Chapter 153 Iatrogenic Spine Destabilization
The instability that exists after a spine operation may arise from pathologic (intrinsic) or iatrogenic (surgical) processes. Iatrogenic destabilization can result from a variety of sources, such as the destruction of ligaments, muscles, or bone, and the denervation of muscles (Table 153-1).1
TABLE 153-1 Spine Destabilization: Etiology and Management
| Surgery | Reason for Instability | Recommended Management |
|---|---|---|
| Extensive cervical laminectomy | Tension band destruction Facet joint destruction |
Laminoplasty or lateral mass plating plus fusion |
| Extensive lumbar laminectomy | Tension band destruction Facet joint destruction |
Controversial Possibly dorsolateral fusion Possibly dorsal instrumentation |
| Cervical corpectomy | Bony destruction ALL/PLL destruction |
Ventral fusion Ventral instrumentation External orthosis |
| Thoracolumbar total corpectomy | Bony destruction ALL/PLL destruction |
Ventral reconstruction plus ventral instrumentation or dorsal instrumentation |
| Corpectomy plus dorsal decompression or total spondylectomy | Extensive bony destruction plus ALL/PLL destruction Facet joint destruction |
Circumferential fusion and instrumentation Equal ventral and dorsal instrumentation |
ALL, anterior longitudinal ligament; PLL, posterior longitudinal ligament.
Biomechanical Considerations
One method that is commonly used for evaluating stability is the three-column method of Denis.2 The anterior column is the ventral half of the vertebral body and the anterior longitudinal ligament (ALL). The dorsal half of the vertebral body and the posterior longitudinal ligament (PLL) constitute the middle column. The dorsal column consists of the facet joints and all ligaments dorsal to the spinal canal.
Using the method of Denis,2 significant instability is considered highly likely if two or more columns have suffered substantial injury. The posterior column has true anatomic boundaries, whereas the anterior and middle columns arbitrarily consider halves of a single vertebral body. Many systems for evaluating stability have been devised, but the method of Denis is an example of such a system that is easy to use and widely accepted for clinical application.
Ligamentous Disruption
Ventral Surgery
The ALL and the PLL, as well as the anulus fibrosus, contribute significantly to the stability of the spine.3–5 The PLL is weaker than the ALL and is often intentionally destroyed during dorsal, ventral, or lateral spine surgery. However, the ALL is often not totally disrupted, even with a wide ventral exposure. A strong and wide ligament, the ALL provides a significant proportion of spinal stability in extension. This function may be considered as a tension band that limits extension. As a result, ventral decompressive spine surgery (e.g., corpectomy), which adequately decompresses the dural sac, generally causes a disruption of the PLL, with preservation of at least a portion of the ALL. The width of the PLL significantly narrows in the middle portions of the vertebral body, thus making it susceptible to surgical disruption. In conjunction with existing bony disruption, surgical decompression usually causes significant instability of the spine. The extent of this destabilization can be assessed via intraoperative manipulation, such as vertebral body distraction. If significant instability is iatrogenically created, an interbody strut graft is necessary, with or without supplementation by instrumentation. The PLL limits flexion and distraction.
Dorsal Surgery
Resection of the interspinous ligaments may lead to instability. Although the interspinous ligaments are relatively weak, their long moment arm (i.e., distance from the instantaneous axis of rotation to the ligament attachment site) provides a mechanical advantage with regard to their function as a tension band.1 The capsular ligaments are strong. Although they function through a short moment arm, their relative strength allows them to provide a significant stabilizing effect, if they are intact.
Bone Destruction
Ventral Surgery
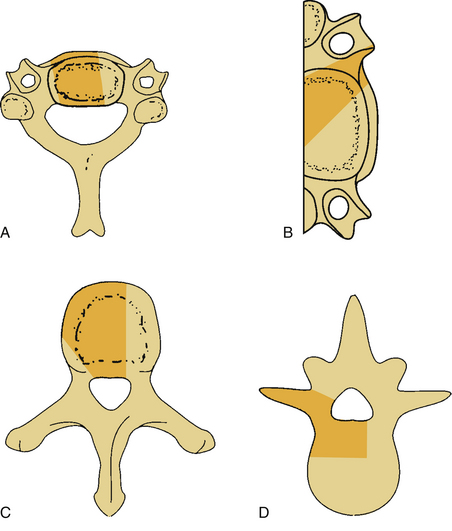
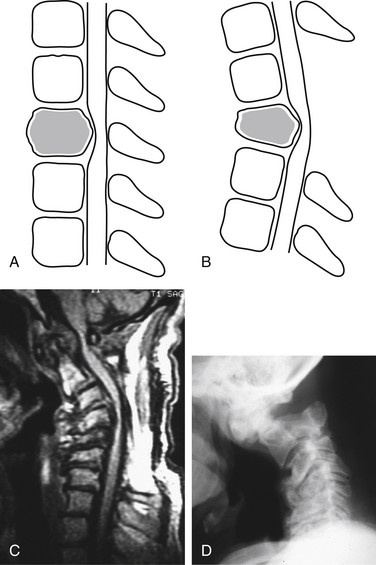
Bone destruction and additional surgical bone removal have a significant impact on spinal stability. Both the amount of vertebral body destruction and its location play an important role in the surgical destabilization process (Fig. 153-1). The first issue is the extent of ventral bony destruction. A complete vertebrectomy causes an obvious instability (see Table 153-1). The extent of instability is closely related to the amount of bone removed.
