Chapter 62
Iliocaval Obstruction
Endovascular Treatment
Peter Neglén, Seshadri Raju
Percutaneous endovenous stenting has emerged during the last decade as the “method of choice” for the treatment of chronic obstruction of the femoroiliocaval venous outflow. The procedure can be performed with low morbidity, no mortality, long-term high patency rate, and low rate of in-stent restenosis. It has replaced bypass surgery as the primary treatment. Open venous reconstruction for chronic femoroiliocaval obstruction should be considered only in cases of unsuccessful or failed endovenous treatment in surgically fit patients with severe symptoms.1 Stenting of the venous outflow tract specifically alleviates pain and swelling and promotes sustained ulcer healing. Most important, the quality of life of the patients is substantially improved. Availability of a relatively simple and effective endovenous treatment has also led to a reappraisal of the role of venous outflow obstruction in the pathophysiologic process of chronic venous disease. The awareness of the possible presence of iliofemoral obstruction is increasing, and consequently the venous outflow is now evaluated more carefully and stenting more frequently considered. Stenting results in marked clinical improvement whether or not an adjunct procedure to control reflux is performed.2,3 In the presence of combined iliac vein obstruction and superficial or deep reflux, therefore, the emerging course of treatment is primary correction of the obstructive component. When significant great saphenous vein reflux is present, the great saphenous vein has increasingly been obliterated by percutaneous technique at the time of the stenting.4
Clinical Findings
Symptoms of obstruction may be any of those associated with chronic venous disease, ranging from moderate swelling and pain to discoloration and stasis ulcer. Venous outflow obstruction plays an important role in the clinical expression of chronic venous disease, especially of pain.5 Remaining obstruction is the principal cause of symptoms in approximately one third of postthrombotic limbs.6,7 The iliac vein is the common outflow tract of the lower extremity, and chronic obstruction of this segment appears to result in more severe symptoms than does lower segmental blockage. The clinical expression is also influenced by any concomitant deep or superficial reflux. It is well recognized that the combination of reflux and obstruction results in the highest levels of venous hypertension and the most severe symptoms compared with either alone.8,9 Negus and colleagues suggested that limb swelling and pain are related to the obstructive component, whereas limb ulceration results from valve reflux.10 It has been shown that ulcers occur only rarely in the presence of isolated iliofemoral obstruction (4%) but more often when obstruction is associated with reflux (30%).5 A substantial number of patients with chronic venous disease complain of disabling pain and swelling of the lower limbs without skin changes. It is possible that these symptoms are mainly attributable to obstruction rather than to reflux. Five years after iliofemoral deep venous thrombosis (DVT) treated conservatively with anticoagulation, 90% of patients suffer symptoms of chronic venous disease. Debilitating “venous claudication” is found in 15% to 44% of patients, and venous ulcer has developed in 15% of limbs.11,12 Venous claudication is a dramatic condition described as an exercise-induced “bursting” pain, which requires several minutes of rest and sometimes leg elevation for relief to be achieved. Certainly patients with significant outflow obstruction may also have less distinct lower extremity pain and discomfort with decreased quality of life and moderate disability.
Patient Selection
Lack of Reliable Tests
Specific symptoms may suggest outflow obstruction, but the varying symptoms in patients with chronic venous disease and the complex underlying pathophysiologic process necessitate further investigations. The major obstacle to appropriate selection of patients for venous outflow stenting is the lack of a reliable test to measure a hemodynamically significant stenosis. Diagnosis of significant outflow obstruction, especially when it involves the common femoral vein to the inferior vena cava (IVC), will therefore be based on morphologic investigations. Physician awareness of the importance of iliofemoral venous obstruction in treating patients with chronic venous disease is essential. Although poor recanalization after acute DVT is presently thought to be the most common cause of chronic venous blockage, the existence of iliac vein compressions without thrombosis is more pathogenic than was previously thought.2,6 Thus, significant iliac venous outflow obstruction may be present without history or morphologic findings suggestive of previous DVT. In our experience in the treatment of iliofemoral obstruction in 938 limbs in 879 patients, 53% of limbs had nonthrombotic compression lesions (defined as absent history of DVT, no venographic or ultrasound findings indicating previous DVT), 40% had postthrombotic obstruction, and 7% had a combined etiology.2
After Deep Venous Thrombosis
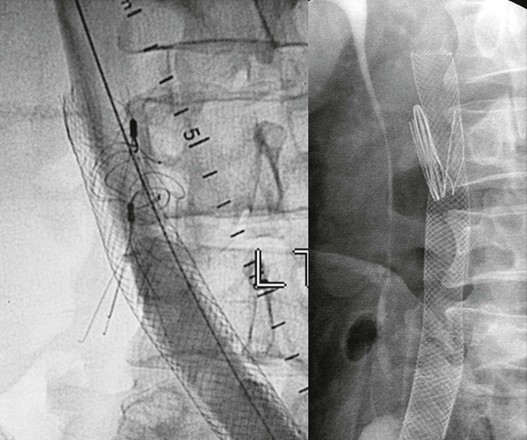
Most symptomatic outflow obstruction occurs after DVT involving the iliac segment. It may be limited to the iliofemoral segment or contiguous from the calf to the iliac veins. Only approximately 20% of these iliac veins will completely recanalize with anticoagulation treatment; the remaining veins recanalize only partially and develop varying degrees of obstruction and collateral formation.11,13 The typical postthrombotic iliofemoral lesion often involves both common and external iliac veins with irregular stenosis or occlusions, and axial, transpelvic, and ascending lumbar collaterals are present. Infrequently, a diffusely narrowed long segment of the iliac vein with no collateral formation is found (Fig. 62-1). We have designated this entity a Rokitansky stenosis, from the 19th century pathologist who described the phenomenon.14 As the severe inflammation of the wall (phlebitis) subsides, a fibrotic cylinder is formed, which impedes any collateral development and expansion of the vein. Thus, significant outflow obstruction cannot be excluded because of lack of collaterals.
Awareness of Iliac Vein Compression
External compression of the iliac veins has been considered a common finding of little clinical importance (Fig. 62-2A and B). Previous studies have established the frequent findings of intraluminal web or band formation and varying degrees of external compression of the iliac vein in the general population (22%-33%15–17 and 66%-88%,18–20 respectively). Symptomatic nonthrombotic iliac vein obstructive lesions have previously been described as May-Thurner syndrome15 or Cockett’s or iliac vein compression syndrome.20 The prevailing concept is that iliac vein compression syndrome typically involves the left proximal common iliac vein and is clinically expressed only in the left lower extremity of predominantly young women of childbearing age. These limitations are not true because compression lesions are not uncommon in men and in elderly patients and may involve the right limb.2,10 Compression of the common iliac vein was seen in 36%, of the external iliac vein in 18%, and of both sites in 46% of limbs in symptomatic patients. The ages of the patients with nonthrombotic blockage ranged from 18 to 90 years (median, 54 years); 20% of patients were men, and 25% of the symptomatic lower limbs were on the right side.
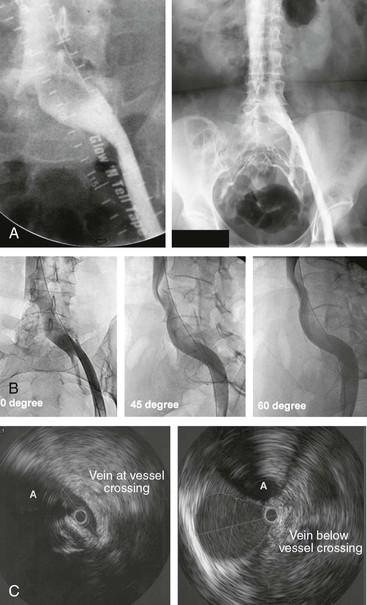
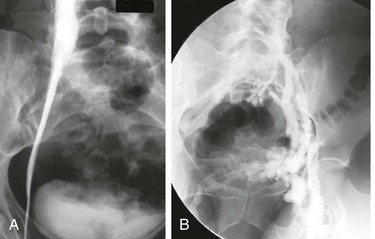
Figure 62-2 A, Single-plane transfemoral venogram showing typical findings suggestive of a left common iliac vein compression: “pancaking” and translucency of vein (left) and widening of the vein and presence of collaterals (right). B, Left transfemoral venogram with multiple projections. The oblique views (45- and 60-degree rotation) reveal stenoses, which are not visualized on the anteroposterior projection (0 degrees). C, Intravascular ultrasound (IVUS) images corresponding to the transfemoral venogram in B. The compression of the left common iliac vein is clearly shown as it is crossed by the right common iliac artery (A) (left) compared with the normal vein below the venous stenosis (right). The black circle inside the vein represents the inserted IVUS catheter.
