Imaging of Intensive Care Patients after
Thoracic Surgery 3
Lung resections vary in their extent from wedge resections of peripheral lesions to complete pneumonectomy. The excision may be extended to include portions of the chest wall, pericardium, diaphragm, or great vessels, depending on the extent of disease.
Pneumonectomy
M. Fuchsjaeger and C. Schaefer-Prokop
A pneumonectomy is performed under standard conditions through a posterolateral thoracotomy.
The most common operation is an intrapleural pneumonectomy, which includes removal of the lung and visceral pleura. It involves opening the pericardium and dissecting the great vessels.
An extrapleural pneumonectomy involves an en-bloc resection of the lung including the parietal and mediastinal pleura, pericardium, and diaphragm.
The mortality rate of pneumonectomy is ca. 5%. Most deaths result from pneumonia, respiratory failure, pulmonary embolism, myocardial infarction, bronchopleural fistula, or pleural empyema.
Normal Postoperative Findings
The volume of the operated hemithorax is reduced after pneumonectomy. The ribs are spaced closer together and are directed at a steeper angle. The contralateral, nonoperated hemithorax often shows increased volume due to compensatory hyperinflation, especially in younger patients, while also showing signs of increased perfusion (Fig. 3.1).
Postoperative chest radiographs or computed tomography (CT) scans usually show pronounced anatomical changes. The radiologist should be aware of previous surgical interventions to understand the changes and interpret them correctly.
Air–fluid level. The pleural space on the operated side fills increasingly with serous fluid, which appears on radiographs as a homogeneous opacity with an air–fluid level (Fig. 3.2). The air–fluid level may persist for months. A double air–fluid level is caused by fibrinous septa. Multiple fluid levels occur in up to 25% of patients with a normal postoperative course and require differentiation from pleural empyema (see under Pneumonia in Chapter 2, p. 49). Once fluid has completely filled the pneumonectomy cavity, it is gradually reabsorbed. This is accompanied by a reduction of hemithoracic volume due to organization of the residual fluid and increasing fibrosis.
Ordinarily the pneumonectomy cavity shows a continuous decrease in air accompanied by a rising fluid level. Any sudden increase in the air volume (sudden fall of the fluid level) is suspicious for a complication such as bronchopleural fistula or a bronchial anastomotic leak.
Mediastinal shift. The mediastinum is shifted from the midline toward the operated side, accompanied by a shift of the trachea. The clamped main bronchus generally does not show a reduction in its diameter.
Fig. 3.1 a, b Increased perfusion of the remaining lung.
Pneumonectomy is followed initially by increased perfusion of the remaining lung, which should not be interpreted as “engorgement.” Compare the pulmonary vessels on postoperative day 1 (a) and postoperative day 7 (b).
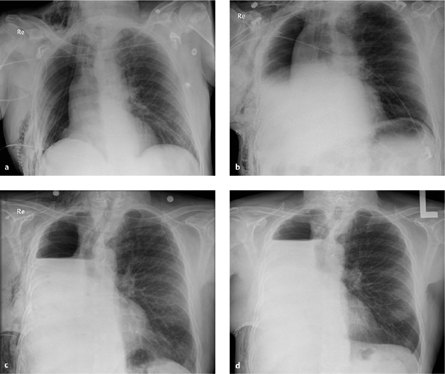
Fig. 3.2 a–d Normal progression of findings after pneumonectomy.
The pneumonectomy cavity is increasingly filled with fluid.
a Postoperative day 1.
b Postoperative day 2.
c Postoperative day 5.
d Postoperative day 10.
Specific Postoperative Complications
Postpneumonectomy Edema
Postoperative edema has an incidence of up to 5% after pneumonectomy (right > left) and less than 1% after lobectomy, typically occurring ca. 2–3 days after the resection. It is marked clinically by rapid progression of dyspnea and hypoxia. The prognosis with treatment is good. The pathophysiology of postpneumonectomy edema may relate to a fluid overload in the remaining lung, left heart failure, a disturbance of capillary membrane permeability due to sepsis or prolonged ventilation, or a surgically induced disruption of lymphatic drainage.
|
Early complications
|
|
Late complications
|
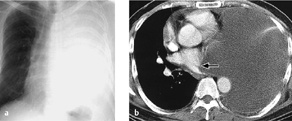
Fig. 3.3 a, b Hemothorax.
A rapidly progressive pleural effusion after surgery (12 hours passed between a and b) is suspicious for a hemothorax in patients with corresponding clinical symptoms.
Radiography. Interstitial edema is visible on chest radiographs and may spread to form patchy areas of alveolar consolidation.
Hemothorax
A hemothorax is usually the result of persistent bleeding from a bronchial artery or a vessel in the chest wall. The bleeding rarely originates from a central pulmonary vessel. The mortality rate is less than 0.1%. Hemothorax is diagnosed clinically in patients who have a chest tube in place.
Radiography. Hemothorax appears radiographically as a rapidly increasing pleural effusion (possibly accompanied by typical clinical signs such as tachycardia, hypotension, and a fall of hematocrit) with an associated mass effect based on its size (Fig. 3.3a, b).
Computed tomography. CT demonstrates a pleural fluid collection, frequently lobulated (loculated), of variable density. The effusion often has biconvex margins and produces a mass effect based upon its size and typical rapid enlargement.
The attenuation values of a hemothorax range from 15 HU to more than 40 HU, depending on the age of the hemorrhage. It is common to find layering effects within the pleural effusion. As the hemorrhage becomes more organized, the effusion may acquire a tumorlike aspect. It is distinguished from a tumor by noting its high attenuation values on plain scans and its lack of enhancement after IV contrast administration.
Chylothorax
A chylothorax results from injury to the thoracic duct or one of its tributaries. Sites of predilection are the left inferior paravertebral hemithorax following extrapulmonary pneumonectomy, the aortopulmonary window region following lymphadenectomy, and the region of the pulmonary ligament on both sides.
Radiography. Like a hemothorax, chylothorax appears radiographically as a rapidly increasing pleural effusion (Fig. 3.4).
Computed tomography. Negative, fat-equivalent CT attenuation values confirm the diagnosis of chylothorax. Negative attenuation values are not always present, however, due to the protein content of the collection.
Pleural Empyema
Extensive infections of the pleural cavity occur in up to 5% of cases, depending on the patient subgroup. A pleural empyema may develop as an early (weeks) or late complication (months to years). The main causative organisms are staphylococci, Pseudomonas, streptococci, and Aerobacter aerogenes.
An empyema occurring in the early postoperative period usually results from the intraoperative spread of infectious organisms or a bronchial leak. The main cause of a late infection is bronchopleural fistulation.