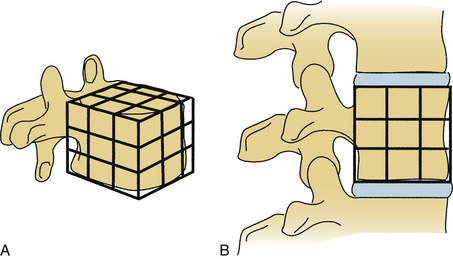
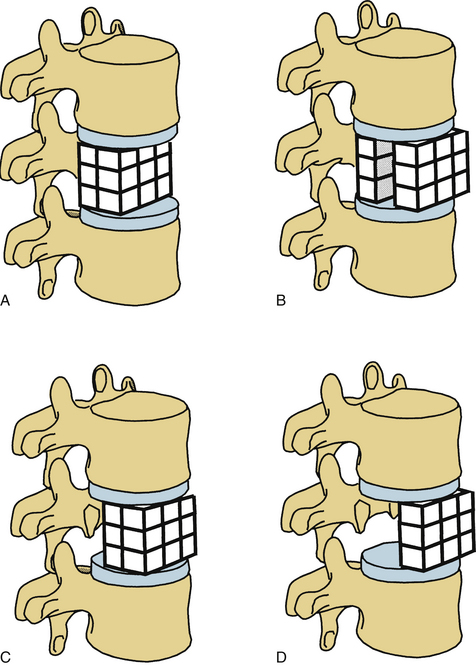
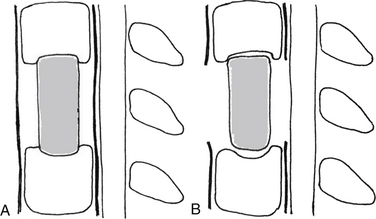
White and Panjabi5 used a three-column model to explain the effects of element disruption on spinal column stability. To determine the effect of a partial vertebral body resection on spinal stability, Benzel1 used a hypothetical design that divides the vertebral body into 27 equal, small cubes (Fig. 153-2). In this regard, resection of the ventral portion of the vertebral body affects spinal stability more than a corresponding resection of the middle or dorsal portion of the vertebral body (Fig. 153-3), because the largest force to which the spine is subjected is that of flexion. The more ventral portion of the vertebral body is farther from the instantaneous axis of rotation, and it therefore exerts its resistance through a longer moment arm in resisting flexion. Also, resection of the middle horizontal section of the vertebral body affects stability more than does resection in the middle vertical sections (Fig. 153-4).
Minimizing bone removal helps decrease postoperative instability. To attain this goal, vertebral body resection in cervical corpectomy should be carefully determined. In this regard, oblique corpectomy is an approach that does not significantly interfere with the stability of the spine.6,7 This approach protects the ventral portion of the vertebral body but sacrifices the dorsal and lateral aspects (see Fig. 153-1B).
As an aside, the uncovertebral joints add stability during extension, lateral bending, and torsion.8 In general, if the (1) ALL, (2) ventral section of the vertebral body, (3) dorsal column integrity, and (4) dorsal column ligaments remain intact, a significant instability does not develop.
Dorsal Surgery
A laminectomy can cause instability because of destabilization of the spine. The frequency of iatrogenic instability is proportional to the width of the laminectomy.9,10 Often, the extent of the injury is not readily apparent shortly after surgery. The prediction of its subsequent occurrence is even less obvious. If a ventral (vertebral body) lesion already exists, the incidence of postlaminectomy kyphosis is even higher.
