CHAPTER 6 Imaging Evaluation of Common Pediatric Emergencies
NEONATAL EMERGENCIES
Surfactant Deficiency Disease
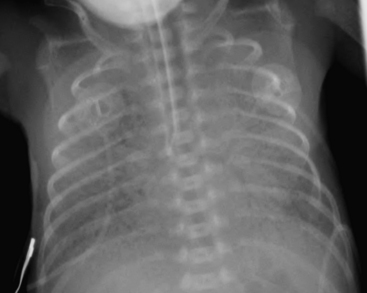
SDD, also termed hyaline membrane disease, almost exclusively affects premature infants (i.e., less than 36 to 38 weeks gestation). The lack of surfactant in the lungs of premature infants causes collapse of pulmonary alveoli, which leads to poor oxygenation. Infants with SDD demonstrate signs and symptoms of respiratory distress at birth, manifested by grunting, nasal flaring, retracting, and tachypnea. The radiographic findings of SDD may be present at birth, although in some cases the findings evolve after 12 to 24 hours. Classic radiographic findings of SDD are low lung volumes with diffuse, bilateral, granular opacities (Fig. 6-1). These opacities improve after treatment with exogenous surfactant. Infants with SDD are often in severe respiratory distress and require mechanical ventilation.
Air Block Complications
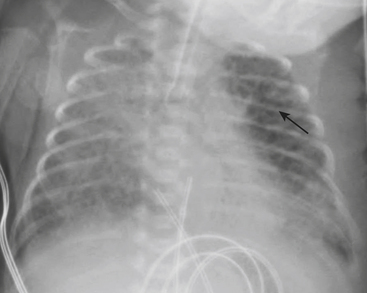
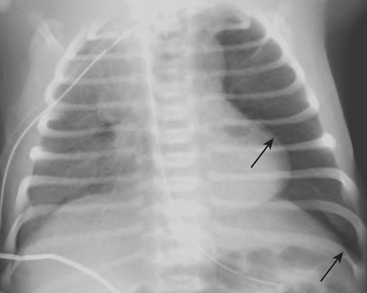
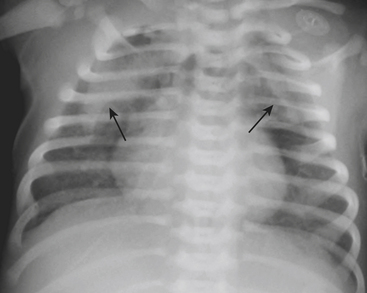
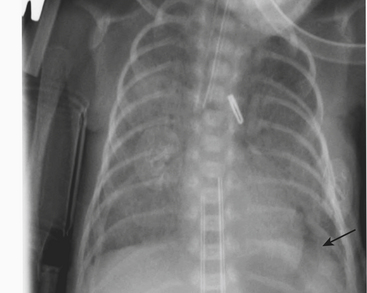
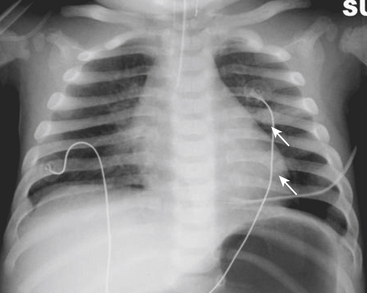
Infants on mechanical ventilators are at risk of developing complications secondary to barotrauma or volutrauma. This is especially true for infants with underlying lung disease such as SDD. Air may rupture outside of the alveoli, entering the perivascular or peribronchiolar spaces and giving rise to interstitial lucencies termed pulmonary interstitial emphysema (Fig. 6-2). The air may also dissect into the mediastinum, pleural space, or pericardium. On a supine chest radiograph, a pneumothorax tends to collect anteriorly along the heart border or inferiorly above the diaphragm (Fig. 6-3), and is manifested as a paracardiac lucency (for a medial pneumothorax) or a deep costophrenic sulcus (for an inferior pneumothorax). A cross-table lateral view is often necessary to demonstrate the anterior location of the air (Fig. 6-4). Pneumomediastinum causes anterior displacement of the thymus in a “spinnaker sail” configuration (Fig. 6-5). Pneumopericardium outlines the heart border without extending superior to the great vessels (Fig. 6-6).
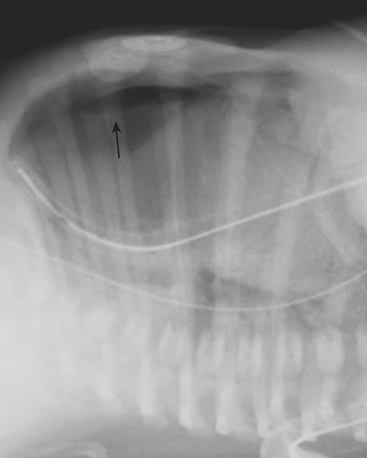
Figure 6-4 A cross-table lateral chest radiograph in the same infant as in Figure 6-3 demonstrates the anterior location of the pneumothorax (arrow).
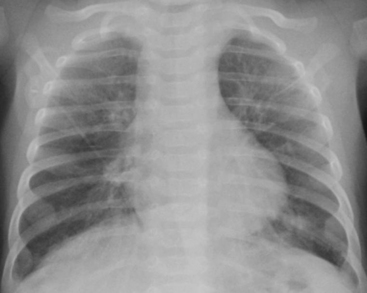
Meconium Aspiration Syndrome
MAS is diagnosed by the presence of meconium below the level of the vocal cords at birth. Infants with MAS are usually post-mature, or have experienced intrauterine stress. The thick, tenacious meconium causes obstruction of small- and medium-sized airways, which leads to areas of both atelectasis and overinflation. The meconium can cause a chemical pneumonitis and also inactivates surfactant within lung alveoli. Chest radiographs in infants with MAS reveal coarsened interstitial densities and bilateral parenchymal opacities interspersed with areas of hyperaeration (Fig. 6-7). MAS is the most common respiratory disease to cause a pleural effusion in the first few days of life.
Posterior Urethral Valves
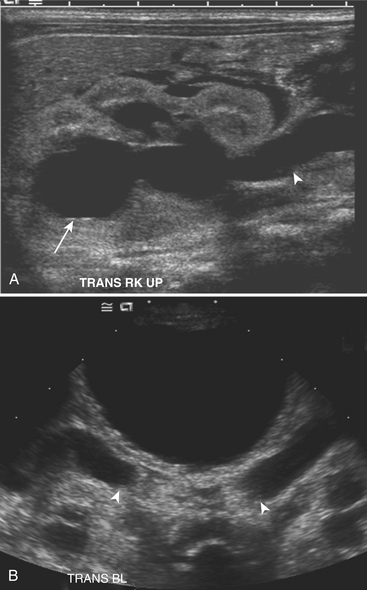
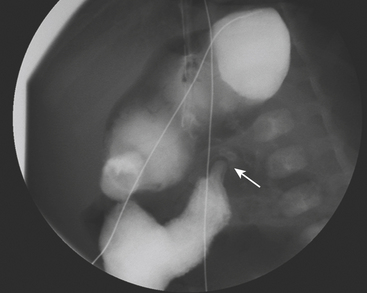
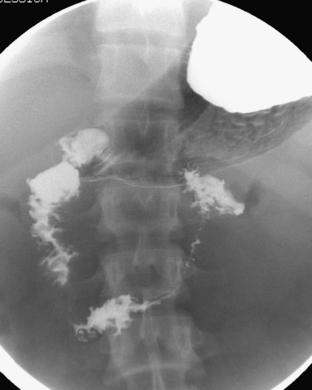
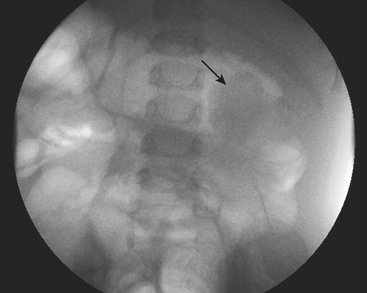
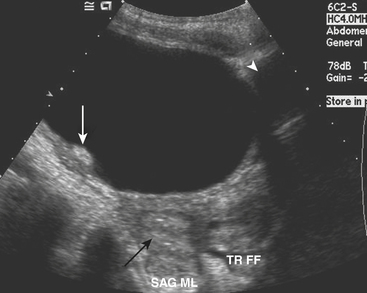
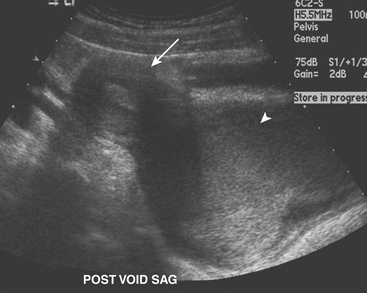
Ultrasound (US) examination of patients with PUVs reveals dilated renal collecting systems and ureters bilaterally (Fig. 6-8). The bladder is often greatly distended, and the bladder wall may appear sacculated, thickened, or trabeculated. The dilated posterior urethra has a “keyhole” configuration at ultrasound. Voiding cystourethrogram (VCUG) is the standard of care for the diagnosis of PUVs. The bladder is usually of large caliber and may require a larger than expected volume of contrast to reach capacity. Bladder wall trabeculations and diverticula are often present. When reflux is present, the refluxed contrast will be diluted by the preexisting urine within the dilated ureter and renal collecting system. The degree of hydronephrosis is often massive. Images of the urethra acquired during voiding demonstrate an abrupt change in caliber between the dilated posterior urethra and the normal-caliber anterior urethra (Fig. 6-9). In some cases the obstructing membrane is identified at the point of transition. The verumontanum is often enlarged and is identified as an intraluminal filling defect along the posterior wall of the urethra just proximal to the valves.
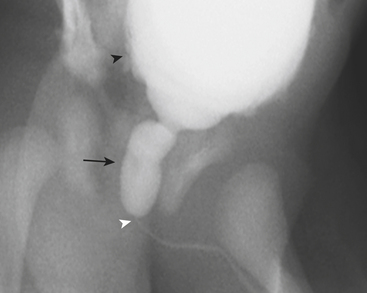
Figure 6-9 Lateral-oblique voiding fluoroscopic image acquired during voiding cystourethrogram (VCUG) on same patient as in Figure 6-8 demonstrates a distended urinary bladder with several small diverticulae (black arrowhead). There is an abrupt change in caliber from the dilated posterior urethra to the anterior urethra (white arrowhead). The enlarged verumontanum can be identified as a filling defect in the posterior urethra (arrow).
Intestinal Obstruction
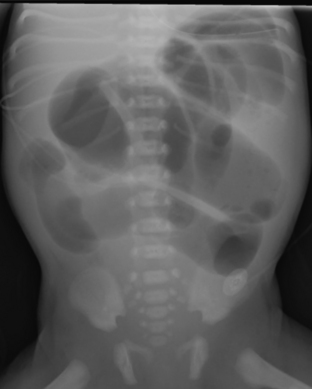
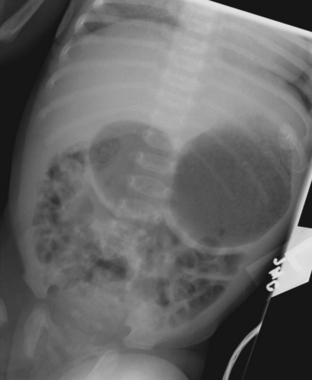
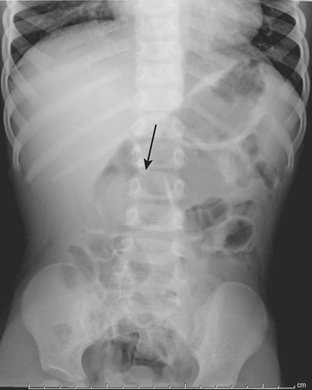
Intestinal obstruction is one of the most common neonatal abdominal emergencies. The obstruction is classified as either a high or low obstruction depending on whether it occurs above or below the level of mid-ileum. Babies typically present with abdominal distention, vomiting, and failure to pass meconium within 24 to 48 hours. The distinction between a high and a low obstruction is often made on the basis of plain abdominal radiographs. If the radiograph demonstrates one or few dilated loops of bowel, the obstruction is likely a high obstruction. If the radiograph reveals multiple dilated loops of bowel, the obstruction is a low obstruction (Fig. 6-10). In a baby with intestinal obstruction the correct interpretation of the plain film is critical in directing the most appropriate next course of action.
