163 Hypoglycemia
• Every patient with an acute neurologic abnormality must be evaluated for hypoglycemia by rapid bedside measurement of the blood glucose concentration.
• The most common cause of hypoglycemia is exogenous insulin or sulfonylurea use as a treatment of diabetes, although hypoglycemia can be the initial feature of a large array of serious illnesses, including sepsis, liver disease, renal disease, and cancer.
• A blood glucose concentration lower than 60 mg/dL in a symptomatic patient should be corrected immediately with the administration of glucose, either orally or intravenously, or with intramuscular glucagon.
• Patients treated for hypoglycemia in the emergency department should be discharged only if a readily identifiable cause of the hypoglycemia was identified and if the risk for recurrence is minimal.
Epidemiology
Patients who use insulin or oral hypoglycemic medications are at the greatest risk for hypoglycemia. These patients experience mild, self-treated hypoglycemic episodes about twice per week.1 Severe hypoglycemia, which requires the assistance of another person to regain euglycemia, is experienced at least once per year by 27% of patients treated with intensive insulin regimens.2 Hypoglycemia is the cause of death in approximately 3% of people with insulin-dependent diabetes.3 Patients taking oral hypoglycemic agents also commonly experience hypoglycemia. Although these episodes are generally associated with milder symptoms, they occur in more than 30% of patients each year.4 Sulfonylureas are the most widely used oral hypoglycemic agents. In 2004, 4148 sulfonylurea overdoses were reported to American poison control centers; of these, 36% occurred in children younger than 6 years, 21% required treatment of hypoglycemia, 2.5% were life-threatening, and 0.22% were fatal.5 The incidence of hypoglycemia is expected to rise as tight glycemic control continues to be emphasized for the 17 million Americans with diabetes.
Normal Glycemic Control
Counterregulatory Hormones
Glycogenolysis increases blood glucose within minutes and can maintain euglycemia in a well-nourished person for 24 hours. Gluconeogenesis requires several hours to raise blood glucose levels and is the principal mechanism responsible for maintaining euglycemia if fasting is extended beyond 24 hours. Secretion of cortisol from the adrenal cortex and growth hormone from the anterior pituitary gland is a delayed response to blood glucose falling below 60 to 65 mg/dL; these hormones are not involved in the correction of acute hypoglycemia but act to maintain euglycemia over a period of days to weeks.6,7
Patients with type 1 and advanced (insulin-dependent) type 2 diabetes may have an impaired counterregulatory reaction. In these patients, the glucagon response is often nonexistent and the epinephrine response is greatly attenuated.8 This impaired counterregulatory response predisposes patients to severe hypoglycemia by blunting the glycemic response to falling blood glucose levels and thereby leaving the patient with no early warning symptoms of hypoglycemia. Furthermore, even one episode of hypoglycemia blunts the epinephrine response to future hypoglycemia and can result in hypoglycemia-associated autonomic failure.9 In this manner a vicious cycle of recurrent hypoglycemia can develop. Avoidance of hypoglycemia for several weeks improves hypoglycemia awareness and restores the epinephrine component of the counterregulatory response.8
Causes of Hypoglycemia
Hypoglycemia occurs in patients with a relative excess of insulin in comparison with the hormones of the counterregulatory response. Such excess can occur through the administration of exogenous insulin, an increase in endogenous insulin, or inhibition of the counterregulatory response. This section highlights the most important causes of hypoglycemia, the mechanisms by which they act, and the context in which they are likely to be observed in the emergency department (ED) (Table 163.1).
| CAUSE (EXAMPLES) | COMMENT |
|---|---|
| Exogenous insulin (treatment of diabetes or hyperkalemia, factitious disorder, Munchausen by proxy) | Hypoglycemia caused by excessive insulin administration. Most common cause of hypoglycemia |
| Oral hypoglycemic agents (sulfonylureas, meglitinides) | Induce secretion of insulin from pancreatic beta cells |
| Alcohol (ethanol) | Inhibition of hepatic gluconeogenesis. Hypoglycemia usually requires concomitant fasting |
| Sepsis | Inhibition of hepatic gluconeogenesis and increased peripheral glucose utilization |