Laminectomy often creates distortion of the dura mater and spinal cord, with flexion and distraction over the ventral fulcrum (Fig. 153-5). Even in the absence of the ventral pathology, the disruption of the laminae, facet joints, and dorsal ligamentous complex may result in progressive deformity, the so-called postlaminectomy kyphosis (Fig. 153-6). Postlaminectomy kyphosis occurs more commonly in the more mobile portion of the spine—the cervical spine. Laminoplasty may preserve a portion of the dorsal tension band and thereby diminish the instability observed after laminectomy.11 Another alternative that minimizes the destabilizing effect of laminectomy is the addition of a stabilization strategy such as dorsal fusion or external orthosis.
The contribution of the facet joints to dorsal column stability is very important. With axial loading, the anterior and middle columns transmit only 36% of the applied load, whereas each pillar (facet) transmits 32% of the total applied load.12 Therefore, regardless of the region of the spine involved, excessive facet joint resection can result in instability. In the cervical spine, the tolerable limit of resection is one third to one half of the facet joint.10 In the lumbar spine, facet resection may often result in glacial instability. However, the value of fusion and instrumentation after partial facetectomies for spinal stenosis is controversial.13
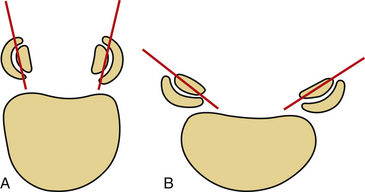
The shape and angulation of the facet joints are also important. A ventral translational deformity is more likely to result if vertically oriented joints and a hyperlordotic posture are present. The L4 and L5 facet joints are sagittally oriented, whereas L5-S1 joints are coronally oriented (Fig. 153-7). Therefore, L5-S1 joints resist translational deformity, whereas L4-5 joints can easily glide on a sagittal plane. Degenerative listhesis frequently involves this level.
Clinical Considerations
Tumor Surgery
An important issue that the spine surgeon cannot avoid is the iatrogenic instability created by radical tumor surgery. Until recently, the surgical treatment of spinal tumors was accomplished predominantly via laminectomy, despite the fact that the neural compression was often ventral to the spinal cord. However, laminectomy was often ineffective. Ventral surgery is often the procedure of choice for treating most bone tumors of the spine.14–16 Because the pathology in most patients with these bone tumors lies ventral to the spinal canal, attempts at tumor resection can cause loss of ventral spinal integrity.
Tumors involving both ventral and dorsal elements cause even greater instability. For oncologic tumor surgery, extramarginal resection of bone tumors of the spine is desirable. Although the spinal cord and nerve roots do not allow such a resection in many instances, there is an increasing trend toward accomplishing total spondylectomies by only a dorsal approach,14,15,17,18 or by circumferential surgery.19,20 Because the iatrogenic destabilization of the spine is so significant in these cases, radical measures to reconstruct and stabilize the spine are mandatory.
Adjacent-Level Spondylosis
It is common to observe degenerative changes above or below the level of a multilevel fusion. This type of instability is obviously iatrogenic. To avoid this, the use of more flexible (dynamic) fixation devices has been proposed.1 Short-segment fixation and fusion may minimize the incidence of this complication. Another technique for dealing with this problem is to create a “transitional” level by using instrumentation that is less rigid at the first segment adjacent to the fused segments. For example, an L3 burst fracture might be treated by the use of rigid screw instrumentation, along with bony fusion from L2 to L4. The “transitional” segment might then be created by the use of laminar hooks (hooks are less rigid than screws) at L1.
Segmental fixation with the use of pedicle fixation systems can be very rigid. Although increasing rigidity may improve fusion rates, it also increases the rate of degeneration of adjacent segments.21,22 Rahm and Hall22 have reported an incidence of adjacent-segment degeneration of 35% in cases with lumbar fusion and internal fixation. They also noted that the degeneration was associated with increasing patient age, use of interbody fusion, and worsening of clinical results with time.