The following factors predispose to the development of a (late) postpneumonectomy empyema:
 secondary pneumonectomy after an initial lobectomy
secondary pneumonectomy after an initial lobectomy
 preoperative radiation
preoperative radiation
 sepsis
sepsis
 mediastinal lymphadenectomy
mediastinal lymphadenectomy
 prolonged mechanical ventilation
prolonged mechanical ventilation
The drainage of an empyema is described under Pneumonia in Chapter 2 (complications, empyema, p. 67).
Radiography. The following signs are noted, depending on the timing of the infection:
 Unusually rapid filling of the hemithorax with fluid
Unusually rapid filling of the hemithorax with fluid
 Shift of the mediastinum toward the healthy side (mass effect from the pleural fluid) (Fig. 3.5).
Shift of the mediastinum toward the healthy side (mass effect from the pleural fluid) (Fig. 3.5).
 Sudden drop of the air–fluid level in the operated hemithorax via a communication with the main bronchus or a fistula in the pleural wall
Sudden drop of the air–fluid level in the operated hemithorax via a communication with the main bronchus or a fistula in the pleural wall
 Reappearance of air in a completely opaque hemithorax due to a bronchopleural fistula or gas-forming bacteria
Reappearance of air in a completely opaque hemithorax due to a bronchopleural fistula or gas-forming bacteria
Fig. 3.4 a, b Basal chylopneumothorax after lobectomy.
Note extension of the air–fluid level along the full sagittal thoracic diameter (unlike an abscess) (a) and the anterior loculated pneumoserothorax, which also contains an air–fluid level (b).
Fig. 3.5 a, b Pyoserothorax with mass effect.
The collection has caused a contralateral shift of the mediastinum.
 Appearance of multiple air–fluid levels at various locations (caution: multiple air–fluid levels are a normal finding in up to 25% of patients; they are suspicious when multiple new air–fluid levels appear after initial homogeneous fluid filling of the hemithorax) (Fig. 3.6).
Appearance of multiple air–fluid levels at various locations (caution: multiple air–fluid levels are a normal finding in up to 25% of patients; they are suspicious when multiple new air–fluid levels appear after initial homogeneous fluid filling of the hemithorax) (Fig. 3.6).
Fig. 3.6 a, b Postoperative pleural empyema in two different patients.
The pleura is thickened and shows increased contrast enhancement. Multiple air inclusions are seen at different levels.
Ultrasonography. Ultrasound scanning can detect septations earlier and with greater clarity than CT. Echogenicity that is increased or variable due to septations and reverberations from air inclusions are typical sonographic features of a “complicated” effusion (see Fig. 2.91).
Computed tomography. CT signs of pleural empyema are a thickened pleura showing increased contrast enhancement and dense exudate with attenuation values greater than 20 HU. CT is better than radiographs for revealing associated mass effects (Fig. 3.6).
Bronchopleural Fistula
Small air leaks in the main bronchus are not uncommon and will generally heal spontaneously with conservative therapy. Large or persistent leaks give rise to bronchopleural fistulas (BPFs). The origin of the fistula is usually located in the bronchial stump. Generally the diagnosis is confirmed by bronchoscopy.
BPFs rarely occur in the early postoperative period. They more commonly develop as a delayed complication after a bronchial stump infection, bronchial ischemia, extensive lymphadenectomy, tumor recurrence, or in cases where the bronchial stump has been ligated at a tooproximal level. Predisposing factors are preoperative radiation and preoperative pleuropulmonary infection.
BPF is the leading cause of pleural empyema.
Radiography. The following radiographic signs may be noted at varying intervals after a pneumonectomy:
 Persistent or increasing pneumothorax with a chest tube in place
Persistent or increasing pneumothorax with a chest tube in place
 Increasing subcutaneous or mediastinal emphysema
Increasing subcutaneous or mediastinal emphysema
 More than a 2-cm drop in the air–fluid level with a mediastinal shift toward the healthy side
More than a 2-cm drop in the air–fluid level with a mediastinal shift toward the healthy side
 Sudden onset of pneumoserothorax with a preexisting serothorax (Fig. 3.7)
Sudden onset of pneumoserothorax with a preexisting serothorax (Fig. 3.7)
 Consolidated areas in the contralateral lung caused by fluid overflow from the pneumonectomy cavity or by direct communication through a BPF
Consolidated areas in the contralateral lung caused by fluid overflow from the pneumonectomy cavity or by direct communication through a BPF
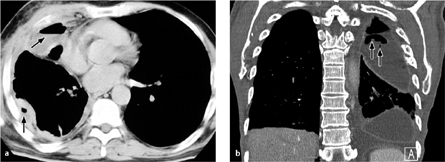
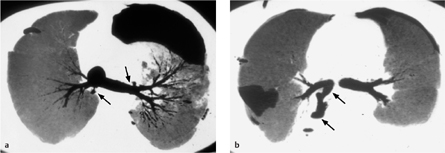
Computed tomography. In up to 50% of cases, thin-slice CT can directly visualize a BPF between the airway (bronchial stump) and pleural space, or between the lung parenchyma and pleural space (Fig. 3.8).
If the fistula cannot be directly visualized, persistent air and fluid collections in the mediastinum or pleural space may suggest the correct diagnosis.
Esophagopleural Fistula
This rare complication (< 1%) occurs most commonly after a right pneumonectomy (direct contact between the esophagus and right pleura).
An esophagopleural fistula (EPF) of early onset (within 2 weeks) may be caused by direct esophageal injury or decreased blood flow to the lower esophagus. Later occurrence of the fistula (from 2–6 weeks postoperatively) may result from an empyema, extensive lymphadenectomy, or BPF. An EPF caused by tumor recurrence may develop more than 2 years after the operation.
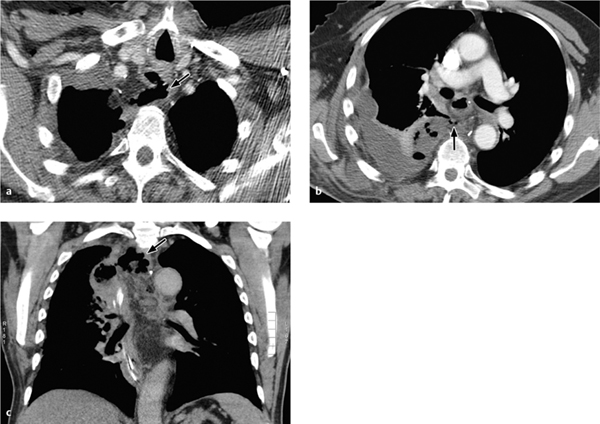
Fig. 3.7 a–c Anastomotic leak after pneumonectomy.
The pneumonectomy cavity is completely filled with fluid (a). This is followed by secondary air inclusions with air–fluid levels (b), which continue to increase during follow-up (c).
