Infantile Hypertrophic Pyloric Stenosis: Epidemiology, Genetics, and Clinical Update
Infantile hypertrophic pyloric stenosis (IHPS), the most common surgical condition producing emesis in infancy, was first described by Hirschsprung [1] in 1888. Ramstedt performed the first successful pyloromyotomy in 1912; however, most cases were lethal at that time. Although advances in medical knowledge and care have resulted in minimal mortality and morbidity today, the cause of IHPS remains unclear [2]. This article provides an update on IHPS, focusing on pathogenesis, diagnosis and treatment, descriptive epidemiology and associated risk factors, and current understanding of the role of genetics.
Pathogenesis
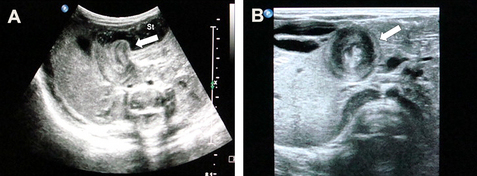
In infants with IHPS, the pyloric portion of the stomach becomes abnormally thickened, resulting in narrowing and elongation of the pyloric channel (Fig. 1). A gastric outlet obstruction is produced, with compensatory dilation, hypertrophy, and hyperperistalsis of the stomach. Most infants with IHPS present in the first 2 to 12 weeks of life with forceful or projectile nonbilious vomiting after feeding. The emesis may become blood-tinged because of gastritis. The timing of the presentation is likely related to increasing volumes of enteral feeding acting on abnormal pyloric tissue. Many infants with IHPS are initially believed to have a food allergy or gastroesophageal reflux [2,3]. The narrow window of diagnosis between approximately 2 and 12 weeks may be due to the introduction of enteral feeding, which acts on abnormal pyloric tissue.
As reviewed by Panteli [4], the pyloric sphincter contracts tonically and phasically to effect gastric emptying. Sphincter function is controlled by a complex system involving the enteric nervous system, gastrointestinal hormones, and interstitial cells of Cajal. Abnormalities in hormonal control, extracellular matrix, smooth muscle fibers, growth factors, interstitial cells of Cajal, and pyloric innervations have been implicated in the pathogenesis of IHPS. The muscular layer of the pylorus in IHPS is reportedly characterized by abnormal distribution of nerve terminals [5], reduced intramuscular nerve supporting cells [6], altered peptidergic innervation [7], altered nitric oxide production [8], ultrastructural abnormalities of enteric nerves and the interstitial cells of Cajal [9], and increased insulin-like growth factor production [10]. This constellation of abnormalities leads to failure of the pyloric muscle to relax, increased synthesis of growth factors, and subsequent hypertrophy [11].
Evaluation
Traditionally, the diagnosis of IHPS was based on a history of projectile vomiting and palpation of the hypertrophied pyloric muscle, which is referred to as an olive because of its size and shape. This method, known as palpating the olive, has a 99% positive predictive value [12]. Positive test feeds can also be used diagnostically; however, this approach has unacceptably high false-positive and false-negative rates and is not used extensively [13,14]. Ultrasound imaging is usually used as a substitute or complement to physical examination or test feeds. Although imaging is more costly, it is highly sensitive, with accuracy and sensitivity approaching 100% [2,12,14]. Measurements of canal length and muscle thickness can support the ultrasonographic diagnosis [14].
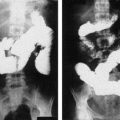
The increasing reliance on imaging has resulted in diagnoses being made before alkalosis has developed, and in a shorter clinical course, less morbidity, and a shorter postoperative hospital stay [15]. Fluoroscopic upper gastrointestinal contrast studies can also be used for diagnosis, because the pyloric canal in IHPS is outlined by a string of contrast material. However, fluoroscopy is time consuming, involves radiation exposure, and its sensitivity is dependent on the skill of the examiner. Consequently, real-time ultrasonography has supplanted fluoroscopy as the diagnostic procedure of choice [2,3]. Although ultrasonography is the standard diagnostic procedure, the pylorus is difficult to visualize in patients with gastric overdistension because of displacement of the pyloric muscle dorsally by the gas-filled and fluid-filled stomach. This problem can usually be averted by turning the patient to a right lateral decubitus position, which causes the pylorus to rise to an anterior position, thus allowing it to be imaged [16]. Borderline cases can also be problematic, because the muscle typically does not measure more than 3 mm in thickness [16]. Repeating the ultrasound several days later may confirm the diagnosis [17]. Dehydration resulting from the protracted vomiting can also cause a spuriously low measurement of muscle thickness, which may increase after fluid resuscitation [18]. In borderline cases, it may also be helpful to visualize the relaxation of the pyloric canal following introduction of fluid into the stomach; this finding reliably excludes IHPS [16]. Although radiologists most often make the diagnosis, Copeland and colleagues [16] report that surgeons who have undergone focused training can diagnose the condition without confirmatory testing by a radiologist.