Why a silent lesion should suddenly become significant in the pathophysiologic process is not fully understood. It has been suggested that the nonthrombotic iliac vein obstructive lesion is a so-called permissive lesion that does not become clinically significant until other components of the venous circulation of the lower limb fail. Correction of a permissive lesion alone often results in cure, which may explain the surprisingly good results of venous stenting in chronic venous disease even in the presence of untreated reflux.2
Evaluation of Venous Hemodynamics
Critical Venous Stenosis
The degree of hemodynamic venous obstruction depends on multiple factors: the number, location, degree of narrowing, and length of the lesions; the development of collaterals; and the volume flow varying at rest and during exercise. The venous circulation is a low-pressure, low-velocity, large-volume, and low-resistance converging vascular system (“sewage draining system”) compared with the high-pressure, high-velocity, small-volume, and high-resistance diverging arterial system (“water supply system”).21 The major obstacle in diagnosis of venous obstruction is that it is presently not known at what degree a venous stenosis is hemodynamically significant. Consequently, there is no accurate hemodynamic test (“gold standard”) available to properly assess venous outflow obstruction and its improvement after stenting in individual limbs.
Plethysmography
Plethysmographic outflow fraction determination and pressure tests (hand-foot pressure differential, hyperemia-induced dorsal foot venous pressure increase) are global hemodynamic tests and may suggest obstruction to the venous outflow at any anatomic site and level, but significant blockage may exist in the presence of a normal result.22–24 Positive test results may support further investigation and intervention, but a negative test result does not exclude clinically significant venous outflow obstruction.
Femoral Venous Pressure
Femoral venous pressure is a test for focal outflow obstruction. A pull-through pressure differential over a lesion or a pressure increase peripheral to the lesion with augmentation of venous inflow may be indicative of a significant stenosis.25–27 The venous pressure not only is a function of resistance to the flow but also depends to a high degree on the flow velocity and magnitude of volume flow. It is not known to what degree the resting venous flow must be increased to detect a functionally significant stenosis, nor is a method available to reproduce this flow rate consistently. Pressure gradients recorded in the venous system are much lower than in the arterial system, and only small pressure differentials may indicate significant obstruction. Studies suggest that a prestenotic pressure rise in the supine position greater than 2 to 4 mm Hg on provocation, a slow return to base level (>30s), or a gradient compared with the contralateral femoral pressure exceeding 2 to 5 mm Hg indicates a hemodynamically significant obstruction.25–27 It has been suggested that a pressure differential on exercise should be at least 5 mm Hg to warrant intervention, but none of these pressure limitations has been validated. Good clinical results have been obtained in the treatment of morphologic obstruction with normal pressure findings.28
The accuracy of these hemodynamic tests is insufficient to detect borderline obstructions. Thus, they play only a limited role in the management of obstructive disease. A positive hemodynamic test result may indicate hemodynamic significance, but a normal finding does not necessarily exclude it.
Investigations of the Venous Morphology
Venography
In lieu of an adequate hemodynamic test, morphologic tests must be used. Ascending venography after injection of contrast dye in a foot vein or antegrade transfemoral venography reveals the distribution and nature of the morphologic changes, including occlusion, stenosis, and the presence of collateral circulation, but is unable to indicate any hemodynamic impact of visualized lesions. Ascending venography usually insufficiently visualizes the iliac vein to permit assessment of any obstruction of that segment. It is mainly used today as a preoperative mapping tool to delineate the inflow to a postthrombotic iliac vein segment considered for stenting. To increase accuracy, all antegrade transfemoral venography should be performed by arterial angiographic techniques with subtraction imaging, multiple oblique projections, and pressure injectors (see Fig. 62-2B). With this technique, the quality of the images will improve and the contrast medium load will be minimal. A single-plane venogram was actually considered “normal” in at least one fourth of limbs despite the fact that intravascular ultrasound (IVUS) showed more than 50% obstruction.29 Interestingly, Cockett and colleagues made similar observations.10,19,20 Venography was diagnostic in only 65% of obstructed limbs in their experience, and collaterals were visualized in only 63%. It was noted that in 54% of symptomatic patients, findings on transfemoral venography appeared “normal” with smooth contours of contrast material in the iliac vein and without collaterals. The authors noted that absence of collateral formation should not negate consideration of the disease.10,20
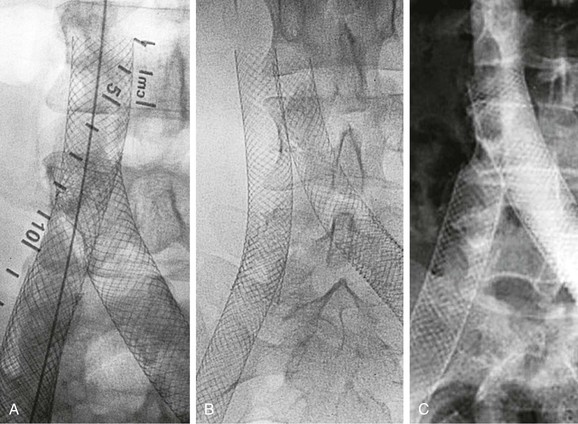
Although the formation of collaterals is classically regarded as a compensatory mechanism to bypass and thus alleviate an obstruction, the precise mechanism and inducement of collateral formation are unknown. Collateral circulation shown before stenting is often not visualized after stenting of a venous stenosis (Fig. 62-3). The flow through the stent is obviously favored. Limbs with collateral formation have been shown to have a significantly tighter stenosis than limbs with no collaterals, as measured by IVUS.30 The rate of limbs with femoral pressure increase on intra-arterial injection of papaverine was three times greater in patients with collaterals. These observations support the concept of pelvic collateralization as an indicator of obstruction and that collaterals poorly compensate for the blockage in symptomatic patients.
Ultrasound Scanning
Ultrasound scanning of the iliac vein is under development but still lacks sufficient accuracy to detect partial chronic obstruction. It can be used to evaluate patency of inserted stents. Computed tomographic venography and magnetic resonance venography techniques are improving and may replace transfemoral venography for screening in the future.31–35 Like ultrasound scanning, none of these tests have been validated in chronic obstructive disease.
Intravascular Ultrasound
IVUS is superior to single-plane and multiplane venography in detection of the extent and type of morphologic lesion of the vein (Fig. 62-2C).30,36–40 It is the most accurate test in this aspect and should be used to validate findings of other morphologic imaging methods. IVUS has proved superior in showing intraluminal details (e.g., trabeculations and webs) that may be hidden in the contrast medium. Venous wall thickness, in-stent layering, and movement can be adequately assessed. An external compression with the resulting deformity of the venous lumen or postthrombotic remodeling can be directly visualized (Fig. 62-4). The degree of stenosis can be precisely calculated by measurement of the cross-cut areas and diameters of the normal and compressed or diseased veins with the software built into the IVUS apparatus. In addition to being a diagnostic tool, it is also a crucial aid to guide stent insertion.
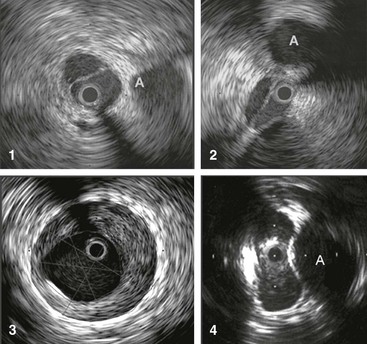
Figure 62-4 Images obtained by venous intravascular ultrasound (IVUS). 1, Intraluminal septa. 2, Trabeculation with multiple lumina. 3, In-stent restenosis precisely identifying the stent, neointimal hyperplasia, and remaining lumen. 4, Compression of the vein by the artery at the iliac vessel crossing, creating an hourglass appearance. The adjacent artery is marked with an A. The black circle inside the vein represents the inserted IVUS catheter.