Patients typically present with fever, chest pain, and significant dysphagia. The presence of food constituents in the drained pleural fluid provides direct evidence of an EPF.
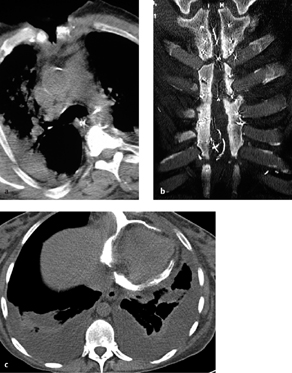
Radiography and CT. Direct visualization of the fistula (e. g., after oral contrast administration) may be difficult. Visualization is aided by moving the patient to a lateral decubitus position with the operated side down (Fig. 3.9). Imaging can be done by conventional esophagography or by CT after the oral administration of water-soluble contrast medium.
Cardiac Herniation
The heart may herniate through the pericardial defect created by an intrapericardial pneumonectomy. This complication occurs within the first few hours after an intrapericardial pneumonectomy and is extremely rare in cases where the pericardial defect has been closed. Cardiac herniation may occur through a pericardial defect of any size.
A right-sided herniation is manifested clinically by obstructed venous return leading to a rise of central venous pressure (due to counterclockwise rotation of the heart about the superior vena cava). A left-sided herniation leads to shock symptoms with hypotension and tachycardia (caused by strangulation of the left ventricle in the pericardial defect).
Radiography. The cardiac silhouette is markedly displaced toward the side of the pneumonectomy, with the cardiac apex touching the lateral chest wall. An empty, air-filled pericardial space may also be seen.
(Right) Pneumonectomy Syndrome
Pneumonectomy syndrome is a delayed complication usually following a right-sided pneumonectomy. Marked compensatory hyperinflation of the left lung shifts the mediastinum toward the right side, obstructing the left main bronchus and promoting intermittent pneumonia and dyspnea. In rare cases cardiac problems may develop due to compression of the (inferior) left pulmonary vein with damming back of blood into the pulmonary circulation.
Fig. 3.8 a–c Bronchopleural fistula.
This patient has a chronic bronchopleural fistula and persistent pneumothorax caused by a communication between the bronchus and pleura.
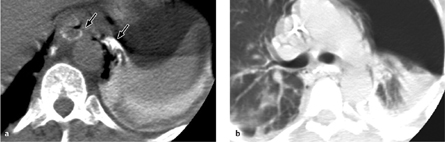
Fig. 3.9 a, b Esophagopleural fistula.
CT shows direct contrast extravasation from the esophagus into the left pleural space through an esophagopleural fistula (here, an iatrogenic fistula caused by balloon dilation of the esophagus). Pneumothorax and mediastinal emphysema are also present.
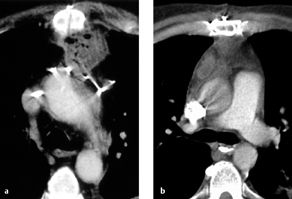
Fig. 3.10 a–c “Pneumonectomy syndrome”following a right pneumonectomy.
a, b Chest radiograph and CT show complete displacement of mediastinal structures toward the right side with compression of bronchial or vascular structures (in this case, the left lower lobe bronchus) by the spinal column.
c Postoperative CT. The mediastinum has been repositioned with a prosthesis implanted into the pneumonectomy space.
Computed tomography. CT demonstrates rotation of the mediastinum with stretching of the left main bronchus and/or inferior pulmonary vein across the spinal column (Fig. 3.10). Signs of intrapulmonary congestion may also be found (ground-glass opacity and thickened interlobular septa).
Nonspecific Complications after Pneumonectomy
Besides the specific complications of pneumonectomy described above, patients who have undergone thoracic surgery are highly susceptible to nonspecific complications such as atelectasis and pneumonia.
Atelectasis may result from the retention of secretions, postoperative swelling of the airways, impaired ciliary motility, and partial denervation. Superinfection and/or aspiration are not uncommon. The prevalence of pneumonia after pneumonectomy is ca. 20%; prolonged mechanical ventilation is a predisposing factor. Causes include superinfected atelectasis and the aspiration of gastric contents (gram-negative bacteria). The most serious complication is necrotizing pneumonia.
Lobectomy
The principal indications for lobectomy are the resection of:
 bronchial carcinoma
bronchial carcinoma
 large emphysematous bullae
large emphysematous bullae
 centrally located (obstructive) benign tumors
centrally located (obstructive) benign tumors
 focal lesions such as fungal infiltrates, pulmonary cysts, bronchopulmonary sequestration, or arteriovenous malformations
focal lesions such as fungal infiltrates, pulmonary cysts, bronchopulmonary sequestration, or arteriovenous malformations
Bronchoplastic techniques are used if the tumor involves only one lobe but has infiltrated the main bronchus central to the lobar bronchus. Tumors of this kind are removed by a lobectomy with a sleeve resection of the infiltrated portion of the main bronchus. Bronchial continuity is restored by reanastomosis.
Normal Postoperative Findings
The chest radiograph shows a moderate mediastinal shift toward the side of the lobectomy with a diminished lung volume on the operated side, ipsilateral elevation of the diaphragm, and compensatory hyperinflation of the remaining pulmonary lobes.
A pneumothorax or hydrothorax may develop, even in patients with a well-positioned chest tube. A mild pneumomediastinum or soft-tissue emphysema may also occur. A small pleural effusion or residual pneumothorax may persist for several days after removal of the chest tube. The mediastinum will later return to its midline position, but the remaining lobes will continue to be hyperinflated.
Arrangement of the pulmonary lobes. The arrangement of the remaining lobes follows a predictable pattern. Since the more anterior lobes are more mobile than the posterior lobes, the middle lobe and lingula tend to leave their anatomical position following an upper or lower lobectomy, occupying and filling the vacant space. On the other hand, resection of the lingula or middle lobe is not followed by a significant displacement of the remaining upper and lower lobes.
Lobectomy is followed by a rotation of the remaining bronchi, which increases the distance between the bronchi and artery. The ipsilateral hilum is generally twisted and appears smaller than the contralateral hilum on projection radiographs.
Specific Postoperative Complications
Generally speaking, the complications described for pneumonectomy, such as bronchopleural fistula and empyema, may also occur after a lobectomy.
Lobar Torsion
True lobar torsion is a rare complication. It is most common to find the middle lobe twisted about the true bronchovascular axis following an upper lobectomy. The central vascular obstruction initially compromises venous blood flow, causing an ischemia that culminates in infarction and lobar gangrene. This condition is marked by rapid clinical deterioration and has a poor prognosis, even when promptly diagnosed.
Fully developed lobar torsion is less common than atelectasis, which may be associated with superinfection and a variable ischemic response caused by vascular kinking of the middle lobe bronchus, which is directed sharply upward after the surgery. A typical presentation is “middle lobe syndrome” following right upper lobectomy (Fig. 3.11).
