Chapter 5 Imaging of the Elbow
Background – imaging modalities
Digital, computed or conventional radiographs
Radiographic imaging is the first-line investigation of many elbow pathologies as it provides a cost-effective, reliable, easily accessible, quick and safe method of assessing acute and chronic elbow conditions. Frontal and lateral views are considered standard and further imaging with oblique radiographs is now rarely requested, as CT, US or MRI is usually better placed to provide further insight.1
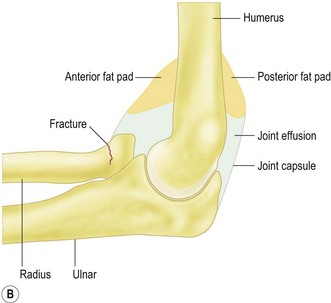
Sensitivity of elbow radiographs to bone injury and pathology is relatively high, although sensitivity falls when the clinician reviewing the film is inexperienced.2 While most of the information available from radiographs relates to bone, the value of the soft tissue signs visible on many radiographs should not be underestimated. Elevation of the anterior and posterior fat pads may indicate a joint effusion or haemarthrosis, which in turn may predict the presence of an occult fracture in the context of trauma. Alternatively, the elevated fat pad may indicate an effusion or synovitis secondary to an erosive arthropathy, infection or loose intra-articular bodies. Periarticular mineralization is often present in crystal arthropathies, hydroxyapatite deposition or in relation to ligaments and muscle following trauma.
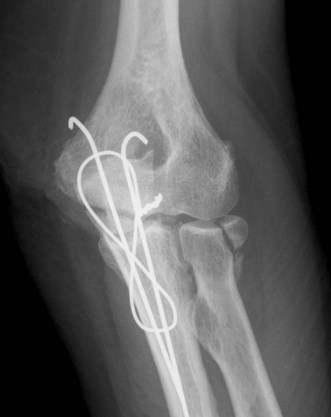
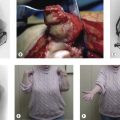
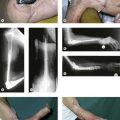
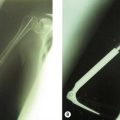
Radiographic imaging is often the only imaging that is required in the follow-up of fractures, dislocation and after surgical intervention (Fig. 5.1).
Magnetic resonance imaging
In many ways magnetic resonance imaging (MRI) provides the ideal modality for investigating the elbow, where it gives excellent anatomical resolution, particularly of ligaments,3 tendons, articular cartilage and subchondral bone and neurovascular structures.4 Multi-planar imaging allows these structures to be clearly identified from several ‘viewpoints’, usually the axial, coronal or sagittal plane. MRI holds the greatest advantage over ultrasound where multiple structures are thought to be abnormal and where the suspected pathology is deep within the joint, rather than superficial.
Where possible the elbow should be imaged using a surface or circular coil with the joint extended and forearm supinated, although in practice this may not be possible because of pain or deformity. In order to maximize MR image quality, the patient should be as comfortable as possible. Careful positioning of the upper limb and elbow during MRI may be used to better demonstrate structures that present an imaging challenge, such as the distal biceps tendon,5 where the elbow is flexed, the arm abducted and the forearm supinated (FABS). Oblique structures may be better visualized by additional imaging with a microscopy coil applied close to the ligament or joint region in question and acquiring thin-slice (1–2 mm) volume data with a T2* gradient echo (GRE), STIR or PD FS TSE sequence, although image acquisition time is significantly increased with this technique.3
Bone scintigraphy
Bone scintigraphy (or a bone scan) may be used to identify metabolically active bone lesions in the elbow, particularly where MRI, CT, plain films and radiographs have not produced a definitive diagnosis, or where one or more of these modalities cannot be utilized. The bone scan uses a radioactive tracer (technetium 99m) attached to methylene diphosphonate (MDP); the MDP is taken up by osteoblasts and is therefore a marker of physiological bone activity. The gamma radiation emitted by decay of technetium 99m is used by a gamma camera to form an image. Single-positron emission computed tomography (SPECT) produces a CT image of the joint, allowing more exact anatomical resolution of pathology. While bone scanning involves the injection of radioactive material into the body, it produces a relatively low effective radiation dose of the order of 0.008 mSv in an adult and 0.025 mSv in a child,6 compared with 2 mSv for a standard appendicular CT scan.
Elbow arthrography
Arthrography may be used in conjunction with CT, MRI and US in the investigation of elbow disorders.7–9 While there are several approaches to intra-articular injection, I find that a posterolateral approach into the radiocapitellar joint, immediately posterior to the radial collateral ligament, is the most reliable method of safely accessing the joint space.
In CT arthrography 2–3 mL of dilute low osmolar contrast medium (LOCM), followed by 5–10 mL of air, as tolerated by the patient, may be injected under ultrasound, fluoroscopic or CT guidance to produce a double-contrast CT arthrogram. Alternatively, 8–15 mL of dilute LOCM (50 : 50 mix with 0.9% saline solution) may be injected to produce a single-contrast CT arthrogram. Axial slices are acquired, preferably with ultra-thin multi-slice CT, and then reconstructed in the coronal and sagittal plane (or any plane required). CT arthrography is most useful when looking for loose bodies and osteophyte impingement, although it may be used to demonstrate chondral and osteochondral fractures.7
MR arthrography is a useful adjunct to conventional MRI, particularly in the assessment of the collateral ligaments, synovial and capsular pathology, and articular cartilage and osteochondral injuries.7,8 Direct arthrography involves injection of LOCM under ultrasound or fluoroscopic guidance, followed by 8–15 mL of either saline or, more commonly, a 1 : 200 solution (2 mmol/L) of gadolinium DTPA, into the elbow joint, followed by MRI.
US arthrography is mainly used to look for the presence of osteochondral loose bodies and involves the injection of 8–15 mL of saline into the joint under US guidance.9
Musculotendinous pathology
Anterior muscle group
The biceps brachii and brachialis muscles are found anteriorly and insert into the radial and ulnar tuberosities, respectively. The distal biceps tendon is made up of a weak aponeurosis, the lacertus fibrosus, which inserts into the antebrachial fascia of the ulnar aspect of the proximal forearm, and a strong lateral tendon that passes through the deep part of the antecubital fossa to insert into the medial aspect of the radial tuberosity. The lacertus fibrosus and the lateral tendon components of the distal biceps tendon correspond to the short and long head of the biceps muscle and may be found as separate bifurcated muscles in a significant minority of normal individuals.10
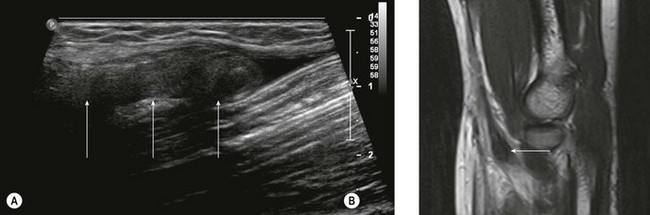
While the majority of biceps injuries involve the proximal, long head of the biceps tendon, the distal tendon is also prone to injury and accounts for approximately 5% of biceps injuries. US provides a good first-line investigation of the biceps tendon.11,12 Complete tendon rupture can be confirmed with US, which will also define the extent of tendon retraction (Fig. 5.2). When looking at the tendon in longitudinal section, the US probe should be oriented with inferolateral angulation, with the elbow flexed and the forearm supinated. When looking at the tendon in transverse section, the probe is held at right angles to the long axis of the forearm. If the heel of the probe is gently pressed into the interosseous groove towards the radial tuberosity, better imaging of the tendon insertion is possible.