“Adjacent-level degeneration” and “adjacent-level disease” (both adjacent-segment degeneration [ASD]) are different entities. In a study by Sugawara et al.,23 asymptomatic adjacent-disc degeneration was detected in 50% of the patients by their measurement methods. However, symptomatic adjacent-disc degeneration occurred in 5% of the patients, and only 2% required additional surgery. ASD may also cause a worsening of the clinical outcomes,24 especially with the ASD after multiple-segment fusion. The most sensitive technique to evaluate ASD is MRI.25
Risk Factors for Adjacent-Segment Degeneration
Type of Surgery
Fusion surgery is associated with a greater incidence of adjacent-level problems than others. The location and extension of the fusion are also important. Patients who underwent fusions of the L5-S1 segment showed a significantly lower risk of ASD than patients with L4-5 fusions (20% vs. 46%). Compared with L4-5 fusions, bisegmental L4-S1 fusions showed a similar trend with a lower risk of ASD (24%).26
Because fusion may precipitate adjacent-level problems, the recommendation may be to not perform multiple-segment fusion in cases such as degenerative lumbar deformity.24 In a retrospective study,27 it was found that even a cervical posterior foraminotomy is associated with a low rate of same- and adjacent-segment disease (4.9%). Adjacent-segment disease following expansive lumbar laminoplasty was observed to occur in 11% of patients, who showed degeneration at the segment adjacent to the laminoplasty.28
Type of Fusion
It is not yet known whether the type of fusion affects adjacent-level degeneration. In a recent randomized study with very long follow-up,29 there was no effect of anterior column support on the ASD after lumbar spinal fusion.
Similar adjacent-level degenerations happen after ventral cervical fusion. In a cadaveric study, a simulated C5-7 anterior cervical discectomy and fusion (ACDF) caused a significant increase in intradiscal pressure and segmental motion in the rostral adjacent-level during physiologic motion.30 After ventral cervical plate fusion, adjacent-level degenerative changes commonly occur, and this may cause an ossification on the upper and lower ends of the plate.31 Some of these postoperative radiographic changes may be related to the technique used. This ossification can be avoided by using the shortest possible plate so that extension of the plate into adjacent healthy discs does not occur.32
Osteoporosis
Adjacent-level degenerative changes may also occur in patients with osteoporotic vertebral body fractures after augmentation with vertebroplasty. As a long-term complication, failure of the adjacent vertebral body may develop. However, a biomechanical study has shown that there is no benefit of prophylactic vertebral reinforcement adjacent to vertebroplasty.33
Avoiding Adjacent-Level Disease
There have been many implant designs to lower the risk of adjacent-level degeneration and related disease. Artificial discs, pedicle-based dynamic implants,35 and ligamentoplasty36 implants are the main groups of these devices, and they are known as motion preservation implants.
Artificial Discs
Because these discs have special complications37 and require ventral surgery in the lumbar spine, their use has not increased in recent years.
Lumbar artificial discs have been shown to preserve the loads across the implanted and adjacent segments.38 In a finite element analysis, Charité artificial disc placement slightly increased motion at the implanted level, with a resultant increase in facet loading when compared with the adjacent segments, whereas the motions and loads decreased at the adjacent levels.
In vitro investigation of cervical adjacent-level intradiscal pressures following a total disc replacement arthroplasty has shown that it did not change significantly after arthroplasty in accordance to the fusion.39
Pedicle-Based Systems and Ligamentoplasty
The Dynesys system (Zimmer, Warsaw, IN) is a prototype of these systems; it has reportedly provided substantial stability in cases of degenerative spinal pathologies.40
However, Rohlmann et al.35 have used a finite element analysis to compare the effects of bilateral dorsal dynamic and rigid fixation devices on the loads in the lumbar spine. They have demonstrated that a dynamic implant does not necessarily reduce axial spinal loads compared to an uninstrumented spine. Hence, one might criticize pedicle-based dynamic systems as not truly dynamic.
In a retrospective study, ligamentoplasty, a motion-preserving surgery, has caused less ASD (9.2%) than posterior lumbar interbody fusion (14.1%) and dorsolateral fusion (13.3%).36
Postlaminectomy Instability
After cervical laminectomy in children, the incidence of kyphosis is very high.41,42 The important factors that affect cervical instability after laminectomy are (1) patient’s age, (2) number of laminae excised, (3) curvature of the cervical spine, and (4) degree of facet joint violation.41 Although the relative incidence of instability after cervical laminectomy is controversial, there is a tendency to not perform multilevel laminectomies in pediatric patients and to provide additional stabilization measures in patients with cervical spondylotic myelopathy undergoing laminectomy.6,41 In patients with cervical spondylotic myelopathy, these measures may either be a laminoplasty or fixation (e.g., lateral mass plating) and fusion after laminectomy.