Current Indications for Stenting
The major obstacle to improving the selection of patients for venous outflow stenting is the lack of a reliable test to measure a hemodynamically significant stenosis. The key for the physician is to be aware of the importance and possibility of venous blockage. Patients with previous DVT, patients with limb symptoms (especially pain out of proportion to detectable disease), patients not improving with conservative treatment, and patients with no other detectable pathologic changes to explain their symptoms are specifically targeted. More than half of patients with leg ulcers that do not improve with conservative treatment and saphenous vein ablation or phlebectomy have been found to have iliac vein outflow obstruction.41 Although a positive result of a noninvasive or invasive test may support further studies, a negative test result should not exclude it. The diagnosis and treatment must presently be based on invasive morphologic investigations of the iliac venous outflow, although hemodynamic criteria would be preferred. IVUS investigation is the ultimate test and should be generously used in symptomatic patients in whom outflow obstruction is suspected. Morphologic obstruction of more than 50% as measured by IVUS has arbitrarily been chosen for stenting.42,43 Limiting workup of patients with significant chronic venous disease to only duplex ultrasound will not suffice, especially not when it is restricted to the infrainguinal vein segments.
Technique
The technical details of percutaneous endovenous stenting of the venous outflow tract have been described in several reports.30,37,42,44 Although venous stenting may appear a simple procedure, attention to details is important to ensure an optimal outcome (Box 62-1). Venous balloon angioplasty with stenting is a different procedure from that employed in the arterial system. Experience acquired from arterial dilatation and stenting may not necessarily be extrapolated to the venous system. Balloon angioplasty alone is insufficient in the venous system. Stent insertion is mandatory. Severe recoil of the vein is observed intraoperatively in the majority of limbs, and simple balloon dilatation leads to early restenosis.45–48 The procedure may be performed under local infiltration analgesia in combination with monitored sedation or general anesthesia. Because the balloon dilatation may be painful, general anesthesia is recommended when tight stenoses or occlusions are stented. Attempts to recanalize occluded veins are also often time-consuming. The procedure should be performed in a fully equipped endovascular or angiographic suite, and availability of IVUS and external ultrasound for cannulation guidance is essential.
Access
Access to the iliac segment can be achieved retrogradely through the jugular or contralateral femoral vein, but an antegrade approach through an ultrasound-guided access distal to the obstruction in the thigh portion of the femoral vein or through the popliteal vein is preferred. Popliteal vein access is rarely used and often is not possible because of segmental occlusion of the proximal femoral vein. The midthigh access facilitates recanalization of occlusions from below, evaluation of the inflow, and precise placement of the stent in relationship to distal tributaries when necessary. Ultrasound guidance is particularly helpful in this situation to avoid inadvertent arterial puncture as the femoral vein occupies a variable posterolateral or posteromedial location in reference to the femoral artery. In contrast to arterial access at the thigh level, control of the venipuncture site by manual pressure is not a problem because of the low venous pressure. Ultrasound-assisted guidance of the cannulation is a necessity and has largely eliminated access complications.
Traversing the Lesion
Nonocclusive Obstruction
A hydrophilic guide wire (0.035 inch) is inserted and the cannula replaced by a 9F to 11F sheath, which will accommodate the appropriate balloon and stent sizes. A stiff guide wire and predilatation with serial dilators may be necessary to facilitate sheath placement in case of perivenous fibrosis of the postthrombotic femoral vein. Except in the event of occlusions, the guide wire can usually be passed through the stenosis and into the IVC with ease. An initial contrast venogram is then obtained. Multiplane venograms (anteroposterior, 45- and 60-degree oblique projections) are necessary to delineate a stenosis, which may not be revealed in the anteroposterior projection, especially if IVUS is not available (Fig. 62-5). IVUS is superior to venography in delineating any obstructive lesion and is recommended for diagnosis of the extent and degree of obstruction.
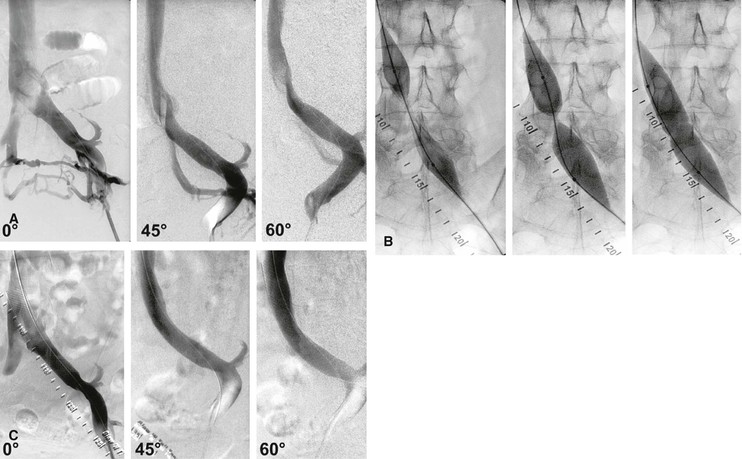
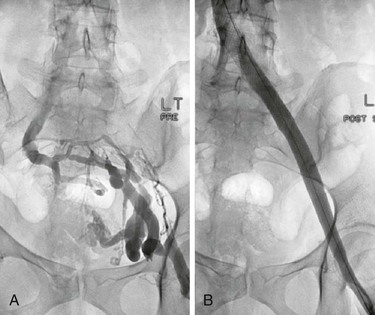
Figure 62-5 Balloon dilatation and stenting of a left common iliac vein compression (May-Thurner syndrome). A, Multiplane projections show the absence of obvious stenosis, a translucent area of the common iliac vein, and the presence of collaterals in the anteroposterior view before venous stenting. The stenosis is detected by rotation. B, Waisting of balloon during inflation by the stenosis. C, Post-stent venogram revealing no stenosis.
Occlusion
When an occlusion is present, the initial venogram may suggest the direction of manipulation of the guide wire beyond the point of occlusion. Recanalization is a challenging procedure and can be time-consuming (Fig. 62-6A to F). Success or failure in recanalization of occlusions cannot be predicted by the duration or extent of the lesion or its venographic appearance. The initial venogram may appear discouraging, but more often than not, a guide wire can be passed through the complete occlusion by sight and feel (see Fig. 62-6A). Patience is the key. In the event of initial failure, one or more additional attempts at reopening of the occlusion are warranted in all cases. Surprisingly, the guide wire tracks in the occluded lumen of the vein, which can be verified with IVUS after predilatation (see Fig. 62-6D). Multiple small-volume injections of contrast material and multiple oblique projections are performed to ensure that the guide wire stays in the vessel and progresses in the correct direction in the pelvis. Limited extravasations of contrast material can be safely ignored and the procedure continued. Larger dye extravasation calls for cessation of the procedure, which can be reattempted a few weeks later. A combination of soft and stiff guide wires with straight, angled, and J tips of different sizes (0.018 to 0.035 inch) and with supporting catheters (straight or angled) is required. Once the correct plane has been entered, rapid progress can usually be achieved without perforation by developing a loop or extended J at the end of a semistiff guide wire or catheter-supported soft wire during manipulation. Once the guide wire has traversed the occluded common iliac vein, passage through the iliocaval junction usually meets with additional resistance, again requiring extended manipulation in the area with coaxial catheter support. Specialized techniques, such as those described to cross the occluded aortoiliac junction, are usually not used.49 Successful vena cava entry is indicated by further easy passage of the guide wire into the right atrium and is confirmed by injection of contrast material through a general purpose catheter (5F) exchanged for the guide wire (see Fig. 62-6B).