Radiography. The chest radiograph initially shows increasing opacity of the twisted lobe. A temporary volume increase due to hyperinflation is followed later by volume loss and complete opacity of the affected lobe.
Computed tomography. Postoperative CT shows increasing consolidation of the affected lobe. Hyperinflation, infection, or atelectasis may increase the lobar volume or, more commonly, may cause a volume decrease. Multiplanar reformatting of 3D-CT data sets can demonstrate the angled, narrowed, or twisted condition of the bronchi and the vessels that supply them.
Fig. 3.11 a–c Postoperative middle lobe syndrome.
The middle lobe bronchus is angled sharply upward following upper lobectomy. Poor poststenotic ventilation and recurrent infections have led to complete atelectasis (with kind permission of D. Wormanns, Berlin).
Segmental Lung Resection
The indications for segmental pneumonectomy include benign neoplasms, bronchiectasis, pulmonary cysts and abscesses, and metastases.
Patient positioning and surgical approach are the same as for lobectomy. A segmental lung resection is more technically demanding than a lobectomy due to anatomical variations in lung segmentation, but surgical mortality is low at only 0.5%.
Normal Postoperative Findings
The chest radiograph following a segmental lung resection is comparable to that after a lobectomy, except that the effects caused by a reduction in lung volume are less pronounced than after lobectomy.
Specific Postoperative Complications
Since the surgical defects created by a segmental resection are not covered by visceral pleura, postoperative air leaks are relatively common. Persistent pneumothorax is another frequent occurrence.
Postoperative intrapulmonary opacities are generally attributable to small intraparenchymal hemorrhages. These opacities will resolve in a matter of days or weeks leaving nothing more than focal parenchymal scars.
After the removal of a nodular mass, fluid may collect in the operative area and form a “pseudotumor” in place of the excised nodule. This change is usually transitory, although rarely a “permanent nodule” may persist and mimic the appearance of a recurrent tumor.
Sleeve Resection
In this operation, portions of the central respiratory tract are resected and continuity restored by reanastomosis. This is illustrated by the resection of an advanced bronchial carcinoma with tracheobronchial invasion, in which the contralateral main bronchus is reanastomosed to the distal trachea.
Specific Postoperative Complications
A leaky bronchial stump may cause a tension pneumothorax to develop during the early postoperative period. Bronchial anastomotic leak occurring at a later stage usually results in empyema. Anastomotic stricture is the most common late complication, occurring in up to 20% of cases.
Radiography. The appearance of a new air–fluid level on the chest radiograph is suspicious for empyema formation due to an anastomotic leak.
Computed tomography. The most sensitive CT sign is the direct visualization of a wall defect. The detection of extrabronchial air close to the anastomosis is sensitive but not very specific. Bronchial wall irregularities are another nonspecific finding. The diagnosis is confirmed by bronchoscopy. Anastomotic stricture is detected by thin-slice CT with multiplanar reformatting.
Lung Transplantation
Single lung transplantation is performed through a posterolateral thoracotomy. Bilateral lung transplantation can be performed as a sequential unilateral transplantation or via a median sternotomy. The bronchial anastomosis is sometimes covered with peribronchial lymphatic tissue to reduce the risk of dehiscence. The pulmonary arteries are joined by end-to-end anastomosis. Postoperative immunosuppression is generally indicated.
Normal Postoperative Findings
The position of the chest tubes is checked immediately after the operation. One tube should be placed near the apex and another near the base.
Pleural effusion. A variable ipsilateral pleural effusion will develop due to impaired lymphatic drainage and should resolve within 2 weeks. If the effusion increases during that period, the possibility of infection should be considered. Effusion may also occur in the setting of a hemothorax or chylothorax.
Reperfusion edema. Noncardiogenic pulmonary edema, known also as reperfusion edema or reimplantation response, consistently develops after lung transplantation. Causal factors are an ischemic increase in capillary permeability (after transient hypoxia), impaired lymphatic drainage, sympathetic denervation, and surgical trauma. Hypertension and consequent increased edema may develop in the first lung of a double-lung transplantation, depending on the sequence of intraoperative implantation.
Radiography. The radiographic features of reperfusion edema are like those of interstitial and alveolar lung edema (Fig. 3.12). They invariably appear during the first 2 days after lung transplantation, and one-third of recipients manifest these changes during the first 12 hours. The edema initially increases until the fifth postoperative day and then clears by the 10th postoperative day or the 14th day at the latest.
Fig. 3.12 Reperfusion edema.
Significant reperfusion edema is found in a 24-year-old woman who received one lobe in each hemithorax (split lung transplantation) because of her small size. Slight mediastinal emphysema and pneumothorax are also noted on the right side.
Computed tomography. CT shows edematous thickening of the bronchial walls and interlobular septa, accompanied by reticular markings and ground-glass opacity or even consolidations of the lung parenchyma dependant on the amount of intrapulmonary fluid.
Specific Postoperative Complications
Despite its lack of specificity, the routine chest radiograph is important in the detection of complications such as pneumonia and allograft rejection. Clinical or radiographic suspicion of pneumonia or rejection should be investigated by bronchoscopy with bronchoalveolar lavage. Bronchoscopic biopsy may be added if required.
Early complications may consist of pneumothorax, acute allograft rejection, pneumonia, bronchial ischemia or dehiscence, bleeding, or phrenic nerve palsy. Perioperative organ injury, ischemia, or vascular stenosis may lead to initial graft dysfunction.
Possible late complications are infection, acute or chronic rejection, bronchial stenosis, and post-transplant lymphoproliferative disease.
Initial Dysfunction
Initial dysfunction describes a condition in which reperfusion edema does not resolve within the first two weeks and the graft shows increasing opacity and functional deterioration.
Radiography and CT. The chest radiograph displays features of DAD/ARDS: diffusely increased density of the lung parenchyma (ranging from ground-glass opacity to consolidation) with superimposed intralobular and interlobular reticular changes (see page 37).
Acute and Chronic Progressive Rejection
An acute rejection response during the first 3 months is manifested clinically by cough, fever, dyspnea, and decreased oxygen saturation.
Radiography. Radiographic changes are subtle or even absent in many cases. Generally speaking, they resemble the radiographic signs of rejection, reperfusion edema, cardiogenic lung edema, or pneumonia. The most common radiographic changes are thickened interlobular septa and increasing pleural effusions.
Computed tomography. CT and especially HRCT may demonstrate a variety of changes, which are ultimately nonspecific (Table 3.2, Fig. 3.13a). It can be difficult to distinguish between acute rejection and infection (especially with CMV and Pneumocystis jirovecii).
