CHAPTER 101 HYPOTHERMIA AND TRAUMA
Human beings, as homeotherms, normally maintain their body temperature within a narrow range around a core temperature of 37° C. A variety of built-in mechanisms work to either preserve or lose heat. The failure of these mechanisms can result in abnormal temperatures and associated pathophysiologic consequences.
Hypothermia, defined as a core temperature of 35° C or less, is a strong predictor of mortality after injury.1–3
INCIDENCE
A recent analysis of the National Trauma Data Bank (NTDB) provides the most comprehensive perspective on the incidence of hypothermia among trauma patients.4 Of 1,126,238 injured patients, the admission body temperature was recorded in 701,491 (62.3%). A total of 11,026 patients (1.57% of all patients with a recorded temperature) were hypothermic, defined as a core temperature lower than 35° C.
MECHANISM OF INJURY
Trauma patients are disrobed in the emergency department, where most heat loss occurs, and are frequently administered cold intravenous (IV) fluids. Hypothermia is more common and more profound in the more seriously injured patients. Therefore, there is uncertainty over whether the increase in mortality is primarily attributable to the hypothermia itself, or to the underlying injuries. Some have proposed that hypothermia is actually protective in trauma patients, and that mortality rates would not be higher in cold patients if comorbid factors were equal.5 However, recent studies have documented an adverse effect of hypothermia on outcome, and a significantly improved likelihood of surviving initial resuscitation when hypothermia is aggressively treated.1,3,4,6,7
EFFECTS ON COAGULATION
Perhaps the most serious effect of hypothermia in the trauma victim is its effect on coagulation. Uncontrollable hemorrhage, often compounded by coagulopathy, is the most frequent cause of early death in these patients. Dilutional thrombocytopenia is usually cited as the primary cause of coagulopathic bleeding when trauma victims undergo massive transfusion.8 However, a prospective, randomized, double-blind controlled clinical trial indicated that dilutional thrombocytopenia is relatively infrequent and that prophylactic administration of platelets was not beneficial.9 Consumptive coagulopathy appeared to be the more common problem associated with massive transfusion.
EFFECTS ON PLATELET COUNT AND FUNCTION
During development of hypothermic cardioplegia for cardiac surgery, there was a surge of research interest in the effects of hypothermia on coagulation in the late 1950s. Experimental studies in dogs at that time demonstrated a reversible thrombocytopenia associated with systemic hypothermia.10,11 However, the thrombocytopenia observed actually occurred at very deep levels of hypothermia, well below that typically seen in a trauma setting.11–15 Yoshihara et al.16 reported that platelet counts dropped by only 20%-30% at an esophageal core temperature of 30° C.
In contrast, levels of hypothermia commonly encountered in clinical practice have been shown to have a significant effect on platelet function. Platelets experience a reversible inhibition of their function under conditions of local or systemic hypothermia, mediated at least in part through the temperature dependence of thromboxane B2 by platelets.17 Thromboxane B2 is a potent vasoconstrictor and platelet aggregating agent. Valeri et al.17 demonstrated this when they induced systemic hypothermia to 32° C in baboons, but kept one forearm warm using heating lamps and a warming blanket. Simultaneous bleeding time measurements in the warm and cold arm were 2.4 and 5.8 minutes, respectively. The authors concluded that warming to restore wound temperature to normal should be tried before resorting to transfusion therapy with platelets and clotting factors when treating hypothermic patients with nonsurgical bleeding.
EFFECT ON CLOTTING FACTOR LEVELS AND FUNCTION
Several studies performed on humans undergoing hypothermic open-heart surgery failed to demonstrate significant alterations in clotting test times except at extreme degrees of hypothermia (i.e., <20° C).16,18–21 Yet, clinical experience suggests otherwise. Many patients with less severe degrees of hypothermia will have a serious coagulopathy that appears related to the presence of the hypothermia. This apparent inconsistency has been resolved by the realization that coagulation during mild hypothermia is disturbed more from enzymatic dysfunction than it is from altered clotting factor levels in blood. This explains the inability for the experimental data from the 1950s and 1960s to correlate with the clinical experience, as the clotting tests performed by the early experimenters were routinely performed at 37° C instead of at hypothermic temperatures.
