Chapter 31 Hypoplastic Left Heart
In 1952, Lev1 called attention to congenital hypoplasia of major components of the left side of the heart. In 1958, Noonan and Nadas2 referred to these malformations as the hypoplastic left heart syndrome. At the severe end of the spectrum, the aortic and mitral valves are atretic, and the left ventricle is virtually nonexistent (Figure 31-1).3,4 At the mild end of the spectrum, the aortic and mitral valves are patent, and there is a lesser degree of left ventricular hypoplasia (Figure 31-2).4 The hypoplastic left heart syndrome is a genetically heterogeneous disorder that affects 1 in 5000 live births5 and accounts for 7.5% of infants with congenital heart disease.6
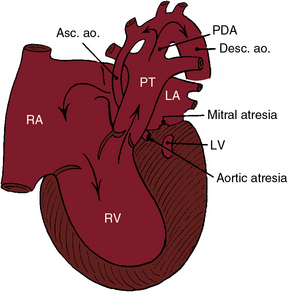
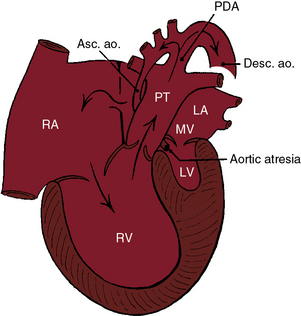
Figure 31-1 Illustration of the essential anatomic and physiologic derangements of a hypoplastic left heart with aortic atresia and mitral atresia. The left ventricular cavity (LV) is a rudimentary blind slit. The physiologic derangements are as described in Figure 31-2. (PDA = patent ductus arteriosus; ASC. ao. = ascending aorta; Desc. ao. = descending aorta.)
Aortic atresia is accompanied by a hypoplastic ascending aorta that serves as a common coronary artery (see Figures 31-1 and 31-2).7 A hypoplastic but patent mitral valve is accompanied by a hypoplastic but patent left ventricle (see Figure 31-2). Mitral atresia is accompanied by a blind slit-like left ventricular cavity embedded in the ventricular muscle (see Figure 31-1). An experimental model in chick embryos is represented by atresia or hypoplasia of the mitral valve and of the left ventricle, aortic valve, and thoracic aorta.8 Hypoplasia or atresia of the mitral valve leaves the left atrium without an exit except a restrictive patent foramen ovale (see Figures 31-1 and 31-2), which is further compromised by hypoplasia of the limbus that is rotated and deviated close to the orifice of the superior vena cava.9 An intact atrial septum is accompanied by either a thick muscular septal wall and a small left atrium or by a thick septum secundum, a thin septum primum, and an enlarged left atrium.10 Alternative decompression pathways for the obstructed left atrium consist of vascular channels from a levoatrial cardinal vein to the innominate vein, an accessory vein from left atrium to the superior vena cava, a venous connection from left atrium to hepatic veins, a coronary venous connection from left atrium to coronary sinus, and a coronary sinoseptal defect.10
Vasoconstriction during early embryogenesis leads to decreased growth and development of pulmonary veins and to alveolar capillary dysplasia, which forces arterial blood to bypass the deficient capillary bed and drain through anomalous bronchial veins.11 Lymphatics are strikingly enlarged.10 Dilated pulmonary veins are thick and arterialized with multiple elastic laminae.10
The subject of the first section of this chapter is aortic atresia with a hypoplastic but perforate mitral valve (see Figure 31-2). The second section deals with aortic atresia and mitral atresia (Figure 31-3).