The hallmark of (chronic progressive) allograft rejection is bronchiolitis obliterans. Approximately 10% of all lung transplant recipients develop bronchiolitis obliterans during the first year after surgery, and ca. 20% by the second year. Classic bronchiolitis obliterans without other superimposed parenchymal changes produces only a mosaic pattern with air trapping, appreciated more clearly in expiration than in inspiration. This is followed by increasing airway changes with a tree-in-bud pattern, bronchiectasis, bronchiolectasis, bronchial wall thickening, and progressive signs of interstitial fibrosis (Fig. 3.13b). Similar changes are also found in pulmonary graft-versus-host disease (GvHD) after bone marrow transplantation.
Airway Complications, Anastomotic Leak
Anastomotic leak is a typical complication during the initial days after lung transplantation. It has an ischemic cause, as the bronchial tissues close to the anastomosis receive only a retrograde blood supply via pulmonary arterioles or by diffusion, since the bronchial arteries are not reanastomosed after the transplantation. Although the incidence of anastomotic leak was still quite high a few years ago, today it has been drastically reduced through improved suturing techniques, better immunosuppression to prevent acute rejection, adjunctive rheologic measures (heparin, prostaglandin 2), and more efficient prophylaxis of infection.
Small defects will generally heal without sequelae. Larger defects lead to necrosis resulting in anastomotic dehiscence. These defects can be managed by bronchoscopic stent insertion if necessary, and a worst-case scenario may necessitate retransplantation. Bronchial strictures may develop as a late complication.
Computed tomography. HRCT with a slice thickness of 1–2 mm will detect even small extraluminal air collections. Small, diverticulum-like peribronchial air collections associated with small wall defects are distinguished from large, pouch-like protrusions with extensive bronchial wall defects, which predispose to bronchial scarring and stenosis (Fig. 3.14).
|
CT in acute rejection
|
|
CT in chronic progressive rejection (bronchiolitis obliterans)
|
Fig. 3.13 a, b Allograft rejection.
a Acute rejection response is marked by extensive, bilateral pleural effusions and focal pulmonary opacities as in DAD/ARDS. These findings are nonspecific, however, and may also result from infection.
b Chronic rejection with bronchitis, bronchiolitis, and fibrotic parenchymal changes (similar to GvHD in the lung after bone marrow transplantation).
Fig. 3.14 a, b Anastomotic leak.
Anastomotic leak is manifested by a small diverticulum-like air collection (a) and a larger extrabronchial air collection (b). While the former usually resolve without sequelae, the latter predis-pose to infection and subsequent bronchial strictures (stent implantation).
Infection
Infections are the most frequent complication of lung transplantation and are the leading cause of death in lung recipients. The risk of infection is increased not only by immunosuppression but also by transplantation-induced ischemic changes, the disruption of lymphatic pathways, and a diminished cough reflex.
The most frequent causative organisms are bacteria such as Pseudomonas and staphylococci, CMV and other viruses, and fungi. Bacterial infections generally occur within the first two months after transplantation. CMV infections occur within the first 4 months (immunosuppression). Fungal infections are rare but are associated with a high mortality.
Radiography and CT. Bacterial infections cause more or less pronounced areas of consolidation that are difficult to distinguish from an acute rejection response or edema.
CMV infections are characterized by an interstitial pattern of reticulonodular opacities, ground-glass opacities, or consolidated areas, often accompanied by small pleural effusions.
Fungal infections most commonly produce multiple nodular or focal opacities.
Cardiovascular Surgery
Most cardiac operations are performed through a median sternotomy or extrapleural approach. Similar postoperative problems and complications arise, regardless of whether the surgery involved a coronary bypass or the correction of valvular heart disease (Table 3.3).
Normal Postoperative Findings
In an accurate anteroposterior projection of the chest, the aortic valve is projected over the spinal column while the mitral valve is displayed lower and to the left of the spinal column. The pulmonary valve is located at a higher level than the aortic valve and slightly to the left of the midline.
Almost all postcardiac surgery patients display atelectatic changes in the left lower lobe. Although cardiac surgery is performed through a median extrapleural sternotomy, the pleural space will have been opened in some patients (postoperative pleural drainage tube). Small amounts of mediastinal or pericardial air are a normal postoperative finding, as are transient pleural effusions.
Specific Postoperative Complications
Mediastinal Hematoma
Small mediastinal fluid collections, hematomas, and air collections are a normal finding up to 3 weeks after cardiac surgery.
Radiography and CT. Postoperative widening of the mediastinum due to hemorrhage or edema is a common finding after sternotomy. Up to 35% widening of the mediastinum (compare mediastinal widths on preoperative PA and postoperative AP chest radiographs) is of no concern if the patient is stable and there is no clinical evidence of hemorrhage.
More than 50% widening relative to the preoperative radiograph (at the same depth of inspiration) is at least suspicious for hemorrhage (Fig. 3.15). The indication for reexploration is based on clinical criteria (> 1500 mL drainage of blood, sudden increase in drainage volume, tamponade or hypotension due to blood loss). More than 60% mediastinal widening is considered a critical radio-graphic finding.
|
|
CT scans show diffuse, sometimes patchy or streaky hyperdense infiltration (> 40 HU) of the mediastinal fat. The mass effect of the collection depends on the extrava-sated blood volume (Fig. 3.16).
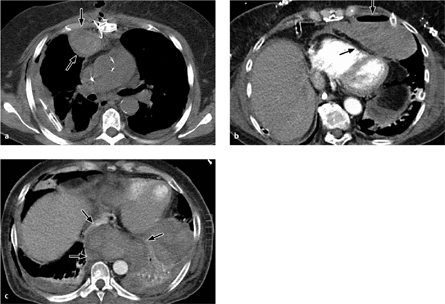
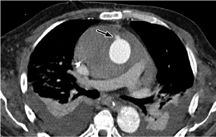
Rare complications include vascular lesions (pseudoaneurysms, Fig. 3.17) at the access site for cardiopulmonary bypass as well as hemorrhagic complications.
Mediastinitis
Postoperative mediastinitis has an incidence of 0.4–5% and is usually associated with sternal dehiscence. It may also result from esophageal perforation or the spread of a cervical infectious process. Even today, mediastinitis has a high mortality rate that exceeds 60%. If conservative antibiotic therapy is unsuccessful, surgical debridement may be considered depending on the extent of the process.
Computed tomography. CT shows stranding or complete obliteration of the mediastinal fat by fluid. Circumscribed abscess formation is uncommon (Fig. 3.18). Mediastinitis has a high association with bilateral pleural effusions. Sometimes a communicating process is found between the substernal soft tissues and the infiltrated subcutaneous soft tissues. Aspiration cytology is used to confirm the diagnosis and identify the causative organism.