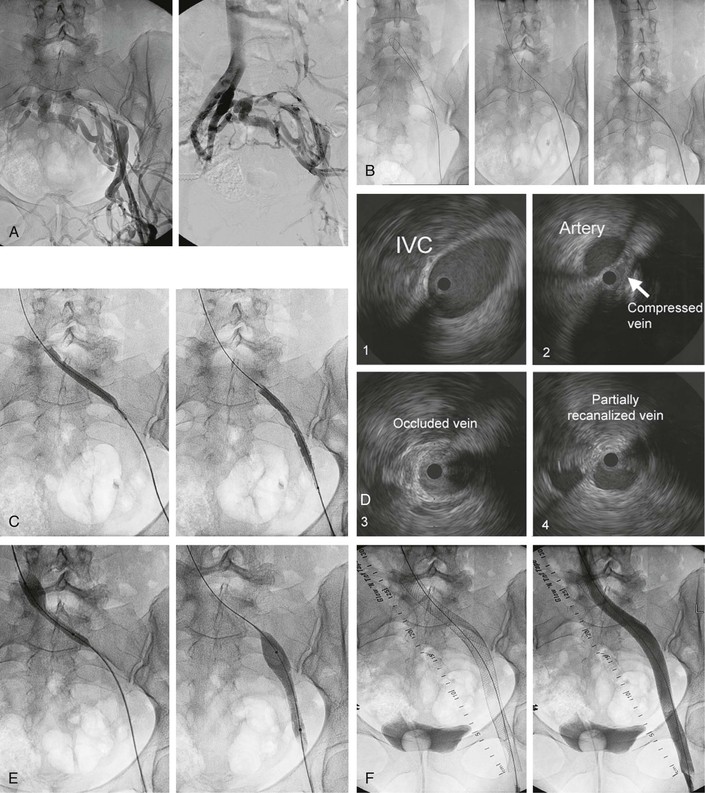
Figure 62-6 A, Transfemoral venogram performed through a sheath inserted after ultrasound-guided cannulation of the left femoral vein. A complete postthrombotic chronic occlusion of the left common iliac vein and severe stenosis of the external and common femoral veins are shown. Huge transpelvic collaterals fill a normal right iliocaval venous system. B, The obstruction has been traversed by manipulation with soft and stiff guide wires supported by guiding catheters (left). A cavogram obtained by contrast dye injection through an inserted general purpose catheter ensures that the inferior vena cava (IVC) has been penetrated. C, The occluded vessel is recanalized by a smaller balloon to allow passage of the intravascular ultrasound (IVUS) catheter for assessment of extent of lesion and subsequent insertion of larger balloons and stents. D, Images obtained by venous IVUS. 1, Normal IVC proximal to stenosis. 2, The common iliac vein severely compressed by the crossing iliac artery. 3, The completely occluded common iliac vein. Despite the chronic nature of the occlusion, the recanalizing guide wire has followed the course of the original vein and is clearly intraluminal on this image. 4, The partially recanalized external iliac vein. The black circle inside the vein represents the inserted IVUS catheter. E, Sequential ballooning of the entire iliofemoral venous segment to final diameter size (in this case, 14-mm width). F, Left, Image showing the entire stent system comprising three stents, proximally placed well into the IVC and then telescoped over each other to cover the entire iliofemoral vein, with the distal end placed at the inflow of the profunda vein. Right, Final transfemoral venogram, which shows uninterrupted venous outflow and no visualization of any collaterals.
Iliocaval Balloon Venoplasty and Stent Placement
Stenting of the Iliofemoral Vein
Predilatation.
After a significant stenosis has been diagnosed, it is predilated by an appropriately sized balloon, usually 16 mm for the distal cava and common iliac veins, 14 mm for the external iliac vein, and 12 mm for the common femoral vein. Predilatation of the track over the wire with 4- to 6-mm balloons is usually required when an occlusion has been recanalized to allow the passage of larger balloons. The entire track is ultimately dilated with 14- to 18-mm balloons (see Fig. 62-6C and E). There may be hesitation to dilate the obstructive vein because of fear of rupture. Clinical hemorrhage and pelvic hematoma have not been observed with the recommended balloon sizes in more than 1500 patients in our experience. If rupture should indeed occur, bleeding would probably be contained owing to the comparatively low prevailing venous pressure and the constraining influence of perivenous fascia, surrounding fibrosis, and retroperitoneal cover. Computed tomographic scanning performed because of postoperative back pain, which occurs infrequently, has failed to show rupture as causative. Limiting dilation to an 8- to 10-mm diameter is likely to result in residual stenosis in the stented segment and subsequent stent malfunction or thrombosis.
Placement of the Stent.
As pointed out before, IVUS is an invaluable diagnostic tool, but it is also essential at this point in the procedure to determine the extent of the lesion for appropriate placement of the stent because the diseased vein segment is frequently more extensive in reality than indicated by venography (see Fig. 62-6D).30,36 Insertion of one or several large-diameter stents (14 to 18 mm wide) corresponding to the size of the dilating balloon is recommended. It is important to redilate the stent after insertion to ensure a good apposition to the vessel wall and to avoid possible migration. Adequate placement is assessed by repeated IVUS.
To ensure a sufficient venous inflow, it is vital to cover the entire obstruction. Initially we chose to minimize the length of the stented area to avoid stent occlusion, fearing that the stent in itself was a risk factor for stent occlusion. If too short a stent is used, it may migrate proximally into the atrium during the stenting procedure.43 Stents were inserted covering only the common iliac vein, with the lower end placed just proximal to the internal-external iliac vein confluence in early cases of left iliac vein compression syndrome. This site is usually the lowest point of the iliac vein in the pelvis, where the vein turns anteriorly on its course toward the groin. In more than half of limbs with a compression lesion (primary disease), this site is involved because of compression of the external iliac vein by the internal iliac artery’s “diving” into the pelvis.2 Secondary stenting procedures for distal recurrent stenosis were primarily due to either an overlooked compression lesion at this site or a predilection of this site for de novo stenosis. The iliac vein confluence should be carefully assessed by IVUS at the time of stenting and the stenting commonly extended “around the curve” well into the external iliac vein. Skip areas between two stents should be avoided. When multiple stents are placed, they should be sufficiently overlapped to prevent separation. It appears that the occlusion rate and the frequency of in-stent recurrent stenosis are not related to stent length or metal load; on the contrary, failure to support the entire diseased vein segment with a stent commonly results in early in-stent recurrent stenosis or occlusion of the stent system.
Adequate Inflow.
As emphasized previously, adequate inflow into the stent system is essential for future patency. In extensive lesions, especially when recanalization of an occluded vein is obtained, it may be necessary to extend the stent below the level of the inguinal ligament (see Fig. 62-6F). The great saphenous, circumflex, and profunda veins are easily identified by IVUS, and the stent can be extended caudad to the inguinal ligament into the common femoral vein just above the inflow from these and other tributaries. Blood flow, especially from a patent profunda vein, appears to provide sufficient inflow into the newly recanalized segment, even when the femoral vein is severely diseased or occluded. Contrary to arterial stenting, it has been shown that braided stainless stents can be safely placed in the venous system across the inguinal crease with no risk of stent fractures, narrowing due to external compression, or focal development of severe in-stent restenosis and no effect on long-term patency.50
Extension into the Inferior Vena Cava.
Most often, self-expanding stents (braided stainless steel or Nitinol mesh stents) are inserted, but sometimes balloon-expanded stainless steel stents with strong radial force must be used. Stenting of a focal obstruction adjacent to the confluence of the common iliac veins requires that the stent be placed well into the IVC to avoid early recurrence of the stenosis. This is especially important when a braided stent is used. Owing to its inherent property, this stent may be “squeezed” (migrate) caudally when it is placed slightly beyond the stenosis to avoid extension of the stent into the IVC. A cephalad recurrent stenosis has been shown to develop in 40% of such limbs.37 The placement of the stent into the IVC does not appear to significantly impair the flow from the contralateral limb to result in thrombosis. Cases of contralateral limb thrombosis have been observed and raised concern. These clots, however, appear to be caused by recurrent attacks of thrombosis rather than obstruction by the contralateral stent. The “kissing” balloon technique used at the aortic bifurcation is unnecessary at the confluence of the common iliac veins, and bilateral stents are not inserted at this location in the treatment of unilateral lesions.
Stenting of the Inferior Vena Cava
Focal obstruction of the suprarenal IVC is most commonly caused by benign membranous or segmental obstruction. The lack of symptoms, including the nearly universal absence of renal or hepatic dysfunction in this subset, is undoubtedly related to excellent collateral development and function. In association with thrombosis of hepatic veins (Budd-Chiari syndrome), the segmental IVC obstructions may result in liver failure and portal hypertension.51,52 Infrarenal obstruction is seen in patients with previous spontaneous thrombosis (usually as an extension of an iliofemoral vein DVT), thrombosis after IVC interruption, or infrequently retroperitoneal fibrosis or malignant disease. Most iliac vein occlusions appear to remain symptomatic despite the presence of tributary collaterals on venography.53 In cases of combined iliac and IVC obstruction, the collateral compensation is usually poor. This group of patients is therefore more likely to develop limb symptoms.
Essentially the same technique for access—traversing the obstruction with the guide wire, venoplasty, and stenting—is used in the IVC as described for the iliofemoral vein obstruction. Larger balloons and stents (18- to 24-mm diameter) are now available and should be used at this site. Both self-expandable and balloon-expandable stents have been placed with success in the IVC because flexibility in this straight vessel is not of importance. As in all instances, all diseased segments should be covered by the stent to ensure adequate inflow and outflow of the stent. In pursuit of these principles, we have extended the stent across patent renal veins, across contralateral iliac veins, and below the inguinal ligament when necessary. Even extensive stenting of the IVC from the atrium to the confluence of the iliac veins does not appear to have any adverse effect on the splanchnic outflow (Fig. 62-7).54,55
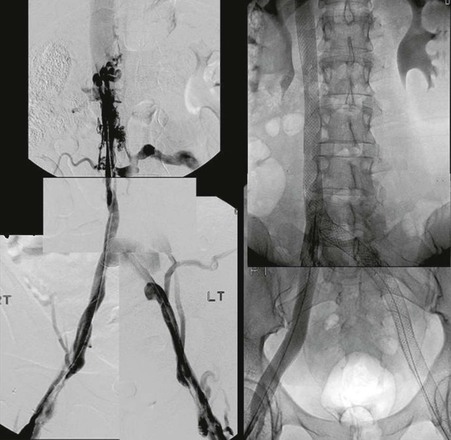
Figure 62-7 Stent placement for segmental occlusion at the renal level and severe stenotic lesions of the infrarenal caval and iliofemoral veins bilaterally. The stent system was placed from the cephalad part of the suprarenal inferior vena cava (IVC) and extending caudally into the femoral veins bilaterally. A Y fenestration allowed stenting of the IVC confluence.