| Liver disease (hepatitis from infections or toxins, cirrhosis, Reye syndrome, HELLP syndrome, hepatoma, metastatic tumors) | Inhibition of hepatic gluconeogenesis and glycogenolysis |
| Renal disease | Decreased clearance of insulin and reduced mobilization of gluconeogenic precursors |
| Congestive heart failure | Hepatic congestion causes inhibition of gluconeogenesis and glycogenolysis |
| Starvation (prolonged fasting, anorexia nervosa, pyloric stenosis, pediatric gastroenteritis) | Depletion of glycogen stores and gluconeogenic precursors |
| Hormone deficiency (cortisol, growth hormone, epinephrine, glucagon, hypopituitarism) | Failure of the counterregulatory mechanism of glucose metabolism. The hormone deficiency may be either congenital or acquired |
| Medications not used for the treatment of diabetes mellitus (ACE inhibitors, acetaminophen, acetazolamide, aluminum hydroxide, beta-blockers, benzodiazepines, Bordetella-pertussis vaccine, chloroquine, chlorpromazine, cimetidine, ciprofloxacin, colchicine, diphenhydramine, disopyramide, doxepin, ecstasy, EDTA, etomidate, ethionamide, fluoxetine, furosemide, haloperidol, imipramine, indomethacin, isoniazid, lidocaine, lithium, maprotiline, mefloquine, monoamine oxidase inhibitors, nefazodone, orphenadrine, pentamidine, phenytoin, propoxyphene, quinine, quinidine, ranitidine, ritodrine, selegiline, terbutaline, tetracyclines, trimethoprim-sulfamethoxazole, warfarin) | Induce hypoglycemia rarely and unpredictably, usually in otherwise healthy individuals |
| Insulinoma | Excessive, unregulated endogenous insulin secretion from a tumor of pancreatic beta-cell origin |
| Nesidioblastosis | Excessive insulin secretion by hypertrophic pancreatic beta cells |
| Non–islet cell tumors (sarcoma, carcinoid, melanoma, leukemia, hepatoma, teratoma, colon, breast, prostate, stomach, mesothelioma) | Various mechanisms, including secretion of insulin-like growth factors, increased metabolic demand, production of insulin autoantibodies |
| Post–gastric surgery status (gastric bypass, gastrectomy, pyloroplasty) | Rapid dumping of glucose into the small intestine causes an exaggerated insulin response; nesidioblastosis may have a role |
| Inborn errors of metabolism (errors in glycogen synthesis, glycogenolysis, gluconeogenesis, mitochondrial beta oxidation, amino acid metabolism) | Congenital defect prevents normal metabolism from maintaining euglycemia |
| Idiopathic ketotic hypoglycemia | Fasting intolerance, possibly caused by deficiency in alanine as a gluconeogenic precursor |
| Autoimmune | Antibodies against insulin or the insulin receptor augment the effects of insulin |
| Akee fruit | Unripe akee, a fruit found in Jamaica, contains toxins that inhibit hepatic gluconeogenesis |
| Vacor rat poison | Damages pancreatic beta cells, which initially causes release of insulin and hypoglycemia but eventually results in impaired insulin secretion and diabetes mellitus. Banned in the United States |
| Transient neonatal hypoglycemia (prematurity, intrauterine growth retardation, severe infant distress syndrome, perinatal asphyxia, maternal hyperglycemia, erythroblastosis fetalis, beta-agonist tocolytic agents) | Occurs in the immediate newborn period. Rarely seen in the ED |
| Persistent neonatal hypoglycemia (mutation in the sulfonylurea receptor gene, glutamate dehydrogenase gene, glucokinase gene) | Occurs in the immediate newborn period. Rarely seen in the ED |
ACE, Angiotensin-converting enzyme; ED, emergency department; EDTA, ethylenediaminetetraacetic acid; HELLP, hemolysis elevated liver enzymes, and low platelet count.
Exogenous Insulin and Oral Hypoglycemic Agents
Administration of insulin or an oral hypoglycemic medication is the most common cause of hypoglycemia. In a patient with diabetes who is being treated with an established regimen of insulin or an oral hypoglycemic medication, hypoglycemia can develop for a number of reasons (Table 163.2).
Table 163.2 Potential Causes of Hypoglycemia in a Diabetic Patient with an Established Regimen of Insulin or an Oral Hypoglycemic Agent
| MECHANISM | EXAMPLES |
|---|---|
| Decreased glucose availability |
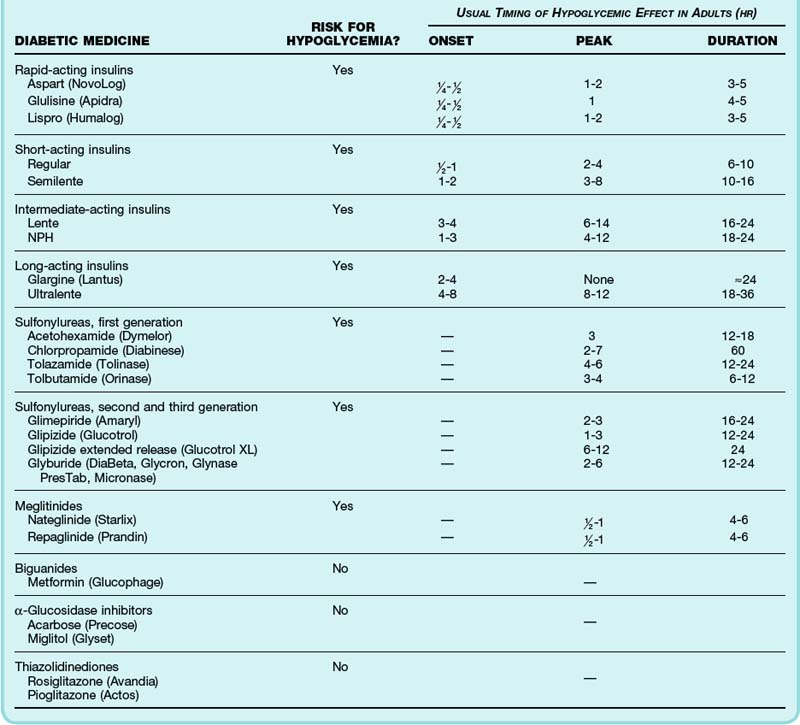
The time to peak effect and duration of action of insulin preparations and oral hypoglycemic medications dictate management and disposition (Table 163.3). Patients often cannot reliably recall which type of insulin they use; a helpful characteristic is that glargine and all rapid- and short-acting insulins are clear liquids whereas neutral protamine Hagedorn (NPH) and Ultralente appear cloudy.