Progression of fluid retention combined with bilateral pleural effusions and more than 3 mm of sternal dehiscence later than 2 weeks after surgery are suspicious for an infectious process.
Fig. 3.15 a, b Mediastinal widening.
Some degree of mediastinal widening is always present after cardiac surgery. More than 50% widening relative to the preoperative width or a marked increase in mediastinal widening over time is suggestive of a complication (here, mediastinal hematoma after bypass surgery).
Fig. 3.16 a–c Hematomas.
a Retrosternal hematoma at a typical location after sternotomy.
b Pleural (not mediastinal) hematoma after lung transplantation.
c Hematoma in the posterior mediastinum after gastrectomy.
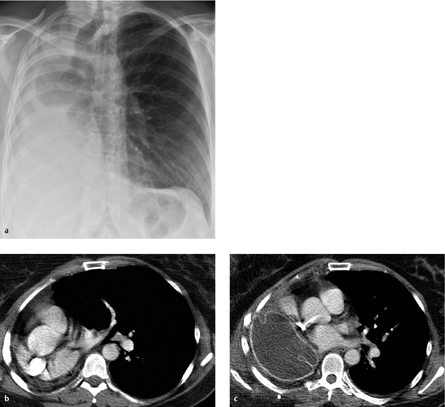
Fig. 3.17 Small aortic pseudoaneurysm at the cannulation site for cardiopulmonary bypass.
Note the large pleural effusions and the (hemorrhagic) pericar-dial effusion.
Sternal Dehiscence and Osteomyelitis
Sternal dehiscence and osteomyelitis are a rare (1–2%) but serious complication of sternotomy. The diagnosis is generally based on clinical findings. Normal postoperative radiographs should show no more than a slight displacement of the fragments and should not show an actual osteotomy gap. A gap larger than 3 mm has been cited in the literature as a criterion for sternal dehiscence, but this finding has proven nonspecific in routine practice (Fig. 3.19). Discontinuity and displacement of the sternal suture wires are a more common and sensitive indicator of sternal dehiscence.
Fig. 3.18 a, b Mediastinitis.
Mediastinitis is a clinical rather than CT diagnosis. Multiple air inclusions (a) are strongly suspicious for infection. Densities alone (b) may be a normal postoperative finding (up to 20 days or more) and do not always signify infection.
Fig. 3.19 a–c Sternal dehiscence and osteomyelitis.
a, b Wide sternal dehiscence (a) and loose suture wires (b) in secondary osteomyelitis.
c Rarely, a fistula may communicate with the pericardium, demonstrated here by contrast instillation.
Fractured sternal suture wires can be identified on chest radiographs. Sternal and chondral infections present in the second or third postoperative week with fever, pain, and purulent discharge. Infections are often associated with sternal dehiscence but rarely spread to involve the mediastinum.
Computed tomography. CT is much more sensitive than conventional radiography and tomography but may be negative in the early stage. Sternal dehiscence is best appreciated on multiplanar reformatted scans, which show decreased structural density and bone destruction along with inflammatory soft-tissue infiltration. The posterior sternal border is often ill-defined and irregular on postoperative scans even in the absence of inflammation, so this is considered a nonspecific sign. CT fistulography can detect bony fistula formation after instillation of a 1 : 10 mL dilution of contrast medium.
Postpericardiotomy Syndrome
Postpericardiotomy syndrome is characterized by fever, pericarditis, pleurisy, and pneumonitis. It develops in up to 25% of patients 2–4 weeks after cardiac surgery but may also occur a few days or even months after the operation.
The chest radiograph shows widening of the cardiac silhouette, a left-sided pleural effusion, and basilar infiltrates. Generally the disease is self-limiting and does not affect the long-term prognosis. Treatment consists of anti-inflammatory medications. Rare complications (1– 2%) are pericardial tamponade and constrictive pericarditis.
Fig. 3.20 Periprosthetic leak in a patient who underwent aortic valve replacement.
This diagnosis was actually confirmed by echocardiography and is more of an “incidental finding” on multidetector CT.
Complications after Valvular Surgery
Infection is a potentially life-threatening complication that may occur during both the early and late postoperative periods. Infections occur at a rate of 1% per year and have a high mortality. The main causative organisms are staphylococci, gram-negative bacteria, and Candida. Infected valves may shed septic emboli into the pulmonary or systemic circulation. Infection leads to valvular dehiscence with periprosthetic leakage (Fig. 3.20) and may spread to the lung.
Computed tomography. CT angiography can very accurately define pseudoaneurysmal dilatations, which most commonly occur in the supravalvular ascending aorta at the access site for cardiopulmonary bypass. Paravalvular leaks can be confirmed by CT angiography but are diagnosed primarily by echocardiography.
Heart Transplantation
In an orthotopic heart transplantation, the recipient heart is removed through a median sternotomy, leaving the atria in place along with the distal ends of the ascending aorta and pulmonary trunk. The donor heart is introduced and anastomosed at the level of the atria, aorta, and pulmonary artery.
Normal Postoperative Findings
The postoperative chest radiograph typically shows an enlarged cardiac silhouette caused by dilatation of the recipient pericardial sac. The cardiac silhouette dwindles in size during subsequent months. The superimposed donor and recipient atria may cause the right atrium to present a double outline.
CT may demonstrate a notch at the anastomosis of the ascending aorta and right atrium (Fig. 3.21). The inferior vena cava is frequently dilated. The distance between the superior vena cava (recipient) or pulmonary artery (recipient) and the ascending aorta (donor) may be increased.
Fig. 3.21 Visible notch in the border of the markedly dilated right atrium after cardiac transplantation.
A pericardial effusion, often loculated, may persist for weeks or months. Steroid medication may lead to mediastinal lipomatosis.
Specific Postoperative Complications
The main sites of predilection for anastomotic hemorrhage are the aortic anastomosis and the anastomotic site of the right ventricle. An aortic dissection or aortic pseudoaneurysm develops in less than 1% of cases.
Infection
Infections are responsible for 40% of all deaths following cardiac transplantation. The most frequent causative organisms are Aspergillus, CMV, and Pseudomonas. Most lung infections occur within the first 3–4 months after heart transplantation. When associated with empyema or mediastinitis, they are an extremely poor prognostic sign. Bacterial pneumonia may develop during the first 2–3 weeks after transplantation.
Acute Allograft Rejection
Acute allograft rejection occurs within 2 weeks to 3 months after transplantation. The risk of allograft rejection declines markedly after the sixth postoperative month. Cardiomegaly is neither sensitive nor specific for rejection.
Acute graft rejection is diagnosed by myocardial biopsy. Possible complications of myocardial biopsy, in which myocardial tissue is taken from the right ventricular septum, are pneumothorax and hemothorax.