Despite the high specificity and sensitivity of diagnostic methods, the current guidelines may not be sufficient for accurate diagnosis of IHPS in infants younger than 3 weeks because of the thin pyloric muscle thickness [19] and equivocal clinical and biochemical variables [14]. Young infants should be observed and reevaluated in 1 to 2 days when the lesion may be more clinically or radiologically evident [20].
Treatment/management
The vomiting associated with IHPS leads to depletion of sodium, potassium, and hydrochloric acid, resulting in hypokalemic, hypochloremic metabolic acidosis. Because IHPS is not a surgical emergency, fluid and electrolyte losses should be corrected before surgical intervention. This correction typically requires hospitalization and intravenous fluid replacement therapy [2,3].
The practice of evidence-based medicine requires physicians to review evidence to justify medical decisions and treatments. Although general pediatric surgeons perform a great variety of different operations, the number of surgeries performed is small relative to comparable procedures performed in adults. In addition, surgery has lagged behind other disciplines in the conduct of randomized clinical trials. As a result, the evidence base for many pediatric and neonatal surgical procedures is weak [21,22]. There is a paucity of randomized, controlled trials for IHPS treatment, and most are based on collaborations between pediatric surgical units, thus highlighting the importance of creating a network of centers that promote multicenter prospective studies [21].
Extramucosal longitudinal pyloromyotomy, first described in 1908 [23], has been the standard treatment of IHPS for decades. The pyloric muscle is split longitudinally, which allows the submucosal layer to bulge out to the level of the serosa. Most patients have excellent short-term and long-term outcomes, and mortality has been virtually eliminated with the use of appropriate fluid resuscitation, improvements in anesthesia, and a standard surgical approach. Muscle thickness returns to normal within 4 weeks, and is associated with healing of the pyloric muscle and return of function [3].
Laparoscopic pyloromyotomy (LP) was first described in 1990 [24], and it is increasingly the surgical treatment of choice as laparoscopic technology has improved. However, it remains controversial whether LP is superior to open pyloromyotomy. Sola and Neville [25] performed a systematic review and meta-analysis of laparoscopic versus open pyloromyotomy. Six prospective studies with 625 patients (303 laparoscopic, 322 open surgery) met selection criteria. Patients who underwent LP had lower total complication rates (odds ratio [OR] 0.58; 95% CI 0.35, 0.97), mostly because of fewer wound complications, shorter time to full feedings (mean difference −11.52 hours, 95% CI −12.77, −10.27) and shorter postoperative lengths of stay (mean difference −5.71 hours, 95% CI −8.90, −2.52). There were no significant differences in the rates of mucosal perforation, wound infection, postoperative emesis, or operating time. Incomplete pyloromyotomy occurred in 6 patients who underwent LP. As Hall and colleagues [21] point out, major complications such as incomplete pyloromyotomy are rare and individual studies are not powered to detect them. Thus, although rare complications are important, they may not pose a clinically significant risk for individual patients. Because LP is associated with shorter postoperative recovery period and duration of pain, it should be considered a valid technique as long as care and attention are paid to avoiding incomplete pyloromyotomy [21].