IVC filters, when incorporated in the occlusion, are simply dilated like a rigid lesion up to 16 atm pressure. The filter is either pushed to the side or fractured, depending on the type of filter, including those with prongs, and the course of the guide wire. This maneuver may be safely performed without rupture of the IVC and with no subsequent bleeding (Fig. 62-8).56 Patency does not appear to be influenced by the fact that an IVC filter is crossed by a stent.
Stenting of the Iliocaval Confluence
Chronic iliocaval confluence obstruction is best managed by double-barrel stenting when it is feasible. The apposition technique requires a high reintervention rate owing to restenosis of the unsupported area. The Y fenestration is the only choice in delayed contralateral stenting or when the IVC is extensively involved, although the funnel techniques can be used in the latter situation. There is no optimal solution to the treatment of the iliocaval confluence presently, and the choice of technique is decided by the extent, site, and type of obstruction.
Double-Barrel Technique.
When both limbs are symptomatic with bilateral iliac vein disease, stenting may have to include the caval confluence.57 A double-barrel stent placement into the IVC may be used when simultaneous bilateral stenting is performed (Fig. 62-9). This is possible only when the caval lesion is limited to the distal infrarenal IVC or when cephalad bilateral lesions of the iliac veins require stent extension into the IVC. The healthy IVC must be wide enough to accommodate two parallel stents. The caval confluence is initially dilated with balloons inserted from both sides and inflated simultaneously, similar to the kissing balloon technique used at the aortic bifurcation. Two braided stainless steel stents (9 cm long and 14 mm wide) are then placed and released concurrently, starting at the same level in the IVC. As is the routine, the stents are redilated after placement. Any additional caudad stenting is then performed as described before.
Y Fenestration Technique.
The double-barrel technique cannot be used when iliocaval stenting has previously been performed or when bilateral iliac vein stenting is necessary in the presence of extensive cephalad caval obstruction. In those situations, a so-called Y fenestration must be performed, which configures the stents in the form of an inverted Y (see Fig. 62-9).57 The IVC stents are initially placed and extended into one of the iliac veins. The guide wire is introduced through the contralateral side and manipulated through the mesh of the side of the stent. This maneuver may be difficult and is facilitated by pushing a stiff needle through the mesh side of the stent (a transjugular intrahepatic portosystemic shunt needle).58,59 A low-profile balloon is placed over the guide wire and inflated, creating a fenestrum (window) in the side of the stent. An additional stent is inserted over the wire through the window. A generous overlap is necessary to prevent foreshortening and retraction of the stent caudally through the fenestrum on dilatation. The inflow of the contralateral iliac vein will be covered with this technique, but no restriction of the flow has been observed with its use.
The IVC Funnel Technique.
An alternative to fenestration in cases with extensive obstruction of the IVC has been described.60 A large-diameter stent (at least 24 mm) is placed like a funnel in the IVC with its lower end at the confluence. Two iliocaval stents are then inserted into the funnel proximally with the double-barrel technique as described before. A systematic outcome evaluation of this technique has not been published.
Apposition of Stents.
In the event that a penetration of the side of the stent during Y fenestration fails, the contralateral stent may be placed as close to the primary stent as possible (appositioned) (see Fig. 62-9).57 This always creates a skip area between the stents and may be performed only if the cephalad common iliac vein is relatively healthy. This alternative technique is poor and often results in later restenosis in the uncovered area, which necessitates placement of a bridge stent.
Perioperative Anticoagulation
The perioperative thrombosis prophylaxis may vary but is fairly standardized in our hands. The perioperative anticoagulation management is important, especially when postthrombotic occlusions are recanalized, balloon dilated, and stented. In these patients, full-dose low-molecular-weight heparin treatment is often given for 10 to 14 days before oral anticoagulation is started. The management outlined here has been successful in our experience. Anticoagulation for patients with postthrombotic disease should be provided per guidelines for the thrombotic disease regardless of any stenting procedure. For example, patients receiving warfarin preoperatively owing to prior DVT, unprovoked DVT with significant thrombophilia, or history of recurrent thrombosis are anticoagulated postoperatively. Other patients are discharged on a regimen of oral aspirin, 81 to 100 mg daily.
The preoperatively anticoagulated patients are instructed to discontinue the warfarin 3 days before the intervention and to self-inject dalteparin sodium, 5000 units daily subcutaneously. A slight increase of preoperative prothrombin time (international normalized ratio ≤1.7) is not a contraindication to proceeding with the procedure. On the day of intervention, all patients are given 2500 units of dalteparin sodium subcutaneously before and after surgery. Patients taking warfarin resume the medication the same day of the intervention. Dalteparin sodium, 5000 units, is self-injected subcutaneously daily in combination with the warfarin treatment for 4 days postoperatively, at which time the international normalized ratio level is usually found to be therapeutic. All patients receive intravenous unfractionated heparin, 3000 to 5000 units, after placement of the sheath in the femoral vein. The dalteparin sodium injections are repeated the next morning before discharge. In general, the patients are hospitalized less than 23 hours.
Complications and Thrombotic Events After Stenting
Venous stenting is performed with low morbidity and no mortality.43,61,62 The nonthrombotic complication rate related to the endovascular intervention is minimal and usually related to the cannulation site, although a few cases of retroperitoneal hematoma requiring blood transfusions have been described.37,43 After introduction of ultrasound-guided access of the femoral vein, the morbidity is virtually nil.
The early thrombosis rate is low after stenting for chronic iliofemoral venous obstruction without prior thrombolysis.2 Early thrombotic events occurred in only 15 of 982 stented limbs (1.5%). All thrombi involving iliac veins (12/982, 1.2%) were found in limbs stented for chronic postthrombotic obstruction (12/464, 2.6%). Thrombolysis of the newly formed clot should be attempted in initially technically successful limbs to reveal and to treat unknown additional obstructions to ensure adequate outflow and inflow.
The overall rate of thrombotic events after stenting was found to be 4% to 5%.43,61 Late thrombosis of the 982 stented iliac veins occurred in 3% of limbs 2 to 77 months after stenting and, interestingly, only in limbs treated for thrombotic obstruction. These thrombi were difficult to dissolve, and thrombolysis was successful in only approximately one third, leaving the majority occluded. Stenting of primary iliac compression lesions (nonthrombotic iliac vein obstructive lesions) with the technique presented has favorable long-term results with no thrombotic occlusions.
The extension of the stent into the IVC has raised concerns about relative obstruction to the venous outflow of the contralateral limb and subsequent thrombosis. The thrombosis rate was, however, low (1%), and three fourths of patients were stented for thrombotic obstruction. This should be compared with the approximately 40% rate of proximal restenosis when a braided stainless steel stent is not extended into the IVC.37 Probably because of acute onset of significant symptoms, the patients sought immediate treatment, and the success rate of thrombolysis or mechanical thrombectomy was high (82%). Thus, contralateral iliac vein thrombosis related to the IVC stent extension was benign and occurred infrequently.