Aortic atresia with hypoplastic but perforate mitral valve
The pathway to the systemic circulation is a single arterial trunk represented by pulmonary artery/ductus/descending aortic continuity (see Figure 31-2). A hypoplastic ascending aorta serves as a common coronary artery.3,12 Fifty percent to 75% of cases are accompanied by moderate coarctation of the aorta13 located either proximal to the ductus (preductal) or distal to the junction of the ductus and aortic arch (paraductal; see Figure 31-3).13 The right ventricle is hypertrophied because it is the sole pumping chamber for the systemic and pulmonary circulations.3 It harbors histologic changes of ischemia and infarction.14 The blind hypoplastic left ventricle is thick-walled and lined with endocardial fibroelastosis.2,3 Rarely, the left ventricle is characterized by isolated apical hypoplasia with fatty replacement.15 Isovolumetric contraction causes myofiber disarray but does not cause direct ventriculocoronary artery communications.16 Pinpoint neonatal aortic stenosis with small left ventricular cavity (see Chapter 7) is associated with intramyocardial sinusoids but not with direct ventriculocoronary arterial communications. The left ventricle is adequately formed in the presence of a ventricular septal defect and a patent aortic valve.3,17
The tricuspid valve is abnormal in a distinct minority of patients.18 The leaflets may be dysplastic with nodular free edges, shortened chordae tendineae, obliterated interchordal spaces, and an accessory orifice, and only two leaflets may be identifiable.18
A major concern in hypoplastic left heart syndrome is the brain that is smaller and structurally less mature than normal.19 The scimitar syndrome has been reported in a child with a hypoplastic left heart.20
The coronary circulation has been a matter of lively interest.2,3,7,14,21,22 A hypoplastic ascending aorta functions as a common coronary artery that receives retrograde systolic and diastolic flow from the patent ductus (see Figures 31-2 and 31-3).2,3,14 The tubular, hypoplastic ascending aorta is not an impediment to retrograde flow into the common coronary artery, but the preductal coarctation (see Figure 31-3) is an impediment.14 Intramyocardial coronary abnormalities analogous to those of pulmonary atresia with intact ventricular septum can be anticipated (see Chapter 24) because isovolumetric contraction generates excessive systolic pressure that acts as a driving force for direct ventriculocoronary arterial communications.7 However, the differences between pulmonary atresia with intact ventricular septum and aortic atresia with a hypoplastic but patent mitral valve are as great as the similarities.7 Myocardial sinusoids consist of restrictive vascular networks that spare the coronary arteries from the impact of high ventricular systolic pressure delivered through direct ventriculocoronary arterial communications.7 In aortic atresia with a hypoplastic but patent mitral valve (see Figure 31-2), the intramyocardial communications are sinusoidal.7 Accordingly, epicardial and subepicardial coronary arteries do not receive the impact of high isovolumetric systolic pressure and are spared the luminal obliterative features of pulmonary atresia with intact ventricular septum (see Chapter 24).7
The physiologic consequences of hypoplastic left heart with aortic atresia and a hypoplastic but perforate mitral valve are determined by the size of the ductus arteriosus, the pulmonary vascular resistance, and the condition of the atrial septum. Constriction of the ductus compromises flow into the systemic circulation and into the hypoplastic ascending aorta, which functions as a common coronary artery (see previous). Right ventricular function suffers because of the ischemic effects of inadequate coronary blood flow,14,23 because of pulmonary hypertension caused by the nonrestrictive ductus, because of high pressure in the obstructed left atrium, and because a large left ventricular mass has disadvantageous effects on right ventricular end-diastolic volume and right ventricular wall motion. Competence of the tricuspid valve is important for survival, yet tricuspid dysplasia with a multiple papillary muscles is common (see previous). Low pulmonary vascular resistance permits increased pulmonary arterial blood flow that is received by the obstructed left atrium from which the only effective egress is a restrictive patent foramen ovale (see previous; see Figure 31-2). In the presence of an adequate interatrial communication, increased pulmonary blood flow makes a large volume of oxygenated left atrial blood available for mixing in the right atrium, so systemic arterial oxygen saturation is relatively high. However, preferential blood flow into the lungs through the ductus is accompanied by a reciprocal fall in systemic blood flow and a shock-like state. When pulmonary vascular resistance is high, systemic blood flow is maintained at the price of increasing cyanosis.