Additional Causes of Hypoglycemia
Alcohol ingestion (ethanol), the second most common cause of hypoglycemia in the ED, inhibits the counterregulatory response by suppressing hepatic gluconeogenesis. It has minimal effects on glycogenolysis. Therefore, alcohol typically requires concomitant fasting to deplete glycogen stores before hypoglycemia ensues. The classic example of alcohol-induced hypoglycemia is a malnourished alcoholic who undertakes a prolonged binge. However, fasting for only 6 hours before significant alcohol consumption in an otherwise healthy person can cause hypoglycemia. Although hypoglycemia is rare (less than 1%) in intoxicated patients in the ED,12,13 the hypoglycemic episodes seen in EDs in lower socioeconomic urban areas involve alcohol 50% of the time.14
Starvation, as in the case of anorexia nervosa, depletes glycogen stores and gluconeogenic precursors and can eventually lead to hypoglycemia. Hypoglycemia as a complication of anorexia nervosa is a late finding and implies a very grave prognosis.15
Insulinomas are tumors of pancreatic beta-cell origin that secrete insulin without the normal feedback mechanisms, thus producing unexplained hyperinsulinemia and hypoglycemia in otherwise healthy people. Insulinomas are rare, with an incidence of 4 per 1 million per year.16 Early diagnosis is important because these tumors are curable with surgery before they lead to potentially fatal hypoglycemia. Nesidioblastosis is characterized by hypertrophied (nonneoplastic) beta-cell tissue that oversecretes insulin and can also be manifested as unexplained hypoglycemia.
Vague autonomic symptoms after eating were once labeled postprandial hypoglycemia, alimentary hypoglycemia, or functional hypoglycemia. This poorly understood mimic of hypoglycemic symptoms has never been linked to depressed blood glucose concentrations.17 However, after gastric surgery, including gastric bypass, gastrectomy, and pyloroplasty, patients can experience a true postprandial or alimentary hypoglycemia. Traditionally, the mechanism was thought to be rapid emptying of carbohydrates into the small intestine causing an exaggerated insulin response, or dumping syndrome. Recent evidence suggests that nesidioblastosis (beta-cell hyperplasia causing hyperinsulinism) plays a role in postprandial hypoglycemia following gastric surgery.18
Medications other than those used for the treatment of diabetes have been associated with hypoglycemia (see Table 163.1).19 Quinine, quinidine, pentamidine, and disopyramide stimulate release of insulin from pancreatic beta cells and have the potential to cause severe hypoglycemia. Commonly used drugs that may cause hypoglycemia include salicylates (mechanism unknown), acetaminophen (hypoglycemia occurs only in overdose with liver damage), angiotensin-converting enzyme (ACE) inhibitors (the mechanism may involve increased insulin sensitivity), and beta-blockers (inhibit the counterregulatory response of epinephrine). ACE inhibitors and beta-blockers typically cause hypoglycemia only when used concomitantly with insulin or oral hypoglycemic agents. Nonselective beta-blockers (e.g., propranolol) have more hypoglycemic potential than do selective beta1-blockers (e.g., metoprolol) and can occasionally cause hypoglycemia in the absence of antidiabetic medications, especially in children.
Causes of Hypoglycemia in Pediatric Populations
Gastroenteritis increases the risk for hypoglycemia by decreasing intestinal absorption of carbohydrates and by increasing metabolic demands of the illness. Reid and Losek20 found that 9% (18 of 196) of children aged 1 month to 5 years seen in an ED with gastroenteritis and dehydration had hypoglycemia, although none of the children exhibited altered mental status.
Idiopathic ketotic hypoglycemia is the most common cause of clinically significant hypoglycemia in nondiabetic children aged 7 months to 5 years. This syndrome is characterized by fasting intolerance and may be caused by a deficiency of alanine, an amino acid substrate for gluconeogenesis.21 During a 10- to 16-hour fast, the counterregulatory response converts triglycerides to ketone bodies and causes a mild metabolic acidosis and ketonuria, but it fails to maintain euglycemia. Affected children tend to be slender but not malnourished, with weight percentile below height percentile. These children often have a concurrent illness and are seen in the ED in the morning with a blood glucose concentration of 35 to 60 mg/dL, bicarbonate value of 14 to 19 mmol/L, and ketonuria. The syndrome typically remits by 6 years of age as lean body mass and alanine levels increase.