Partial tears of the distal biceps tendon may be diagnosed by identifying thickening of the tendon close to the radial tuberosity, together with a wavy appearance of the distal tendon on longitudinal scanning.13 The tendon is most prone to tendinosis and tears close to the radial tuberosity as a result of distal tendon impingement between the radius and ulna and relative hypovascularity of the tendon in this region (Fig. 5.3). Unfortunately, it is also this region of the tendon that is most difficult to visualize with US and most prone to anisotropic artefact as a result of its oblique route. If there is doubt about the US findings, further imaging with MRI is advised.
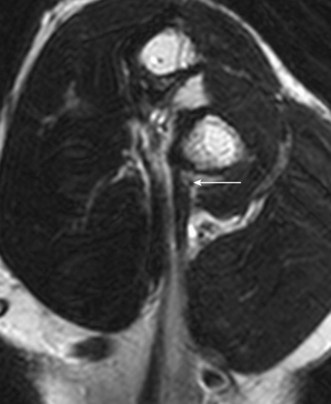
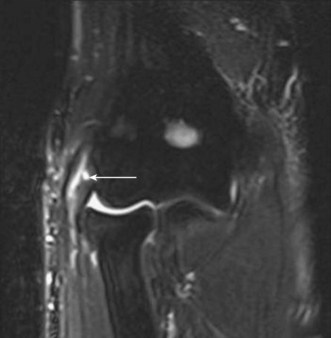
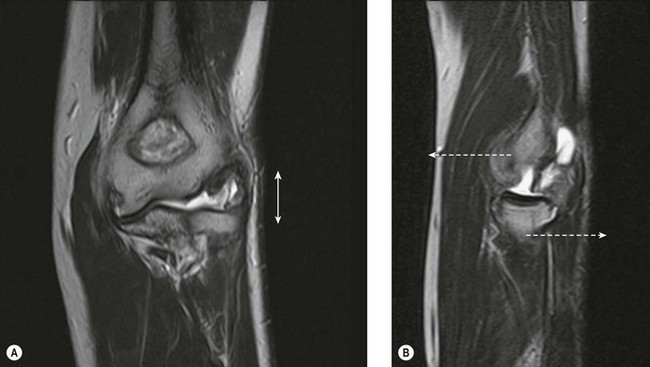
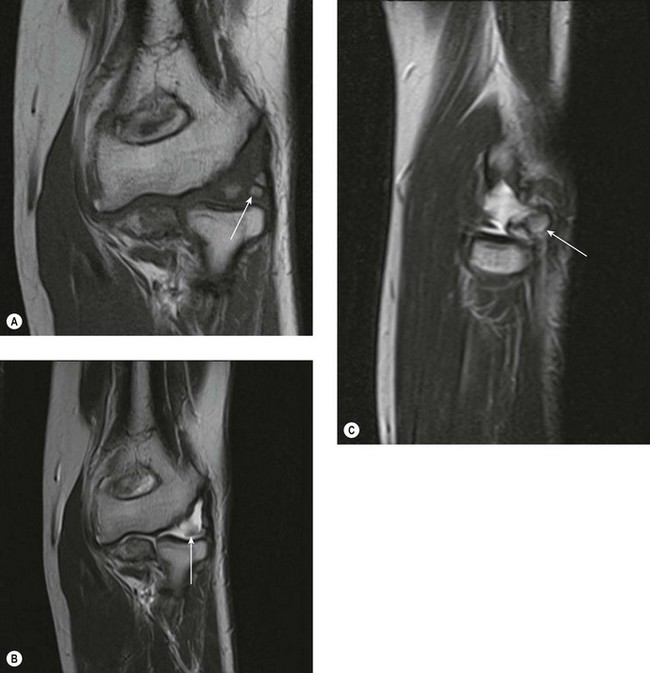
MRI of the distal biceps tendon is best performed with axial and sagittal imaging. However, the oblique course of the tendon often makes standard MRI imaging less than satisfactory. Improved visualization of the distal tendon and insertion can be achieved with the FABS view, where the elbow is flexed, the arm abducted and the forearm supinated.5 The images are acquired in the coronal plane of the elbow, perpendicular to the radius, with proton density with and without fat suppression, or T1 SE and T2 TSE with fat suppression, in addition to standard elbow sequences. This method of imaging the biceps tendon is particularly useful in detecting partial tears of the tendon (Fig. 5.4).5
Lateral muscle group
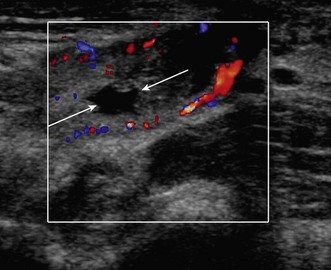
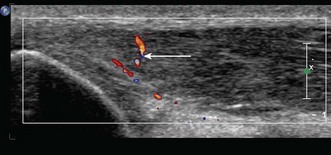
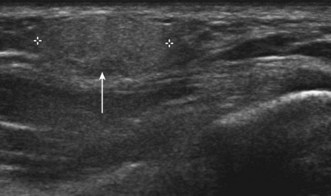
Lateral epicondylitis or tennis elbow is the most frequent cause of elbow discomfort.14 The diagnosis is often made from the history and clinical examination alone. Activities that may lead to overuse injury include throwing and racket sports and occupations involving repetitive forearm movement. It is most common in people aged 30–50, smokers and weekend warriors, who undertake intermittent bursts of high activity. Where there is clinical doubt about the diagnosis, or where the patient is not responding to treatment, the diagnosis may be confirmed with ultrasound or MRI. US will show a thickened common extensor origin, often with areas of hypoechoic change or hypervascularity on Doppler imaging (Fig. 5.5).15
US has been shown to be less sensitive than MRI in demonstrating epicondylitis, but has a similar specificity.16 However, US has a number of advantages over MRI. The tendon origin is often tender to US probe pressure, allowing a degree of clinical correlation with the imaging findings. Comparison can be made with the contralateral elbow, particularly where the findings are subtle. Gentle valgus stress during US imaging may demonstrate gaping of partial- or full-thickness tears.
MRI will demonstrate ill-defined, increased T2 signal on fluid-sensitive sequences, (STIR or fat saturation), within the common extensor origin, often with oedema surrounding the tendon or within the lateral epicondyle. Partial or complete tears may show a better-defined focus of high T2 signal within the tear, with partial or complete disruption of muscle fibres (Fig. 5.6). Partial tears can show both a thickened or thinned appearance. MRI may also be used to assess an associated lateral ulnar collateral ligament injury.17
Medial muscle group
Injuries of the medial muscle group are less common, with lateral epicondylitis occurring 10 times more frequently than medial epicondylitis (tendinosis of the common flexor origin). Medial epicondylitis or golfer’s elbow usually involves the pronator teres, flexor carpi radialis and palmaris longus muscle origins. Precipitating factors include an acute injury with valgus strain and sudden deceleration, such as when a golf club hits a tree root, and chronic repetitive valgus strain in racket sport, golf and during throwing activities. Ultrasound examination will show a thickened, hypoechoic tendon origin with increased vascularity on Doppler imaging. There may be enthesial change within the cortex of the medial epicondyle, particularly where the injury is chronic or where there is a history of long-term overuse or valgus stress (Fig. 5.7).
Posterior muscle group
Injury to the triceps muscle is relatively infrequent and usually follows a fall onto an outstretched arm or a direct blow to the muscle.18 The diagnosis of triceps rupture can be made with US, with the probe oriented in the sagittal plane. The tendon appears corrugated and is often outlined by fluid, with haematoma and debris in the rupture defect. Triceps rupture is often associated with bony avulsion of the insertion and in this situation the retracted bony avulsion fragment can be easily identified with US. In addition, the presence of a large haematoma that may lead to compartment syndrome and radial nerve compression19 can be identified quickly and easily. Compression of the ulnar nerve has also been reported,20,21 and fluid or haematoma within the cubital tunnel is visible with US.