Results
Stent-Related Outcome
Patency Rates
A number of reports describing patency rates after stenting of chronic obstructions of the femoroiliocaval venous outflow are now available (Table 62-1). Stenting was not preceded by thrombolysis. The largest report consists of 982 limbs stented under IVUS guidance for chronic postthrombotic obstructions (464 limbs) and nonthrombotic iliac vein obstructive lesions (518 limbs).61 Iliofemoral venography or external ultrasound was performed once or several times in 610 of these limbs. The overall cumulative primary, assisted-primary, and secondary patency rates found in this study at 72 months were 67%, 89%, and 93%, respectively (Fig. 62-10). A similar result at 8 years was reported by Hartung and coworkers after iliocaval stenting in 89 patients with chronic obstruction of mixed etiology.62
Table 62-1
Cumulative Patency Rates after Femoroiliocaval Stenting in Limbs with Obstruction of Different Etiology
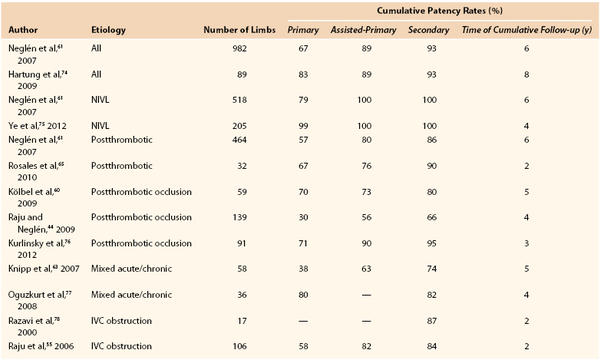
IVC, Inferior vena cava; NIVL, nonthrombotic iliac vein obstructive lesions.
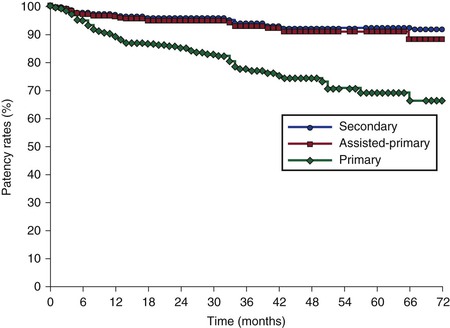
Figure 62-10 Cumulative primary, assisted-primary, and secondary patency rates of 603 limbs after iliofemoral stenting. The lower numbers represent limbs at risk for each time interval (SEM < 10%).
The stent-related outcome appears to be associated mainly with presence and severity of thrombotic disease (see Table 62-1). Neglén and colleagues reported that primary and secondary patency rates dropped markedly from limbs with nonthrombotic iliac vein obstructive lesions, to thrombotic limbs with obstruction, to thrombotic limbs with occlusion (79% and 100%, 57% and 86%, and 54% and 74%, respectively) (Fig. 62-11).61 Initial analysis of possible contributing factors suggested that tight long lesions of thrombotic etiology requiring multiple stents reaching distally into the common femoral vein were of greatest risk to occlude. This suggested that extension into the common femoral vein beneath the inguinal ligament should not be performed. However, a later study has shown that length of stented vein or extensions beneath the inguinal ligament into the common femoral vein are not associated with decreased patency when braided stainless steel stents are used.50 Stent occlusions will be seen more frequently when the venous lesions are incompletely covered. Arterial Nitinol mesh stents placed in the artery or vein below the inguinal ligament often fracture and occlude. Fractures and compression of braided stainless steel stent placed in this position have never been reported and were not observed during the quoted study, nor are they related to venous stent occlusion at this site.50
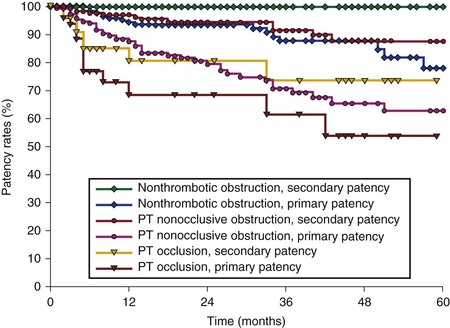
Figure 62-11 Cumulative primary and secondary patency rates for stented limbs with nonthrombotic iliac vein obstruction, nonocclusive thrombotic obstruction, and thrombotic occlusion (requiring recanalization). The lower numbers represent total limbs at risk for each time interval (SEM < 10%). PT, Postthrombotic.
Despite the fact that the thrombotic state was such a high risk factor and thrombophilia was more frequent in limbs with thrombotic disease, the presence of thrombophilia in itself was not significantly associated with occlusion. The operation side and gender did not influence stent outcome in that study, but younger age appeared to do so. Knipp and colleagues found that gender, recent trauma, and age younger than 40 years were predictive of decreased primary patency.63 This different finding may be explained by selection of patients because half of the patients were enrolled with acute DVT in that study. Only 10% of the limbs presented without current or previous DVT, possibly too few to detect the thrombotic state as being predictive.
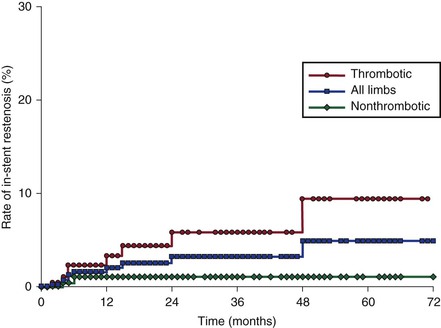
In-Stent Recurrent Stenosis
In our study, the cumulative severe (>50%) in-stent recurrent stenosis rate was assessed in 464 limbs and remained low in the long term, 5% at 72 months (Fig. 62-12).61 Factors associated with in-stent recurrent stenosis are similar to those associated with stent occlusion, except for age. The presence of thrombotic disease is the dominating factor. The cumulative rate of in-stent recurrent stenosis was higher in thrombotic limbs than in nonthrombotic limbs (10% and 1%, respectively). Despite this observation, it has not been conclusively proved that progressive in-stent recurrent stenosis results in occlusion.64 Stent occlusion appears to be caused by a recurrent thrombotic event rather than by slowly evolving narrowing of the stent. Hartung and colleagues reported a 13% restenosis rate, but these authors appear to have included stenosis at the lower stent-vein border area, which is not considered true in-stent recurrent stenosis.43 The nature and mechanism of development of in-stent recurrent stenosis are not yet known.
Clinical Outcome
Patients with chronic venous disease are younger, will live longer, and have a better prognosis compared with patients with arterial atherosclerotic disease. Chronic venous disease rarely threatens the survival of limb or patient, so the goal is to improve symptoms and the quality of life. The clinical results are gratifying, with substantial decrease in Venous Clinical Severity Score and Venous Disability Score, high cumulative ulcer healing rate, and improved quality of life. The median Venous Clinical Severity Score has been reported to markedly decrease in patients with obstruction of all etiology from 9 to 1 or 2 after iliofemoral stenting43,65,66 and in ulcerated limbs from 21 to 7.66 The median Venous Disability Score decreased from 2 to 1.43
Neglén and coworkers observed 918 of 982 patients (93%) for up to 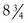 years (mean, 24 months; range, 1-107 months).61 This study reported the degree of swelling assessed by physical examination (grade 0, none; grade 1, pitting, not obvious; grade 2, ankle edema; and grade 3, obvious swelling involving the limb) and level of pain measured by the visual analogue scale.67 The preoperative and postoperative mean pain and swelling scores improved substantially: 3.7 (range, 0-9) and 0.8 (range, 0-10), and 1.7 (range, 0-3) and 0.8 (range, 0-3), respectively (P < .0001). The rate of limbs with severe pain (≥5 on a visual analogue scale) fell from 41% to 11% after intervention; gross swelling (grade 3) in limbs decreased from 36% to 18%. After 5 years, overall 62% and 32%, respectively, remained completely free of pain and swelling. This analysis was based on complete relief of swelling and pain (grade 0 swelling and 0 level of pain) and does not reflect partial improvement (Fig. 62-13). The incidence of ulcer healing after stent placement in 148 limbs with active ulcer was 68%, and the cumulative ulcer recurrence-free rate at 5 years was 58% (Fig. 62-14). Ulcers recurred in only 8 limbs of 101 healed ulcers during the follow-up period. Thus, if healing of the ulcer was achieved after stenting, ulcer recurrence was rare within the study period. These limbs frequently had remaining reflux, which was untreated during the observation period. Long-term ulcer healing was the same in limbs with primary (nonthrombotic iliac vein obstructive lesions) and thrombotic obstruction (62% and 55%, respectively; P = .2819). A recent report of 158 patients with stented ulcers of mixed etiology showed a greater cumulative ulcer-free rate at 5 years of 75% after iliofemoral stenting. In this study, healing was better in nonthrombotic limbs compared with postthrombotic limbs (87% vs 66% at 5 years, respectively; P < .02).68 The majority of patients with combined reflux and obstruction have sustained clinical improvement, including ulcer healing rate, after iliofemoral stenting alone despite the presence of reflux.2,3
years (mean, 24 months; range, 1-107 months).61 This study reported the degree of swelling assessed by physical examination (grade 0, none; grade 1, pitting, not obvious; grade 2, ankle edema; and grade 3, obvious swelling involving the limb) and level of pain measured by the visual analogue scale.67 The preoperative and postoperative mean pain and swelling scores improved substantially: 3.7 (range, 0-9) and 0.8 (range, 0-10), and 1.7 (range, 0-3) and 0.8 (range, 0-3), respectively (P < .0001). The rate of limbs with severe pain (≥5 on a visual analogue scale) fell from 41% to 11% after intervention; gross swelling (grade 3) in limbs decreased from 36% to 18%. After 5 years, overall 62% and 32%, respectively, remained completely free of pain and swelling. This analysis was based on complete relief of swelling and pain (grade 0 swelling and 0 level of pain) and does not reflect partial improvement (Fig. 62-13). The incidence of ulcer healing after stent placement in 148 limbs with active ulcer was 68%, and the cumulative ulcer recurrence-free rate at 5 years was 58% (Fig. 62-14). Ulcers recurred in only 8 limbs of 101 healed ulcers during the follow-up period. Thus, if healing of the ulcer was achieved after stenting, ulcer recurrence was rare within the study period. These limbs frequently had remaining reflux, which was untreated during the observation period. Long-term ulcer healing was the same in limbs with primary (nonthrombotic iliac vein obstructive lesions) and thrombotic obstruction (62% and 55%, respectively; P = .2819). A recent report of 158 patients with stented ulcers of mixed etiology showed a greater cumulative ulcer-free rate at 5 years of 75% after iliofemoral stenting. In this study, healing was better in nonthrombotic limbs compared with postthrombotic limbs (87% vs 66% at 5 years, respectively; P < .02).68 The majority of patients with combined reflux and obstruction have sustained clinical improvement, including ulcer healing rate, after iliofemoral stenting alone despite the presence of reflux.2,3
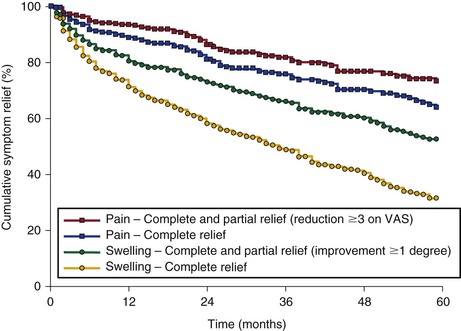
Figure 62-13 Cumulative complete relief and improvement of pain and swelling after iliofemoral venous stenting alone (with no subsequent reflux repair). The lower numbers represent total limbs at risk for each time interval (all SEM < 10%). VAS, Visual analogue scale.