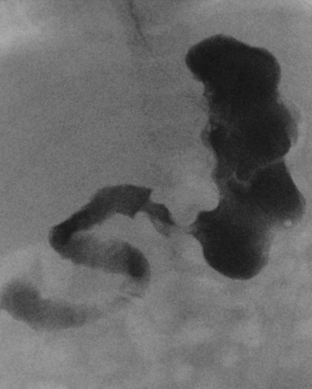
A high intestinal obstruction may be secondary to duodenal atresia or stenosis, jejunal atresia or stenosis, or malrotation. An upper gastrointestinal (UGI) examination is often requested in order to identify the level of obstruction. The UGI also assists the surgeon in determining the urgency of surgery. In duodenal atresia or stenosis, the obstruction almost always occurs at the level of the ampulla of Vater. Abdominal radiographs in duodenal atresia often reveal the classic “double bubble” sign in an otherwise gasless abdomen. The dilated gas-filled “bubbles” represent the stomach and duodenal bulb. This is virtually diagnostic of duodenal atresia in the correct clinical setting. In cases of incomplete duodenal obstruction, radiographs will reveal gas in distal bowel loops. At UGI examination, there is often a focal area of narrowing within the duodenum through which a tiny amount of contrast may pass. In some cases, the stenosis may be secondary to a duodenal web, which appears as a curvilinear filling defect extending across the duodenal lumen (Fig. 6-11). Coexistent congenital anomalies are not uncommon, most often in the form of congenital heart disease. Approximately 30% of babies with duodenal atresia or stenosis have Down syndrome.
Low intestinal obstruction may be secondary to meconium ileus, ileal atresia, small left colon syndrome (functional immaturity of the colon), colonic atresia, and Hirschsprung’s disease. A contrast enema is the study of choice to elucidate the diagnosis. Meconium ileus almost always occurs in infants with cystic fibrosis. On contrast enema, a microcolon is present. A microcolon is a colon of universally small caliber (1 cm or less in diameter). Its presence implies that the colon has never been used. Bowel atresia proximal to the distal ileum does not lead to a microcolon because the succus entericus produced by the distal small bowel allows the colon to achieve a normal caliber. In patients with meconium ileus refluxed contrast in the distal ileum reveals multiple filling defects within the distal small bowel. These filling defects represent inspissated meconium. In ileal atresia, on the other hand, it is not possible to reflux contrast past the atresia into the terminal ileum. In functional immaturity of the colon, the descending colon and sigmoid colon are small in caliber compared with the normal ascending and transverse colon. These patients are often infants of diabetic mothers. In colonic atresia the visualized colon at contrast enema will be a microcolon, and contrast will be unable to pass proximal to the atresia. In Hirschsprung’s disease there is a transition from normal bowel that is of normal caliber to aganglionic bowel that is small in caliber (Fig. 6-12). Classically, this transition occurs at the level of the rectosigmoid. In normal infants the rectal diameter should be greater than the sigmoid diameter, although in Hirschsprung’s disease this relationship is reversed. Ultimately, a biopsy is required to confirm the diagnosis.
Esophageal Atresia
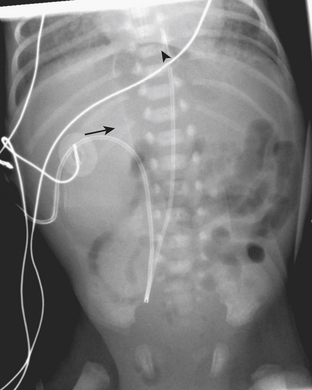
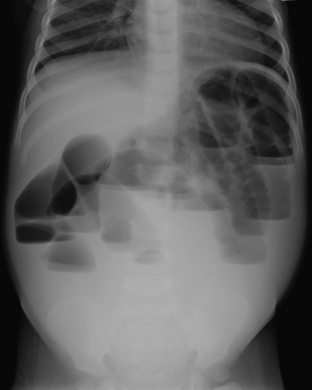
Congenital atresia of the esophagus is a rare abnormality that may occur as an isolated abnormality or as part of a larger spectrum of abnormalities. In most cases there is an associated fistula to the trachea. The diagnosis is almost always suspected clinically when an infant has trouble feeding, or there is difficulty passing a nasogastric tube into the stomach. Radiographs help to confirm the diagnosis when there is no gas identified within any bowel loops in the abdomen. If placement of a nasogastric tube has been attempted, the tube will be identified above the diaphragm on the radiograph, often in a dilated, gas-filled, esophageal pouch (Fig. 6-13).
Necrotizing Enterocolitis
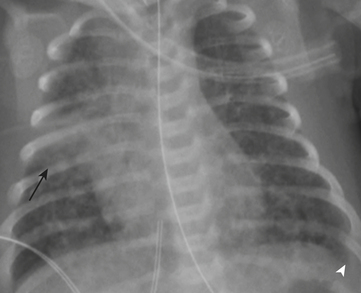
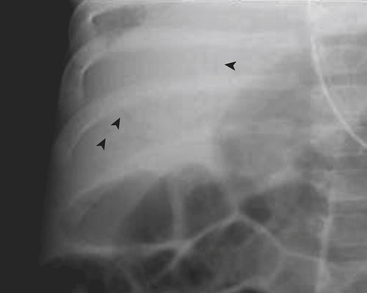
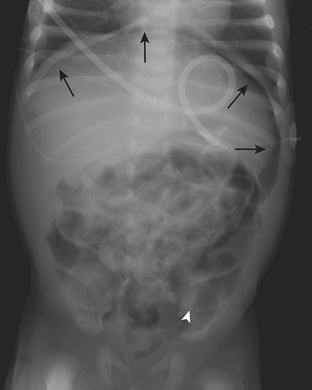
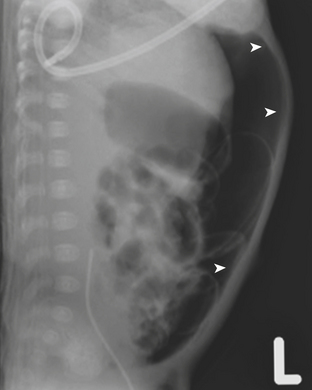
Necrotizing enterocolitis (NEC) is a gastrointestinal condition that occurs in 2% to 3% of neonates, most commonly premature infants. Mortality rates in infants with NEC range from 15% to 40%. The pathogenesis of the disease is thought to be related to relative immaturity of intestinal motility and barrier function, as well as abnormal bacterial colonization. Neonates with NEC often present within the first 2 weeks of life with feeding intolerance, vomiting, abdominal distention, blood in the stool, and/or generalized lethargy. Abdominal radiographs are the modality of choice for the initial evaluation of neonates with suspected NEC and for monitoring its progression. An early sign of NEC is a mildly dilated and fixed loop (or loops) of bowel on serial abdominal radiographs. Pneumatosis intestinalis, or air within the wall of the bowel, is considered a hallmark of NEC (Fig. 6-14). Pneumatosis represents by-products of the metabolism of bacteria within the intestinal wall. While the bubbly lucencies of pneumatosis can often be mistaken for stool on an abdominal radiograph, it is uncommon for an infant younger than 2 weeks of age to have stool within the colon. The presence of portal venous gas and pneumoperitoneum on an abdominal radiograph usually indicates more severe disease and/or perforation of the bowel. Portal venous gas appears on abdominal radiographs as lucent, branching, linear structures within the liver (Fig. 6-15). When pneumoperitoneum is present the falciform ligament is visualized on a radiograph secondary to the presence of outlining air on both sides (Fig. 6-16). Both sides of the bowel wall are also visible (Fig. 6-17). The abdomen may appear abnormally lucent when large amounts of gas collect anteriorly within the abdomen on a supine film (Fig. 6-18). A cross-table lateral view of the abdomen is helpful in confirming the presence of free air under the diaphragm (Fig. 6-19).
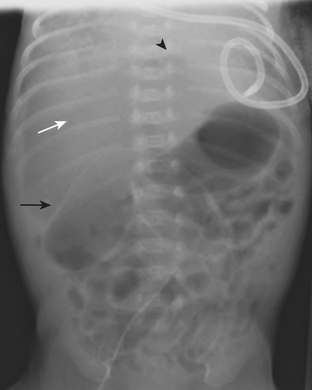
Figure 6-18 Abdominal radiograph in the same patient as in Figure 6-16 taken several hours later demonstrates hyperlucency of the liver (white arrow) and free air under the diaphragm (arrowhead), as well as air outlining loops of bowel (black arrow).
GASTROINTESTINAL
Hypertrophic Pyloric Stenosis
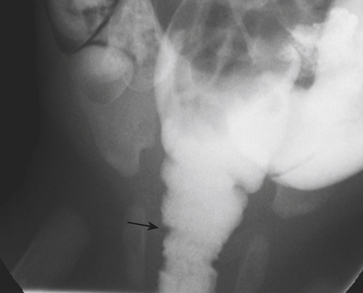
Abdominal radiographs in infants with HPS often demonstrate a dilated, gas-filled stomach (unless the stomach has been decompressed with a nasogastric tube). Peristaltic contractions within the stomach give the appearance of a “caterpillar stomach” (Fig. 6-20). Ultrasound is the imaging study of choice for the diagnosis of HPS. When HPS is present, the pyloric channel is nearly always visualized and appears both thickened and elongated (Fig. 6-21). The hypertrophied pyloric channel fails to relax despite vigorous peristaltic contractions in the stomach. The hyperechoic mucosa is redundant and crowded and often protrudes into the antrum, a finding termed the “nipple” sign. The length of the pyloric channel is variable and can range between 14 and 20+ mm. Measurements of muscle thickness are more reliable for the diagnosis of HPS. A muscle thickness greater than 3 mm is consistent with pyloric stenosis. It is important to document the passage of fluid from the gastric antrum into the duodenal bulb. In normal patients the normal pyloric ring bridges these two structures (Fig. 6-22). Careful observation of this region over the course of the examination reliably allows differentiation between a normal and an abnormal study.
Prior to the advent of ultrasound, UGI examination was performed to confirm the diagnosis of HPS. While the thickness of the pylorus muscle is not discernible at UGI examination, there are other imaging signs that suggest the diagnosis. The pyloric channel appears elongated at UGI examination as wispy, linear tracts of contrast pass through the canal, a finding called the “string” sign (Fig. 6-23). The “shoulder” sign is the name given to the appearance of the thickened pylorus impression on the dilated stomach antrum. Vigorous peristaltic contractions in the stomach occur without associated relaxation of the pyloric channel. Imaging over time allows reliable diagnosis of pyloric stenosis, as contrast is never able to pass through the channel in more than tiny wisps at a time.