Triceps tendinosis shows US changes identical to those seen in epicondylitis. The difficulty lies in differentiating a partial tendon tear from tendinosis, as both may have a similar appearance on US, with hypoechoic change within a thickened tendon. Disruption of the usual striated, fibrillar pattern indicates a tear rather than tendinosis.18,22 If doubt persists, sagittal T2 FS MRI will show a better-defined focus of altered T2 signal change within a partial tear and ill-defined or diffuse oedema with tendinosis.
A snapping triceps tendon, caused by subluxation of the medial head of triceps over the medial malleolus, may be demonstrated with US if the probe is held over the distal triceps while the elbow is flexed.23 This may be associated with a swollen ulnar nerve indicating neuropathy, or occasionally with coexistent subluxation of the ulnar nerve.23
Entrapment neuropathy, neural tumours and trauma
Ulnar nerve
Ulnar nerve compression is relatively common, with the incidence of ulnar nerve entrapment at the elbow second only to entrapment of the median nerve in the carpal tunnel.24 The ulnar nerve lies immediately posterior to the medial malleolus and reaches the forearm through the cubital tunnel. From proximal to distal the nerve courses through the condylar groove and then deep to the tunnel retinaculum and arcuate ligament, before passing between the ulnar and humeral heads of flexor carpi ulnaris. Compression of the ulnar nerve is most common at two points in the cubital tunnel: proximally, within the condylar groove; and distally, at the point where the nerve reaches the flexor carpi ulnaris.
US examination of the ulnar nerve is best performed with the arm abducted and externally rotated. A high-frequency probe with a narrow footprint should be held at right angles to the long axis of the arm, allowing the ulnar nerve to be identified in cross-section posterior to the medial malleolus before it enters the cubital tunnel. Ulnar nerve dislocation may be demonstrated if the probe is held over the ulnar nerve at the cubital tunnel while the patient moves the elbow from extension to flexion.23
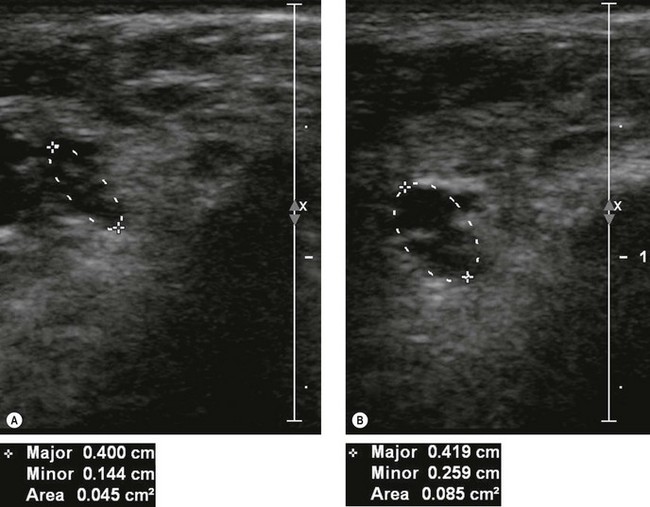
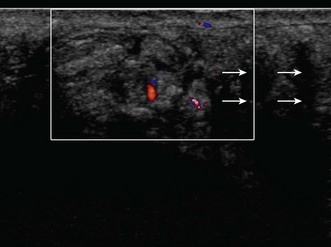
If US shows a thickened nerve proximal to the cubital tunnel,25 with a cross-sectional area greater than 7.5 mm2 (0.075 cm2) at the level of the medial epicondyle this is diagnostic of entrapment neuropathy (Fig. 5.8).26 It is helpful to compare the cross-sectional diameter with the normal, contralateral ulnar nerve to confirm that the nerve is swollen. US may also be used to look for extrinsic causes of compression such as an accessory medial anconeus muscle, the anconeus epitrochlearis, ganglion cysts, synovial thickening and osteophytes arising from the posteromedial margin of the ulnar trochlear joint.27
When MRI is used to examine the ulnar nerve, the nerve is most easily identified with axial T1 SE or PD sequences. The ulnar nerve will appear enlarged at the level of the cubital tunnel, with increased T2 signal in the nerve on T2 FSE or T2 FSE with fat suppression imaging.24
The ulnar nerve is also more prone to injury at the elbow and proximal forearm than the wrist or upper arm, typically as a result of a laceration, blunt trauma or in association with an elbow fracture or dislocation.28 Many injuries will show improvement in neurological function over time and will not need surgical repair, particularly where the trauma is blunt.28 In addition, there is frequently distortion of soft tissue anatomy in the acute phase, which makes both US and MRI less reliable. For these reasons it may be advisable to delay imaging until the swelling has subsided and to limit further investigation to those patients who show no sign of improvement of clinical signs or electromyography (EMG).
Median nerve and anterior interosseous branch
Distally the median nerve can be compressed as it passes between the ulnar and humeral heads of the pronator teres, leading to pronator syndrome. MRI can be used to demonstrate denervation myositis, with increased fluid signal in the pronator teres, flexor carpi radialis, flexor digitorum superficialis and flexor digitorum profundus muscles with median nerve injury, most clearly seen with axial T2 FS or PD FS imaging. Chronic denervation myopathy shows muscular atrophy and increased fat signal within the muscle belly, best appreciated on axial T1 images. With entrapment neuropathy of the anterior interosseous nerve the pathology is purely motor and there will be an absence of sensory symptoms or signs. MRI of the forearm can be used to help differentiate compression of the median nerve proper from isolated compression of the anterior interosseous branch if clinical examination and EMG studies are inconclusive. Anterior interosseous denervation will selectively involve the radial aspect of the flexor digitorum profundus and flexor pollicis longus muscles in the forearm and the pronator quadratus muscle at the wrist.29
Radial and posterior interosseous nerve
Radial nerve entrapment most frequently involves the deep branch of the radial nerve at or close to the origin of the supinator muscle (the arcade of Frohse) or as it passes through the supinator muscle. As this causes neuropathy of the posterior interosseous nerve (the continuation of the deep branch of the radial nerve beyond the supinator), the patient presents with weakness of wrist extension.30 In this situation MRI is advisable as the course of the radial nerve can be identified and any extrinsic or intrinsic radial nerve pathology identified. Denervation myositis, with increased fluid signal within the supinator muscle and extensor compartment muscles of the forearm, or muscular fatty atrophy, are the most frequently identified abnormality on MRI.24,31
Nerve tumours
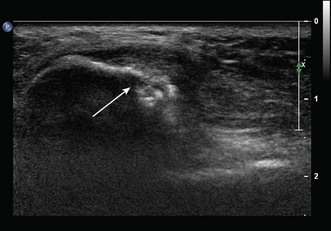
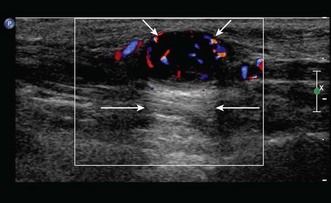
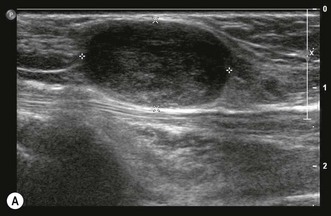
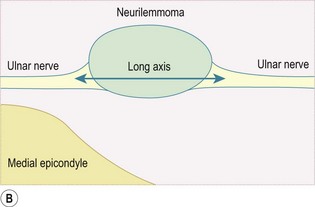
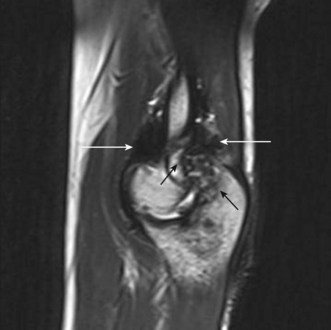
Any peripheral nerve, including superficial and deep branches, may develop a neuroma, either as a primary tumour or occasionally in response to direct trauma; this is true of the nerves that cross the elbow. Ultrasound can be used to identify a mass that is contiguous with the nerve or, when the nerve cannot be clearly identified, lies within the path of the nerve. Neurilemmomas, or Schwannomas, are hypoechoic tumours that are frequently capsulated and may show areas of cystic degeneration or hypervascularity (Fig. 5.9). Acoustic enhancement is a common feature. The nerve typically lies eccentric to the mass as the tumour arises from the nerve sheath. Neurofibromas also have a hypoechoic echotexture but rarely show a capsule, and hypervascularity is not seen. The nerve will usually run through the centre of the lesion.32 US is useful in detecting extrinsic compression by ganglia, which most commonly involves the ulnar nerve, although this can also result in compression neuropathy of the median and posterior interosseous nerves.