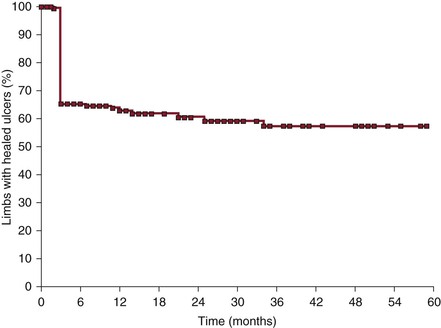
Figure 62-14 Cumulative ulcer recurrence-free rate after iliofemoral venous stenting alone (with no subsequent reflux repair). The steep fall at 3 months can be explained by the fact that limbs that failed to heal the ulcers during the observation period were considered not healed at 3 months. Leg ulcers that healed rarely recurred.
Obstruction of the common femoral and iliocaval venous outflow is more likely to cause decreased quality of life than are femoropopliteal vein blockages, particularly after previous DVT.12,69–71 A validated health-related quality of life questionnaire (CIVIQ)72 assessing subjective leg pain, sleep disturbance due to leg problems, work-related leg problems, and effect of leg symptoms on morale and social activities was filled out by stented patients prospectively before and after intervention (mean follow-up, 5 months; range,1-79 months; n = 381).61 There was significant improvement in all five problem categories after stenting of both nonthrombotic iliac vein obstructive lesions and thrombotic outflow obstructions. Chronic venous disease regardless of etiology affects quality of life adversely, and stenting in patients with chronic venous outflow obstruction frequently markedly improved it.
Hemodynamic Outcome
As previously discussed, there is no accurate hemodynamic test available to properly assess venous outflow obstruction and its improvement after stenting in individual limbs. It might, therefore, also be in vain to expect other hemodynamic parameters to significantly improve. The ultimate result of stenting is therefore better assessed by the clinical outcome as outlined before. Changes in the results of conventional tests, such as ambulatory venous pressure (percentage drop) with venous filling time, air plethysmography (venous filling index [VFI90], venous volume, and ejection fraction), and arm-foot pressure differential/hyperemia-induced pressure increase, have been found to be relatively minor compared with the clinical improvement.61 Significant decrease of the mean hand-foot pressure differential was found in stented limbs and occurred with and without remaining reflux and no adjunct procedures, and ambulatory venous pressure improved in most subsets of limbs in that study. Although numerically small, these changes were statistically significant and indicated that the outflow obstruction was alleviated and the global hemodynamics improved after stenting.73
After successful stenting of 23 selected limbs with iliofemoral postthrombotic obstruction and reflux, Delis and associates reported a significant increase in reflux matched by a greater venous filling time and decrease of residual volume fraction measured by strain-gauge plethysmography.12 It has been suggested that alleviation of proximal obstruction by stenting would increase distal reflux, a “protection” against reflux would be lost, and the clinical condition would perhaps be worsened. We found that better reflux-related parameters (venous filling time, VFI90, venous volume) after treatment were observed only when adjunct saphenous procedures were combined with the stenting.61 In no subset of patients was there observed a deterioration of venous reflux. The analysis of stented limbs with reflux and no adjunct procedure showed neither deterioration nor improvement of these parameters in this study. The presence of axial deep reflux before stenting did not worsen the global reflux measurably after stenting. Although increased retrograde flow measured by VFI90 may increase in individual patients, it was not found to be a dominant or constant phenomenon in this larger stented group of limbs.
Before stenting, limbs with thrombotic obstruction clearly had more extensive venous disease with more severe obstruction and reflux more frequently involving multiple systems and levels than did limbs with nonthrombotic iliac vein obstructive lesions.61 Despite this observation, stenting improved clinical symptoms and quality of life substantially and similarly in both groups of patients. The positive clinical outcome was achieved with an improvement of the calf muscle pump function in the limbs with nonthrombotic iliac vein obstructive lesions, whereas the thrombotic limbs had no measurable hemodynamic improvement in these parameters. The hemodynamic response in patients who became completely free of pain and swelling or in whom ulcers healed was no different from that in those with residual pain or swelling or nonhealing ulcers.
Follow-Up
Patients having had iliocaval stenting should be observed on a regular basis. Transfemoral venography, ascending venography, or iliocaval duplex ultrasound scanning should be performed before the patient is discharged, 6 weeks to 3 months later, 6 months after intervention, and then annually. Patients with high risk for development of early thrombosis, mainly limbs with recanalization of postthrombotic occlusion, may be seen more often. Adequate anticoagulation is vital for stent patency in this group of patients. When a recurrent in-stent stenosis of more than 50% is found on routine surveillance, balloon angioplasty should be performed to maintain stent patency whether or not the patient is symptomatic. On emergence of significant interval symptoms, even with normal venography or ultrasound findings, IVUS should be performed generously to ensure that an in-stent restenosis or extrastent de novo stenosis does not go undetected and untreated.
Selected Key References
Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990.
A comprehensive long-term study of clinical and stent-related outcome after venous stenting.
Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694–700.
The “gold standard” of IVUS to measure morphologic outflow obstruction is established.
Neglén P, Thrasher TL, Raju S. Venous outflow obstruction: an underestimated contributor to chronic venous disease. J Vasc Surg. 2003;38:879–885.
Study stressing that outflow obstruction, not only reflux, plays a major role in chronic venous disease.
Raju S, Neglén P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44:136–143.
The importance of the previously overlooked nonthrombotic iliac vein lesions (external compression) is pointed out.
Raju S, Darcey R, Neglén P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51:401–408.
Shows unexpected efficacy of stenting, even in patients with combined outflow obstruction and reflux.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Garg N, et al. Factors affecting outcome of open and hybrid reconstructions for nonmalignant obstruction of iliofemoral veins and inferior vena cava. J Vasc Surg. 2011;53:383–393.
2. Raju S, et al. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44:136–143.
3. Raju S, et al. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51:401–408.
4. Neglén P, et al. Combined saphenous ablation and iliac stent placement for complex severe chronic venous disease. J Vasc Surg. 2006;44:828–833.
5. Neglén P, et al. Venous outflow obstruction: an underestimated contributor to chronic venous disease. J Vasc Surg. 2003;38:879–885.