Extensive lumbar laminectomy is often necessary for patients with spinal stenosis. Because of the nature of the compression, this procedure often includes a partial facetectomy. Although the exact incidence of instability after extensive lumbar laminectomy for lumbar stenosis is not well known, some surgeons have suggested the use of bilateral hemilaminectomies and partial facetectomies, without destruction of interspinous ligaments, while preserving the majority of the laminal and spinous process. Performing a fusion, with or without instrumentation, is controversial, however.1,43,44
Prevention and Management of Iatrogenic Spinal Instability
Thoracic and Lumbar Spine
Because of the widespread use of ventral surgery for spinal tumors, more reconstruction materials and stabilization problems are discussed. In general, if the lesion is strictly ventral, and the operation has destroyed only the anterior column and part of the middle column, ventral reconstruction and stabilization would be adequate. For single- or two-level lesions in the upper and middle thoracic spine, only a ventral reconstruction can be used. However, for lesions below level T10, ventral instrumentation, in addition to reconstruction, may be necessary. If, however, two or three columns are involved in lesions below level T10, supplemental dorsal instrumentation may be used after ventral reconstruction and ventral stabilization.46
Some surgeons perform en bloc resections of the dorsal and ventral elements of the spine from a dorsal route.44 Tomita et al.17 have described this approach as “total en bloc spondylectomy.” From an oncologic point of view, this method is superior to traditional spinal tumor surgery, curettage, and intralesional removal. The investigators have used a reconstruction material and additional dorsal fixation with instrumentation. It can be used in one stage and one position only. However, its disadvantage is the risk of spinal cord ischemia, resulting from bilateral occlusion of the segmental arteries.
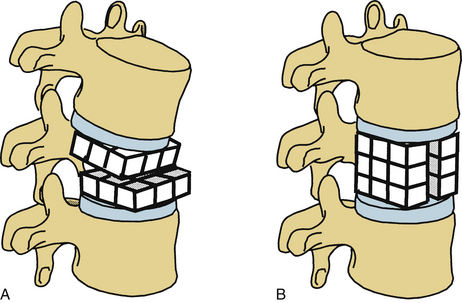
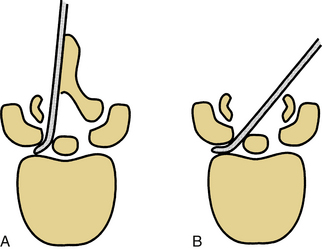
Limiting the extent of dorsal element removal or disruption during laminectomy can minimize spine destabilization by limiting the disruption of the dorsal tension band (e.g., by performing multilevel laminotomies instead of laminectomy). Laminotomy, theoretically, restricts the amount of important bone and soft tissue removed. However, laminectomy provides a trajectory advantage that most likely outweighs the laminotomy advantage (Fig. 153-8).
Lumbosacral Spine
The sacrum is a transitory structure between the vertebral column and pelvis. The sacroiliac joint is the immobile joint that functions as a shock absorber for the spine. The pelvis itself transfers loads from the sacroiliac joints to the hip joints, with the help of strong ligamentous structures. White and Panjabi examined the weight-bearing capacity of the sacroiliac joint. They found that, despite the lower half of the S1 vertebral body and half of the sacroiliac joint being resected, only 50% of weight-bearing capacity was lost. This, in fact, is adequate for ambulation, and there is no need for stabilization in most cases (Table 153-2).5 If the sacral lesion is a primary tumor, and complete tumor removal with extra marginal resection is desired, combined ventral and dorsal approaches, with a significant amount of bone and ligamentous tissue destruction, is necessary. In this case, an adequate reconstruction is required.18,47–49
TABLE 153-2 Resection Levels and the Need for Reconstruction in Sacral Tumor Surgery
| Resection Level | Reconstruction |
|---|---|
| One sacroiliac joint | Can usually compensate well with external support |
| Partial sacrectomy below S2 (most of the sacroiliac joints are preserved) | Not necessary |
| Total or subtotal sacrectomy above S2 (most of the sacroiliac joints are lacking) | Obviously necessary with instrumentation crossing the sacroiliac joint |
The key point for reconstruction is whether the sacroiliac joints are involved.50,51 If the tumor is not large and the sacroiliac joints are preserved, stability is not lost. However, involvement of one or both joints dictates the need for internal fixation and fusion. Kostuik and Esses15 suggest surgical reconstruction if 50% or more of the sacroiliac joint is removed.
If the sacrum above the S2 segment is resected with some of the ilium, the L5 vertebral body drops into the pelvis.18,52 In this case, an implant connecting the lumbar spine to both sides of the ilium is necessary. Three alternatives for the implant procedure are described in Box 153-1. Because the defect is so massive, the patient’s own bones do not allow an autograft fusion, and allograft bone is usually used. The forces from the lumbar spine are distributed to both lower extremities via the sacrum and pelvis. This complicates the lumbopelvic instrumentation decision-making process.53,54
BOX 153-1 Indications for Reconstruction of the Sacroiliac Joints
Resection of more than 50% of the sacroiliac joints
Bilateral resection of the sacrum above S2
Total resection of the sacroiliac joint on one side associated with ventral pelvic disruption or deficiency
From Bridwell KH: Management of tumors at the lumbosacral junction. In Marguiles JY, Floman Y, Farcy J-PC, et al, editors: Lumbosacral and spinopelvic fixation, Philadelphia, 1996, Lippincott-Raven, pp 109-122.
