al. Gadoxate-enhanced T1-weighted MR cholangiography: comparison of 1.5 T and 3.0 T. Rofo. 2009;181(6):587–592.
73. Lee, NK, et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29(6):1707–1724.
74. Seale, MK, et al. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29(6):1725–1748.
75. Takao, H, et al. MR imaging of the biliary tract with Gd-EOB-DTPA: effect of liver function on signal intensity. Eur J Radiol. 2011;77(2):325–329.
76. Tamrazi, A, Vasanawala, SS. Functional hepatobiliary MR imaging in children. Pediatr Radiol. 2011;41(10):1250–1258.
77. Meyers, AB, et al. Characterization of pediatric liver lesions with gadoxetate disodium. Pediatr Radiol. 2011;41(9):1183–1197.
78. Maki, JH, et al. Steady-state free precession MRA of the renal arteries: breath-hold and navigator-gated techniques vs. CE-MRA. J Magn Reson Imaging. 2007;26(4):966–973.
79. Katoh, M, et al. Free-breathing renal MR angiography with steady-state free-precession (SSFP) and slab-selective spin inversion: initial results. Kidney Int. 2004;66(3):1272–1278.
80. Reeder, SB, et al. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 34(4), 2011.
81. Yokoo, T, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258(3):749–759.
82. van Werven, JR, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256(1):159–168.
83. Ford, PV, et al. Procedure guideline for gastrointestinal bleeding and Meckel’s diverticulum scintigraphy, Society of Nuclear Medicine. J Nucl Med. 1999;40(7):1226–1232.
84. Gelfand, MJ, Parisi, MT, Treves, ST. Pediatric radiopharmaceutical administered doses: 2010 North American consensus guidelines. J Nucl Med. 2011;52(2):318–322.
85. Vijay, BB, et al. Spontaneous biliary perforation in an infant: an unusual chronic presentation. Clin Nucl Med. 2008;33(4):273–275.
86. Majd, M. Radionuclide imaging in clinical pediatrics. Pediatr Ann. 1986;15(5):396–402. [407-408].
87. Seibert, JJ, Byrne, WJ, Euler, AR. Gastric emptying in children: unusual patterns detected by scintigraphy. AJR Am J Roentgenol. 1983;141(1):49–51.
88. Signer, E. Gastric emptying in newborns and young infants. Measurement of the rate of emptying using indium-113m-microcolloid. Acta Paediatr Scand. 1975;64(3):525–530.
89. Singh, SJ, et al. Gastric emptying of solids in normal children—a preliminary report. J Pediatr Surg. 2006;41(2):413–417.
90. Cook, BJ, et al. Radionuclear transit to assess sites of delay in large bowel transit in children with chronic idiopathic constipation. J Pediatr Surg. 2005;40(3):478–483.
91. Shin, YM, et al. Signs and symptoms of slow-transit constipation versus functional retention. J Pediatr Surg. 2002;37(12):1762–1765.
92. Notghi, A, et al. Use of geometric center and parametric images in scintigraphic colonic transit studies. Gastroenterology. 1994;107(5):1270–1277.
93. St-Vil, D, et al. Meckel’s diverticulum in children: 20-year review. J Pediatr Surg. 1991;26(11):1289–1292.
94. American College of Radiology. ACR-SNM-SPR practice guideline for the performance of gastrointestinal scintigraphy (website). http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/GI_Scintigraphy.pdf, 2012. [Accessed October 31].
95. Schneider, P, Duren, C, Reiners, C. SPECT-CT image fusion could enhance Meckel scan. World J Pediatr. 2010;6(3):281.
96. Papathanassiou, D, et al. SPECT-CT of Meckel diverticulum. Clin Nucl Med. 2007;32(3):218–220.
97. Rypins, EB, Kipper, SL. 99mTc-hexamethylpropyleneamine oxime (Tc-WBC) scan for diagnosing acute appendicitis in children. Am Surg. 1997;63(10):878–881.
98. Charron, M, Di Lorenzo, C, Kocoshis, S. CT and 99mTc-WBC vs colonoscopy in the evaluation of inflammation and complications of inflammatory bowel diseases. J Gastroenterol. 2002;37(1):23–28.
99. Stathaki, MI, et al. Role of scintigraphy in inflammatory bowel disease. World J Gastroenterol. 2009;15(22):2693–2700.
100. Skehan, SJ, et al. 18F-fluorodeoxyglucose positron tomography in diagnosis of paediatric inflammatory bowel disease. Lancet. 1999;354(9181):836–837.
101. Lemberg, DA, et al. Positron emission tomography in the investigation of pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):733–738.
102. Loffler, M, et al. High diagnostic value of 18F-FDG-PET in pediatric patients with chronic inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:379–385.
103. Park, HL, et al. Acute appendicitis secondary to metastatic small cell lung cancer incidentally found on F-18 FDG PET/CT. Clin Nucl Med. 2012;37(1):e19–e21.
104. Jasper, N, et al. Diagnostic value of [(18)F]-FDG PET/CT in children with fever of unknown origin or unexplained signs of inflammation. Eur J Nucl Med Mol Imaging. 2010;37(1):136–145.
105. de Lonlay, P, et al. Congenital hyperinsulinism: pancreatic [18F]fluoro-L-dihydroxyphenylalanine (DOPA) positron emission tomography and immunohistochemistry study of DOPA decarboxylase and insulin secretion. J Clin Endocrinol Metab. 2006;91(3):933–940.
106. Otonkoski, T, et al. Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes. 2006;55(1):13–18.
107. Heneweer, C, Grimm, J. Clinical applications in molecular imaging. Pediatr Radiol. 2011;41(2):199–207.