Clinical Presentation
The signs and symptoms in patients with hypoglycemia are divided into two categories: those caused by elevated levels of glucagon and epinephrine as part of the counterregulatory response (autonomic or hyperepinephrinemic symptoms) and those cause by insufficient supply of glucose to the brain (neuroglycopenic symptoms). Autonomic symptoms typically appear when the blood glucose concentration drops below 60 mg/dL and include anxiety, irritability, nausea, vomiting, palpitations, trembling, flushing, hunger, and sweating. Neuroglycopenic symptoms emerge below a blood glucose concentration of 50 mg/dL and include inability to concentrate, inattention, headache, lethargy, dizziness, blurry vision, agitation, confusion, and focal neurologic deficits.5 When the blood glucose concentration drops below 30 mg/dL, seizures and coma may ensue.1 Permanent brain damage and death are rare but occur when the hypoglycemia is severe and left untreated. Predicting who will have permanent neurologic impairment is difficult in the ED, but elderly patients, those with concurrent hypoglycemia, and in particular those with previous strokes are vulnerable to incomplete recovery.
The signs and symptoms of hypoglycemia are not uniformly present with each episode. Recurrent episodes in the same person tend to be symptomatically similar, but hypoglycemia can be manifested differently among individuals. Variations include the blood glucose threshold at which symptoms develop, the severity of symptoms at a given glucose concentration, the predominance of certain symptoms, and their order of appearance. The severity of neuroglycopenic symptoms depends on many factors other than the nadir of blood glucose, including the patient’s general state of health and age; integrity of the counterregulatory response; and the severity, duration, timing, and number of previous hypoglycemic episodes. For example, Osoria et al. showed that nondiabetic, neurologically normal people who were administered exogenous insulin to decrease their blood glucose concentration to 30 mg/dL experienced only subtle neuroglycopenic symptoms and no alteration in consciousness.22 Hypoglycemic episodes blunt the symptoms of future hypoglycemia. Therefore, lower blood glucose concentrations with recurrent episodes will be symptomatically silent and predispose the patient to repeated severe hypoglycemia, which can cause cumulative cognitive decline.23
Other than previous hypoglycemic episodes, additional features that increase the risk for hypoglycemia in diabetic patients include the use of insulin, longer duration of insulin use, higher doses of insulin, glycosylated hemoglobin values that are initially high and fall rapidly with therapy, adolescent age, and male gender.2 Use of an insulin pump does not alter the risk for hypoglycemia when compared with multiple daily insulin injections.
Differential Diagnosis
The signs and symptoms of hypoglycemia are associated with a long list of diagnoses. The causes of hypoglycemia create an equally long list (see Tables 163.1 and 163.2).
Furthermore, the blood glucose concentration should be checked in all patients requiring ED resuscitation because critical illness can cause hypoglycemia. Losek24 found that 18% (9 of 49) of pediatric patients undergoing nontraumatic ED resuscitation were hypoglycemic. Additionally, hypoglycemia should be considered in all accidental and intentional drug ingestions, in children with prolonged vomiting and diarrhea, and in all diabetics encountered in the ED.
Diagnostic Testing
Glucometry
The accuracy of bedside glucometry and laboratory tests of serum glucose concentration can be compromised by several mechanisms.25 Bedside glucometers are less accurate with hematocrit levels above 55 or below 30 because of variations in the relative amount of blood cells and plasma. Artificially low serum glucose measurements are seen with hemolytic anemia (nucleated red blood cells consume glucose in the collection tube26), with leukemia (leukocytes consume glucose27), and when blood is collected in a tube lacking a glycolysis-suppressing agent such as fluoride. An artificially high glucose concentration may be reported by bedside glucometry during an acetaminophen overdose because of interference with the measuring technique.28
Extended Laboratory Testing
Other diagnostic tests that are useful in selected cases, especially for critically ill hypoglycemic patients, include liver function tests, blood alcohol concentration, salicylate level, acetaminophen level, random cortisol level, chest radiograph, urine cultures, blood cultures, electrocardiogram, and cardiac markers. Recent evidence suggests that hypoglycemia may be associated with cardiac ischemia. Desouza et al. found that ischemic electrocardiographic changes were more common during hypoglycemia than during euglycemia or hyperglycemia in diabetic patients with coronary artery disease.29 Thus an evaluation for ischemic heart disease is indicated for patients with hypoglycemia and risk for coronary artery disease.
Critical Blood Sample
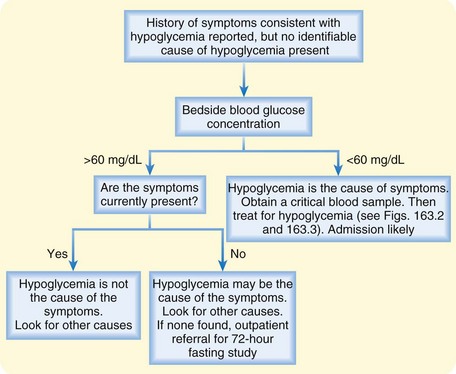
In nondiabetic patients without an immediately identifiable cause of hypoglycemia who have symptoms consistent with hypoglycemia, a normal glucose reading on a bedside glucometer excludes hypoglycemia irrespective of symptoms (Fig. 163.1). No further testing for hypoglycemia is indicated—other possible causes of the symptoms should be suspected.