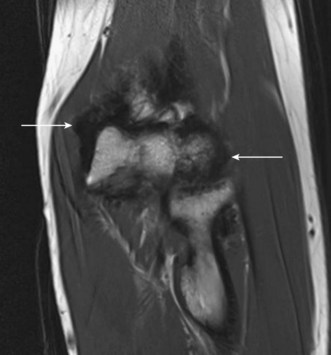
Peripheral nerve tumours usually show low to intermediate signal on T1 sequences and high T2 signal on MRI. Following gadolinium contrast, the lesions typically demonstrate avid, uniform post-contrast enhancement. The mass will often be shown as a fusiform swelling arising from within a nerve (neurofibromas) or from the nerve sheath, eccentric to the path of the nerve (neurilemmoma; Fig. 5.10).
Elbow instability
Approximately 50% of the stability of the elbow joint is inherent in the congruence of the bony articulations, with the remaining constraint provided by the ulnar and radial collateral ligaments and anterior capsule, contributing to medial, lateral and anterior stability respectively.33 After trauma, it follows that reduction of a fracture or dislocation as close as possible to normal bony anatomy will improve the chance of long-term joint stability. Fine-slice MDCT of the injured joint will help with preoperative assessment of the acute injury, identifying the configuration of fractures, particularly intra-articular components, as well as identifying any unsuspected additional injury.34 MDCT is particularly useful in identifying fractures of the coronoid process that may indicate transient dislocation at the time of injury. Multi-slice CT imaging with sub-millimetre collimation allows high-quality images to be reconstructed in any plane, which is an advantage in the context of acute trauma, as the patient may not be cooperative with regard to ideal positioning during scanning.
Ulnar collateral ligament
The ulnar or medial collateral ligament (UCL) is composed of anterior, posterior and oblique bands that are embedded in the medial joint capsule. The anterior and posterior bands arise from the medial epicondyle and insert into the sublime tubercle of the medial aspect of the coronoid process and olecranon, respectively, with the oblique transverse band lying in between. Of the three components of the ligament, the anterior band has the greatest clinical importance as it provides resistance to valgus strain,33,35 whereas the posterior band only becomes taught between 60° and 90° of flexion. The transverse band does not cross the joint and is not generally considered to contribute to elbow stability. Injury to the anterior band is particularly common amongst participants in throwing sports as a result of fatigue from chronic valgus stress. In children and adolescents, injury to the UCL may occur with avulsion of the medial apophysis.36
Clinical differentiation of UCL injury from medial epicondylitis, and between a UCL sprain and complete tear, can be difficult. Radiographs with the elbow under valgus stress may show medial joint laxity with a UCL injury, although this has been demonstrated to be a normal finding in the throwing arm of 25% of asymptomatic athletes.36 Tears of the UCL can be demonstrated with both US and MRI, and both imaging modalities have advantages. With US it is possible to compare with the unaffected side and to use dynamic scanning with valgus stress to confirm the presence of a complete tear.37,38 MRI demonstrates the anterior band of the UCL extremely well, especially in the axial and coronal planes.39 In addition, ulnar collateral ligament injury may be identified in combination with a lateral compression injury to the capitellum or radial head.40 This may not be visible with US, and for this reason MRI holds an overall advantage in imaging of the UCL.
In order to visualize the UCL with US the elbow should be flexed to approximately 30° and the forearm supinated. If the US probe is held in the coronal plane over the medial joint between the medial epicondyle and the medial aspect of the coronoid process the usual linear, hyperechoic, fibrillar structure of the anterior band of the UCL should be seen. UCL ligament tears usually occur in the mid ligament, compared with the lateral ligament injuries, which are more commonly seen close to the humeral origin. Complete tears will show discontinuity or complete absence of the ligament, whereas a partial tear or sprain will demonstrate ligament thickening.37 Both may show surrounding oedema, particularly within the flexor digitorum superficialis muscle, which is frequently injured at the same time as a UCL injury. The posterior and oblique bands are poorly demonstrated with ultrasound.22
With MRI, the anterior band of the UCL is best demonstrated in the coronal plane with the elbow extended and forearm supinated, although the ligament may also be followed in the axial plane from proximal to distal. UCL anatomy will generally be better demonstrated with a T1 TSE or proton density sequence, with abnormal signal better demonstrated on fluid-sensitive imaging, (PD FS, T2 FS and STIR). A combination of these sequences is advised. It is essential to review the ligament on axial and sagittal sequences to ensure the ligament is truly disrupted, as false reporting of a tear may occur if the ligament lies oblique to the coronal plane or there is partial volume artefact. Complete tears will show discontinuity of the ligament, usually in the mid substance, whereas a partial tear or sprain will typically involve the deep fibres of the anterior bundle. MRI may demonstrate the T sign, where joint fluid extends into the torn deep fibres of the ligament between the intact superficial fibres of the anterior bundle and the ulnar attachment.39 Both may show surrounding oedema, or a stress response within the medial epicondyle or medial ulnar attachments, particularly in the acute phase.
Lateral collateral ligaments
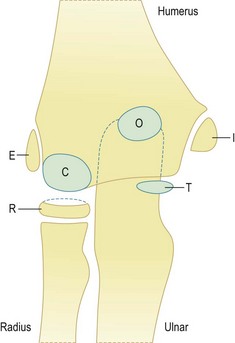
The lateral or radial ligament complex comprises the radial collateral, lateral ulnar collateral (LUCL) and annular ligaments, and a variably present accessory lateral collateral ligament. The major resistance to varus stress is provided by the joint articulation and anterior capsule,33 although posterolateral rotatory instability occurs when the lateral ulnar collateral ligament is deficient.