Esophageal Surgery
Antireflux operations can be performed through a thoracic or abdominal approach. Minimally invasive laparoscopic techniques are most widely used (see Chapter 5, p. 169). The Heller technique of modified esophageal myotomy, performed through a left posterolateral thoracotomy, is the method of choice for the treatment of achalasia.
Various surgical techniques are employed:
 Complete resection of the esophagus with a gastric pull-up reconstruction (Akiyama procedure). The anastomosis is placed at the cervical (extrathoracic) or intrathoracic level.
Complete resection of the esophagus with a gastric pull-up reconstruction (Akiyama procedure). The anastomosis is placed at the cervical (extrathoracic) or intrathoracic level.
 Esophageal reconstruction with interposed small bowel or colon. In this case the cervical anastomosis is accompanied by multiple intra-abdominal anastomoses.
Esophageal reconstruction with interposed small bowel or colon. In this case the cervical anastomosis is accompanied by multiple intra-abdominal anastomoses.
Specific Postoperative Complications
The complication rate after esophageal surgery is higher than after other gastrointestinal surgery because it is more difficult for the esophagus to retain sutures and staples, due partly to the absence of a serosal layer, and because the gastric pull-up or interposed bowel segment may compromise blood flow.
Anastomotic Leak
The principal complication, known as the “Achilles heel” of esophageal surgery, is anastomotic leak, which occurs in up to 20% of cases even at experienced centers. The cervical anastomosis is more commonly affected than the mediastinal anastomosis, although a leaky thoracic anastomosis is associated with greater morbidity (Fig. 3.22).
Radiography and CT. The leak itself is diagnosed by oral (intraluminal) contrast administration. CT is best for evaluating the degree of mediastinal infiltration, abscess formation, and involvement of the pleural space (empyema). In most cases a direct communication exists between the mediastinum and pleural space, resulting in empyema (more common on the left side than the right). The detection of contrast medium in the pleural space after oral contrast administration confirms an esophageal perforation. Mediastinal emphysema is frequently present.
Treatment. While leaks accessible to external drainage can sometimes be successfully managed by conservative therapy, large leaks with associated mediastinitis require reoperation. Oversewing the leak or revising the esophagogastrostomy is appropriate only if the esophagus and gastric tube have an adequate blood supply. Otherwise the only option is resection of the gastric tube, blind closure of the stomach, and the creation of a cervical esophagostomy. A catheter jejunostomy is constructed for enteral feeding. Alimentary continuity is restored secondarily with an interposed colon segment.
Fig. 3.22 a–c Anastomotic leaks.
Anastomotic leaks at the cervical level (a, c) and thoracic level (b) after esophageal resection usually result from ischemic complications. They may lead to sizable leaks with mediastinitis and pleural empyema.
Pulmonary and Tracheal Complications
Postoperative pulmonary complications such as atelectasis, pneumonia, and respiratory failure are common (up to 30%), regardless of the specific operative technique, and may have serious consequences.
There is an ca. 5% incidence of circumscribed tracheal necrosis, usually involving the membranous part, following an extensive lymphadenectomy in the upper media-stinum. Tracheal necrosis is a very serious complication with a high mortality rate. Less frequent complications are chylothorax and recurrent laryngeal nerve palsy.
Summary
Following pneumonectomy, the pleural space on the operated side initially fills continuously with serous fluid, which is gradually reabsorbed. The mediastinum is shifted toward the operated side. The principal complications are postpneumonectomy edema, hemothorax and chylothorax, and pleural empyema, which always requires the exclusion of a bronchopleural fistula. Rare complications are esophagopleural fistula, cardiac herniation, and (right-sided) pneumonectomy syndrome.
Lobectomy is occasionally complicated by postoperative atelectasis and a variable ischemic response caused by vascular kinking of the middle lobe bronchus, which is directed sharply upward after the surgery. Anastomotic stricture develops as a late complication in up to 20% of sleeve resections.
Lung transplantation is usually followed by the development of a pronounced ipsilateral pleural effusion, which should clear within 2 weeks, and by reperfusion edema. Initial graft dysfunction presents the radiographic features of DAD/ARDS, whereas chronic progressive rejection after lung transplantation presents as bronchiolitis obliterans. Infections are the most frequent early complication of lung transplantation and are the leading cause of death
The principal complication of cardiovascular surgery is mediastinal hematoma. Postoperative widening of the mediastinum by 50% is suspicious for hemorrhage. Mediastinitis is a clinical diagnosis. It is marked by increasing fluid retention with bilateral pleural effusions and more than 3 mm of sternal dehiscence noted later than 2 weeks after surgery.
The principal complication of esophageal surgery is anastomotic leak. With an incidence of up to 20% even at experienced centers, this complication is detected by oral contrast administration. CT is best for evaluating the degree of mediastinal infiltration, abscess formation, and involvement of the pleural space (empyema).

 The radiologist should be aware of previous surgical interventions when interpreting postoperative chest radiographs and CT scans.
The radiologist should be aware of previous surgical interventions when interpreting postoperative chest radiographs and CT scans. A continuous decrease of air and increase of fluid is normal after pneumonectomy. A sudden increase in air volume is suspicious for a complication.
A continuous decrease of air and increase of fluid is normal after pneumonectomy. A sudden increase in air volume is suspicious for a complication.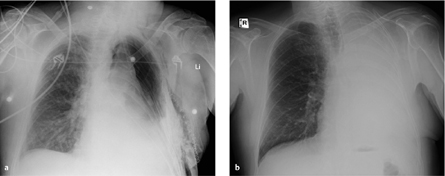
 Empyema
Empyema Cardiac herniation
Cardiac herniation Pulmonary edema (hydrostatic, permeability edema)
Pulmonary edema (hydrostatic, permeability edema) Chylothorax
Chylothorax Hemothorax
Hemothorax Bronchopleural fistula
Bronchopleural fistula Wound infection
Wound infection Esophagopleural fistula
Esophagopleural fistula Postpneumonectomy syndrome
Postpneumonectomy syndrome Tumor recurrence
Tumor recurrence Esophagopleural fistula
Esophagopleural fistula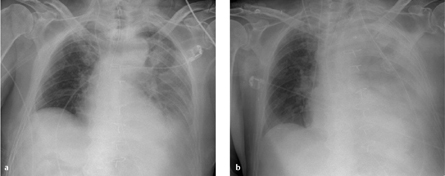
 The attenuation values of a hemothorax range from 15 to 40 HU, depending on the age of the hemorrhage.
The attenuation values of a hemothorax range from 15 to 40 HU, depending on the age of the hemorrhage. Negative, fat-equivalent CT densities confirm a chylothorax but are not always present.