Treatment
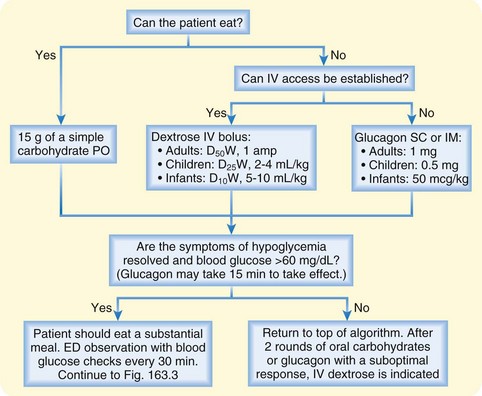
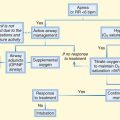
Treatment of hypoglycemia involves repletion of blood glucose via oral or IV administration of exogenous glucose or glucagon administration to stimulate endogenous glucose production. The specific treatment modality depends on the patient’s age, whether the patient’s mental status allows safe oral ingestion, and whether IV access can be established (Fig. 163.2).
Methods of Administration
Intravenous Administration
If a patient’s mental status prohibits oral repletion and IV access can be obtained, an IV dextrose bolus is the treatment of choice. The IV dose in adults is 1 ampule of 50% dextrose in water (D50W), which contains 25 g of glucose it may be repeated up to three times. The increase in blood glucose concentration in response to 1 ampule of D50W is unpredictable; it ranges from about 40 to 350 mg/dL and averages about 160 mg/dL. The glycemic response to a bolus of dextrose peaks within 5 minutes and lasts about 30 minutes.30,31
Adjunctive Treatments
Octreotide
Octreotide is used as a supplemental treatment of sulfonylurea- and insulinoma-induced hypoglycemia. It is a synthetic analogue of the hormone somatostatin and a potent inhibitor of pancreatic insulin secretion. Although octreotide does not treat existing hypoglycemia, it can prevent recurrent hypoglycemia and reduce the amount of dextrose supplementation needed.32,33 In sulfonylurea-induced hypoglycemia, octreotide should be used instead of continuous dextrose infusions and glucagon whenever possible because dextrose and glucagon stimulate further insulin secretion from the sulfonylurea-primed pancreatic beta cells.
Diazoxide
Diazoxide, a rarely used antihypertensive medication, also inhibits pancreatic insulin secretion and can be used for sulfonylurea-induced hypoglycemia. It is considered a second-line therapy in comparison with octreotide because of lower efficacy and greater risk for toxicity.34 Side effects include hypotension and sodium retention. The IV adult dose is 300 mg of diazoxide given over a 1-hour period; the IV pediatric dose is 1 to 3 mg/kg given over a 1-hour period. Doses can be repeated every 4 hours as needed.
Other Adjunctive Treatments
When hypoglycemia is caused by a massive overdose of SC insulin, one case report suggests that needle aspiration or surgical excision of the SC reservoir may reduce the systemic absorption of insulin.35
Urine alkalization can reduce the half-life of chlorpropamide from 49 hours to 13 hours through increased renal excretion. However, urine alkalization does not appear to be useful in reducing the duration of effect of other hypoglycemic agents.36
Risks Associated with Presumptive Treatment
It has been suggested previously that dextrose be given to all patients with undifferentiated altered mental status as part of the “coma cocktail,” which consists of oxygen plus IV administration of 1 ampule of D50W, 100 mg of thiamine, and 0.4 mg of naloxone. This practice was challenged in the 1980s when animal and retrospective human studies suggested that hyperglycemia may be detrimental to the injured brain, particularly in the setting of acute stroke, cardiac arrest, hypotension, and head trauma.37 The true risk of administering a small glucose load to a euglycemic or hyperglycemic patient with a brain injury has not been established but is thought to be small. Hypoglycemia, in contrast, is known to be detrimental, especially to the injured brain. Therefore, the current recommendation is to obtain a bedside glucose measurement in patients with altered mental status and administer dextrose only when the blood glucose level is lower than 60 mg/dL. Treatment should be initiated empirically only when a glucometer is not immediately available.
Traditional teachings also warn that dextrose should never be given to patients with altered mentation without a preceding dose of thiamine because of the risk of precipitating Wernicke encephalopathy. Thiamine is a cofactor for two essential reactions in the glycolysis–tricarboxylic acid cycle (conversion of pyruvate to acetyl coenzyme A and α-ketoglutarate to succinate). Theoretically, without adequate supplies of thiamine, the energy contained in glucose cannot be converted to adenosine triphosphate, so substrates for the thiamine-dependent reactions accumulate in the brain and cause Wernicke encephalopathy. Administering dextrose to a thiamine-deficient patient increases the production of these substrates, but no clinical evidence has shown that a dextrose load dosed to reverse hypoglycemia precipitates Wernicke encephalopathy if thiamine is not given first.38 When treating a patient with altered mental status and confirmed hypoglycemia or with possible hypoglycemia without access to a bedside glucometer, EPs should administer thiamine and dextrose simultaneously if possible. Dextrose administration should not be delayed if thiamine is not immediately available, even in alcoholic patients. Hypoglycemia should be corrected immediately, and thiamine should be administered as soon as possible.