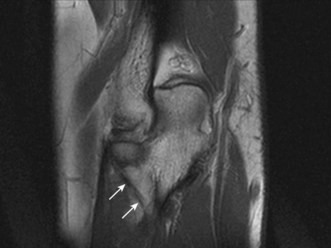
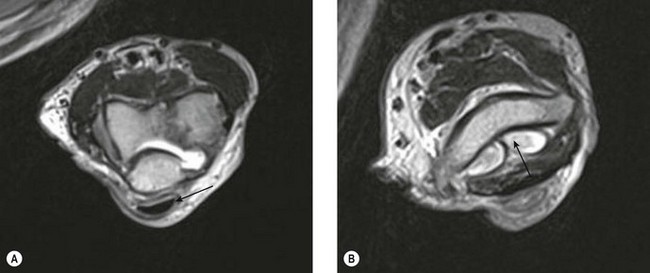
The radial or lateral collateral ligament (RCL) arises from the anterior aspect of the lateral epicondyle and inserts into the lateral fibres of the annular ligament, provides resistance to varus strain and approximates the radial head to the capitellum, thereby reinforcing bony stability. The annular ligament arises from the radial border of the proximal ulna and wraps around the radial head, acting as the primary stabilizer of the proximal radial ulnar joint, with no resistance to varus stress. The accessory lateral collateral ligament, when present, acts to stabilize the annular ligament. Finally, the lateral ulnar collateral ligament (LUCL) extends from the posterior aspect of the lateral epicondyle, passing posterior to the radial head to insert on the radial aspect of the proximal ulna (Fig. 5.11). The RCL acts to prevent posterior subluxation of the radial head and deficiency of the ligament is thought to result in posterolateral rotatory instability.41
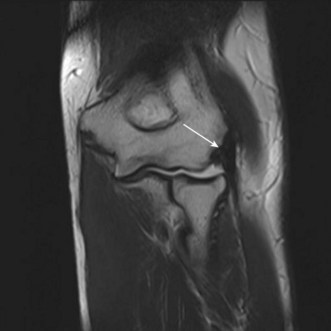
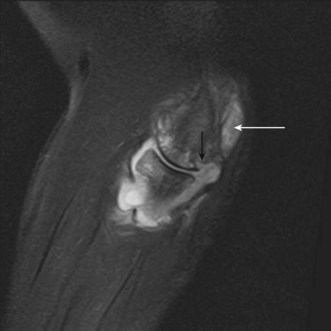
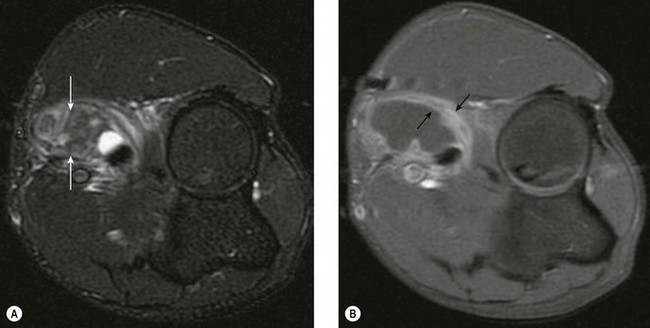
Both the LUCL and RCL are best imaged using coronal plane MRI17,39 with the elbow extended and supinated. The RCL lies immediately anterior to the LUCL and the normal ligament appears as a low-signal stripe deep to the common extensor tendon origin. The LUCL passes obliquely in the coronal plane from proximal to distal and from lateral to medial, where it is visible as a thin low-signal stripe passing immediately posterior to the radial neck (Fig. 5.11). MRI evidence of ligament rupture includes discontinuity of either ligament with a fluid-filled gap, usually close to the lateral epicondyle (Fig. 5.12).17,42 A thickened, intact ligament indicates a sprain (Fig. 5.13).17 Increased T2 signal may be present surrounding the ligament in the acute phase, and it is common to find abnormal signal within the extensor tendon origin consistent with an associated lateral epicondylitis.17
Acute trauma – fractures and dislocation
Childhood elbow injuries
The most common bony injuries of the elbow in children are the supracondylar fracture, typically in children less than 10 years old, radial neck fractures and medial or lateral epicondyle avulsion, which most often occur in the 10–15 age range. The normal angle between the long axis of the humeral shaft and capitellum is approximately 40° and the anterior humeral line should bisect the anterior third of the capitellum. Displacement of the capitellum posteriorly indicates an extension supracondylar fracture, whereas anterior displacement indicates the less common flexion injury. Where the fracture is undisplaced and not visible with radiographs, elevation of the posterior fat pad in particular has been shown to predict the presence of an occult fracture in 75% of cases where the fat pad is visible, with approximately half of these representing undisplaced supracondylar fractures.43
The radiographic diagnosis of elbow fractures in children is made more complicated by the secondary ossification centres, which have particular relevance to avulsion of the medial epicondyle, as the avulsion fragment may be dismissed as a normal ossification centre in error. Knowledge of the timing of ossification around the elbow is essential. The normal age of onset of ossification around the elbow is given in Table 5.1.
Table 5.1 The normal age of onset of ossification around the elbow
| Capitellum | 1 year |
Ossification is usually slightly earlier in girls compared to boys, and the onset of ossification varies considerably between individuals, although the order of ossification remains the same. The commonest pitfall is to mistake an avulsed and displaced medial epicondyle for a trochlear ossification centre; the medial epicondyle ossification centre should always be present before the trochlea ossifies. In this situation a comparison radiograph of the contralateral side may be justified in order to confirm the presence of a normal medial epicondyle. If doubt persists about the presence of an occult fracture or the presence of an avulsion versus normal ossification centre, an MRI is advised, with coronal T1 SE, T2 FS TSE or PD FS sequence to look for bone bruising and any associated ligament or muscle injury.44 Elbow fractures, and in particular supracondylar fractures, account for a large proportion of fractures in children, and the rate of misdiagnosis, particularly in emergency medicine, is disproportionately high.45
Adult elbow injuries
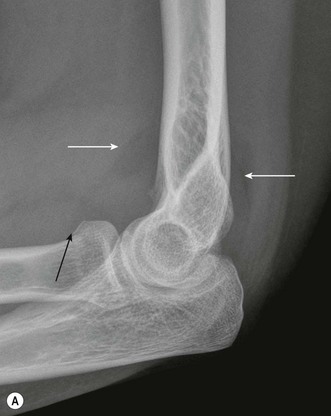
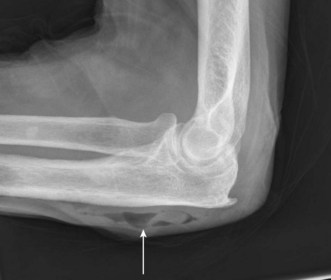
Radiographic identification of elbow fractures is more straightforward in the skeletally mature patient, although pitfalls still exist. Undisplaced or minimally displaced fractures of the radial head may be missed with simple radiographs, and soft tissue signs such as elevation of the anterior and posterior fat pads should be actively looked for (Fig. 5.14). In the context of acute trauma, the presence of an effusion has a strongly positive predictive value for occult fracture: 75% of elbows with raised fat pads have occult fractures, of which nearly 90% are radial head fractures.46
Comminuted (Mason III) or displaced (Mason II) radial head fractures are frequently associated with osteochondral injuries to the capitellum (29%), loose bodies (92%), and medial (54%) and lateral (80%) collateral ligament injury, even in the absence of a documented history of dislocation.47 Further imaging with MRI, including coronal plane imaging to assess collateral ligament integrity, osteochondral fractures and bone bruising, and sagittal imaging, which will demonstrate radial capitellar alignment and loose bodies within the olecranon and coronoid fossae, is recommended.40 Occult fractures will appear as well-defined linear low T1 signal with more diffuse surrounding high T2 signal.
A coronoid process fracture may be the only clue that the patient has suffered a transient dislocation. Isolated coronoid process fractures are often only visible on a true lateral radiographic view of the elbow and may be missed if poor radiographic positioning occurs as a result of pain or immobility. Where there is strong clinical suspicion of a fracture in the presence of an effusion and an apparently normal radiograph, further imaging with CT is advised.48
While CT can be used to identify occult fractures, it is best utilized for the preoperative assessment of a known fracture or reduced dislocation.34 CT will clearly show the fracture lines, as well as the number and position of comminuted fragments, demonstrate the alignment of radial capitellar, ulnar trochlear and proximal radial ulnar joints, and identify any loose bone fragments. CT is particularly useful in the context of trauma where the terrible triad of radial head fracture, coronoid process fracture and posterior dislocation is suspected. The degree of displacement and the size of fracture fragments can be assessed with CT. While it would be intuitive to assume that a large coronoid process fracture fragment is associated with a greater degree of instability, there is actually a closer correlation between small coronoid fracture fragments (which may be more easily missed with radiographs) and the terrible triad; conversely, larger fragments (>50%) are more correlated with a simple posterior dislocation.49
Osteochondrosis and osteochondritis dissecans
Osteochondrosis of the capitellum or Panner’s disease is a self-limiting abnormality of the osteochondral bone, which usually occurs in children between the ages of 5 and 12 before the capitellum is fully ossified. It is thought that the process is caused by avascular necrosis of subchondral bone secondary to a lateral compression injury of the elbow.50 Osteochondrosis is often visible in plain radiographs that will show fragmentation of the ossification centre. MRI shows decreased T1 and increased T2 fluid signal with epiphyseal fragmentation. Loose body formation is rare and the articular cartilage typically remains intact. It is usual for the lesion to resolve over time with conservative management, in contrast to osteochondritis dissecans.