History
Hypoplastic left heart comprises 7% to 8% of symptomatic heart disease in the first year of life24 and is responsible for 25% of cardiac deaths in the first week of life.6 The malformation has been called the most malignant form of congenital heart disease, a conclusion underscored by an average lifespan of only 5 to 14 days.3,6,25 Precarious survival depends on three tenuous variables: patency of the ductus arteriosus, pulmonary vascular resistance, and an adequate interatrial communication.26 Tachypnea, tachycardia, and cyanosis are present during the brief interval of ductal patency.24 Risk is greatest during the period of normal ductal closure when systemic blood flow and coronary blood flow decrease or cease altogether. A fall in pulmonary vascular resistance diverts blood from the systemic circulation into the pulmonary circulation and augments flow into the obstructed left atrium. A rise in pulmonary vascular resistance improves systemic blood flow, but at the price of hypoxemia. Ninety-five percent of afflicted infants die within the first month of life.6,27 Two extraordinary survivals include a 22-year-old woman with a large patent ductus arteriosus and an adequate-sized atrial septal defect and a 24-year-old man with a patent ductus arteriosus and a ventricular septal defect.28
There is male prevalence of 55% to 70% in aortic atresia with a hypoplastic but perforate mitral valve.2,3,29 Maternal age tends to be above average, with a mean of 31 years. First-degree relatives of probands have an increased prevalence of congenital heart disease.24 The malformation is occasionally familial30,31 and has been reported in siblings as an autosomal recessive inheritance.32 A mosaic chromosomal 22q11 deletion is associated with hypoplastic left heart,33,34 and genetic disorders include Turner’s syndrome (see subsequent), trisomy 13, trisomy 18, and trisomy 21.35–37 Concordance has been reported in a monochromic twin pregnancy.38
Reports of geographic clustering implicate environmental factors.39 Eastern Wisconsin has increased rates of hypoplastic left heart syndrome,40,41 a region in Baltimore has twice the expected frequency,42 and the island of Malta has a decreased incidence.43
Hypoxemia and hypotension are associated with hypoxic-ischemic cerebral injury and intracranial hemorrhage.44 Major and minor congenital central nervous system abnormalities include microcephaly, microencephaly, abnormal cortical mantle, agenesis of the corpus callosum, and holoprosencephaly.45 The overall frequency of extracardiac anomalies, including central nervous system abnormalities, is 12% to 37%.35
Physical Appearance
The hypoplastic left heart syndrome can be associated with Turner’s syndrome (see Chapter 8)35–37,46; the Rubenstein-Taybi syndrome, which is a Mendelian dominant disorder characterized by mental retardation, growth retardation, typical facies, cognitive defects, broad thumbs and toes, and short stature47,48; the abdominal distension of Hirschsprung’s disease49; and the Kubuki syndrome, characterized by eversion of the lower lateral eyelids, arched eyebrows with the lateral one third dispersed or sparse, depressed nasal tip, prominent ears, skeletal anomalies, short stature, and mental retardation.50
Arterial Pulse, Jugular Venous Pulse, and Precordial Palpation
Brachial and carotid arterial pulses remain palpable because the ascending aortic tubular hypoplasia does not include the arch (see Figures 31-1 and 31-2).2 Femoral artery pulses are palpable because of pulmonary trunk/ductal/descending aortic continuity (see Figures 31-1 and 31-2) and because coarctation is usually paraductal or mild preductal (see Figure 31-3). When low pulmonary vascular resistance diverts blood from the systemic circulation, all arterial pulses diminish, if not vanish.
Auscultation
Auscultatory signs are unimpressive in contrast to the dramatic clinical picture. Murmurs are usually absent (Figure 31-4),2,24 or a soft midsystolic murmur is generated by ejection into the dilated hypertensive pulmonary trunk. A long systolic murmur is likely to be caused by tricuspid regurgitation. The second heart sound is single because the aortic valve is atretic and is loud because of pulmonary hypertension (see Figure 31-4). A triple rhythm is caused by summation of right ventricular third and fourth heart sounds (see Figure 31-4).
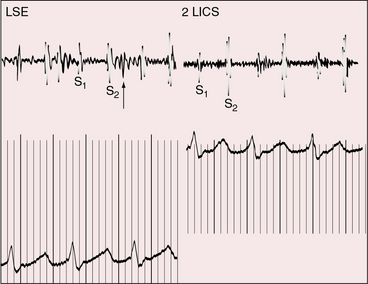
Figure 31-4 Phonocardiograms from a 9-day-old male with aortic atresia and a hypoplastic but perforate mitral valve. Necropsy findings were as illustrated in Figure 31-2. A loud filling sound (unmarked arrow) with after-vibrations at the lower left sternal edge (LSE) was caused by summation of right ventricular third and fourth heart sounds. The second heart sound (S2) in the second left intercostal space (2 LICS) was loud because of pulmonary hypertension and single because the aortic valve was atretic. (S1 = first heart sound.)