Persistent Altered Mental Status
If hypoglycemia is the sole cause of the altered mental status, restoration of euglycemia should lead to reversal of the neurologic symptoms within minutes. In the case of a seizure caused by hypoglycemia, the altered mentation may persist for a short period while the patient recovers from the postictal state. If a patient’s mental status remains altered for more than 15 minutes after return to euglycemia and there is no history of seizure, a secondary cause must be considered. The EP should return to the primary assessment and consider a broad differential diagnosis, including traumatic brain injury, anoxic brain injury, stroke, alcohol intoxication, opioid intoxication, central nervous system infection, liver failure, and renal failure. The coma cocktail should be given in full, if not administered earlier. Rapid-sequence intubation, computed tomography of the brain, and lumbar puncture may be indicated. If a lumbar puncture is performed, a low cerebrospinal fluid glucose value is not necessarily a sign of central nervous system infection because this value remains depressed for several hours after a low blood glucose concentration has been corrected.39
Disposition
Discharge
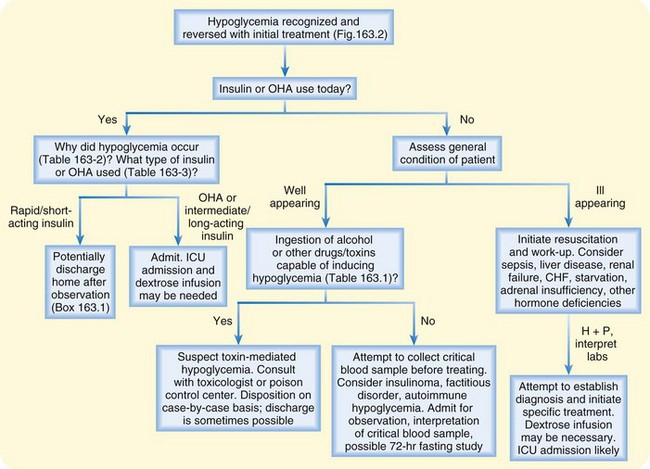
Patients with hypoglycemia caused by a short-acting, easily reversed mechanism that is unlikely to recur may be considered for discharge (Box 163.1). A history of recurrent episodes of hypoglycemia may be an indication for hospital admission to adjust medications. When taken in massive doses, even short-acting insulin preparations can cause delayed, recurrent hypoglycemia because of altered pharmacokinetics. Renal failure also increases the half-lives of insulin preparations and can cause unexpected recurrent hypoglycemia.
Box 163.1 Minimum Requirements for Discharge from the Emergency Department (Ed) after a Hypoglycemic Episode
Hypoglycemia fully and rapidly reversed without continuous infusion of dextrose
Uneventful 4-hour ED observation with serial blood glucose measurements in the normal range and not trending downward
Cause of the hypoglycemia known and recurrence unlikely (i.e., short-acting insulin)
Isolated hypoglycemic episode (no other recent severe hypoglycemic events or frequent mild hypoglycemic events)
Patient understands how to prevent future episodes
Patient can accurately monitor blood glucose at home
Responsible adult will monitor the patient for the next several hours
![]() Patient Teaching Tips
Patient Teaching Tips
Know the symptoms of hypoglycemia. Train yourself to estimate your blood glucose concentration through your symptoms.
Carry fast-acting carbohydrates with you at all times. Immediately take 15 to 30 g when you experience symptoms of hypoglycemia. Do not delay to check your blood glucose level. After the symptoms resolve, eat a small snack of complex carbohydrates and protein (cheese or peanut butter and crackers).
Stop driving immediately if you experience symptoms of hypoglycemia.
Even moderate alcohol consumption increases the risk for hypoglycemia.
Train friends and family when and how to administer glucagon. These people should know where the glucagon kit is and should practice mixing the glucagon mixture. Know the expiration date on your glucagon kit.
The risk for hypoglycemia is increased in the hours to days after an initial hypoglycemic episode. You should increase your vigilance and check your blood glucose more frequently in the 24 hours following a hypoglycemic episode.
Call your doctor for a hypoglycemic episode requiring fast-acting carbohydrates that you cannot readily explain and the use of glucagon.
Call 911 for an incomplete response to glucagon or oral fast-acting carbohydrates or a seizure.
More information is available through the American Diabetes Society (www.diabetes.org).
Observation and Admission
Patients who do not meet the criteria outlined in Box 163.1 require admission or monitoring in an observation unit for at least 24 hours (Fig. 163.3). Because of the risk for recurrence, hospitalization is recommended for those with hypoglycemia caused by an oral hypoglycemic medication or an intermediate- or long-acting insulin. Hypoglycemia caused by sulfonylureas tends to be prolonged and severe, and intensive care unit admission may be necessary, especially if a continuous dextrose infusion is needed in addition to octreotide to maintain euglycemia. Experience with hypoglycemia caused by the meglitinides is limited, so these patients should be managed similar to those with sulfonylurea-induced hypoglycemia.