In recent years, a number of studies have been performed wherein the clotting tests were performed at hypothermic temperatures. Bunker and Goldstein,18 in a study previously mentioned of controlled hypothermia in 10 patients, measured clotting tests at the hypothermic temperature of the patients as well as at 37° C. While they found no significant changes in clotting times when performed at 37° C, they state that “prolongation of the clotting times for all coagulation tests except whole blood clotting times was consistently observed when performed at the hypothermic temperatures.”
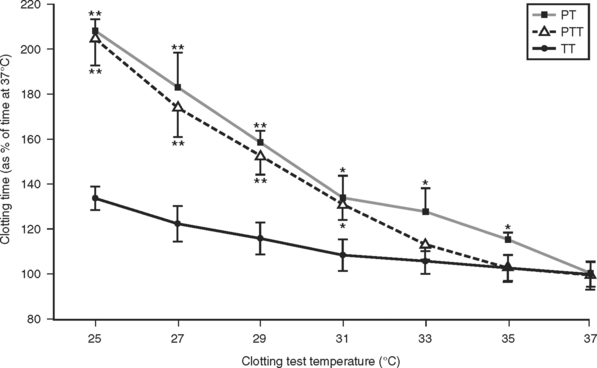
A detailed study of the kinetic effects of hypothermia on clotting factor function was undertaken by Reed et al.22 These studies were done by performing standardized clotting tests in a modified coagulation timer (fibrometer). Because the heat block of fibrometers used clinically are set by the manufacturer at 37° C, an external digital temperature controller was connected to the heat block power source to enable measurement of clotting times at the range of hypothermic temperatures typically encountered in trauma patients. Measurement of the prothrombin time, partial thromboplastin time, and thrombin time performed on assayed reference human plasma containing normal levels of all the clotting factors at temperatures ranging from 25° C to 37° C showed a significant slowing of clotting factor function that was proportional to the degree of hypothermia (Figure 1).
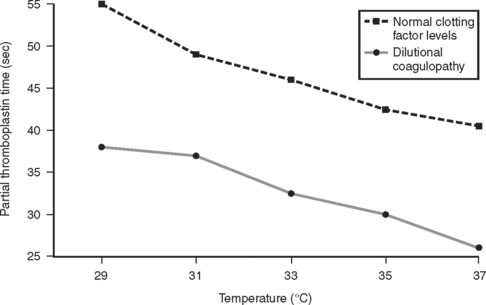
These results were later confirmed by Gubler et al.,23 in a study using a similar modified fibrometer that demonstrated an additive effect of hypothermia on dilutional coagulopathy (Figure 2).
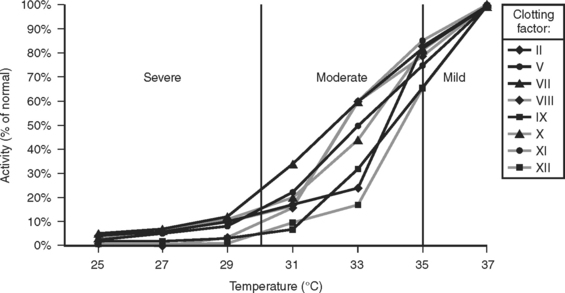
A subsequent study demonstrated that hypothermia could produce a coagulopathy functionally equivalent to a severe clotting factor deficiency, even at intermediate levels of hypothermia and even though there was no actual deficiency of clotting factors24 (Figure 3).
In summary, hypothermia does little to affect platelet and clotting factor levels, but it does a great deal to affect the function of these coagulation components. A recent analysis indicates that at mild temperature reductions between 33° C and 37° C, platelets are more profoundly affected than are clotting factors, although clotting factor dysfunction becomes increasingly severe as temperature cools further.25 Because of the potent effect that severe hypothermia has on platelet and clotting factor function, it is essential that body temperature be normalized before exogenous platelets or clotting factors are administered. Even though clotting studies may demonstrate severe clotting factor deficiencies, there is no value in transfusing coagulation components to severely hypothermic patients. This is because normal levels of clotting factors fail to clot effectively in the setting of severe hypothermia. Thus, administration of platelets or clotting factors to moderately or severely hypothermic patients is essentially futile, as the coagulation components will not function in a hypothermic environment (i.e., below 34° C).