Electrocardiogram
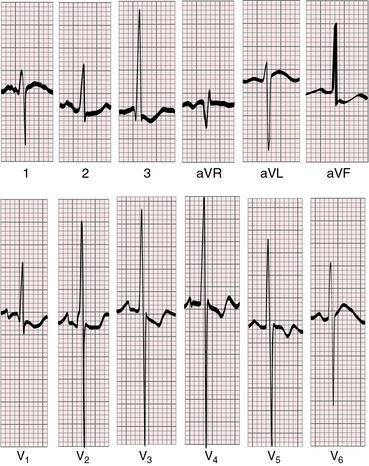
Tall peaked right atrial P waves are common (Figures 31-5 and 31-6) but not invariable (Figure 31-7). The PR interval is occasionally prolonged (see Figure 31-5). Left axis deviation is uncommon despite abnormalities of the left bundle branch, and left bundle branch block is unknown.2,51 Alterations in the branching portion of the His bundle and the left bundle branch depend on the size of the left ventricular cavity, with major changes reserved for absence or virtual absence of a cavity.52 When the left ventricular cavity is small but not slit-like, the left bundle branch has a peripheral Purkinje network and the right bundle branch is normal in size.52 Wolff-Parkinson-White bypass tracks are rare even though there are persistent connections of the left bundle branch to ventricular septal musculature with abundant Mahaim fibers.52
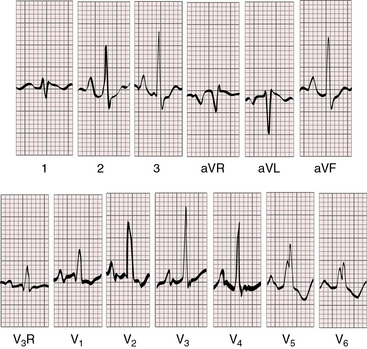
Figure 31-5 Electrocardiogram from the 9-day-old male with aortic atresia and a hypoplastic but perforate mitral valve. The phonocardiogram is shown in Figure 31-4. Tall peaked right atrial P waves are present in leads 2, 3, aVF, and V2-4. Right atrial enlargement is indicated by qR complexes in leads V3R and V1. The PR interval is prolonged. Right ventricular hypertrophy is reflected in the tall monophasic R waves in right and mid precordial leads and the vertical QRS axis. The splintered QRS in leads V5-6 is the result of a right ventricular conduction defect. The left ventricle is not represented.
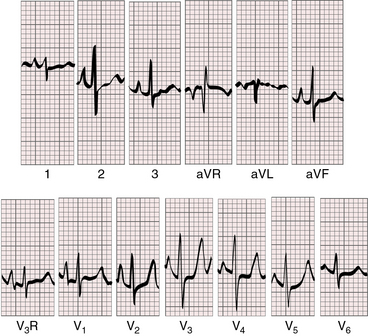
Figure 31-6 Electrocardiogram from a 3-week-old male with aortic atresia and a hypoplastic but perforate mitral valve. Necropsy findings were as illustrated in Figure 31-2. Tall peaked right atrial P waves are present in leads 2, 3, aVF, and V1-3. Right ventricular dominance is indicated by the prominent R waves in leads V2-4, the upright T waves in right precordial leads, and the rightward QRS axis.
The QRS reflects pure right ventricular hypertrophy (see Figures 31-5 and 31-6). Left ventricular hypertrophy is infrequent despite an increase in mass because of the effect of a low end-diastolic volume on the magnitude of the QRS.53 Left ventricular forces are absent when the cavity is small but may be present when the cavity is more adequately developed (see Figure 31-7).
X-Ray
Dilation of the right atrium and right ventricle is responsible for cardiac enlargement (Figure 31-8).54 The shadow of the hypoplastic ascending aorta is necessarily absent, and the shadow of the dilated hypertensive pulmonary trunk is necessarily prominent (see Figure 31-8). Pulmonary venous congestion is the rule,54 but the amount of lung available for assessment may be limited by remarkable cardiomegaly, even in the first 24 to 48 hours of life.
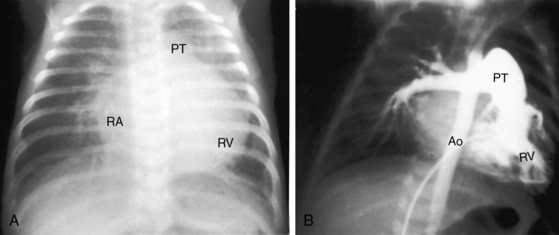
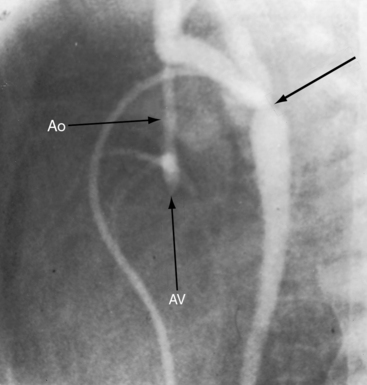
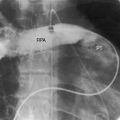
Figure 31-8 X-ray and angiocardiogram from a 2-day-old male with aortic atresia and a hypoplastic but perforate mitral valve. Necropsy findings were as illustrated in Figure 31-2. A, Pulmonary venous congestion is prominent. The right atrium (RA) and right ventricle (RV) are enlarged, the pulmonary trunk (PT) is dilated, and the ascending aorta is conspicuously absent. B, The right ventricular (RV) angiocardiogram visualizes the large pulmonary trunk (PT) with pulmonary artery/ductus /descending aortic continuity. (Ao = descending aorta.)