Osteochondritis dissecans (OCD) or avascular necrosis of the elbow joint is typically found in adolescents and young adults51 and most often occurs within the capitellum, but may also be seen in the radial head or trochlea. While the process may be idiopathic, there is often a history of trauma involving a valgus or axial compression injury, repetitive valgus stress or a risk factor for avascular necrosis such as high levels of endogenous or therapeutic steroids, marrow pathology (e.g. leukaemia, sickle cell disease and Gaucher’s disease) or alcohol abuse.
Radiographs are the first-line investigation of OCD, although it should be stressed that the sensitivity of plain radiographs in detecting early OCD is low and radiographs may appear normal at initial presentation.51 By the time that OCD is visible with radiographic examination, the pathology will already be well advanced. Plain radiographs will show subchondral lucency, often with cortical irregularity, and a defect may be present within the capitellum once fragmentation has occurred. Early detection of suspected OCD should therefore be made using MRI when the initial radiograph is normal,40 or with scintigraphy when MRI is contraindicated.
MRI will show a focus of low to intermediate T1 signal within the subchondral bone, with a low T1 signal margin. The lesion may have variable signal on fluid-sensitive sequences, with high T2 fluid signal progressing to low T2 signal when sclerosis is present. Fragment instability is indicated by well-defined high T2 fluid signal separating the osteochondral fragment from the underlying bone marrow, a cleft in the articular cartilage at the lesion margin and a high T2 cystic structure deep to the lesion.52,53 Instability is more often associated with lesions with a largest dimension greater than 1 cm in size52 or greater than 0.8 cm2 in area.54
Direct MR arthrography can be used to clearly demonstrate the more sharply defined margin seen in unstable lesions, with contrast outlining the loose fragment.55 Indirect MR arthrography will show enhancement of granulation tissue around an unstable lesion, as well as demonstrating contrast-enhanced synovial fluid extending between the unstable fragment and the adjacent marrow.
When MRI is contraindicated and the initial radiograph appears normal or does not clearly show whether the fragment is stable or not, other modalities may be used to make the diagnosis. CT arthrography will show mixed sclerosis and lucent change that confirms the diagnosis of OCD, and contrast will outline an unstable fragment.56 CT arthrography may also be used to look for intra-articular loose bodies and to assess bony congruence. Alternatively, the rarely utilized investigation of technetium 99 MDP bone scanning with dynamic triple-phase imaging (immediate blood flow, 10-minute blood pooling and delayed bone uptake) can also be used to detect occult unstable lesions. Unstable OCD lesions show increased uptake during blood pooling and delayed phases of scintigraphy.54 Conversely, with stable OCD increased uptake during the blood pool phase is rarely seen and there is less marked uptake of the technetium tracer during the delayed phase, which allows differentiation between stable and unstable lesions.
Loose bodies, locking and restricted range of movement
Synovial osteochondromatosis
Idiopathic synovial osteochondromatosis is caused by benign subsynovial connective tissue metaplasia, which results in intrasynovial proliferation and chondral body formation. Eventually osteochondral loose bodies will appear within the elbow joint, or occasionally within adjacent bursae or tendon sheaths.57,58 The elbow joint is a relatively common site for this rare disorder, which is usually monoarticular.57 It is more common in men than women and typically presents in middle age. In contrast, secondary synovial osteochondromatosis is characterized by the absence of subsynovial metaplasia and the presence of an underlying aetiology, such as an osteochondral fracture, osteoarthritis or osteochondritis dissecans, which results in multiple loose bodies
During the early stages of idiopathic synovial osteochondromatosis radiographic examination will show a widened joint space with elevation of the fat pads and articular erosion. Loose bodies may not be present at this stage or may not be calcified or ossified and therefore not visible with radiographs. A significant minority of chondral bodies are not calcified or ossified.59
MRI is also useful in demonstrating associated nerve compression secondary to osteochondral loose bodies. This most commonly affects the ulnar nerve59 but has also been reported to involve the radial nerve and posterior interosseous nerve.
Locking and restricted range of movement
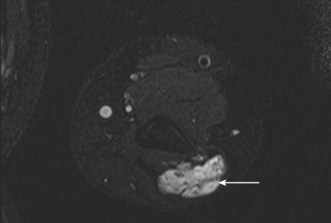
The first-line investigation when looking for a suspected loose body is the standard AP and lateral radiograph. It has been argued that radiographic examination has a similar sensitivity and specificity for loose bodies compared to CT or MR arthrography.60 However, MR arthrography offers far greater sensitivity for any associated soft tissue pathology and to the underlying cause for the loose body.7,8 Non-arthrographic MRI may clearly identify intra-articular loose bodies, particularly when a joint effusion is present (Fig. 5.15).
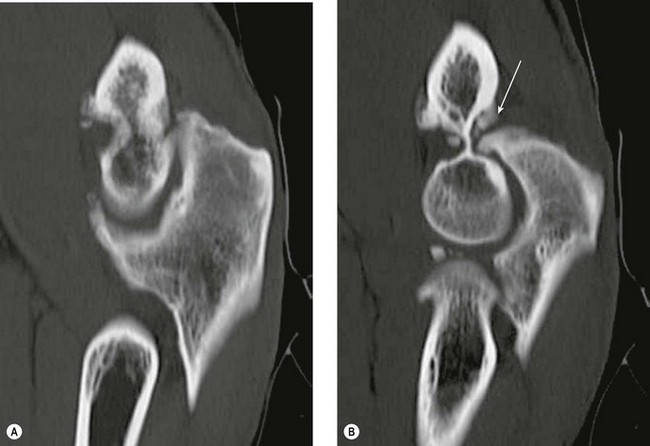
CT1 and CT arthrography7,8 have been shown to provide a sensitive method of demonstrating loose bodies (Fig. 5.16). CT is particularly useful in assessing the size of any associated bony defect or osteophyte impingement, although sensitivity to soft tissue pathology is low.
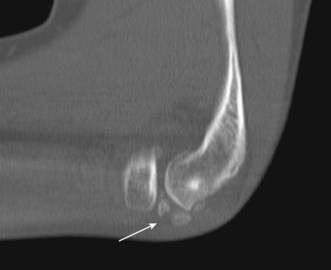
Figure 5.16 Loose osteochondral bodies (white arrow) in the radiocapitellar joint. CT, sagittal MPR.
Loose bodies may also be demonstrated with ultrasound,8 with the advantage of reduced cost. The sensitivity of US to loose bodies is increased in the presence of an effusion. Saline arthrography has been used to artificially create an effusion, making identification of loose bodies easier.9
Osteophyte impingement most often occurs as a result of osteoarthritis, with abnormal new bone formation at the joint margins. Olecranon or olecranon fossa new bone may present with restricted extension (Fig. 5.17), whereas coronoid fossa or coronoid osteophyte will restrict flexion. Pronosupination will be restricted by osteophytes involving the proximal radio ulnar joint. CT may be used in preoperative planning for arthroscopic resection of osteophytes.