EFFECTS ON OTHER ORGANS
The organ systems that are most commonly affected by hypothermia include the circulatory, immunologic, neurologic, and coagulation systems. Cardiac function can be affected by hypothermia in the form of bradyarrhythmias and, at a core temperature below 28° C–30° C, ventricular fibrillation. The body’s attempt at restoring normothermia results in an elevation of oxygen that takes place primarily in muscles through shivering. Because of the excessive oxygen consumption required to maintain or restore normothermia in an environment with significant cold stress, organ dysfunction can occur due to a relative undersupply of oxygen, with a resultant increased risk of cardiac complications in elderly patients.26
The potential immunologic consequences of hypothermia have been extensively studied. Because of the enzymatic nature of most immunologic functions, it makes sense that hypothermia would inhibit many of these processes. Moreover, our immunologic system is often pitted against bacteria that are not homeotherms as humans are, and may therefore not suffer as severe a functional deterioration in the presence of a hypothermic environment. Some relatively well-done clinical studies provide evidence that mild hypothermia is associated with increased risk for surgical site infection.27,28 Laboratory studies of the neuroprotective effects of hypothermia appeared promising, but clinical trials have been disappointing, and the immunologic effects of hypothermia were associated with an increase in pneumonia and septic complications.29–31
MANAGEMENT
The relatively high specific heat of the body makes hypothermia very difficult to treat. The rapidity and aggressiveness with which treatment is provided should be based on how severely the hypothermia is affecting the patient. There are a number of clinical studies that describe the efficacy of currently available rewarming techniques. However, many were conducted on healthy, nonvasoconstricted volunteers, and most did not take into account the patient’s initial body temperature and mass, the rate of endogenous heat production, and the presence or absence of anesthetics, vasodilating agents, shock, or shivering, all of which are important determinants of the rewarming rate.
Active External Rewarming
Standard fluid-circulating heating blankets are a commonly used external rewarming technique. Based upon observed rewarming rates in hypothermic patients, it has been estimated that roughly 2.5 kcal/hr per degree Celsius temperature difference between blanket and skin occurs.32 Roughly 25–35 kcal/hr of heat transfer can be expected, which is enough to rewarm body temperature by approximately 0.5° C/hr. Convective air rewarmers provide a larger surface area for heat exchange than fluid circulating heating blankets. However, the density of air is so low that it contains very little thermal energy. For example, one can tolerate a 150° F sauna for 10 minutes, but inserting a hand in 150° F water for 10 seconds results in an immediate scald injury.
The very low heat-carrying capacity of air means that little heat can be transferred to a patient by blowing warm air over the skin. However, an additional consequence of the laws of thermodynamics is that when two masses are in contact with one another, heat always flows from the area of higher temperature to the area of lower temperature, regardless of differences in heat content (law of entropy). The purpose of a convective warmer is to establish a microenvironment around the patient that is warmer than skin temperature. This prevents heat loss from the skin (except through sweating). These devices may be used to minimize heat loss from covered areas, but are ineffective means of treating hypothermia, and most of the actual warming that is observed is due to the patient’s own heat generation. In a randomized treatment study hypothermic patients did not warm faster with a convective heating blanket than with a standard cotton hospital blanket.33
Overhead radiant warmers can produce intense local heat in vasoconstricted patients if there is not enough circulation to carry the heat away, which can cause severe thermal injury. Patients must be fully exposed for radiant warming to occur. A blanket is often placed over the patient to diminish the risk of thermal injury, but radiant heat is then supplied only to the blanket, and the patient is warmed in a very inefficient manner by the air trapped underneath the blanket. Based on observed rewarming rates in hypothermic patients, Henneberg et al.34 have calculated an approximate heat transfer of 17.7 kcal/hr with the use of an overhead radiant warmer.