Malrotation
Abdominal radiographs and gastrointestinal contrast studies (UGI) are of paramount importance in making the diagnosis of malrotation. The bowel gas pattern is often normal in the presence of malrotation. A normal abdominal radiograph should not preclude further investigation in a child with clinical concern for malrotation. A UGI examination evaluates the course of the duodenum and the position of the duodeno-jejunal junction (DJJ). In normal patients the duodenum has a characteristic C-sweep that consists of four portions: the duodenal bulb and the descending, transverse, and ascending portions (Fig. 6-24). The descending through ascending portions of the duodenum are fixed in the retroperitoneum. The DJJ is normally located to the left of midline at the level of the duodenal bulb. When the DJJ is inferior or medial to this position, it is considered abnormal (Fig. 6-25). There are a few rare exceptions to this rule, which include previous surgery in the abdomen, abdominal masses that displace bowel, and marked gastric or colonic distention. A “corkscrew” configuration of the duodenum implies that a midgut volvulus is present. Midgut volvulus causes a closed loop bowel obstruction. The bowel may appear tapered or “beaked” at the level of obstruction, with proximally dilated bowel loops. Despite all of these findings, the diagnosis of malrotation remains difficult to make with certainty in up to 30% of cases in light of the subtlety of the findings and the overlap with normal anatomy. If the course of the duodenum is not straightforward, serial abdominal radiographs can be performed to follow the contrast distally to the colon. When malrotation is present, the small bowel is commonly located on the right side of the abdomen, and the cecum may also be abnormally positioned.
Intussusception
Intussusception is one of the most common causes of bowel obstruction in young children. Intussusception occurs when one segment of the bowel (the intussusceptum) telescopes into the bowel immediately distal to it (intussuscipiens). This occurs most often in children between 6 months and 3 years of age. Common presenting symptoms are vomiting, lethargy, and bouts of irritability where the child draws his or her legs to the chest. The more classic symptoms of a palpable abdominal mass and “red currant jelly stool” are present in only the minority of cases and should not be relied on to suggest the diagnosis. Bloody stool is a manifestation of sloughed intestinal mucosa, which is a late finding in the disease and is present in less than half of all cases. The implications of delayed diagnosis may be devastating, as a persistent intussusception leads to bowel ischemia and perforation. Most intussusceptions in children are idiopathic, while the remaining cases are secondary to the presence of a pathologic lead point (PLP). PLPs include Meckel’s diverticulum, colonic polyps, lymphoma, duplication cyst, or underlying diseases of the bowel such as Henoch-Schönlein purpura. The most common site for intussusception to occur is at the hepatic flexure, of which ileocolic intussusceptions are the most common type. Small bowel intussusceptions also may occur. Small bowel intussusceptions are usually transient phenomena that may not cause symptoms. They are likely to reduce spontaneously, and air reduction enema is not therapeutic. Imaging evaluation of the child with suspected intussusception begins with abdominal radiographs. Supine and left lateral decubitus views are recommended. The decubitus film, by allowing the cecum and ascending colon to assume a nondependent position in the abdomen, allows air to fill the cecum when an ileocolic intussusception is not present or if the cecum is not filled with stool. The sigmoid colon is often located within the right lower quadrant in young children, and one should be careful when excluding intussusception based on gas-filled bowel loops in this location. The “meniscus” sign and “target” sign are terms used to describe the radiographic appearance of a soft tissue mass in the colon (Fig. 6-26). Small bowel dilatation and air–fluid levels may be present signifying the presence of a small bowel obstruction (Fig. 6-27). Despite these signs, half of all abdominal radiographs are diagnostically unhelpful in the diagnosis of intussusception, and further evaluation is required.
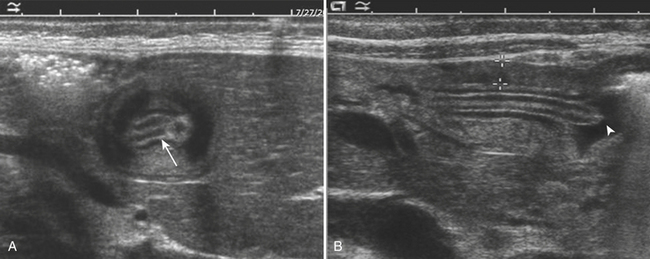
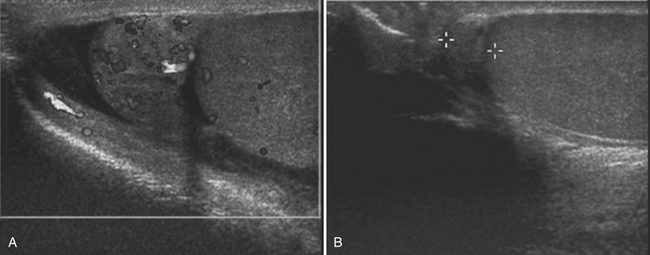
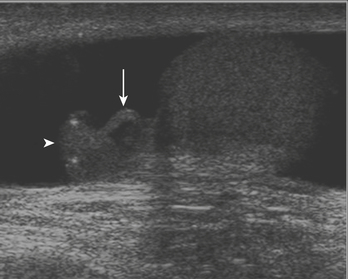
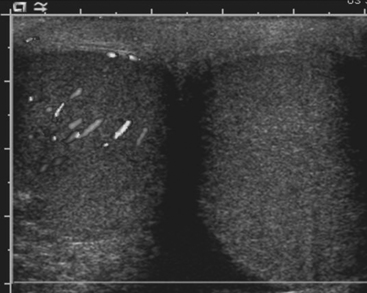
For confirmation of intussusception, ultrasound is the preferred modality. When the sonographer is familiar with its imaging appearance, intussusception is identified in all cases. Intussusception has a characteristic appearance in both transverse and sagittal planes. In the transverse plane, an intussusception appears as a mass usually between 3 and 5 cm in diameter that has a “target” or “doughnut” configuration of alternating hypo- and hyperechoic layers (Fig. 6-28A). In the sagittal plane the mass assumes a “pseudo-kidney” configuration. Color Doppler is used to evaluate the vascularity of the bowel and is a promising predictor of bowel viability (Fig. 6-28B). Enlarged mesenteric lymph nodes may be present at the site of intussusception, which may function as a lead point. Imaging pitfalls in the diagnosis of intussusception include stool in the cecum, thickened loops of small bowel in the right lower quadrant, and the normal psoas muscle. Each of these structures may be mistaken for an intussusception. It is also important to appreciate the difference between small bowel intussusceptions and ileocolic intussusception. Small bowel intussusceptions are usually treated conservatively, whereas ileocolic intussusceptions proceed to enema reduction or surgery. The diagnosis of small bowel intussusception is suggested by the location of the intussusception (outside of the right lower quadrant), small diameter (less than 2 cm), and short-segment involvement (less than 5 cm).
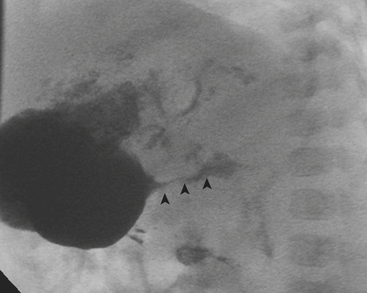
Fluoroscopically guided contrast reduction enema is a widely accepted and utilized method of treatment for ileocolic intussusception. Contraindications to performing an enema include shock, peritonitis, or radiographic evidence of perforation. Although different contrast agents are used, air is preferred at our institution. After inserting a rectal catheter into the buttocks air is insufflated into the colon. The intussusception is visualized fluoroscopically as it is reduced back through the colon (Fig. 6-29). A successful reduction involves the reduction of the intussusception beyond the ileocecal valve (often with a visible “pop”) with reflux of air into the small bowel. Air reduction enema is successful in over 80% of cases. When the enema is not successful, surgery is curative. A rare, but serious, complication of the air reduction enema is bowel perforation leading to tension pneumoperitoneum, which necessitates emergent needle decompression. Perforation occurs in approximately 1.5% of cases.
GENITOURINARY
Urinary Tract Infection
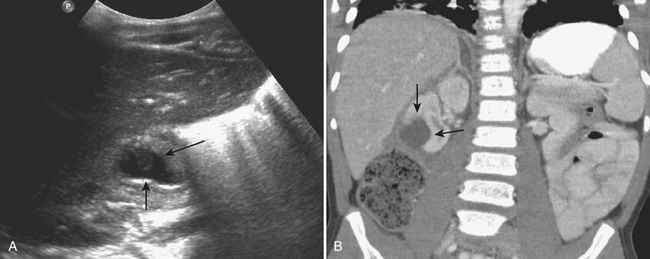
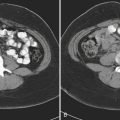
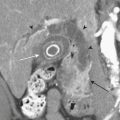
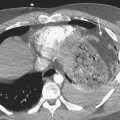
In patients with acute pyelonephritis, ultrasound may reveal focal areas of decreased attenuation. These same areas demonstrate decreased vascularity with color Doppler US. Urothelial thickening is often present. A severe infection may evolve into renal abscess or perinephric abscess. At US these collections appear as hypoechoic or anechoic masses with increased through-transmission (Fig. 6-30A). A renal abscess may simulate a solid mass at US. In these instances, contrast-enhanced CT is performed. At CT an abscess will appear as a focal, hypoattenuating mass (Fig. 6-30B). While the wall of the abscess enhances after contrast administration, the central portion of the abscess will not enhance. A perinephric abscess is suggested by fluid or soft tissue attenuation within the perinephric space. These collections may penetrate through Gerota’s fascia and involve surrounding structures, such as the psoas muscle. Urine cultures in patients with renal abscess are negative in up to 20% of cases.
Ureteropelvic Junction Obstruction
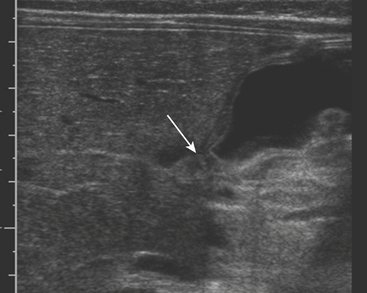
The ultrasound appearance of UPJO is a dilated renal collecting system on the patient’s symptomatic side during the acute pain crisis (Fig. 6-31). The proximal ureter will appear dilated with an abrupt tapering at the level of the crossing vessel. US should be performed during the pain crisis since the hydronephrosis is present only when the patient is experiencing symptoms. Unilateral hydronephrosis is not specific for UPJO, nor does US evaluate the functional status of the kidney. While additional investigation is often required before treatment can be initiated, US is critical in the preliminary imaging of patients with suspected urinary tract obstruction.
Urolithiasis
The imaging evaluation of a child with suspected urolithiasis is performed with US, CT, or both. US is an appealing first-line imaging modality because it detects complications of urolithiasis such as hydronephrosis or perinephric fluid without the use of ionizing radiation. At US, renal calculi appear as echogenic foci with posterior acoustic shadowing (Fig. 6-32). US also has the advantage of evaluating the renal parenchyma for changes of medical renal disease or evidence of scarring. US is less sensitive than CT for detection of urinary tract calculi and is poorly accurate at diagnosing calculi that are confined to the ureter. CT is often performed in children who have persistent urolithiasis symptoms despite a normal US examination.