Generalized joint pathology
Osteoarthritis
Primary osteoarthritis of the elbow is typically found in middle-aged men employed in strenuous manual labour,61 whereas secondary osteoarthritis is seen as a result of trauma, particularly fracture dislocation involving the coronoid process. In addition, synovial osteochondromatosis and osteochondritis dissecans have been implicated in secondary osteoarthritis.62
For the most part good-quality radiographs are sufficient to diagnose osteoarthritis in the elbow. CT is helpful in assessing the site and size of osteophytes and may help with the identification of loose bodies.1
Inflammatory arthropathy
The early accurate diagnosis of rheumatoid and seronegative arthropathy is now essential if patients are to receive effective intervention with disease-modifying antirheumatic drugs before joint damage occurs. Traditionally, plain radiographs are used to look for erosions, effusion and periarticular soft tissue swelling, although this method is insensitive to the early changes found with inflammatory arthropathy, and irreversible damage to the joints is often present by the time these radiographic signs become visible. Both ultrasound and MRI provide an effective method of detecting early erosions, enthesitis and synovitis around the elbow.63,64
Ultrasound has the advantage of wide availability and ease of access, with high spatial resolution of bone surfaces and soft tissues. Disadvantages include poor imaging of deep and inaccessible structures, with little or no intra-articular imaging, and difficulty in comparing lesion progress over time. US will demonstrate thickened, vascularized synovium and tenosynovitis.63 In addition, superficial and accessible erosions can be demonstrated if the defect in the cortex is greater than 2 mm in width. This allows sound waves to penetrate the defect.63 Intra-articular or medullary lesions will usually be missed with US, although these lesions are visible with MRI.63,64
MRI is useful in detecting erosions early in the disease process, as well as inflammatory change within bones. Fluid-sensitive sequences such as STIR or T2 with fat suppression will show high T2 signal within erosions and bone oedema (Fig. 5.18). Non-fat suppressed T1 imaging is useful, as the contrast between high signal medullary fat and low signal erosions and inflammatory change makes lesions conspicuous. Medullary and subcortical bone lesions can be detected, in contrast to US, where only superficial lesions are readily identified. Gadolinium-enhanced T1 imaging with fat suppression demonstrates highly vascularized synovium within erosions and increases the conspicuity of inflammatory lesions.64 Taking this further, a thin-slice T1W VIBE sequence before and after intravenous gadolinium contrast may be reconstructed in multiple planes using an MPR viewer to identify subtle erosion, cartilage defects and synovitis early in the disease process.
Pigmented villonodular synovitis
Pigmented villonodular synovitis (PVNS) is a benign and indolent disorder caused by locally invasive synovial proliferation, characterized on histology by haemosiderin-loaded multinuclear giant cells. While PVNS is more frequently found in the knee or hip, disease involving the elbow is well reported. Haemosiderin has a paramagnetic effect which causes very low signal on T1, T2 and PD weighted sequences resulting in black synovium (Fig. 5.19). This effect is most pronounced with T2* gradient echo. Similar appearances may also be found with inflammatory arthropathies, beta-2 microglobulin amyloid deposition arthropathy related to long-term haemodialysis, and following recurrent haemarthroses, particularly in haemophilia (Fig. 5.20).
Olecranon bursitis and joint infection
The large majority of cases of olecranon bursitis are related to repetitive trauma or an acute injury, or to inflammatory or crystal arthropathies. Approximately 20% of cases are due to infection, occasionally spontaneous, but often due to trauma of the overlying skin or cellulitis, or, finally, following steroid injection of the bursa. Plain films will demonstrate soft tissue swelling and occasionally soft tissue calcification. Features that may indicate the presence of infection include periosteal reaction, cortical erosion and gas within the bursa or adjacent soft tissues (Fig. 5.21).
US will demonstrate a fluid collection within the bursa. When the underlying diagnosis is a crystal arthropathy, US will often show highly echogenic foci that have an acoustic shadow (Fig. 5.22). The MRI appearances of infected versus non-infected bursitis often overlap, and MRI is often unable to discriminate between the two. However, the lack of soft tissue or bursal enhancement following intravenous gadolinium contrast effectively excludes infective bursitis.65
Spontaneous septic arthritis of the elbow is rare, and infection is more common after trauma, joint injection or occasionally after surgery. It should, however, be stressed that the patient’s history, clinical signs of joint infection, raised inflammatory markers and white cell count should be used to confirm a suspected diagnosis of joint infection, as the early MR signs of infection, such as effusion or reactive synovitis, are not specific (Fig. 5.23). US or fluoroscopy may be used to guide accurate joint aspiration.
Swelling and masses arising in and around the elbow joint
The majority of soft tissue masses that arise at the elbow will be superficial, which means that ultrasound will generally provide a good first-line investigation, discriminating between solid and cystic masses and demonstrating increased vascularity within the lesion.66 Common lesions include ganglia (Fig. 5.24), lipomas (Fig. 5.25), post-traumatic haematomas (Fig. 5.26) and bursae; neuromas and vascular malformations (Fig. 5.27) are uncommon and malignant masses are rare.
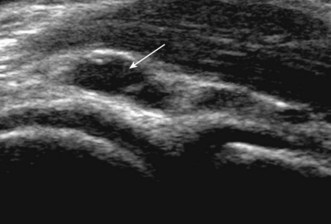
Figure 5.24 Ganglion cyst (white arrow) arising from the radial margin of the radiotrochlear joint. Ultrasound.
MRI provides complementary information, and is particularly useful with deeper masses, providing clearer definition of the margins of the mass with respect to muscle compartments, blood vessels and neural structures (Fig. 5.26). Gadolinium may be used to differentiate solid from cystic masses or haematomas (Fig. 5.27) and to show high vascularity.
1 Miller TT. Imaging of elbow disorders. Orthop Clin N Am. 1999;30(1):21-36.
2 Lee C, Bleetman A. Commonly missed injuries in the accident and emergency department. Trauma. 2004;6(1):41-51.
3 Yoshioka H, Ueno T, Tanaka T, et al. High-resolution MR imaging of the elbow using a microscopy surface coil and a clinical 1.5 T MR machine: preliminary results. Skel Radiol. 2004;33(5):265-271.
4 Melloni P, Valls R. The use of MRI scanning for investigating soft-tissue abnormalities in the elbow. Eur J Radiol. 2004;54(2):303-313.
5 Giuffrè BM, Moss MJ. Optimal positioning for MRI of the distal biceps brachii tendon: flexed abducted supinated view. Am J Roentgenol. 2004;182(4):944-946.
6 Donohoe KJ, Henkin RE, Royal HD, et al. Procedure guideline for bone scintigraphy: 1.0. J Nucl Med. 1996;37(11):1903-1906.
7 Steinbach L, Schwartz M. Elbow arthrography. Radiol Clin North Am. 1998;36(4):635-649.
8 Bianchi S, Martinolli C. Detection of loose bodies in joints. Radiol Clin North Am. 1998;37(4):679-690.
9 Miller JH, Beggs I. Detection of intra-articular loose bodies of the elbow with saline arthrosonography. Clin Radiol. 2001;56(3):231-234.
10 Dirim B, Brouha SS, Pretterklieber ML, et al. Terminal bifurcation of the biceps brachii muscle and tendon: anatomic considerations and clinical implications. Am J Roentgenol.. 2008;191(6):W248-W255.
11 Weiss C, Mittelmeier M, Gruber G. Do we need MR images for diagnosing tendon ruptures of the distal biceps brachii? The value of ultrasonographic imaging. Ultraschall Med. 2000;21(6):284-286.
12 Chew M, Giuffrè BM. Disorders of the distal biceps brachii tendon. RadioGraphics. 2005;25:1227-1237.
13 Miller TT, Adler RS. Sonography of tears of the distal biceps tendon. Am J Roentgenol. 2000;175(4):1081-1086.
14 Bennett JB. Lateral and medial epicondylitis. Hand Clin. 1994;10:157-163.
15 Connell D, Burke F, Coombes P, et al. Sonographic examination of lateral epicondylitis. Am J Roentgenol. 2001;176(3):777-782.
16 Miller TT, Shapiro M, Schultz E, Kalish P. Comparison of sonography and MRI for diagnosing epicondylitis. J Clin Ultrasound. 2002;30:193-202.
17 Bredella M, Tirman P, Fritz R, et al. MR imaging findings of lateral ulnar collateral ligament abnormalities in patients with lateral epicondylitis. Am J Roentgenol. 1999;173(5):1379-1382.