6. Johnson BF, et al. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg. 1995;21:307–312.
7. Johnson BF, et al. The site of residual abnormalities in the leg veins in long-term follow-up after deep vein thrombosis and their relationship to the development of the post-thrombotic syndrome. Int Angiol. 1996;15:14–19.
8. Nicolaides AN, et al. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414–419.
9. Nicolaides AN, et al. Investigations of patients with deep vein thrombosis and chronic venous insufficiency. Med-Orion Publishing Co: Los Angeles, Calif; 1991.
10. Negus D, et al. Compression and band formation at the mouth of the left common iliac vein. Br J Surg. 1968;55:369–374.
11. Akesson H, et al. Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg. 1990;4:43–48.
12. Delis KT, et al. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118–126.
13. Plate G, et al. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg. 1990;4:483–489.
14. Rokitansky C, et al. Sydenham Society: London; 1852. A manual of pathological anatomy. vol 4.
15. May R, et al. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419–427.
16. Ehrich WE, et al. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J. 1943;26:737–750.
17. McMurrich JP. The occurrence of congenital adhesions in the common iliac veins, and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci. 1943;135:342–346.
18. Kibbe MR, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39:937–943.
19. Cockett FB, et al. Iliac vein compression. Its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J. 1967;2:14–19.
20. Cockett FB, et al. The iliac compression syndrome. Br J Surg. 1965;52:816–821.
21. Strandness DE Jr, et al. The effect of geometry on arterial blood flow. Hemodynamics for surgeons. Grune & Stratton: New York; 1975.
22. Neglén P, et al. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583–589.
23. Labropoulos N, et al. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch Surg. 1997;132:46–51.
24. Hurst DR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106–113.
25. Albrechtsson U, et al. Femoral vein pressure measurements for evaluation of venous function in patients with postthrombotic iliac veins. Cardiovasc Intervent Radiol. 1981;4:43–50.
26. Negus D, et al. Femoral vein pressures in post-phlebitic iliac vein obstruction. Br J Surg. 1967;54:522–525.
27. Rigas A, et al. Measurement of the femoral vein pressure in oedema of the lower extremities. Report of 50 cases. J Cardiovasc Surg (Torino). 1971;12:411–416.
28. Raju S, et al. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35:8–15.
29. Neglén P, et al. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20:560–571.
30. Neglén P, et al. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694–700.
31. Arnoldussen CW, et al. An imaging approach to deep vein thrombosis and the lower extremity thrombosis classification. Phlebology. 2012;27(Suppl 1):143–148.
32. Wolpert LM, et al. Magnetic resonance venography in the diagnosis and management of May-Thurner syndrome. Vasc Endovascular Surg. 2002;36:51–57.
33. Sampson FC, et al. The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007;17:175–181.
34. Thomas SM, et al. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol. 2008;63:299–304.
35. Lindquist CM, et al. Utility of balanced steady-state free precession MR venography in the diagnosis of lower extremity deep venous thrombosis. AJR Am J Roentgenol. 2010;194:1357–1364.
36. Forauer AR, et al. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13:523–527.
37. Neglén P, et al. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2000;7:79–91.
38. Ahmed HK, et al. Intravascular ultrasonographic findings in May-Thurner syndrome (iliac vein compression syndrome). J Ultrasound Med. 2001;20:251–256.
39. Satokawa H, et al. Intravascular imaging methods for venous disorders. Int J Angiol. 2000;9:117–121.
40. Hingorani A, et al. Role of IVUS versus venograms in assessment of iliac-femoral vein stenosis. J Vasc Surg. 2011;52:804.
41. Alhalbouni S, et al. Iliac-femoral venous stenting for lower venous stasis symptoms. Ann Vasc Surg. 2012;26:185–189.
42. Neglén P, et al. Proximal lower extremity chronic venous outflow obstruction: recognition and treatment. Semin Vasc Surg. 2002;15:57–64.
43. Hartung O, et al. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42:1138–1144.
44. Raju S, et al. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009;50:360–368.
45. Marzo KP, et al. Early restenosis following percutaneous transluminal balloon angioplasty for the treatment of the superior vena caval syndrome due to pacemaker-induced stenosis. Cathet Cardiovasc Diagn. 1995;36:128–131.
46. Neglén P, et al. Iliofemoral venous thrombectomy followed by percutaneous closure of the temporary arteriovenous fistula. Surgery. 1991;110:493–499.
47. Wisselink W, et al. Comparison of operative reconstruction and percutaneous balloon dilatation for central venous obstruction. Am J Surg. 1993;166:200–204.
48. Walpole HT Jr, et al. Superior vena cava syndrome treated by percutaneous transluminal balloon angioplasty. Am Heart J. 1988;115:1303–1304.
49. Murphy KD. Mechanical thrombectomy for DVT. Tech Vasc Interv Radiol. 2004;7:79–85.
50. Neglén P, et al. Venous stenting across the inguinal ligament. J Vasc Surg. 2008;48:1255–1261.
51. Rector WG Jr, et al. Membranous obstruction of the inferior vena cava in the United States. Medicine (Baltimore). 1985;64:134–143.
52. Lee BB, et al. Primary Budd-Chiari syndrome: outcome of endovascular management for suprahepatic venous obstruction. J Vasc Surg. 2006;43:101–108.
54. van der Laan L, et al. [The central-venous compression syndrome: rare, but adequately treatable with endovascular stenting.]. Ned Tijdschr Geneeskd. 2004;148:433–437.
55. Raju S, et al. Obstructive lesions of the inferior vena cava: clinical features and endovenous treatment. J Vasc Surg. 2006;44:820–827.
56. Neglén P, et al. Stenting of chronically obstructed inferior vena cava filters. J Vasc Surg. 2011;54:153–161.
57. Neglén P, et al. Bilateral stenting at the iliocaval confluence. J Vasc Surg. 2010;51:1457–1466.
58. Farrell T, et al. Sharp recanalization of central venous occlusions. J Vasc Interv Radiol. 1999;10:149–154.
59. Honnef D, et al. Sharp central venous recanalization by means of a TIPS needle. Cardiovasc Intervent Radiol. 2005;28:673–676.
60. Kölbel T, et al. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther. 2009;16:483–491.
61. Neglén P, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990.
62. Hartung O, et al. Endovascular management of chronic disabling ilio-caval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38:118–124.
63. Knipp BS, et al. Factors associated with outcome after interventional treatment of symptomatic iliac vein compression syndrome. J Vasc Surg. 2007;46:743–749.
64. Neglén P, et al. In-stent recurrent stenosis in stents placed in the lower extremity venous outflow tract. J Vasc Surg. 2004;39:181–187.
65. Rosales A, et al. Stenting for chronic post-thrombotic vena cava and iliofemoral venous occlusions: mid-term patency and clinical outcome. Eur J Vasc Endovasc Surg. 2010;40:234–240.
66. Wahlgren CM, et al. Endovascular treatment in postthrombotic syndrome. Vasc Endovascular Surg. 2010;44:356–360.
67. Scott J, et al. Accuracy of subjective measurements made with or without previous scores: an important source of error in serial measurement of subjective states. Ann Rheum Dis. 1979;38:558–559.
68. Raju S, et al. Endovenous management of venous leg ulcers. J Vasc Sug. 2013;1:165–172.
69. Comerota AJ. Quality-of-life improvement using thrombolytic therapy for iliofemoral deep venous thrombosis. Rev Cardiovasc Med. 2002;3(Suppl 2):S61–S67.
70. Mavor GE, et al. Collaterals of the deep venous circulation of the lower limb. Surg Gynecol Obstet. 1967;125:561–571.
71. Kahn SR, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112.
72. Launois R, et al. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539–554.
73. Raju S, et al. Recanalization of totally occluded iliac and adjacent venous segments. J Vasc Surg. 2002;36:903–911.
74. Hartung O, et al. Management of pregnancy in women with previous left ilio-caval stenting. J Vasc Surg. 2009;50:355–359.
75. Ye K, et al. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol. 2012;23:497–502.
76. Kurklinsky AK, et al. Outcomes of venoplasty with stent placement for chronic thrombosis of the iliac and femoral veins: single-center experience. J Vasc Interv Radiol. 2012;23:1009–1015.
77. Oguzkurt L, et al. Iliac vein compression syndrome: outcome of endovascular treatment with long-term follow-up. Eur J Radiol. 2008;68:487–492.
78. Razavi MK, et al. Chronically occluded inferior venae cavae: endovascular treatment. Radiology. 2000;214:133–138.