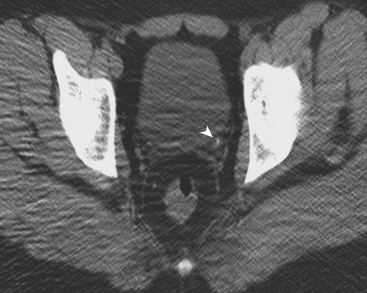
Unenhanced CT (without oral or intravenous contrast) is highly sensitive for the detection of urolithiasis in pediatric patients. Lower-dose CT protocols have been developed that minimize radiation risk to patients. Diagnosis of urolithiasis on CT is facilitated because phleboliths, which often mimic ureteral calculi in older patients, are rarely encountered in children. Secondary CT signs of urolithiasis are helpful in confirming the diagnosis, and include proximal ureteral dilatation, renal enlargement, hydronephrosis, decreased renal attenuation, the tissue rim sign, and perinephric stranding. The tissue rim sign refers to the circumferential rim of soft tissue attenuation surrounding a ureteral calculus (Fig. 6-33). These secondary signs of urinary tract calculi are less commonly encountered in pediatric patients versus their adult counterparts. Proximal ureteral dilatation and renal enlargement are the most commonly detected secondary signs, and occur in approximately half of patients. Perinephric or periureteral stranding, rarely encountered in the pediatric patient, may be secondary to the decreased amount of perinephric fat in this population.
Scrotal Hernia
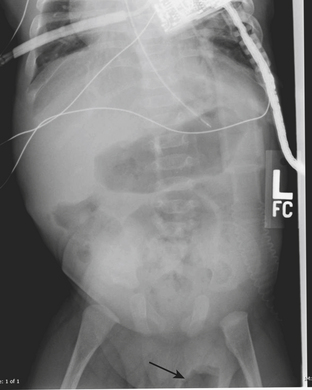
If the testes have not descended into the scrotum by 1 year of age cryptorchidism (failure of the testes to descend into the scrotal sac), inguinal-scrotal hernias, and hydroceles may result. While these conditions are most often detected on physical examination, it is not uncommon to make the diagnosis at ultrasound. Plain films are diagnostic of scrotal hernias in infants when the finding of bowel gas in the scrotum is noted (Fig. 6-34). Since this is not a common finding, US is the imaging modality of choice for evaluation of a scrotal hernia when the physical exam is inconclusive. Herniated omentum appears as an echogenic structure outside of the testis and epididymis. Bowel loops containing both air and fluid may also be visualized within the scrotum. Visualization of bowel peristalsis during imaging is an important finding to note when herniated bowel is identified within the scrotum. If bowel is identified within the scrotum but no peristalsis is visualized during the entire examination, the bowel may be strangulated. An akinetic, dilated loop of bowel observed at US in a hernia sac is reported as a highly sensitive and specific sign of bowel strangulation. A strangulated scrotal hernia is an indication for urgent surgery.
Epididymitis
Ultrasound examination of a child with epididymitis demonstrates enlargement of the epididymis (primarily the head) with heterogeneous echotexture. On color Doppler evaluation there is increased blood flow to the epididymis and/or testis (Fig. 6-35). A reactive hydrocele may be an associated finding. When the entire testis is involved, it is often enlarged and has altered echogenicity. On gray-scale imaging findings alone, the appearance of the testis may mimic a diffusely infiltrative disease such as leukemia or lymphoma, although the clinical presentation should suggest the correct diagnosis.
Torsion of the Testicular Appendages
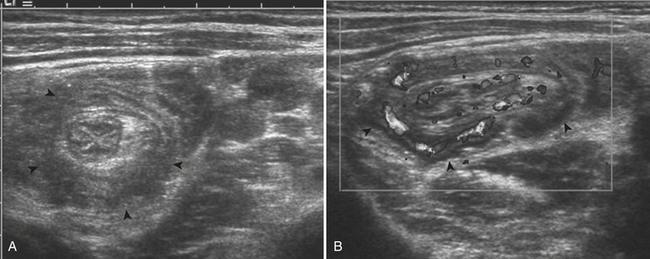
While there are four testicular appendages, only two are commonly visible at ultrasound: the appendix testis and appendix epididymis. These appendages are remnants of embryonic ducts and serve no real function. Because they are attached by a small pedicle, they are prone to torsion. Torsion of these appendages is one of the most common causes of acute scrotal pain in children. The appendix testis is more commonly affected than the appendix epididymis, although it is often difficult to identify the offending appendage. Patients are usually young, prepubertal males who complain of acute-onset scrotal pain. On clinical examination there may be a bluish discoloration of the skin at the site of pain, which is called the “blue dot” sign and is pathognomonic. At ultrasound the torsed appendix is often identified as a round, extratesticular, extraepididymal mass lacking color Doppler flow (Fig. 6-36). A reactive hydrocele may be present, as well as scrotal wall skin thickening.
Testicular Torsion
Ultrasound is the preferred imaging examination for the diagnosis of testicular torsion because of its high sensitivity and specificity. Gray-scale US findings are often completely normal when torsion is present, and the testes may appear symmetric with respect to both size and echogenicity. A small hydrocele may be present on the affected side. Within a few hours of the onset of symptoms, the scrotal wall will appear thickened, and the testis and epididymis appear enlarged and hypoechoic (secondary to inflammation and/or hemorrhage). Color Doppler is crucial for the diagnosis of torsion. The lack of demonstrable blood flow to the affected testis (assuming appropriate US settings are used) is virtually pathognomonic for torsion (Fig. 6-37). In prepubertal patients it is often difficult to demonstrate the presence of blood flow even in normal testes. Testicular scintigraphy is often used as an adjunct to US when a diagnosis of torsion cannot be made with certainty. Given the added delay of scintigraphic examinations, however, some surgeons operate on the basis of an equivocal US. The treatment for testicular torsion is detorsion of the affected testicle, and orchiopexy, where the testis is affixed to the scrotal wall to prevent torsion from recurring in the future.
Ovarian Torsion
Pelvic US is the study of choice for evaluation of ovarian torsion. Normal ovaries often appear hypoechoic relative to the adjacent pelvic tissue, and have an ovoid or ellipsoid shape. While the size of normal ovaries varies, mean ovarian volumes are approximately 1.2 cm3 in prepubertal girls and 5.8 cm3 in pubertal girls. Microcystic follicles are routinely identified in normal ovaries. When the ovaries demonstrate normal gray-scale imaging characteristics and have a normal size and shape, color Doppler evaluation is usually not necessary. If, however, one ovary appears abnormally large relative to the other side, torsion may be present. In nearly all cases of ovarian torsion, the affected ovary is massively enlarged and has a round or globular configuration. In neonates and young girls ovarian torsion is commonly seen as a large cystic mass with fluid–debris levels. In young or adolescent girls the more classic imaging appearance is an enlarged, echogenic ovary with multiple enlarged peripheral follicles. In other cases, the ovary may appear as a complex cystic mass secondary to the presence of an underlying cyst or tumor (Fig. 6-38). Color Doppler evaluation of the ovary will often reveal an absence of blood flow, a finding classically associated with ovarian torsion. However, torsion may be present even if arterial waveforms are demonstrated because the ovary has a dual blood supply (from both the uterine and ovarian arteries). The presence of arterial waveforms within the ovary should not sway the diagnosis away from ovarian torsion if the gray-scale imaging findings and physical examination are consistent with torsion.
Hematocolpos/Hematometrocolpos
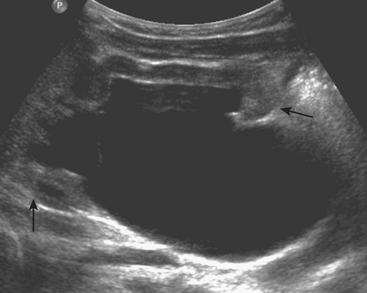
US examination in these patients reveals a round or oblong-shaped midline or paramidline pelvic mass that compresses the bladder anteriorly and rectum posteriorly. The mass appears hypoechoic with posterior acoustic transmission. Internal echoes are present within the mass, reflecting the complex nature of its contents (hemorrhage). If only the vagina is involved, the uterus will be visualized separately from this mass (Fig. 6-39). When the uterus is affected, only a thin rind of normal uterine tissue may be visualized. Patients will most often experience relief of symptoms once hymenotomy is performed.
CHEST AND AIRWAY
Retropharyngeal Abscess
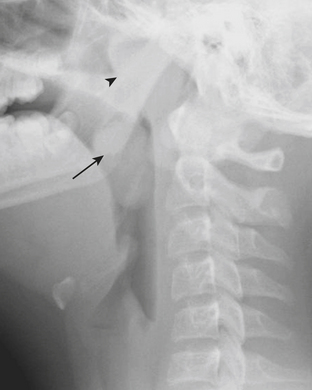
In addition to stridor, patients with retropharyngeal abscess typically also present with symptoms of neck pain and fever. Radiographs of the neck are often requested to evaluate the retropharyngeal soft tissues. Due to the wide range of ages and sizes within the pediatric population, precise measurements of the retropharyngeal soft tissues are not as reliable as relative measurements for determining the presence of soft tissue swelling. The thickness of the retropharyngeal/prevertebral soft tissues should not be greater than the anteroposterior diameter of the adjacent vertebral body (Figs. 6-40). False thickening of the retropharyngeal/prevertebral soft tissues can occur if the child’s neck is in flexion, or if the film was acquired with the child in end-expiration. Although CT can be used to further evaluate positive findings on neck radiographs, it is often prudent to repeat the lateral radiograph of the neck with the patient in a more exaggerated extension or in full inspiration when the initial radiographs are felt to be falsely positive.
Another common cause of acute stridor within the nasopharynx and oropharynx is enlargement of the tonsils and adenoids. This diagnosis can be readily differentiated from retropharyngeal abscess on the basis of findings made on lateral radiograph of the neck (Fig. 6-41). Enlarged adenoids and tonsils cause encroachment on the nasopharyngeal and oropharyngeal airway, without associated thickening of the retropharyngeal soft tissues.
Epiglottitis
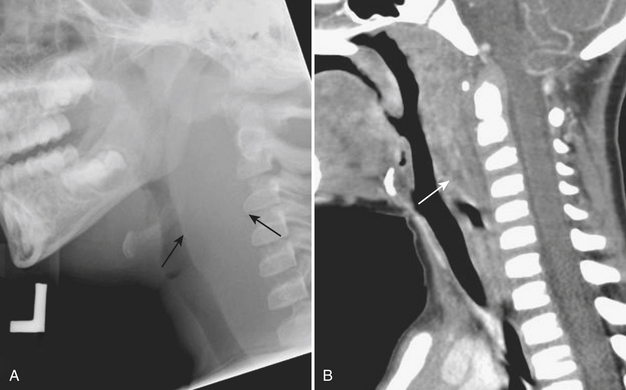
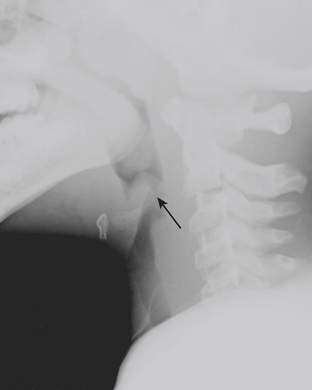
Epiglottitis is inflammation and enlargement of the epiglottis, aryepiglottic folds, and supraglottic tissue. Epiglottitis is a life-threatening condition caused by both viral and bacterial etiologies. Haemophilus influenza B has historically been the most common cause of epiglottitis, although its incidence has decreased since the development of the Haemophilus influenza B vaccine. The epiglottis can be identified at the level of the hyoid bone on lateral radiographs of the neck. On lateral neck radiographs “thumblike” enlargement of the epiglottis and thickening of the aryepiglottic folds are findings consistent with epiglottitis (Fig. 6-42). Children for whom there is high clinical and radiographic concern for epiglottitis are often examined in the operating room with the presence of an anesthesiologist, as airway compromise may be precipitated by manipulation of the hypopharynx, and urgent intubation or tracheostomy may be necessary.