Active Core Rewarming
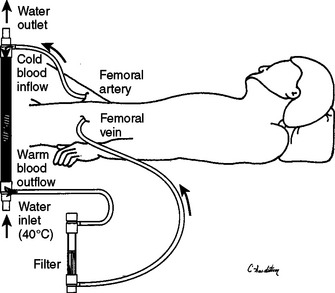
Rewarming with cardiopulmonary bypass is, in effect, a means of rewarming via the provision of a continuous infusion of warmed IV fluids. The limitations imposed by the patient’s fluid requirements are circumvented by recirculating the patient’s own blood. Continuous arteriovenous rewarming (CAVR) is a newly described means of performing extracorporeal circulatory rewarming that does not require a mechanical pump.6,35,36 CAVR uses percutaneously placed 8.5-Fr femoral arterial and venous lines and the patient’s own blood pressure to create an extracorporeal AV fistula through the heating mechanism of a counter current fluid warmer. The tubing circuit is heparin bonded, and no additional heparinization is needed (Figure 4).
Unlike cardiopulmonary bypass, this technique requires an intact circulation, and its effectiveness is limited when arterial pressure falls below 80 mm Hg. However, hypotensive patients generally require additional fluids, which can be “piggybacked” into the heat exchanger to supplement the fistula flow rate. The typical flow rate in normotensive individuals is between 250 and 350 ml/min. If the patient’s temperature is 32° C and blood is reinfused at a temperature of 39° C approximately 6 kcal of heat will be transferred every 3–4 minutes.
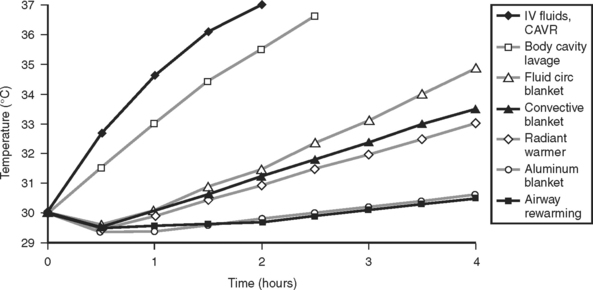
Rewarming efficacy can be analyzed using standard thermodynamic and heat transfer equations to provide a more accurate assessment various rewarming techniques. A mathematical model has been developed which takes into account body mass and surface area, the specific heat of tissues, the various conductivities of body tissues as a function of temperature, endogenous heat production, and the thermophysical properties of air, water, radiation, and other heat transfer media.37 A computer simulation provides the expected rewarming rates based on the properties of the technique used (Figure 5).
MORTALITY
Hypothermia has two well-known clinical effects: to preserve life and to kill. Which one of these properties is most active in the trauma patient has been debated for centuries. Hippocrates recommended packing injured soldiers in snow and ice. Baron de Larrey, a battlefield surgeon during Napoleon’s campaigns, noted that injured soldiers who sat closest to the fire were usually the first to die. Animal studies repeatedly demonstrate that hypothermic animals are better able to survive shock than normothermic counterparts.38,39
Despite these observations, current recommendations for treatment of injured patients call for strict efforts to prevent hypothermia, and for aggressive treatment to reverse it once it has occurred.40 These recommendations are based on findings of repeated clinical studies demonstrating that mortality is significantly higher in trauma patients who develop hypothermia.1,2,4,41 One study controlled for magnitude of injury using the Injury Severity Score (ISS), the presence or absence of shock, and fluid and blood product requirements. Patients who became hypothermic had significantly higher mortality rates than similarly injured patients who remained warm. Mortality was 100% if core body temperature dropped to 32° C, even in mildly injured patients.1 A large study analyzing the NTDB (National Trauma Data Bank) found that hypothermia was an independent predictor of mortality by using stepwise logistic regression (odds ratio 1.54, 95% CI 1.40-1.71) (Figure 6).4
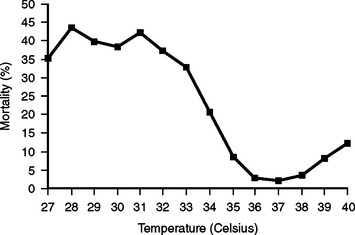
Figure 6 Mortality at each admission body temperature determined from the National Trauma Data Bank (NTDB).