Echocardiogram
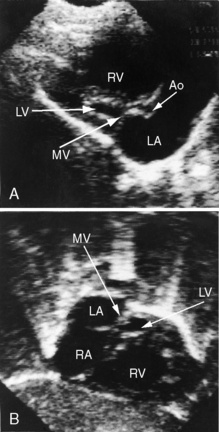
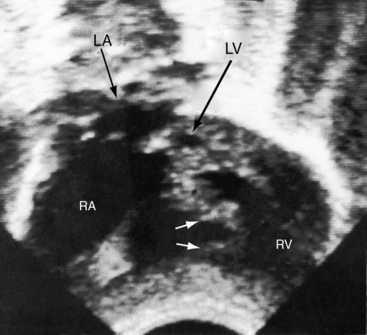
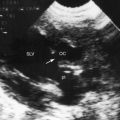
Echocardiography with color flow imaging and Doppler interrogation establishes the diagnosis of hypoplastic left heart with aortic atresia and a hypoplastic but perforate mitral valve.55 Fetal echocardiography permits the diagnosis as early as the 24th week of gestation.56 Flow patterns in the fetal ductus can be monitored,57 and the condition of the atrial septum can be determined. A hypoplastic but perforate mitral valve communicates with a small left ventricle that gives rise to an atretic aortic valve and a tubular hypoplastic ascending aorta (Figure 31-9). The ventricular septum and the free wall of the hypoplastic left ventricle are thick and immobile, and the small cavity is lined with endocardial fibroelastosis (see Figure 31-9). Coarctation is identified as a thin discrete posterior ledge extending across the lumen of the aorta at the level of the ductus arteriosus or as kinking and narrowing at the site of ductal insertion. The left atrium is small and thick-walled (see Figure 31-9). Function of the enlarged hypertrophied right ventricle and competence of the tricuspid valve can be established (see Figure 31-9),58,59 and ductus/pulmonary trunk/descending aortic continuity is confirmed with color flow imaging. Doppler interrogation establishes retrograde flow into the hypoplastic ascending aorta and occasionally identifies biphasic flow in the proximal coronary arteries. Direct ventriculocoronary arterial communications can be identified.60
Hypoplastic left heart with aortic and mitral atresia
The major physiologic derangements are similar to those of aortic atresia with a hypoplastic but perforate mitral valve, but there are anatomic differences and subtle clinical differences. The attachment of the valve of the fossa ovalis may be malaligned relative to the muscular rim of the fossa. Mitral atresia is represented by an imperforate macroscopic membrane on the floor of the left atrium or a microscopic fibrous remnant between the floor of the left atrium and a putative left ventricular cavity represented by a minute blind slit (see Figure 31-1).3,61,62 Myofiber disarray does not occur because isovolumetric contraction does not occur.3,7,62 Because there is no left ventricular cavity to develop excessive systolic pressure, ventriculocoronary arterial connections do not develop, and epicardial and subepicardial coronary arteries are neither thickened nor tortuous.7 A ventricular septal defect or absence of the right ventricular sinus is associated with a well-formed left ventricle.3 The physiologic consequences are the same, and longevity patterns are similar,63 whether aortic atresia is accompanied by mitral atresia or by a hypoplastic but perforate mitral valve (see Figures 31-1 and 31-2). Male gender predominates, although female twins have been reported.29
Subtle clinical points favor hypoplastic left heart with mitral atresia. The electrocardiogram shows pure right ventricular hypertrophy devoid of left ventricular potentials because a left ventricular cavity does not exist (Figure 31-10).53 The left bundle branch does not develop a Purkinje network because there is no left ventricular cavity in which to do so.52 Mahaim fibers are the only extensions of the left bundle branch that maintain continuity with ventricular septal muscle.52 The right bundle branch enlarges because it is responsible for conduction to virtually all of the ventricular muscle mass.52 These unusual pathways of atrioventricular conduction and ventricular activation are not represented in the electrocardiogram. The echocardiogram identifies atresia of both the aortic and mitral valves and occasionally identifies the blind rudimentary left ventricular cavity (Figure 31-11).