Because the sacrum is a transitory structure between the vertebral column and the pelvis and lower extremities, adding the sacrum into the long segment fixations for deformity correction is also an important point.9,55 Achieving strong lumbosacral fixation and fusion poses unique problems because of the shape of the sacrum and the presence of a large amount of cancellous bone. For this reason, different adaptations, such as the use of larger-diameter screws, application of multiple screws, and insertion of the rod into the iliac cristae after significant bending (Galveston technique), have been recommended (Table 153-3).3,9,55,56
TABLE 153-3 Methods of Sacropelvic Fixation and Reconstruction
| Technique | Reference |
|---|---|
| With Sacrum Preserved | |
| Isola plate | Asher and Strippgen3 |
| Galveston technique | Allen and Ferguson9 |
| Isola iliac screws | |
| With Sacrum Resected | |
| Fusion of L5 to the ilium | Shikata et al47 |
| Autogenous grafting of residual lumbar vertebral bodies to residual pelvis and sacrum | Kostuik and Esses15 |
| Massive structural allograft reconstruction (allograft sacropelvis) | |
The modified Galveston L-rod technique is a reasonable reconstructive method after total sacrectomy.53,57 It prevents caudal migration and axial rotation of the spinal column. Adding a threaded transiliac rod can prevent the open-book phenomenon and provide stability around the horizontal axis of the spinal column; at the same time, it also prevents rotation around this axis.
Cooper P.R., Errico T.J., Martin R., et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1-8.
Denis F. The three-column spine and its significance in the classification of acute thoracolumbar spine injuries. Spine (Phila Pa 1976). 1983;8:817-831.
Heller J.G., Whitecloud T.S. Post-laminectomy instability of the cervical spine—etiology and stabilization technique. Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven Press. 1991;vol. 2:1219-1240.
Hsu KY, Zucherman J, White AH, et al: Deterioration of motion segments adjacent to lumbar spine fusions. Presented at the Annual Meeting of the North American Spine Society, Colorado Springs, CO, 1988.
Kostuik J.P., Esses S.I. Sacral destabilization and restabilization—causes and treatment. Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven Press. 1991;vol. 2:2172-2173.
Tomita K., Yoribatake Y., Kawahara N., et al. Total en bloc spondylectomy and circumspinal decompression for solitary spinal metastasis. Paraplegia. 1994;32:36-46.
1. Benzel E.C. Biomechanics of spine stabilization: principles and clinical practice. New York: McGraw Hill; 1995.
2. Denis F. The three-column spine and its significance in the classification of acute thoracolumbar spine injuries. Spine(Phila Pa 1976). 1983;8:817-831.
3. Asher M.A., Strippgen W.E. Anthropometric studies of the human sacrum relating to dorsal transsacral implant designs. Clin Orthop Relat Res. 1986;203:58-62.
4. Mirbaha M.M. Exposure of the vertebral bodies of the proximal lumbar segments. Some anatomic points. Spine (Phila Pa 1976). 1978;3:329-335.
5. White A.A., Panjabi M.M. The problem of clinical instability in the human spine: a systematic approach. In: White A.A., Panjabi M.M., editors. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1990:277-378.
6. George B., Zerah M., Lot G., et al. Oblique transcorporeal approach to anteriorly located lesions in the cervical spinal canal. Acta Neurochir. 1993;121:187-190.
7. Takayasu M., Hara M., Takagi T. Osteoplastic anterolateral vertebrotomy without fusion for multilevel cervical ossification of the posterior longitudinal ligament. Neurosurgery. 1999;45:500-507.
8. Kotani Y., McNulty P.S., Abumi K., et al. The role of anteromedial foraminotomy and the uncovertebral joints in the stability of the cervical spine. A biomechanical study. Spine (Phila Pa 1976). 1998;23:1559-1565.
9. Allen B.L.Jr., Ferguson R.L. The Galveston technique for l rod instrumentation of the scoliotic spine. Spine (Phila Pa 1976). 1982;7:276-284.
10. Raynor R.B., Pugh J., Shapiro I. Cervical facetectomy and its effect on spine strength. J Neurosurg. 1985;63:278-282.