Patients Treated in Prehospital Setting
Emergency medical service (EMS) personnel frequently treat hypoglycemia in the prehospital setting. After treatment and resolution of the symptoms, patients often refuse transport to the ED. Recent studies suggest that the risk for recurrent hypoglycemia is not different in patients transported to the ED and those released on the scene.40,41 Mechem et al.42 reported that 9% (9 of 103) of patients who initially refused transport to the ED contacted EMS again within 3 days because of recurrent hypoglycemia. Similarly, Lerner et al.43 found that 8% (3 of 38) of patients released by EMS experienced recurrent hypoglycemia in 24 to 48 hours. However, caution is advised because fatal cases of hypoglycemia have occurred following release by EMS.42 EMS personnel should encourage all patients with symptomatic hypoglycemia, especially those who do not meet the criteria listed in Box 163.1, to be transported to the ED.
Special Consideration: Accidental Pediatric Sulfonylurea Ingestion
Children are particularly susceptible to the effects of sulfonylureas, and significant hypoglycemia can develop after the ingestion of a single tablet.44,45 Therefore, all pediatric patients with possible sulfonylurea ingestion should be evaluated in the ED. If hypoglycemia is present on arrival at the ED, treatment as outlined previously with dextrose, octreotide, oral feedings, and hospital admission is indicated. The optimal management of a child who ingested a sulfonylurea or is thought to have done so and who has a normal blood glucose concentration is debated in the literature, with recommendations varying from mandatory admission to 8-hour ED observation.45–48
Two studies provide the best available data for directing the management of asymptomatic pediatric sulfonylurea ingestions. In a retrospective study of pediatric patients after sulfonylurea ingestion, Quadrani et al.49 found that 27% (25 of 93) had a blood glucose concentration lower than 60 mg/dL 30 minutes to 16 hours after ingestion, with the mean time to development of hypoglycemia being 4.3 hours. All four patients in whom hypoglycemia developed after 8 hours had received a continuous dextrose infusion while euglycemic during the observation period. It is thought that the dextrose infusions delayed the onset of hypoglycemia in these patients.
In a prospective study of pediatric sulfonylurea ingestions, Spiller et al.50 found that hypoglycemia developed in 29% (54 of 185). In all but one of the hypoglycemic patients, low blood glucose developed within 8 hours of ingestion. The lone patient in whom hypoglycemia developed after 8 hours had been given a continuous dextrose infusion after a blood glucose measurement of 62 mg/dL at 3 hours.
1 Pramming S, Thorsteinsson B, Bendtson I, et al. Symptomatic hypoglycaemia in 411 type 1 diabetic patients. Diabet Med. 1991;8:217.
2 Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271.
3 Laing SP, Swerdlow AH, Slater SD, et al. The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med. 1999;16:466.
4 Miller CD, Phillips LS, Ziemer DC, et al. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161:1653.
5 Watson WA, Litovitz TL, Rodgers GC, et al. 2004 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2005;23:589.
6 Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260:E67.
7 Schwartz NS, Clutter WE, Shah SD, et al. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. 1987;79:777.
8 Cryer PE. Current concepts: diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004;350:2272.
9 Fanelli CG, Paramore DS, Hershey T, et al. Impact of nocturnal hypoglycemia on hypoglycemic cognitive dysfunction in type 1 diabetes. Diabetes. 1998;47:1920.
10 Roberge RJ, Kaplan R, Frank R, et al. Glyburide-ciprofloxacin interaction with resistant hypoglycemia. Ann Emerg Med. 2000;36:160.
11 Leiba A, Leibowitz A, Grossman E. An unusual case of hypoglycemia in a diabetic patient. Ann Emerg Med. 2004;44:427.
12 Sucov A, Woolard RH. Ethanol-associated hypoglycemia is uncommon. Acad Emerg Med. 1995;2:185.
13 Ernst AA, Jones K, Nick TG, et al. Ethanol ingestion and related hypoglycemia in a pediatric and adolescent emergency department population. Acad Emerg Med. 1996;3:46.
14 Malouf R. Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol. 1985;17:421.
15 Rich LM, Caine MR, Findling JW, et al. Hypoglycemic coma in anorexia nervosa. Case report and review of literature. Arch Intern Med. 1990;150:894.
16 Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinoma—incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66:711.
17 Service FJ. Medical progress: hypoglycemic disorders. N Engl J Med. 1995;332:1144.
18 Service GJ, Thompson GB, Service FJ, et al. Brief report: hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249.
19 Marks V, Teale JD. Hypoglycemic disorders: drug-induced hypoglycemia. Endocrinol Metabol Clin. 1999;28:555.
20 Reid SR, Losek JD. Hypoglycemia complicating dehydration in children with acute gastroenteritis. J Emerg Med. 2005;29:141.
21 Daly LP, Osterhoudt KC, Weinzimer SA. Presenting features of idiopathic ketotic hypoglycemia. J Emerg Med. 2003;25:39.