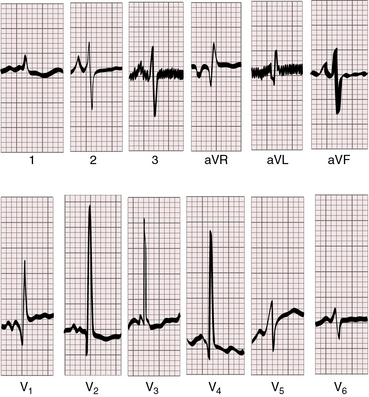
Figure 31-10 Electrocardiogram from a 3-week-old male with aortic atresia and mitral atresia. Necropsy findings were as illustrated in Figure 31-1. Peaked right atrial P waves are present in leads 2 and 3. Right atrial enlargement is indicated by the qR pattern in leads V1. Pure right ventricular hypertrophy is reflected in tall monophasic R waves in leads V2-4 and the vertical and superior QRS axis.
1 Lev M. Pathologic anatomy and interrelationship of hypoplasia of the aortic tract complexes. Lab Invest. 1952;1:61-70.
2 Noonan J.A., Nadas A.S. The hypoplastic left heart syndrome; an analysis of 101 cases. Pediatr Clin North Am. 1958;5:1029-1056.
3 Roberts W.C., Perry L.W., Chandra R.S., Myers G.E., Shapiro S.R., Scott L.P. Aortic valve atresia: a new classification based on necropsy study of 73 cases. Am J Cardiol. 1976;37:753-756.
4 Tchervenkov C.I., Jacobs J.P., Weinberg P.M., et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol Young. 2006;16:339-368.
5 Bondy C.A. Hypoplastic left heart syndrome. N Engl J Med. 2010;362:2026-2028.
6 Fyler D.C. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:375-461.
7 Baffa J.M., Chen S.L., Guttenberg M.E., Norwood W.I., Weinberg P.M. Coronary artery abnormalities and right ventricular histology in hypoplastic left heart syndrome. J Am Coll Cardiol. 1992;20:350-358.
8 Harh J.Y., Paul M.H., Gallen W.J., Friedberg D.Z., Kaplan S. Experimental production of hypoplastic left heart syndrome in the chick embryo. Am J Cardiol. 1973;31:51-56.
9 Remmell-Dow D.R., Bharati S., Davis J.T., Lev M., Allen H.D. Hypoplasia of the eustachian valve and abnormal orientation of the limbus of the foramen ovale in hypoplastic left heart syndrome. Am Heart J. 1995;130:148-152.
10 Rychik J., Rome J.J., Collins M.H., Decampli W.M., Spray T.L. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol. 1999;34:554-560.
11 Rabah R., Poulik J.M. Congenital alveolar capillary dysplasia with misalignment of pulmonary veins associated with hypoplastic left heart syndrome. Pediatr Dev Pathol. 2001;4:167-174.
12 Lopez W. Aortic atresia without significant hypoplasia of the ascending aorta. Am J Roentgenol Radium Ther Nucl Med. 1964;92:888-892.
13 Elzenga N.J., Gittenberger-De Groot A.C. Coarctation and related aortic arch anomalies in hypoplastic left heart syndrome. Int J Cardiol. 1985;8:379-393.
14 Lloyd T.R., Marvin W.J.Jr. Age at death in the hypoplastic left heart syndrome: multivariate analysis and importance of the coronary arteries. Am Heart J. 1989;117:1337-1343.
15 Flett A.S., Elliott P.M., Moon J.C. Images in cardiovascular medicine. Cardiovascular magnetic resonance of isolated left ventricular apical hypoplasia. Circulation. 2008;117:e504-e505.
16 Bulkley B.H., Weisfeldt M.L., Hutchins G.M. Isometric cardiac contraction. a possible cause of the disorganized myocardial pattern of idiopathic hypertrophic subaortic stenosis. N Engl J Med. 1977;296:135-139.