18 Bianchi S, Martinoli C, Abdelwahab I. Ultrasound of tendon tears. Part 1: General considerations and upper extremity. Skel Radiol. 2005;34:500-512.
19 Brumback RJ. Compartment syndrome complicating avulsion of the origin of the triceps muscle: a case report. J Bone Joint Surg Am. 1987;69:1445-1447.
20 Herrick RT, Herrick S. Ruptured triceps in a powerlifter presenting as cubital tunnel syndrome: a case report. Am J Sports Med. 1987;15:514-516.
21 Duchow J, Kelm J, Kohn D. Acute ulnar nerve compression syndrome in a powerlifter with triceps tendon rupture: a case report. Int J Sports Med. 2000;21:308-310.
22 Tran N, Chow K. Ultrasonography of the elbow. Semin Musc Radiol. 2007;11:105-116.
23 Jacobson J, Jebson P, Jeffers A, et al. Ulnar nerve dislocation and snapping triceps syndrome: diagnosis with dynamic sonography: report of three cases. Radiology. 2001;220:601-605.
24 Kim S, Choi J, Huh Y, et al. Role of magnetic resonance imaging in entrapment and compressive neuropathy: what, where, and how to see the peripheral nerves on the musculoskeletal magnetic resonance image. Part 2: Upper extremity. Eur Radiol. 2007;17(2):509-522.
25 Yoon JS, Kim BJ, Kim SJ, et al. Ultrasonographic measurements in cubital tunnel syndrome. Muscle Nerve. 2007;36:853-855.
26 Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648.
27 Park G, Kim J, Lee S. The ultrasonographic and electrodiagnostic findings of ulnar neuropathy at the elbow. Arch Phys Med Rehabil. 2004;85(6):1000-1005.
28 Kim D, Han K, Tiel R, et al. Surgical outcomes of 654 ulnar nerve lesions. J Neurosurg. 2003;98(5):993-1004.
29 Dunn A, Salonen D, Anastakis D. MR imaging findings of anterior interosseous nerve lesions. Skel Radiol. 2007;36:1155-1162.
30 Kleinert J, Mehta S. Radial nerve entrapment. Orthop Clin North Am. 1996;27:305-315.
31 Ferdinand B, Rosenberg Z, Schweitzer M, et al. MR imaging features of radial tunnel syndrome: initial experience. Radiology. 2006;240(1):161-168.
32 Beggs I. Sonographic appearances of nerve tumors. J Clin Ultrasound. 1999;27(7):363-368.
33 Morrey B, An K. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11:315-319.
34 Haapamaki VV, Kiuru MJ, Koskinen SK. Multidetector computed tomography diagnosis of adult elbow fractures. Acta Radiol. 2004;45(1):65-70.
35 Morrey B, Tanaka S, Kai-Nan A. Valgus stability of the elbow: a definition of primary and secondary constraints. Clin Orthop. 1991;265:187-195.
36 Singh H, Osbahr DC, Wickham MQ, et al. Valgus laxity of the ulnar collateral ligament of the elbow in collegiate athletes. Am J Sports Med. 2001;29:558-561.
37 Miller TT, Adler RS, Friedman L. Sonography of injury of the ulnar collateral ligament of the elbow: initial experience. Skel Radiol. 2004;33:386-391.
38 De Smet A, Winter T, Best T, et al. Dynamic sonography with valgus stress to assess elbow ulnar collateral ligament injury in baseball pitchers. Skel Radiol. 2002;31(11):671-676.
39 Blease S, Stoller D, Safran M, et al. The elbow. 3rd ed. Stoller DW, editor. Magnetic resonance imaging in orthopaedics and sports medicine. vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:1519-1523.
40 Brunton L, Anderson M, Pannunzio M, et al. Magnetic resonance imaging of the elbow: update on current techniques and indications. J. Hand Surg. 2006;31(6):1001-1011.
41 O’Driscoll S, Bell D, Morrey B. Posterolateral rotatory instability of the elbow. J. Bone Joint Sur Am. 1991;73:440-446.
42 Teh J. Imaging of the elbow. Imaging. 2007;19:220-233.
43 Skaggs D, Mirzayan R. The posterior fat pad sign in association with occult fracture of the elbow in children. J Bone Joint Surg Am. 1999;81(10):1429-1433.
44 Griffith JF, Roebuck DJ, Cheng JCY, et al. Acute elbow trauma in children: spectrum of injury revealed by MR imaging not apparent on radiographs. Am J Roentgenol. 2001;176:53-60.
45 Skaggs D, Pershad J. Pediatric elbow trauma. Pediatr Emerg Care. 1997;13(6):425-434.
46 O’Dwyer H, O’Sullivan P, Fitzgerald D, et al. The fat pad sign following trauma in adults: its usefulness and reliability in suspecting occult fracture. J Comput Assist Tomogr. 2004;28(4):562-565.
47 Itamura J, Roidis N, Mirayan R, et al. Radial head fractures: MRI evaluation of associated injuries. J Shoulder Elbow Surg. 2005;14(4):421-424.
48 Ring D, Jupiter J, Zilberfarb J. Posterior dislocation of the elbow with fractures of the radial head and coronoid. J Bone Joint Surg Am. 2002;84:547-551.
49 Doornberg J, Ring D. Coronoid fracture patterns. J Hand Surg. 2006;31(1):45-52.
50 Kobayashi K, Burton K, Rodner C, et al. Lateral compression injuries in the pediatric elbow: Panner’s disease and osteochondritis dissecans of the capitellum. J Am Acad Orthop Surg. 2004;12(4):246-254.
51 Kijowski R, Smet A. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skel Radiol. 2005;34(5):266-271.
52 Blease S, Stoller D, Safran M, et al. The elbow. 3rd ed. Stoller DW, editor. Magnetic resonance imaging in orthopaedics and sports medicine. vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:1578-1582.
53 Kijowski R, Smet A. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. Am J Roentgenol. 2005;185(6):1453-1459.
54 Mesgarzadeh M, Sapega AA, Bonakdarpour A, et al. Osteochondritis dissecans: analysis of mechanical stability with radiography, scintigraphy, and MR imaging. Radiology. 1987;165(3):775-780.
55 Elentuck D, Palmer W. Direct magnetic resonance arthrography. Eur Radiol. 2004;14(11):1956-1967.
56 Dotzis A, Galissier B, Peyrou P, et al. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18:e18-e21.
57 Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
58 Giustra P, Furman R, Roberts L, Killoran P. Synovial osteochondromatosis involving the elbow. Am J Roentgenol. 1976;127(2):347-348.
59 Kamineni S, O’Driscoll SW, Morrey BF. Synovial osteochondromatosis of the elbow. J Bone Joint Surg Br. 2002;84-B:961-966.
60 Dubberley J, Faber K, Patterson S, et al. The detection of loose bodies in the elbow. J Bone Joint Surg Br. 2005;87-B(5):684-686.
61 Stanley D. Prevalence and etiology of symptomatic elbow osteoarthritis. J Shoulder Elbow Surg. 1994;3(6):386-389.
62 Gramstad G, Galatz L. Management of elbow osteoarthritis. J Bone Joint Surg Am. 2006;88(2):421-430.
63 Guermazi A, Taouli B, Lynch J, et al. Imaging of bone erosion in rheumatoid arthritis. Semin Musc Radiol. 2004;8:269-285.
64 Jbara M, Patnana M, Kazmi F, et al. MR imaging: arthropathies and infectious conditions of the elbow, wrist, and hand. Radiol Clin North Am. 2006;44(4):625-642.
65 Floemer F, Morrison W, Bongartz G, et al. MRI characteristics of olecranon bursitis. Am J Roentgenol. 2004;183:29-34.
66 Ostlere S, Graham S. Imaging of soft tissue masses. Imaging. 2005;17:268-284.