One study compared the mortality of hypothermic patients (<35° C) admitted over a 10-month period who were treated with a combination of airway rewarming, fluid circulating or convective heating blankets, an aluminized head covering, and warm IV fluids with a consecutive sample of patients who were rapidly rewarmed with CAVR.6 Time to rewarming (T > 35° C) was 3.23 hours with standard rewarming techniques and 39 minutes with CAVR. Rapid rewarming with CAVR resulted in a 57% decrease in blood product requirements, a 67% decrease in crystalloid requirements, and a reduction in mortality in trauma patients. In a more recent randomized, prospective clinical trial comparing slow versus rapid rewarming in critically injured patients, significantly more patients in the rapid rewarming (CAVR) group were able to be successfully resuscitated.7 Two additional prospective, but nonrandomized studies have demonstrated improvements in outcome in trauma patients when protocols designed to minimize heat loss were utilized.42,43
1 Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27:1019-1024.
2 P Psarras, RR Ivatury & M Rohman et al Presented at the Eastern Association for the Surgery of Trauma, Longboat Key, Florida
3 Luna GK, Maier RV, Pavlin EG, Anardi D, Copass MK, Oreskovich MR. Incidence and effect of hypothermia in seriously injured patients. J Trauma. 1987;27(9):1014-1018.
4 Martin RS, Kilgo PD, Miller PR, Hoth JJ, Meredith JW, Chang MC. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24(2):114-118.
5 Britt LD, Dascombe WH, Rodriguez A. New horizons in management of hypothermia and frostbite injury. Surg Clin North Am. 1991;71:345-370.
6 Gentilello LM, Cobean RA, Offner PJ, Soderberg RW, Jurkovich GJ. Continuous arteriovenous rewarming: rapid reversal of hypothermia in critically ill patients. J Trauma. 1992;32:316-325. discussion 325–317
7 Gentilello LM, Jurkovich GJ, Stark MS, Hassantash SA, O’Keefe GE. Is hypothermia in the victim of major trauma protective or harmful? A randomized, prospective study. Ann Surg. 1997;226:439-447. discussion 447–439
8 Counts RB, Haisch C, Simon TL, Maxwell NG, Heimbach DM, Carrico CJ. Hemostasis in massively transfused trauma patients. Ann Surg. 1979;190(1):91-99.
9 Reed RL2nd, Ciavarella D, Heimbach DM, et al. Prophylactic platelet administration during massive transfusion. A prospective, randomized, double-blind clinical study. Ann Sur. 1986;203:40-48.
10 Villalobos T, Adelson E, Barila T. Hematologic changes in hypothermic dogs. Proc Soc Exp Biol Med. 1955;89:192-196.
11 Willson J, Miller W, Eliot T. Blood studies in the hypothermic dog. Surgery. 1958;43:979-989.
12 Helmsworth J, Stiles W, Elstun W. Leukopenic and thrombocytopenic effect of hypothermia in dogs. Proc Soc Exp Biol Med. 1955;90:474-476.
13 Helmsworth J, Stiles W, Elstun W. Changes in blood cellular elements in dogs during hypothermia. Surgery. 1955;38(5):843-846.
14 Couves C, Overton R, Eaton W. Hematologic changes in hypothermic dogs. Surg Forum. 1955;6:102-106.
15 Wensel R, Bigelow W. The use of heparin to minimize thrombocytopenia and bleeding tendency during hypothermia. Surgery. 1959;45:223-228.
16 Yoshihara H, Yamamoto T, Mihara H. Changes in coagulation and fibrinolysis occurring in dogs during hypothermia. Thromb Res. 1985;37:503-512.
17 Valeri C, Feingold H, Cassidy G, Ragno G, Khuri S, Altschule M. Hypothermia-induced reversible platelet dysfunction. Ann Surg. 1987;205(2):175-181.