Negative, fat-equivalent CT densities confirm a chylothorax but are not always present.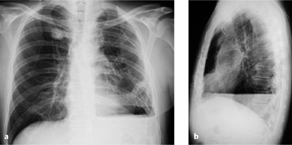
 When the detection of pus confirms a pleural empyema, a bronchopleural fistula should be excluded.
When the detection of pus confirms a pleural empyema, a bronchopleural fistula should be excluded. Thin-slice CT can directly visualize a bronchopleural fistula in up to 50% of cases.
Thin-slice CT can directly visualize a bronchopleural fistula in up to 50% of cases.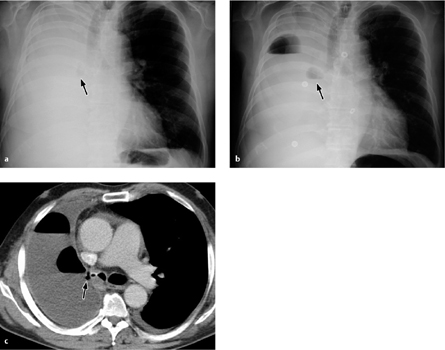
 Cardiac herniation toward the left side leads to shock symptoms with tachycardia and hypotension due to strangulation of the left ventricle in the pericardial defect.
Cardiac herniation toward the left side leads to shock symptoms with tachycardia and hypotension due to strangulation of the left ventricle in the pericardial defect.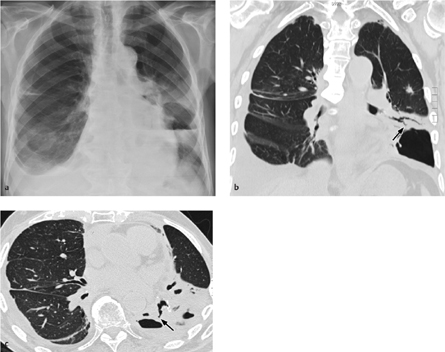
 Upward angulation of the middle lobe bronchus after lobectomy may cause vascular kinking leading to atelectasis and ischemia.
Upward angulation of the middle lobe bronchus after lobectomy may cause vascular kinking leading to atelectasis and ischemia. The middle lobe or lingula tends to migrate after an upper or lower lobectomy, occupying and filling the vacant space.
The middle lobe or lingula tends to migrate after an upper or lower lobectomy, occupying and filling the vacant space.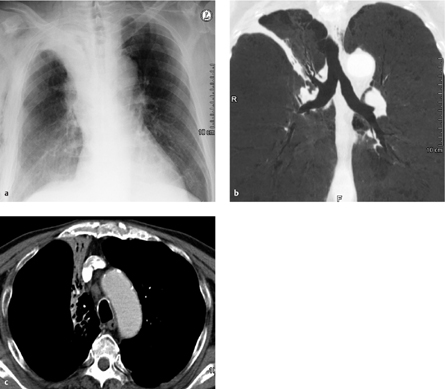
 Anastomotic stricture occurs at a late complication in up to 20% of sleeve resections.
Anastomotic stricture occurs at a late complication in up to 20% of sleeve resections. Reperfusion edema is caused by an ischemic increase in capillary permeability, impaired lymphatic drainage, sympathetic denervation, and surgical trauma.
Reperfusion edema is caused by an ischemic increase in capillary permeability, impaired lymphatic drainage, sympathetic denervation, and surgical trauma.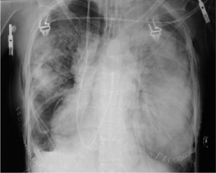
 Chronic progressive rejection after lung transplantation presents as bronchiolitis obliterans.
Chronic progressive rejection after lung transplantation presents as bronchiolitis obliterans. Thickened bronchial walls
Thickened bronchial walls Thickened interlobular septa
Thickened interlobular septa Focal or diffuse alveolar opacities (perihilar)
Focal or diffuse alveolar opacities (perihilar) Ground-glass opacities
Ground-glass opacities Pleural effusion
Pleural effusion Thickened bronchial walls
Thickened bronchial walls Thickened interlobular septa
Thickened interlobular septa Cylindrical bronchiectasis
Cylindrical bronchiectasis Decreased vascularity
Decreased vascularity Air trapping (expiratory views)
Air trapping (expiratory views) Focal peribronchial opacities
Focal peribronchial opacities Fibrosis
Fibrosis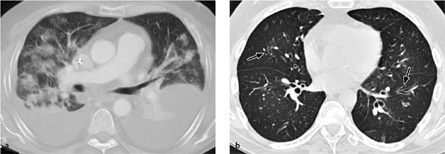
 Infections are the most frequent complication of lung transplantation and the leading cause of death.
Infections are the most frequent complication of lung transplantation and the leading cause of death. Postoperative mediastinal widening by 60% is suspicious for hemorrhage.
Postoperative mediastinal widening by 60% is suspicious for hemorrhage. Increasing fluid retention with bilateral pleural effusions and more than 3 mm sternal dehiscence later than 2 weeks after surgery are suspicious for infection.
Increasing fluid retention with bilateral pleural effusions and more than 3 mm sternal dehiscence later than 2 weeks after surgery are suspicious for infection. Perioperative myocardial infarction, cardiac arrhythmia
Perioperative myocardial infarction, cardiac arrhythmia Decreased cardiac output (myocardial ischemia, arrhythmia, tamponade, hypervolemia, prosthetic valve dysfunction, etc.)
Decreased cardiac output (myocardial ischemia, arrhythmia, tamponade, hypervolemia, prosthetic valve dysfunction, etc.) Postoperative bleeding
Postoperative bleeding Mediastinal tamponade
Mediastinal tamponade Thromboembolism
Thromboembolism Infection
Infection Renal, gastrointestinal, or neurologic complications
Renal, gastrointestinal, or neurologic complications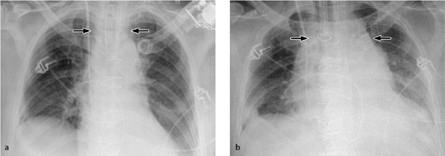
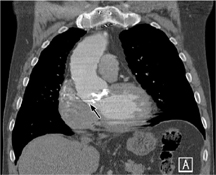
 Infection is a serious complication of valvular heart surgery and may lead to septic embolism and/or valvular dehiscence.
Infection is a serious complication of valvular heart surgery and may lead to septic embolism and/or valvular dehiscence.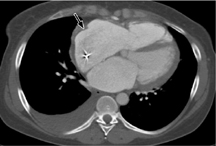
 Acute allo-graft rejection after heart transplantation is diagnosed by myocar-dial biopsy of the right ventricular septum.
Acute allo-graft rejection after heart transplantation is diagnosed by myocar-dial biopsy of the right ventricular septum. Anastomotic leak after esophageal surgery is detected by oral contrast administration.
Anastomotic leak after esophageal surgery is detected by oral contrast administration.