Croup
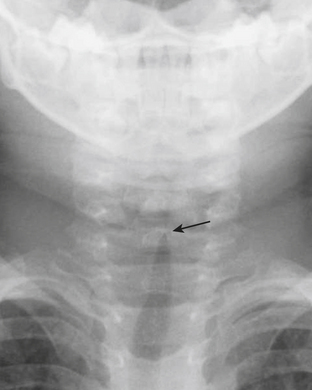
Viral croup (laryngotracheobronchitis) is the most common cause of acute stridor in children. Findings on chest or neck radiographs include narrowing of the subglottic airway. This is best visualized on the anteroposterior projection as the “steepling” or loss of the normal shouldering in the subglottic region (Fig. 6-43). Bacterial tracheitis, also known as membranous croup, is another serious upper airway infection that is seemingly becoming more common as viral croup and epiglottitis have decreased in incidence. The diagnosis of bacterial tracheitis can be suggested on radiographs if subglottic narrowing is seen in conjunction with tracheal irregularity or a soft tissue membrane.
Glottic/Subglottic Masses
Subglottic masses may present in an emergent setting when a superimposed viral or bacterial illness causes exacerbation and acuity of symptoms. The most common glottic mass is a papilloma, and the most common subglottic mass is a hemangioma. Extrinsic mass effect from mediastinal or neck masses can cause airway compromise. Bronchogenic cysts, foregut duplication cysts, and vascular rings can cause airway narrowing and acute symptoms when coexistent with an acute respiratory infection. Any abnormal deviation of the trachea (besides the mild contour deformity from the normally positioned left-sided aortic arch) should be investigated (Fig. 6-44). Fluoroscopy and contrast esophagram can be performed to confirm the presence of mass effect on the airway and adjacent esophagus. If these initial studies demonstrate an abnormality, CT or magnetic resonance imaging (MRI) can better visualize the abnormality.
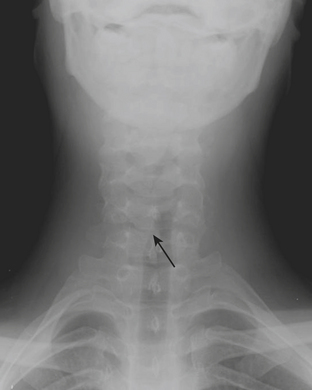
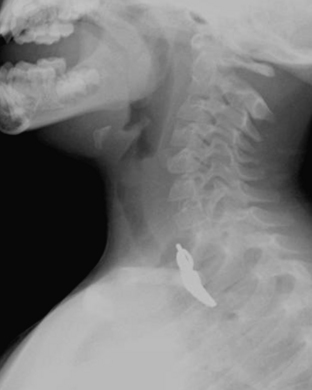
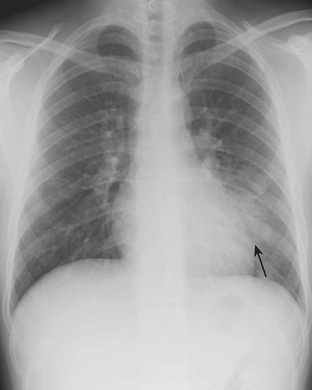
Foreign Body
The majority of patients with an aspirated foreign body (FB) are between the ages of 1 and 3 years. The FB may become embedded anywhere along the airway in children. Symptoms of aspirated FB include choking, wheezing, coughing, and dyspnea. Patients with an aspirated FB require bronchoscopy for removal of the object. Clinical history, radiography, and fluoroscopy can all be used to determine if a FB has been aspirated. If the FB is caught at the level of the vocal cords, the pharynx may appear hyperinflated on neck radiographs. Aspirated FBs are likely to settle into the right mainstem bronchus, given that the latter typically has a more vertical orientation than the left mainstem bronchus. In children with a history of suspected aspirated foreign body, inspiratory and expiratory chest radiographs evaluating for areas of air trapping are performed. Bilateral decubitus views can be performed in place of expiratory films in younger patients who are not able to cooperate for inspiratory and expiratory imaging. In a normal patient, both lungs should collapse equally on an expiratory film, and the dependent lung should collapse on a lateral decubitus film while the nondependent lung expands. When the dependent lung does not collapse on decubitus films, air trapping is implied, and an aspirated FB must be suspected (Figs. 6-45). Radiographs cannot exclude the presence of an aspirated foreign body, and false-negative radiographs are common. Negative radiographs should not preclude bronchoscopy in a child with a convincing history or physical examination consistent with aspirated FB. Occasionally, when the diagnosis of FB aspiration is delayed, postobstructive pneumonia may develop. In some cases, plain films may reveal that the FB has not been aspirated, but rather is lodged within the esophagus (Fig. 6-46).
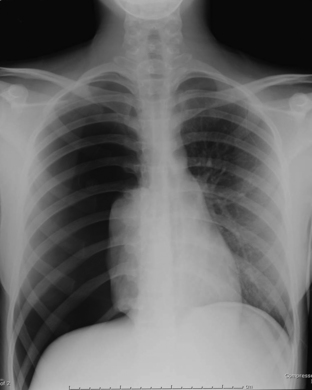
Pneumonia
Lower respiratory tract infections are one of the most common indications for imaging the chest in pediatric patients. Bacterial pneumonia is manifested on a chest radiograph as an area of focal air space opacity without associated volume loss (Fig. 6-47). Pneumonia may be complicated by pleural effusion or parenchymal necrosis. Lobar pneumonias are the most common presentation of a bacterial pneumonia in children. Radiographs are often repeated after completion of antibiotic therapy to assess the resolution of the consolidation. If there has been no significant improvement after appropriate treatment, additional imaging with CT is warranted. If a patient has had multiple, appropriately treated, recurrent pneumonias in the same location, a CT scan of the chest may be warranted to assess for an underlying anomaly predisposing the patient to recurrent illnesses, such as pulmonary sequestration. In young children, bacterial pneumonia may also present on chest radiographs as a round mass, aptly called “round pneumonia.”
Bronchiolitis
Viral bronchiolitis is one of the most frequent causes of infant hospitalization during the winter. Infants with bronchiolitis present with wheezing, cough, and respiratory distress. Nontoxic-appearing infants with classic symptoms usually require no imaging. Chest radiographs are often obtained when the diagnosis is unclear, or when an underlying bacterial pneumonia is suspected. Chest radiographs often reveal evidence of airway disease, which include areas of atelectasis, air trapping, and bronchiolar wall thickening (Fig. 6-48). Atelectasis and air trapping are findings related to underlying bronchial obstruction caused by edema and secretions. Bronchiolar wall thickening is most prominent in the perihilar regions on chest radiographs.
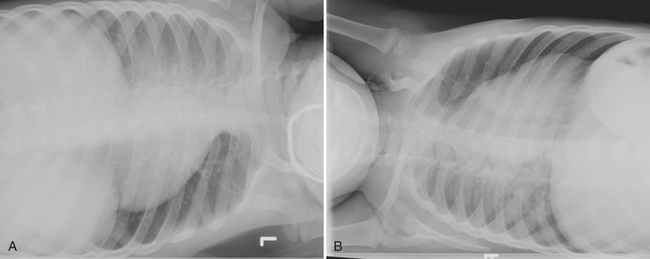
Pneumothorax
Acute shortness of breath in an older child may be secondary to a spontaneous pneumothorax. Pneumothoraces in this age group are often caused by rupture of a small subpleural bleb, typically located at the lung apex. Chest radiographs reveal variable degrees of collapse of the affected lung, and the visceral pleura will be identified as a discrete line paralleling the chest wall. A shift of the heart and mediastinum to the opposite hemithorax is consistent with tension pneumothorax, which requires immediate decompression (Fig. 6-49). Patients with an underlying parenchymal bleb will often have recurrent pneumothoraces if the bleb is not resected or if pleurodesis is not performed. CT is more sensitive than plain radiographs for visualizing tiny blebs. CT is often performed after reexpansion of the affected lung has occurred to best visualize the peripheral lung parenchyma. Patients with underlying pulmonary disorders such as cystic fibrosis and Langerhans cell histiocytosis are at higher risks for spontaneous pneumothoraces due to the presence of blebs and cysts.
CENTRAL NERVOUS SYSTEM
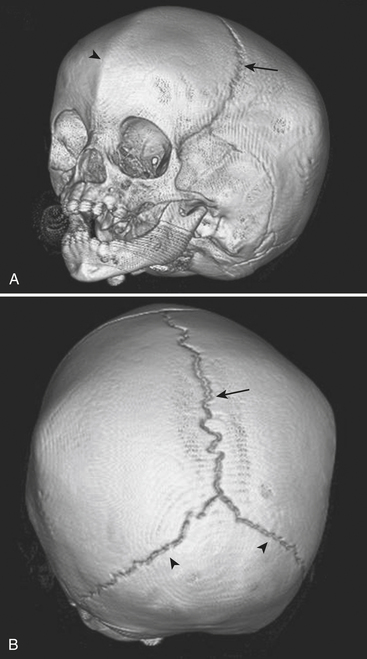
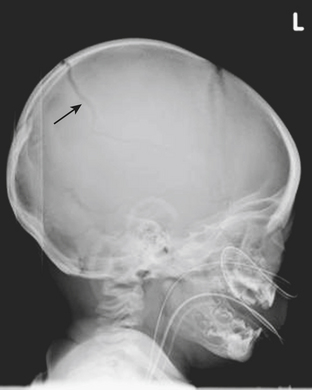
Skull Fractures
The leading cause of death in the pediatric population is traumatic brain injury. Patterns of acute intracranial injury are similar to the adult population and are further discussed in other chapters. There are, however, certain special considerations to keep in mind when evaluating the pediatric brain. Skull fractures in children are often difficult to detect given the presence of open sutures in these young patients. Plain skull radiographs are useful for evaluating fractures in certain situations, although CT has largely supplanted plain radiographs for this diagnosis. This is largely in part due to the superior capability of CT to evaluate for complications of fracture, such as intracranial hemorrhage. The detection of skull fractures can be subtle even on CT. Skull fractures must be distinguished from the normal sutures (Fig. 6-50). Cranial sutures are usually symmetric and lack overlying soft tissue swelling, in contrast to skull fractures (Fig. 6-51). Accessory and anomalous sutures can also be mistaken for skull fractures. If oriented along the axial plane of the CT scan, skull fractures may not be visible on the axial images. It is critical to evaluate the scout image so that these fractures are detected. The degree of comminution of the fracture, depression or displacement of fragments, and additional intracranial findings such as hemorrhage, mass effect, and edema are all important associated findings. Bilateral fractures, complex fractures, or fractures that cross a suture line, especially in patients without an appropriately significant traumatic history, may be signs of nonaccidental injury (NAI). NAI should be suspected when the history does not support the radiographic findings. Although subdural hematomas may be present in victims of NAI, hemorrhage of different ages does not necessarily equate with abuse, especially in children with underlying enlargement of the extra-axial spaces.