22 Osoria I, Arafah BM, Mayor C, et al. Plasma glucose alone does not predict neurologic dysfunction in hypoglycemic nondiabetic subjects. Ann Emerg Med. 1999;33:291.
23 Langan SJ, Deary IJ, Hepburn DA, et al. Cumulative cognitive impairment following recurrent severe hypoglycaemia in adult patients with insulin-treated diabetes mellitus. Diabetologia. 1991;34:337.
24 Losek JD. Hypoglycemia and the ABC’s (sugar) of pediatric resuscitation. Ann Emerg Med. 2000;35:43.
25 Scott PA, Wolf LR, Spadafora MP. Accuracy of reagent strips in detecting hypoglycemia in the emergency department. Ann Emerg Med. 1998;32:305.
26 Macaron CI, Kadri A, Macaron Z. Nucleated red blood cells and artefactual hypoglycemia. Diabetes Care. 1981;4:113.
27 Goodenow TH, Malarkey WB. Leukocytosis and artifactual hypoglycemia. JAMA. 1977;237:1961.
28 Cartier LJ, Leclerc P, Pouilet M, et al. Toxic levels of acetaminophen produce a major positive interference on Glucose Elite and Accu-Chek Advantage glucose meters. Clin Chem. 1998;44:893.
29 Desouza C, Salazar H, Cheong B, et al. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485.
30 Adler PM. Serum glucose changes after administration of 50% dextrose solution: pre- and in-hospital calculations. Am J Emerg Med. 1986;4:504.
31 Balentine JR, Gaeta TJ, Kessler D, et al. Effect of 50 milliliters of 50% dextrose in water administration on the blood sugar of euglycemic volunteers. Acad Emerg Med. 1998;5:691.
32 Fasano CJ, O’Malley GF, Paul F, et al. A prospective trial of octreotide vs. placebo in recurrent sulfonylurea-associated hypoglycemia. Acad Emerg Med. 2006;13:S180-a.
33 McLaughlin ST, Crandall CS, McKinney PE. Octreotide: an antidote for sulfonylurea-induced hypoglycemia. Ann Emerg Med. 2000;36:133.
34 Boyle PJ, Justice K, Krentz AJ, et al. Octreotide reverses hyperinsulinemia and prevents hypoglycemia induced by sulfonylurea overdoses. J Clin Endocrinol Metab. 1993;76:752.
35 McIntyre AS, Woolf VJ, Burnham WR. Local excision of subcutaneous fat in the management of insulin overdose. Br J Surg. 1986;73:538.
36 Neuvonen PJ, Karkkainen S. Effects of charcoal, sodium bicarbonate and ammonium chloride on chlorpropamide kinetics. Clin Pharmocol Ther. 1983;33:386.
37 Browning RG, Olson DW, Stueven HA, et al. 50% dextrose: antidote or toxin? Ann Emerg Med. 1990;19:683.
38 Hack JB, Hoffman RS. Thiamine before glucose to prevent Wernicke encephalopathy: examining the conventional wisdom. JAMA. 1998;279:583.
39 Kaplinsky N, Frankl O. The significance of the cerebrospinal fluid examination in the management of chlorpropamide-induced hypoglycemia. Diabetes Care. 1980;3:248.
40 Carter AJE, Keane PS, Dreyer JF. Transport refusal by hypoglycemic patients after on-scene intravenous dextrose. Acad Emerg Med. 2002;9:855.
41 Socransky SJ, Pirrallo RG, Rubin JM. Out-of-hospital treatment of hypoglycemia: refusal of transport and patient outcome. Acad Emerg Med. 1998;5:1080.
42 Mechem CC, Kreshak AA, Barger J, et al. The short-term outcome of hypoglycemic diabetic patients who refuse ambulance transport after out-of-hospital therapy. Acad Emerg Med. 1998;5:768.
43 Lerner EB, Billittier AJ, Lance DR, et al. Can paramedics safely treat and discharge hypoglycemic patients in the field? Am J Emerg Med. 2003;21:115.
44 Szlatenyi CS, Capes KF, Wang RY. Delayed hypoglycemia in a child after ingestion of a single glipizide tablet. Ann Emerg Med. 1998;31:773.
45 Little GL, Boniface KS. Are one or two dangerous? Sulfonylurea exposure in toddlers. J Emerg Med. 2005;28:305.
46 Harrigan RA, Nathan MS, Beattie P. Oral agents for the treatment of type 2 diabetes mellitus: pharmacology, toxicity, and treatment. Ann Emerg Med. 2001;38:68.
47 Burkhart KK. When does hypoglycemia develop after sulfonylurea ingestion? Ann Emerg Med. 1998;31:771.
48 Robertson WO. Sulfonylurea ingestions: hospitalization not mandatory. J Toxicol Clin Toxicol. 1997;35:115.
49 Quadrani DA, Spiller HA, Widder P. Five year retrospective evaluation of sulfonylurea ingestion in children. J Toxicol Clin Toxicol. 1996;34:267.
50 Spiller HA, Villalobos D, Krenzelok EP, et al. Prospective multicenter study of sulfonylurea ingestion in children. J Pediatr. 1997;131:143.