17 Pellegrino P.A., Thiene G. Aortic valve atresia with a normally developed left ventricle. Chest. 1976;69:121-122.
18 Martinez R. Assessment of the tricuspid valve in hypoplastic left heart syndrome. Cardiol Young. 2004;14(suppl 1):27-33.
19 Licht D.J., Shera D.M., Clancy R.R., et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529-536. discussion 536–527
20 Mcbride M.E., Huddleston C.B., Balzer D.T., Goel D., Gazit A.Z. Hypoplastic left heart associated with scimitar syndrome. Pediatr Cardiol. 2009;30:1037-1038.
21 Raghib G., Bloemendaal R.D., Kanjuh V.I., Edwards J.E. Aortic atresia and premature closure of foramen ovale. Myocardial sinusoids and coronary arteriovenous fistula serving as outflow channel. Am Heart J. 1965;70:476-480.
22 Roberson D.A., Cui W., Cuneo B.F., Van Bergen A.H., Javois A.J., Bharati S. Extensive left ventricular to coronary artery connections in hypoplastic left heart syndrome. Echocardiography. 2008;25:529-533.
23 Van Der Horst R.L., Hastreiter A.R. Ascending aortic obstruction of retrograde coronary blood flow in aortic atresia. Am Heart J. 1981;101:345-346.
24 Bailey L.L., Gundry S.R. Hypoplastic left heart syndrome. Pediatr Clin North Am. 1990;37:137-150.
25 Cohen D.M., Allen H.D. New developments in the treatment of hypoplastic left heart syndrome. Curr Opin Cardiol. 1997;12:44-50.
26 Hoshino K., Ogawa K., Hishitani T., Kitazawa R., Uehara R. Hypoplastic left heart syndrome: duration of survival without surgical intervention. Am Heart J. 1999;137:535-542.
27 Barron D.J., Kilby M.D., Davies B., Wright J.G.C., Jones T.J., Brawn W.J. Hypoplastic left heart syndrome. Lancet. 2009;374:551-564.
28 Maxwell P., Somerville J. Aortic atresia: survival to adulthood without surgery. Br Heart J. 1990;64:336-337.
29 Bjornstad P.G., Michalsen H. Coexistent mitral and aortic valve atresia with intact ventricular septum in sibs. Br Heart J. 1974;36:302-306.
30 Kojima H., Ogimi Y., Mizutani K., Nishimura Y. Hypoplastic-left-heart syndrome in siblings. Lancet. 1969;2:701.
31 Rao S.S., Gootman N., Platt N. Familial aortic atresia. Report of a case of aortic atresia in siblings. Am J Dis Child. 1969;118:919-922.
32 Grobman W., Pergament E. Isolated hypoplastic left heart syndrome in three siblings. Obstet Gynecol. 1996;88:673-675.
33 Grossfeld P.D. The genetics of hypoplastic left heart syndrome. Cardiol Young. 1999;9:627-632.
34 Consevage M.W., Seip J.R., Belchis D.A., Davis A.T., Baylen B.G., Rogan P.K. Association of a mosaic chromosomal 22q11 deletion with hypoplastic left heart syndrome. Am J Cardiol. 1996;77:1023-1025.
35 Natowicz M., Chatten J., Clancy R., et al. Genetic disorders and major extracardiac anomalies associated with the hypoplastic left heart syndrome. Pediatrics. 1988;82:698-706.
36 Natowicz M., Kelley R.I. Association of Turner syndrome with hypoplastic left-heart syndrome. Am J Dis Child. 1987;141:218-220.
37 Van Egmond H., Orye E., Praet M., Coppens M., Devloo-Blancquaert A. Hypoplastic left heart syndrome and 45X karyotype. Br Heart J. 1988;60:69-71.
38 Andrews R.E., Cook A.C., Yates R.W.M. Concordance for hypoplastic left heart syndrome in a monochorionic twin pregnancy. Heart. 2003;89:e13.
39 Morris C.D., Outcalt J., Menashe V.D. Hypoplastic left heart syndrome: natural history in a geographically defined population. Pediatrics. 1990;85:977-983.
40 Davies B., D’Udekem Y., Ukoumunne O.C., Algar E.M., Newgreen D.F., Brizard C.P. Differences in extra-cellular matrix and myocyte homeostasis between the neonatal right ventricle in hypoplastic left heart syndrome and truncus arteriosus. Eur J Cardiothorac Surg. 2008;34:738-744.