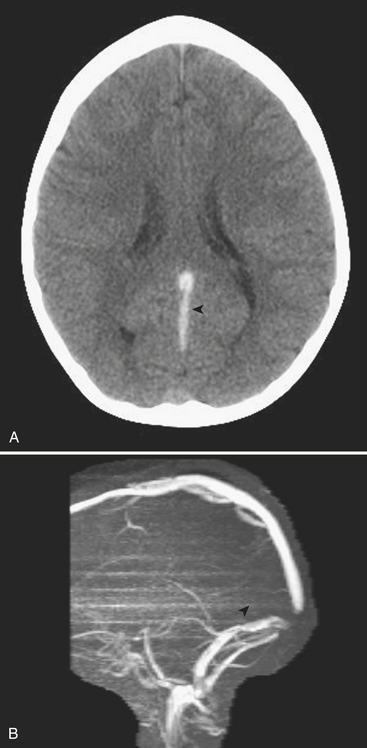
Venous Sinus Thrombosis
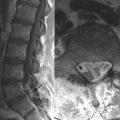
VST can be detected on the basis of findings made on head ultrasound, CT, and MRI. On US, images obtained through the anterior fontanelle of young infants will demonstrate the lack of Doppler flow within the superior sagittal sinus. On noncontrast CT, thrombus within a venous sinus will appear abnormally dense (Fig. 6-52A). This finding may also be seen in dehydrated patients with an elevated hematocrit level. If this is the case the attenuation of the arteries and veins should appear similar, whereas in VST the sinus will appear denser than the arteries. At contrast-enhanced CT the “empty delta” sign describes the appearance of an intraluminal filling defect (i.e., thrombus) within the dural envelope. MRI is more sensitive for detection of venous thrombosis. Findings on MRI consistent with VST include absence of normal flow voids and abnormal signal intensity within the sinus (Fig. 6-52B). Indirect signs of VST include collateral venous channels, cerebral hemorrhage, and signs of increased intracranial pressure. VST may lead to venous infarction and hemorrhage in the cortex and/or basal ganglia, depending on the sinuses involved.
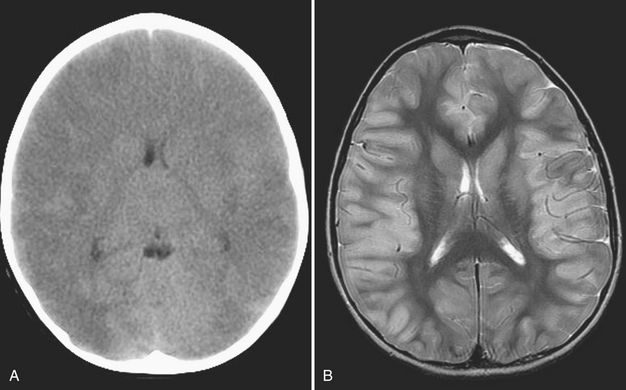
Meningitis/Encephalitis
Meningitis is a clinical diagnosis, often made on the basis of history and laboratory markers, including cerebrospinal fluid analysis. Imaging of patients with meningitis is usually not performed unless complications are suspected, such as encephalitis, epidural abscess, or venous sinus thrombosis. In these instances, contrast-enhanced MRI is the most appropriate imaging evaluation, although the diagnosis can also be suggested based on findings made at CT. In a child with clinical and laboratory findings consistent with intracranial infection, the effacement of sulci and subtle differences in attenuation of the cortical gyri on CT scan suggest parenchymal infection such as encephalitis. On MRI, the involved brain parenchyma will appear abnormally increased in signal on fluid-sensitive sequences (Figs. 6-53). Cerebellitis, or inflammation of the cerebellum, may be suggested on noncontrast head CT by areas of abnormal hypointense foci within the cerebellum, often in a pattern mimicking the longitudinal pattern of the cerebellar folia (Fig. 6-54). Often a recent history of a viral infection or immunization can be recalled. Cerebellitis may be suggested by CT, but abnormalities may be subtle and MRI is usually the diagnostic study.
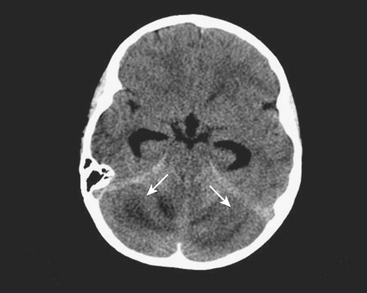
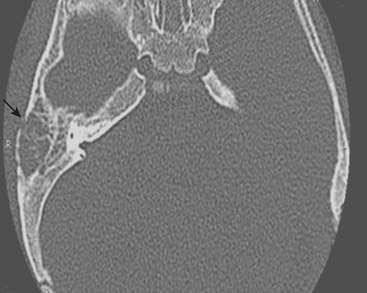
Mastoiditis
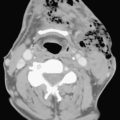
Fever and ear pain are common symptoms of otitis media (OM) in young children. OM is diagnosed on the basis of physical exam findings, and is commonly treated with antibiotics. Imaging is not indicated for uncomplicated OM. Lack of response or progression of symptoms while on antibiotics may prompt further evaluation for complications of OM or other causes for the patient’s symptoms. When mastoiditis or other complications of OM are clinically suspected, a CT through the temporal bones should be performed with intravenous contrast. Fluid within the middle ear on CT scan is consistent with OM. Mastoiditis is often associated with OM and represents extension of infection from the middle ear into the mastoid air cells, as these structures are contiguous. Bony erosion or intracranial extension of infection (such as a subperiosteal abscess) is evidence of coalescent mastoiditis, and surgical intervention may be required (Fig. 6-55). Given the proximity of the dural venous sinuses to the temporal bone, particular attention should be paid in order to exclude secondary venous thrombosis.
MUSCULOSKELETAL
Salter Harris Fractures
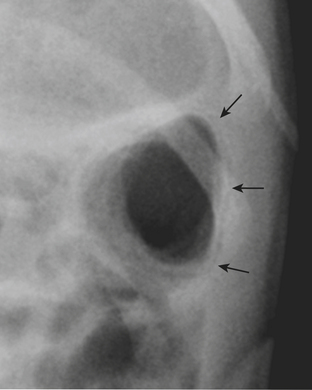
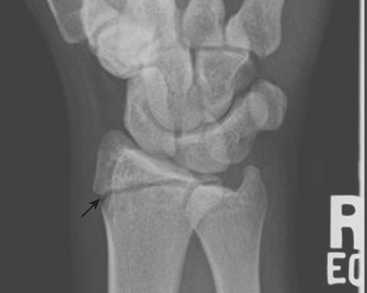
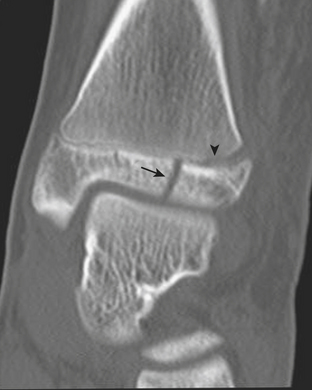
One of the most well known fracture classification systems is the Salter Harris (SH) classification, which describes the different appearance of fractures that occur at the physis of young children. SH1 fractures solely involve the growth plate (physis) (Fig. 6-56). SH2 fractures extend through the metaphysis into the growth plate (Fig. 6-57). SH3 fractures extend through the epiphysis into the growth plate. SH4 fractures extend from the metaphysis, through the growth plate, into the epiphysis. SH5 fractures are crush injuries to the growth plate. The significance of fractures that occur at the physis is that they may cause early fusion of the physis and lead to growth disturbance. Two distinct SH fractures are the triplane and juvenile Tillaux fractures, which typically occur in the older adolescent skeleton and are most common in the distal tibia. The triplane fracture is a complex SH4 injury that has a vertical component through the epiphysis, a horizontal component through the growth plate, and an oblique component through the metaphysis (Fig. 6-58). The Tillaux fracture is an SH3 injury involving fracture of the anterolateral aspect of the growth plate and epiphysis (Fig. 6-59). Fusion of the tibial growth plate begins centrally, proceeds medially, and completes laterally. The Tillaux pattern is secondary to injury after the medial distal tibial growth plate has fused. When Tillaux or triplane injuries are suspected or diagnosed by radiographs, CT is typically performed, because decisions for orthopedic repair hinge on the appearance of the fractures and measurements of distraction.
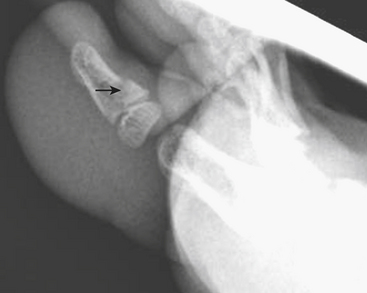
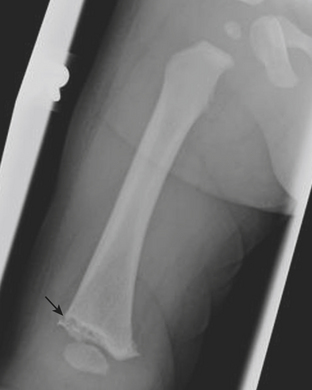
Figure 6-57 Lateral radiograph through the distal phalanx of the great toe demonstrates an SH2 fracture (arrow).
Plastic Bending Fractures
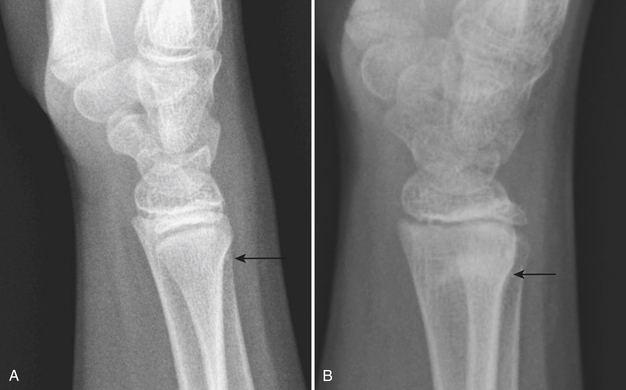
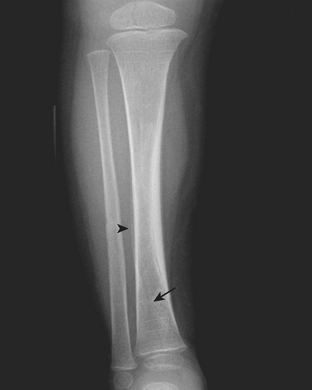
Plastic bending fractures are seen in the long bones of children who have suffered axial load injuries. These fractures appear as bowing contour deformities. Buckle or torus fractures are seen as minimal disruptions or irregularities in the cortical contours, particularly common in the long bones of the forearm (Fig. 6-60). Greenstick fractures demonstrate cortical continuity along one aspect of the bone and obvious fracturing or splintering of the opposite cortical margin. Toddler’s fractures are hairline cortical tibial fractures seen in walking older infants and young children (Fig. 6-61). These pediatric fractures are often initially overlooked, due to the subtle differences in angulation or cortical continuity. Certain buckle fractures, plastic fractures (bowing deformities), hairline fractures, SH1 fractures, and impaction fractures can be subtle and are more evident if a contralateral view is obtained. When clinical suspicion is high but initial radiographs appear normal, follow-up radiographs may demonstrate periosteal reaction, sclerosis, or increased conspicuity of the fracture line as evidence of healing injury.