11. Baisden J., Voo L.M., Cusick J.F., et al. Evaluation of cervical laminectomy and laminoplasty. Spine (Phila Pa 1976). 1999;24:1283-1289.
12. Pal G.P., Sherk H.H. The vertical stability of the cervical spine. Spine (Phila Pa 1976). 1988;13:447-449.
13. Rosenberg N.J. Degenerative spondylolisthesis. Surgical treatment. Clin Orthop Relat Res. 1976;117:112-120.
14. Errico T.J., Cooper P.R. Transthoracic and translumbar decompression and stabilization for spine tumors. Contemp Neurosurg. 1993;15:1-6.
15. Kostuik J.P., Esses S.I. Sacral destabilization and restabilization—causes and treatment. Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven Press. 1991;vol. 2:2172-2173.
16. Sundaresan N., DiGiancinto G.V. Surgical considerations and approaches. In: Sundaresan N., Schmidek H.H., Schiller A.L., et al, editors. Tumors of the spine: diagnosis and clinical management. Philadelphia: WB Saunders; 1990:358-379.
17. Tomita K., Yoribatake Y., Kawahara N., et al. Total en bloc spondylectomy and circumspinal decompression for solitary spinal metastasis. Paraplegia. 1994;32:36-46.
18. Zileli M., Sabah D., Hoscoskun C. Surgery of the sacrum tumors. Presented at the Eurospine 96 Meeting. Zurich: Switzerland; October 1996.
19. Ma Y.Z., Tang H.F., Chai B.F., et al. The treatment of primary vertebral tumors by radical resection and prosthetic vertebral replacement. Clin Orthop Relat Res. 1987;215:78-90.
20. Selby D.K., Henderson R.J. Circumferential (360 degree) spinal fusion. Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven Press. 1991;vol. 2:1989-2006.
21. Hsu KY, Zucherman J, White AH, et al: Deterioration of motion segments adjacent to lumbar spine fusions. Presented at the Annual Meeting of the North American Spine Society, Colorado Springs, CO, 1988.
22. Rahm M.D., Hall B.B. Adjacent-segment degeneration after lumbar fusion with instrumentation: a retrospective study. J Spinal Disord. 1996;9:392-400.
23. Sugawara T., Itoh Y., Hirano Y., et al. Long-term outcome and adjacent disc degeneration after anterior cervical discectomy and fusion with titanium cylindrical cages. Acta Neurochir (Wien). 2009;151(4):303-309.
24. Yang J.Y., Lee J.K., Song H.S. The impact of adjacent segment degeneration on the clinical outcome after lumbar spinal fusion. Spine (Phila Pa 1976). 2008;33:503-507.
25. Neal C.J., Rosner M.K., Kuklo T.R. Magnetic resonance imaging evaluation of adjacent segments after disc arthroplasty. J Neurosurg Spine. 2005;3:342-347.
26. Disch A.C., Schmoelz W., Matziolis G., et al. Higher risk of adjacent segment degeneration after floating fusions: long-term outcome after low lumbar spine fusions. J Spinal Disord Tech. 2008;21:79-85.
27. Clarke M.J., Ecker R.D., Krauss W.E., et al. Same-segment and adjacent-segment disease following posterior cervical foraminotomy. J Neurosurg Spine. 2007;6:5-9.
28. Kawaguchi Y., Ishihara H., Kanamori M., et al. Adjacent segment disease following expansive lumbar laminoplasty. Spine J. 2007;7:273-279.
29. Videbaek T.S., Egund N., Christensen F.B., et al. Adjacent segment degeneration after lumbar spinal fusion: the impact of anterior column support a randomized clinical trial with an eight- to thirteen-year magnetic resonance imaging follow-up. Spine (Phila Pa 1976). 2010;35:1955-1964.
30. Park D.H., Ramarkrsihnan P., Cho T.H., et al. Effect of lower two-level anterior cervical fusion on the superior adjacent level. J Neurosurg Spine. 2007;7:336-340.
31. Park J.B., Watthanaaphisit T., Riew D. Timing of development of adjacent-level ossification after anterior cervical arthrodesis with plates. Spine J. 2007;7:633-636.
32. Goffin J., Loon J.V., Calenbergh F.V., et al. Long-term results after anterior cervical fusion and osteosynthetic stabilization for fractures and/or dislocations of the cervical spine. J Spinal Disord. 1995;8:500-508.
33. Oakland R.J., Furtado N.R., Wilcox R.K., et al. Preliminary biomechanical evaluation of prophylactic vertebral reinforcement adjacent to vertebroplasty under cyclic loading. Spine J. 2009;9:174-181.