18 Bunker J, Goldstein R. Coagulation during hypothermia in man. Proc Soc Exp Biol Med. 1958;97:199-202.
19 von Kaulla K, Swan H. Clotting deviations in man associated with open-heart surgery during hypothermia. J Thorac Surg. 1958;36(6):857-868.
20 Ahmad N, Agarwal GP, Dube RK. Comparative studies of blood coagulation in hibernating and non-hibernating frogs. (Rana tigrina). Thromb Haemostas (Stuttg). 1979;42:959-964.
21 Bahn S, Mursch P. The effects of cold on hemostasis. Oral Surg Oral Med Oral Pathol. 1980;49:294-300.
22 Reed R, Bracey A, Hudson J, Miller T, Fischer R. Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141-152.
23 Gubler K, Gentilello L, Hassantash S, Maier R. The impact of hypothermia on dilutional coagulopathy. J Trauma. 1994;36:847-851.
24 Johnston T, Chen Y, Reed R. Functional equivalence of hypothermia to specific clotting factor deficiencies. J Trauma. 1994;37:413-417.
25 Wolberg A, Meng Z, Monroe D, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56(6):1221-1228.
26 Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127-1134.
27 Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209-1215.
28 Flores-Maldonado A, Medina-Escobedo CE, Rios-Rodriguez HM, Fernandez-Dominguez R. Mild perioperative hypothermia and the risk of wound infection. Arch Med Res. 2001;32(3):227-231.
29 Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
30 Shiozaki T, Hayakata T, Taneda M, et al. A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J Neurosurg. 2001;94:50-54.
31 Shiozaki T, Nakajima Y, Taneda M, et al. Efficacy of moderate hypothermia in patients with severe head injury and intracranial hypertension refractory to mild hypothermia. J Neurosurg. 2003;99(1):47-51.
32 Morrison RC. Hypothermia in the elderly. Int Anesthesiol Clin. 1988;26(2):124-133.
33 Ereth MH, Lennon RL, Sessler DI. Limited heat transfer between thermal compartments during rewarming in vasoconstricted patients. Aviat Space Environ Med. 1992;63(12):1065-1069.
34 Henneberg S, Eklund A, Joachimsson PO, Stjernstrom H, Wiklund L. Effects of a thermal ceiling on postoperative hypothermia. Acta Anaesthesiol Scand. 1985;29(6):602-606.
35 Gentilello LM, Cortes V, Moujaes S, et al. Continuous arteriovenous rewarming: experimental results and thermodynamic model simulation of treatment for hypothermia. J Trauma. 1990;30(12):1436-1449.
36 Gentilello LM, Rifley WJ. Continuous arteriovenous rewarming: report of a new technique for treating hypothermia. J Trauma. 1991;31:1151-1154.
37 Gentilello LM, Moujaes S. Treatment of hypothermia in trauma victims: thermodynamic considerations. J Intensive Care Med. 1995;10(1):5-14.
38 Wu X, Kochanek PM, Cochran K, et al. Mild hypothermia improves survival after prolonged, traumatic hemorrhagic shock in pigs. J Trauma. 2005;59(2):291-299. discussion 299–301
39 Tisherman SA, Safar P, Radovsky A, Peitzman A, Sterz F, Kuboyama K. Therapeutic deep hypothermic circulatory arrest in dogs: a resuscitation modality for hemorrhagic shock with ‘irreparable’ injury. J Trauma. 1990;30:836-847.
40 American College of Surgeons. Advanced Trauma Life Support, 7th ed. Chicago: American College of Surgeons, 2005.
41 Tyburski JG, Wilson RF, Dente C, Steffes C, Carlin AM. Factors affecting mortality rates in patients with abdominal vascular injuries. J Trauma. 2001;50:1020-1026.
42 Satiani B, Fried SJ, Zeeb P, Falcone RE. Normothermic rapid volume replacement in traumatic hypovolemia. A prospective analysis using a new device. Arch Surg. 1987;122:1044-1047.
43 Satiani B, Fried SJ, Zeeb P, Falcone RE. Normothermic rapid volume replacement in vascular catastrophes using the Infuser 37. Ann Vasc Surg. 1988;2:37-42.