Elbow Fractures
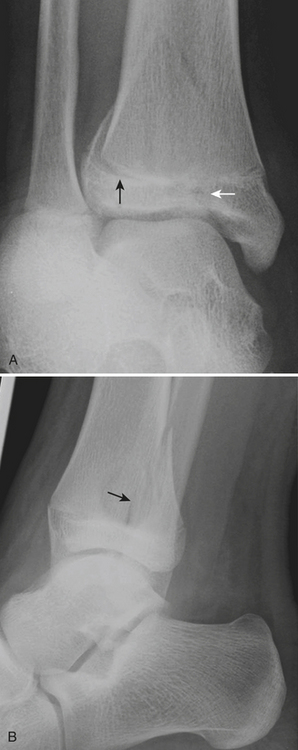
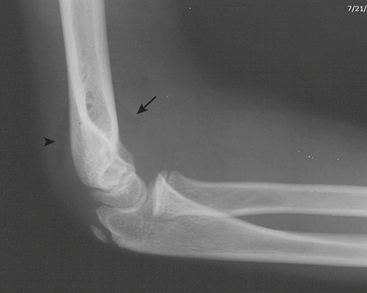
The elbow is a particularly complex joint to evaluate for injury in the pediatric skeleton. Due to the multiple ossification centers, predominance of radiolucent cartilage, and common site of injury, elbow fractures are particularly prone to false negative plain film interpretations. Knowledge of the normal anatomic alignment of the elbow, as well as of the appearance and timing of the ossification centers, is essential when evaluating the elbow. “CRITOE” is a common mnemonic that describes the typical order in which the ossification centers appear (capitellum, radial head, internal epicondyle, trochlea, olecrenon, external epicondyle). Bowing of the anterior fat pad (“sail” sign) and visualization of the posterior fat pad indicate a joint effusion (Fig. 6-62). The presence of an effusion does not necessarily connote a fracture, although it reflects significant traumatic injury to the joint. The radiocapitellar line drawn through the midshaft of the radius should always intersect the capitellum. Absence of this relationship represents dislocation of the radial head from its normal anatomic articulation with the capitellum. The anterior humeral line drawn along the anterior cortex of the humerus should intersect the middle third of the capitellum. If the line intersects the anterior third of the capitellum or passes anterior to the capitellum, a supracondylar fracture should be suspected. This line can lead to false positive assessments in nonlateral projections. Care should also be used in the extremely young patients with minimal ossification of the capitellum. A mnemonic such as FOOL (fat pads, cortical outlines, ossification centers, anterior humeral and radiocapitellar lines) can remind the busy radiologist to look for the presence of effusions, subtle buckle fractures, avulsed or “missing” ossification centers, and radial head dislocations or subtle supracondylar fractures.
Septic Hip
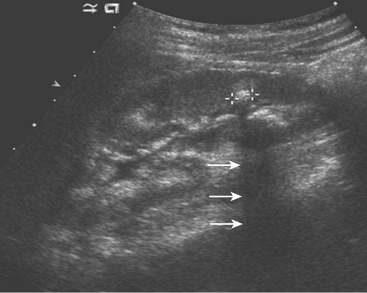
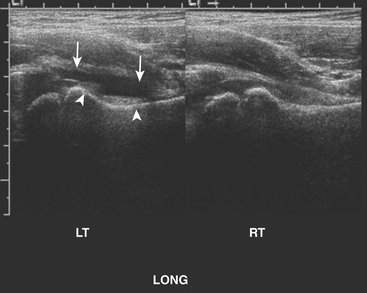
Patients presenting with hip pain may have septic arthritis of the hip joint, which requires emergent diagnosis in order to avoid permanent joint damage. The presence of a hip effusion can be suggested on anteroposterior radiographs of the pelvis by asymmetric widening of the femoral head–acetabular teardrop distance on the affected side, although radiographs are not sensitive for this diagnosis. Ultrasound is an excellent tool for determining the presence of a hip effusion. Using a high frequency linear transducer along the long axis of the femoral metaphysis, an effusion will appear as a hypoechoic collection ballooning outward from the cortex of the femoral head and neck (Fig. 6-63). Imaging the normal asymptomatic side demonstrates the asymmetry in the amount of fluid in the symptomatic joint. Transient synovitis cannot be differentiated from a septic hip sonographically. The diagnosis is determined by the histopathologic evaluation of the aspirated fluid. In a child with hip pain, the presence of fever, elevated white blood count, increased erythrocyte sedimentation rate, and elevated C-reactive protein should help direct the decision to perform hip aspiration.
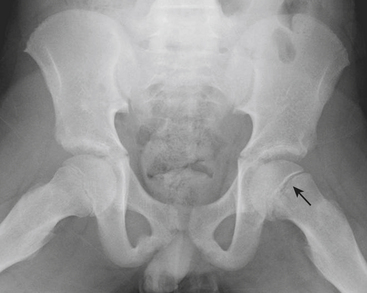
Other causes of hip pain in children include Legg-Calve-Perthes (LCP) disease and slipped capital femoral epiphysis (SCFE). LCP is idiopathic avascular necrosis of the hip. Most commonly occurring in 4- to 8-year-olds, the early radiographic signs may be subtle. Subcortical lucency along the proximal femoral epiphysis may be the first imaging sign of the disease. Cortical collapse, sclerosis, and deformity of the head and neck are seen in more advanced disease. SCFE is often diagnosed in the slightly older age group (ages 8 to 12), and is more common in overweight children. On anteroposterior radiographs, early SCFE can be seen as subtle asymmetry of the growth plates, while more advanced SCFE will have lateral displacement of the proximal femoral metaphysis with respect to the epiphysis. Often the slip is better seen on the frog leg lateral view, where the offset of the epiphysis and metaphysis is better demonstrated (Fig. 6-64).
Nonaccidental Injury
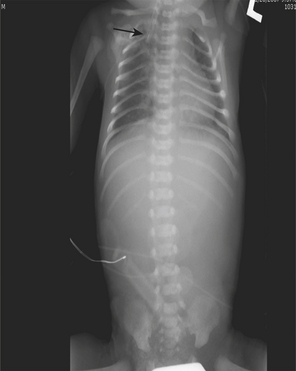
When interpreting pediatric imaging studies, one must be constantly on the lookout for signs of nonaccidental injury. Certain types of osseous injuries have a high specificity for NAI. Posterior rib fractures, for example, are highly specific for NAI and may be detected on chest or abdominal radiographs obtained for unrelated history, such as cough or vomiting (Fig. 6-65). Other fractures highly suggestive of child abuse are classic metaphyseal lesions, sometimes referred to as corner or bucket-handle fractures (Fig. 6-66). Spiral fractures of the long bones in infants who are not ambulatory are also worrisome for NAI. When clinical concern is raised or when a radiographic finding is suggestive of NAI, a full skeletal survey should be performed with high-quality, small-field-of-view technique. Multiple fractures, particularly if they are of varying ages, should alert radiologists to the possibility of abuse. A follow-up skeletal survey in 2 weeks can be suggested to assess for additional injuries that may not be visible on initial radiographs. There are few metabolic disorders that can mimic the osseous injuries seen in NAI, the most common being osteogenesis imperfecta (OI). These disorders are very rare, and often there are other clinical findings or a family history to suggest the diagnosis.
Agrons G.A., Harty M.P. Lung disease in premature neonates: Impact of new treatments and technologies. Semin Roentgenol. 1998;33:101-116.
Anderson R.C.E., Scaife E.R., Fenton S.J., et al. Cervical spine clearance after trauma in children. J Neurosurg. 2006;105(Suppl 5):S361-S364.
Artz G.J., Reilly J.S., Mattei P. Stridor in an infant. Clin Pediatr. 2006;45:578-581.
Atabaki S.M. Pediatric head injury. Pediatr Rev. 2007;28:215-223.
Atkinson G.O.Jr., Patrick L.E., Ball T.I.Jr., et al. The normal and abnormal scrotum in children: Evaluation with color Doppler sonography. AJR Am J Roentgenol. 1992;158:613-617.
Bixby S.D., Lucey B.C., Soto J.A., et al. Perforated versus nonperforated acute appendicitis: Accuracy of multidetector CT detection. Radiology. 2006;241:780-786.
Blanch A.J., Perel S.B., Acworth J.P. Paediatric intussusception: Epidemiology and outcome. Emerg Med Australas. 2007;19:45-50.
Bruce J., Huh Y., Cooney D., et al. Intussusception; Evolution of current management. J Pediatr Gastroenterol Nutr. 1987;6:663-664.
Buonomo C. Neonatal gastrointestinal emergencies. Radiol Clin North Am. 1997;35:845-864.
Choi H., Snyder H.M.III, Duckett J.W. Urolithiasis in childhood: Current management. J Pediatr Surg. 1987;22:158-164.
Choudhary A.K., Sellars M.E.K., Wallis C., et al. Primary spontaneous pneumothorax in children: The role of CT in guiding management. Clin Radiol. 2005;60:508-511.
Cleveland R.H. A radiologic update on medical diseases of the newborn chest. Pediatr Radiol. 1995;25:631-637.
Daneman A., Navarro O. Intussusception part 1: A review of diagnostic approaches. Pediatr Radiol. 2003;33:79-85.
Daneman A., Navarro O. Intussusception part 2: An update on the evolution of management. Pediatr Radiol. 2004;34:97-108.
Dillon P.W., Cilley R.E. Newborn surgical emergencies. Gastrointestinal anomalies, abdominal wall defects. Pediatr Clin North Am. 1994;40:1289-1314.
Donnelly L.F. Traumatic elbow effusions in children are not synonymous with occult fracture—even with evaluation by MR imaging. AJR Am J Roentgenol. 2002;179:531-532.
Donnelly L.F., Emery K.H., Brody A.S., et al. Minimizing radiation dose for pediatric body applications of single-detector helical CT: Strategies at a large children’s hospital. AJR Am J Roentgenol. 2001;176:303-306.
Eich G.F., Superti-Furga A., Umbricht F.S., et al. The painful hip: Evaluation of criteria for clinical decision-making. Eur J Pediatr. 1999;158:923-928.
Ein S.H., Alton D., Palder S.B. Intussusception in the 1990s: Has 25 years made a difference? Pediatr Surg Int. 1997;12:374-376.
Epelman M., Daneman A., Navarro O.M., et al. Necrotizing enterocolitis: Review of state-of-the-art imaging findings with pathologic correlation. Radiographics. 2007;27:285-305.
Evans E.D., Kramer S.S., Kravitz R.M. Pediatric diseases of the lower airways. Semin Roentgenol. 1998;33:136-150.
Fassett D.R., McCall R., Brockmeyer D.L. Odontoid synchondrosis fractures in children. Neurosurg Focus. 2006;20:E7.
Findlay R.D., Taeusch H.W., Walther F.J. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996;97:48-52.
Fiorella D.J., Donnelly L.F. Frequency of right lower quadrant position of the sigmoid colon in infants and young children. Radiology. 2001;219:91-94.
Ford E.G., Senac M.O., Srikanth M.S., et al. Malrotation of the intestines in children. Ann Surg. 1992;215:172-178.
Fragoso A.C., Pereira J.M., Estevao-Costa J. Nonoperative management of omental infarction—a case report. J Pediatr Surg. 2006;41:1777-1779.
Freed H.A., Shields N.N. Most frequently overlooked radiographically apparent fractures in a teaching hospital emergency department. Ann Emerg Med. 1984;13:900-904.
Garel L., Dubois J., Grignon A., et al. US of the pediatric female pelvis: A clinical perspective. Radiographics. 2001;21:1393-1407.
Godbole P., Stringer M.D. Bilious vomiting in the newborn: How often is it pathologic? J Pediatr Surg. 2002;6:909-911.
Goodman T.R., McHugh K. The role of radiology in the evaluation of stridor. Arch Dis Child. 1999;81:456-459.
Graif M., Itzchak Y. Sonographic evaluation of ovarian torsion in childhood and adolescence. Am J Roentgenol. 1988;150:647-649.