34. Hwang S.H., Kayanja M., Milks R.A., et al. Biomechanical comparison of adjacent segmental motion after ventral cervical fixation with varying angles of lordosis. Spine J. 2007;7:216-221.
35. Rohlmann A., Burra N.K., Zander T., et al. Comparison of the effects of bilateral posterior dynamic and rigid fixation devices on the loads in the lumbar spine: a finite element analysis. Eur Spine J. 2007;16:1223-1231.
36. Kanayama M., Togawa D., Hashimoto T., et al. Motion-preserving surgery can prevent early breakdown of adjacent segments: comparison of posterior dynamic stabilization with spinal fusion. J Spinal Disord Tech. 2009;22:463-467.
37. Shim C.S., Shin H.D., Lee S.H. Posterior avulsion fracture at adjacent vertebral body during cervical disc replacement with ProDisc-C: a case report. J Spinal Disord Tech 20(6):. 2007:468-472.
38. Goel V.K., Grauer J.N., Patel T.C.h., et al. Effects of Charité artificial disc on the implanted and adjacent spinal segments mechanics using a hybrid testing protocol. Spine (Phila Pa 1976). 2005;30:2755-2764.
39. Dmitriev A.E., Cunningham B.W., Hu N., et al. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine (Phila Pa 1976). 2005;30:1165-1172.
40. Schmoelz W., Huber J.F., Nydegger T., et al. Dynamic stabilization of the lumbar spine and its effects on adjacent segments: an in vitro experiment. J Spinal Disord Tech 16(4):. 2003:418-423.
41. Heller J.G., Whitecloud T.S. Post-laminectomy instability of the cervical spine—etiology and stabilization technique. Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven Press. 1991;vol. 2:1219-1240.
42. Katsumi Y., Honma T., Nakamura T. Analysis of cervical instability resulting from laminectomies for removal of spinal cord tumor. Spine (Phila Pa 1976). 1989;14:1172-1176.
43. Herkowitz H.N., Kurz L.T. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg [Am]. 1991;73:802-808.
44. Shenkin H.A., Hash C.J. Spondylolisthesis after multiple bilateral laminectomies and facetectomies for lumbar spondylosis: follow-up study. J Neurosurg. 1979;50:45-47.
45. Lichtblau S. Dislocation of the sacro-iliac joint: a complication of bone-grafting. J Bone Joint Surg [Am]. 1962;44:193.
46. Cooper P.R., Errico T.J., Martin R., et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1-8.
47. Shikata J., Yamamuro T., Kotoura Y., et al. Total sacrectomy and reconstruction for primary tumors. Report of two cases. J Bone Joint Surg [Am]. 1988;70:122.
48. Sung H.W., Shu W.P., Wang H.M., et al. Surgical treatment of primary tumors of the sacrum. Clin Orthop Relat Res. 1987;215:91.
49. Stener B., Guntenberg B. High amputation of the sacrum for extirpation of tumors: principles and technique. Spine (Phila Pa 1976). 1978;3:351.
50. Bridwell K.H. Management of tumors at the lumbosacral junction. In: Marguiles J.Y., Floman Y., Farcy J.-P.C., et al, editors. Lumbosacral and spinopelvic fixation. Philadelphia: Lippincott-Raven; 1996:109-122.
51. Gunterberg B., Romanus B., Stener B. Pelvic strength after major amputation of the sacrum. Acta Orthop Scand. 1976;47:635-642.
52. Gennari L., Azzarelli A., Quagliuolo V. A posterior approach for the excision of sacral chordoma. J Bone Joint Surg [Br]. 1987;69:565-568.
53. Gökaslan Z.L., Romsdahl M.M., Kroll S.S., et al. Total sacrectomy and Galveston l-rod reconstruction for malignant neoplasms: technical note. J Neurosurg. 1997;87:781-787.
54. Tomita K., Tsuchiya H. Total sacrectomy and reconstruction for huge sacral tumors. Spine (Phila Pa 1976). 1990;15:1223-1227.
55. Louis R. Fusion of the lumbar and sacral spine by internal fixation with screw plates. Clin Orthop Relat Res. 1986;203:18-33.
56. Baldwin N.G., Benzel E.C. Sacral fixation using iliac instrumentation and a variable angle screw device. J Neurosurg. 1994;81:313-316.
57. Jackson R.J., Gokaslan Z.L. Spinal-pelvic fixation in patients with lumbosacral neoplasms. J Neurosurg. 2000;92:61-70.