41 Cronk C.E., Pelech A.N., Malloy M.E., Mccarver D.G. Excess birth prevalence of Hypoplastic Left Heart syndrome in eastern Wisconsin for birth cohorts 1997–1999. Birth Defects Research. 2004;70:114-120.
42 Kuehl K.S., Loffredo C.A. A cluster of hypoplastic left heart malformation in Baltimore, Maryland. Pediatr Cardiol. 2006;27:25-31.
43 Grech V. Decreased prevalence of hypoplastic left heart syndrome in Malta. Pediatr Cardiol. 1999;20:355-357.
44 Glauser T.A., Rorke L.B., Weinberg P.M., Clancy R.R. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:991-1000.
45 Glauser T.A., Rorke L.B., Weinberg P.M., Clancy R.R. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:984-990.
46 Reis P.M., Punch M.R., Bove E.L., Van De Ven C.J. Outcome of infants with hypoplastic left heart and Turner syndromes. Obstet Gynecol. 1999;93:532-535.
47 Bartsch O., Wagner A., Hinkel G.K., et al. FISH studies in 45 patients with Rubinstein-Taybi syndrome: deletions associated with polysplenia, hypoplastic left heart and death in infancy. Eur J Hum Genet. 1999;7:748-756.
48 Hanauer D., Argilla M., Wallerstein R. Rubinstein-Taybi syndrome and hypoplastic left heart. Am J Med Genet. 2002;112:109-111.
49 Ahola J.-A., Koivusalo A., Sairanen H., Jokinen E., Rintala R.J., Pakarinen M.P. Increased incidence of Hirschsprung’s disease in patients with hypoplastic left heart syndrome—a common neural crest-derived etiology? J Pediatr Surg. 2009;44:1396-1400.
50 Kung G.C., Chang P.M., Sklansky M.S., Randolph L.M. Hypoplastic left heart syndrome in patients with Kabuki syndrome. Pediatr Cardiol. 2010;31:138-141.
51 Soloff L.A. Congenital aortic atresia; report of the first case with left axis deviation of the electrocardiogram. Am Heart J. 1949;37:123-128.
52 Bharati S., Lev M. The conduction system in hypoplasia of the aortic tract complex. Circulation. 1979;59:1324-1332.
53 Voukydis P.C. Effect of intracardiac blood on the electrocardiogram. N Engl J Med. 1974;291:612-616.
54 Bardo D.M., Frankel D.G., Applegate K.E., Murphy D.J., Saneto R.P. Hypoplastic left heart syndrome. Radiographics. 2001;21:705-717.
55 Ludman P., Foale R., Alexander N., Nihoyannopoulos P. Cross sectional echocardiographic identification of hypoplastic left heart syndrome and differentiation from other causes of right ventricular overload. Br Heart J. 1990;63:355-361.
56 Simpson J.M. Hypoplastic left heart syndrome. Ultrasound Obstet Gynecol. 2000;15:271-278.
57 Rychik J., Gullquist S.D., Jacobs M.L., Norwood W.I. Doppler echocardiographic analysis of flow in the ductus arteriosus of infants with hypoplastic left heart syndrome: relationship of flow patterns to systemic oxygenation and size of interatrial communication. J Am Soc Echocardiogr. 1996;9:166-173.
58 Kimball T.R., Witt S.A., Khoury P.R., Daniels S.R. Automated echocardiographic analysis of systemic ventricular performance in hypoplastic left heart syndrome. J Am Soc Echocardiogr. 1996;9:629-636.
59 Altmann K., Printz B.F., Solowiejczky D.E., Gersony W.M., Quaegebeur J., Apfel H.D. Two-dimensional echocardiographic assessment of right ventricular function as a predictor of outcome in hypoplastic left heart syndrome. Am J Cardiol. 2000;86:964-968.
60 Bensky A.S., Covitz W. Echocardiographic demonstration of a ventriculocoronary artery communication in a neonate with hypoplastic left heart syndrome. J Am Soc Echocardiogr. 1994;7:324-326.
61 Gittenberger-De Groot A.C., Wenink A.C. Mitral atresia. Morphological details. Br Heart J. 1984;51:252-258.
62 Kanjuh V., Eliot R.S., Edwards J.E. Coexistent mitral and aortic valvular atresia: a pathologic study of 14 cases. Am J Cardiol. 1965;15:611-621.
63 Ehrlich M., Bierman F.Z., Ellis K., Gersony W.M. Hypoplastic left heart syndrome: report of a unique survivor. J Am Coll Cardiol. 1986;7:361-365.