Greason K.L., Thompson W.R., Downey E.C., et al. Laparoscopic pyloromyotomy for infantile hypertrophic stenosis: Report of 11 cases. J Pediatr Surg. 1995;30:1571-1574.
Guthrie S.O., Gordon P.V., Thomas V., et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:279-285.
Haney P.J., Bohlman M., Sun C.C. Radiographic findings in neonatal pneumonia. AJR Am J Roentgenol. 1984;143:23-26.
Hernanz–Schulman M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227:319-331.
Hernanz–Schulman M., Sells L.L., Ambrosino M.M., et al. Hypertrophic pyloric stenosis in the infant without a palpable olive: Accuracy of sonographic diagnosis. Radiology. 1994;193:771-776.
Hertzberg B.S., Kliewer M.A., Paulson E.K. Ovarian cyst rupture causing hemoperitoneum: Imaging features and the potential for misdiagnosis. Abdom Imaging. 1999;24:304-308.
Hopkins A., Lahiri T., Salerno R., et al. Changing epidemiology of life-threatening upper airway infections: The reemergence of bacterial tracheitis. Pediatrics. 2006;118:1418-1421.
Jagannathan J., Dumont A.S., Prevedello D.M., et al. Cervical spine injuries in pediatric athletes: Mechanisms and management. Neurosurg Focus. 21:E4(1–5), 2006.
Jain K.A. Sonographic spectrum of hemorrhagic ovarian cysts. J Ultrasound Med. 2002;21:879-886.
Karmazyn B., Werner E.A., Rejaie B., et al. Mesenteric lymph nodes in children: What is normal? Pediatr Radiol. 2005;35:774-777.
Kawashima A., Sandler C.M., Goldman S.M. Imaging in acute renal infection. BJU Int. 2000;86(Suppl 1):S70-S79.
Kleinman P.K. Differentiation of child abuse and osteogenesis imperfecta: Medical and legal implications. AJR Am J Roentgenol. 1990;154:1047-1048.
Kleinman P.K., Nimkin K., Spevak M.R., et al. Follow-up skeletal surveys in suspected child abuse. AJR Am J Roentgenol. 1996;167:893-896.
Ko H.S., Schenk J.P., Troger J., et al. Current radiological management of intussusception in children. Eur Radiol. 2007;17:2411-2421.
Kocher M.S., Mandiga R., Zurakowski D., et al. Validation of a clinical prediction rule for the differentiation between septic arthritis and transient synovitis of the hip in children. J Bone Joint Surg Am. 2004;86:1629-1635.
Kornecki A., Daneman A., Navarro O., et al. Spontaneous reduction of intussusception: Clinical spectrum, management, and outcome. Pediatr Radiol. 2000;30:58-63.
Kosloske A.M., Musemeche C.A., Ball W.S., et al. Necrotizing enterocolitis: Value of radiographic findings to predict outcome. AJR Am J Roentgenol. 1988;151:771-774.
Krishnan A., De Souza A., Konijeti R., et al. The anatomy and embryology of posterior urethral valves. J Urol. 2006;175:1214-1220.
Lavocat M.P., Granjon D., Allard D., et al. Imaging of pyelonephritis. Pediatr Radiol. 1997;27:159-165.
Lee S.E., Kim H.Y., Jung S.E., et al. Situs anomalies and gastrointestinal abnormalities. J Pediatr Surg. 2006;41:1237-1242.
Lim H.K., Bae S.H., Lee K.H., et al. Assessment of reducibility of ileocolic intussusception in children: Usefulness of color Doppler sonography. Radiology. 1994;191:781-785.
Lin P.W., Stoll B.J. Necrotizing enterocolitis. The Lancet. 2006;368(9543):1271-1283.
Lonergan G.J., Baker A.M., Morey M.K., et al. From the archives of the AFIP: Child abuse: Radiologic–pathologic correlation. Radiographics. 2003;23:811-845.
Long F.R., Kramer S.S., Markowitz R.I., et al. Intestinal malrotation in children: Tutorial on radiographic diagnosis in difficult cases. Radiology. 1996;198:775-780.
Long W.A., Lawson E.E., Harned H.S., et al. Pleural effusion in the first days of life: A prospective study. Am J Perinatol. 1984;1:190-194.
Lumerman J., Gershbaum M.D., Hines J., et al. Unenhanced helical computed tomography for the evaluation of suspected renal colic in the adolescent population: A pilot study. Urology. 2001;57:342-346.
Macpherson R.I., Leithiser R.E., Gordon L., et al. Posterior urethral valves: An update and review. Radiographics. 1986;6:753-791.
Martin L., Buonomo C. Acute chest syndrome of sickle cell disease: Radiographic and clinical analysis of 70 cases. Pediatr Radiol. 1997;27:637-641.
Mazeh H., Kaliner E., Udassin R. Three recurrent episodes of malrotation in an infant. J Pediatr Surg. 2007;42:E1-3.
McCall R., Fassett D., Brockmeyer D. Cervical spine trauma in children: A review. Neurosurg Focus. 20:E5(1–8), 2006.
McNeely P.D., Atkinson J.D., Saigal G., et al. Subdural hematomas in infants with benign enlargement of the subarachnoid spaces are not pathognomonic for child abuse. Am J Neuroradiol. 2006;27:1725-1728.
Merten D.F., Goetzman B.W., Wennberg R.P. Persistent fetal circulation: An evolving clinical and radiographic concept of pulmonary hypertension of the newborn. Pediatr Radiol. 1984;6:74-80.
Meservy C.J., Towbin R., McLaurin R.L., et al. Radiographic characteristics of skull fractures resulting from child abuse. AJR Am J Roentgenol. 1987;149:173-175.
Milliner D.S., Murphy M.E. Urolithiasis in pediatric patients. Mayo Clin Proc. 1993;68:241-248.
Montenegro M.A., Santos S.L., Li L.M., et al. Neuroimaging of acute cerebellitis. J Neuroimaging. 2002;12:72-74.
Moore T.C. Omphalomesenteric duct malformations. Semin Pediatr Surg. 1996;5:116-123.
Nance M.L., Adamson W.T., Hedrick H.L. Appendicitis in the young child: A continuing diagnostic challenge. Pediatr Emerg Care. 2000;16:160-162.
Navarro O., Daneman A. Intussusception part 3: Diagnosis and management of those with an identifiable or predisposing cause and those that reduce spontaneously. Pediatr Radiol. 2004;34:305-312.
Ogata M., Imai S., Hosotani R., et al. Abdominal ultrasonography for the diagnosis of strangulation in small bowel obstruction. Br J Surg. 1994;81:421-424.
Özokutan B.H., Küçükaydin M., Gündüz Z., et al. Urolithiasis in childhood. Pediatr Surg Int. 2000;16:60-63.
Palmer J.S., Donaher E.R., O’Riordan M.A., et al. Diagnosis of pediatric urolithiasis: Role of ultrasound and computerized tomography. J Urol. 2005;174(4 Pt 1):1413-1416.
Patriquin H.B., Yazbeck S., Trinh B., et al. Testicular torsion in infants and children: Diagnosis with Doppler sonography. Radiology. 1993;188:781-785.
Platzer P., Jaindl M., Thalhammer G., et al. Cervical spine injuries in pediatric patients. J Trauma. 2007;62:389-396.
Rao P.M., Rhea J.T., Novelline R.A. Helical CT technique for the diagnosis of appendicitis: Prospective evaluation of a focused appendix CT examination. Radiology. 1997;202:139-144.
Romano C., Brown T., Frewen T.C. Assessment of pediatric near–drowning victims: Is there a role for cranial CT? Pediatr Radiol. 1993;23:261-263.
Rooks V.R., Lebowitz R.L. Extrinsic ureteropelvic junction obstruction from a crossing renal vessel: Demography and imaging. Pediatr Radiol. 2001;31:120-124.
Rosado W.M., Trambert M.A., Gosink B.B., et al. Adnexal torsion: Diagnosis by using Doppler sonography. AJR Am J Roentgenol. 1992;159:1251-1253.
Rudman D.T., Elmaraghy C.A., Shiels W.E., et al. The role of airway fluoroscopy in the evaluation of stridor in children. Arch Otolaryngol Head Neck Surg. 2003;129:305-309.
Rushton H.G. Urinary tract infections in children. Epidemiology, evaluation, and management. Pediatr Clin North Am. 1997;44:1133-1169.
Sadow K.B., Atabaki S.M., Johns C.M.S., et al. Bilious emesis in the pediatric emergency department: Etiology and outcome. Clin Pediatr. 2002;41:475-479.
Sargent M.A., Babyn P., Alton D.J. Plain abdominal radiography in suspected intussusception: A reassessment. Pediatr Radiol. 1994;24:17-20.
Schuh S., Lalani A., Allen U., et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatrics. 2007;150:429-433.
Servaes S., Zurakowski D., Laufer M.R., et al. Sonographic findings of ovarian torsion in children. Pediatr Radiol. 2007;37:446-451.
Shah R.K., Roberson D.W., Jones D.T. Epiglottitis in the Hemophilus influenza type B vaccine era: Changing trends. Laryngoscope. 2004;114:557-560.
Skibo L., Reed M.H. A criterion for a true lateral radiograph of the elbow in children. Can Assoc Radiol J. 1994;45:287-291.
Smergel E., Greenberg S.B., Crisci K.L., et al. CT urograms in pediatric patients with ureteral calculi: Do adult criteria work? Pediatr Radiol. 2001;31:720-723.
Stark K.E., Siegel M.J. Ovarian torsion in prepubertal and pubertal girls: Sonographic findings. AJR Am J Roentgenol. 1994;163:1479-1482.
Stoodley N. Controversies in non-accidental head injury in infants. Br J Radiol. 2006;79:550-553.
Strouse P.J. Disorders of intestinal rotation and fixation (“malrotation”). Pediatr Radiol. 2004;34:837-851.
St-Vil D., Brandt M.L., Panic S., et al. Meckel’s diverticulum in children: A 20-year review. J Pediatr Surg. 1991;26:1289-1292.
Swaniker F., Soldes O., Hirschi R.B. The utility of technetium 99m pertechnetate scintigraphy in the evaluation of patients with Meckel’s diverticulum. J Pediatr Surg. 1999;34:760-764.
Swischuk L.E., Hernandez J.A. Frequently missed fractures in children (value of comparative views). Emerg Radiol. 2004;11:22-28.
Thornbury J.R. Acute renal infections. Urol Radiol. 1991;12:209-213.
Tsai J.D., Huang F.Y., Lin C.C., et al. Intermittent hydronephrosis secondary to ureteropelvic junction obstruction: Clinical and imaging features. Pediatrics. 2006;117:139-146.
Tung G.A., Kumar M., Richardson R.C., et al. Comparison of accidental and nonaccidental traumatic head injury in children on noncontrast computed tomography. Pediatrics. 2006;118:626-633.
Vane D.W., West K.W., Grosfeld J.L. Vitelline duct anomalies: Experience with 217 childhood cases. Arch Surg. 1987;122:542-547.
Verschelden P., Filiatrault D., Garel L., et al. Intussusception in children: Reliability of US diagnosis—a prospective study. Radiology. 1992;8:343-347.
Vogl T.J., Bergman C., Villriner A., et al. Dural sinus thrombosis: Value of venous MR angiography for diagnosis and follow-up. AJR Am J Roentgenol. 1994;162:1191-1198.
Zerella J.T., Dimier M., McGill L.C., et al. Foreign body aspiration in children: Value of radiography and complications of bronchoscopy. J Pediatr Surg. 1998;33:1651-1654.