Although pyloromyotomy remains the first choice of treatment in Western countries, several investigators, mostly from Asian countries, have reported that some infants respond to intravenous or oral atropine without requiring surgery. The rationale for atropine therapy is that the pathophysiology of IHPS may be due, in part, to impaired function of acetylcholine and muscarinic receptors. Because atropine is an anticholinergic agent with strong antimuscarinic activity, it may decrease intestinal peristalsis by relaxing smooth muscles [26]. In studies conducted in Japan [27–30], Taiwan [31], and India [32], infants with IHPS were treated with intravenous or oral atropine sulfate daily until vomiting ceased, with higher doses given thereafter for varying periods of time. The investigators concluded that medical treatment is a safe and effective treatment option for IHPS, although approximately 10% of patients required pyloromyotomy and prolonged medical treatment was often needed. Meissner and colleagues [33] reported that atropine sulfate was successful in only 25 of 33 IHPS cases treated in Germany, and that clinical improvement was often not observed before the sixth or seventh day of treatment. Because of the persistent vomiting, nearly a quarter of families requested that their child be treated surgically before completing the 7 days of treatment. The investigators conclude that the 75% success rate does not favor atropine in view of the 95% success rate with surgical repair, and that the higher cure rates reported by other investigators were likely caused by prolonged medical therapy. Surgically treated patients are typically discharged within 24 hours of surgery, whereas medical treatment may require 7 days or more of skilled nursing and careful follow-up. Although the investigators conclude that atropine sulfate therapy should not be recommended where standard surgical procedure is available [33], Aspelund and Langer [2] believe it should be considered as an alternative in infants with contraindications to anesthesia or surgery.
Epidemiology
The reported prevalence of IHPS has varied considerably by region and time. Although early case reports date from the eighteenth and nineteenth centuries, from the time of Hirschsprung’s initial report (1888) through the mid-twentieth century very few cases were examined in case series reports [34]. More recent reports have been varied both in prevalence estimates and trends over time. The most recent national report on prevalence of birth defects for 2003 to 2007 shows state-level prevalence of IHPS ranging by almost an order of magnitude, from 0.5 to 4.21 per 1000 live births (excluding states with clearly incomplete case ascertainment) [35].
Although numerous studies have examined trends in prevalence of IHPS with time, the cumulative weight of the evidence suggests that the prevalence has been stable over time. Although contemporary prevalence estimates are in the range of 1 to 2 per 1000 infants, many reports examine trends during periods dating to the mid-twentieth century. Although some reports suggest an increasing trend, most of these observations come from regions with a lower initial prevalence estimate. National estimates for the prevalence of IHPS in the United States are not available; IHPS was not selected for inclusion in the most recent report because it generally has onset after the first day of life [36].
IHPS prevalence varies by maternal race/ethnicity. Studies in the United States generally report higher prevalence of IHPS among white non-Hispanic mothers, with significantly lower prevalence among non-Hispanic black mothers. In a recent Texas study, babies born to Hispanic mothers born in the United States had prevalence of IHPS similar to those born to white mothers, whereas the prevalence was significantly lower among those born outside the United States [37]. We reported a similar association between maternal nativity and risk of another complex disorder: gastroschisis [38]. This finding further supports a cause that involves a genetic/environmental interaction. Crude prevalence rates for births to Asian mothers tend to be lower than among white non-Hispanic mothers; in the most recent national surveillance report, IHPS prevalence was lowest among births to Asian mothers in all but 3 states [35].
MacMahon [34] provided a comprehensive review of the descriptive epidemiology of IHPS; this article compares his findings with more recent reports. Studies have shown no consistent association between maternal age and IHPS. However, the prevalence of IHPS seems to decline with increasing birth order, and most reports find the highest prevalence among first-born infants. IHPS is considerably more common among boys, with 4 to 5 times greater prevalence than in girls in most Western European and North American studies. Evidence for an association of IHPS with birth weight remains enigmatic. A recent study from Texas [37] found reduced prevalence among infants born at greater than 4500 g birth weight, whereas the prevalence among infants born at 3500 to 4499 g did not differ from the reference group. Although Wang [37] found no association with low birth weight, Applegate and Druschel [39] found a decreased risk for IHPS among infants born at less than 1500 g. Patterns of IHPS variation with gestational age mirror those for birth weight to some extent. As MacMahon noted [34], although preterm birth does not seem to be a risk factor, symptom onset in relation to maturity dated from conception rather than birth may explain the observed patterns. However, most birth defects registries in the United States do not record infant age at onset for IHPS. Wang [37] used 37 to 39 weeks of gestational age at birth as the reference group, and found higher adjusted prevalence ratios for IHPS among infants born at 28 to 31, 32 to 34, and 35 to 36 weeks’ gestation, but lower prevalence among infants born after less than 28 or more than 40 weeks of gestation. Data suggest that maternal smoking may be associated with an increased risk for IHPS but studies have been inconclusive [40]. Studies failed to show increased risk for IHPS associated with Helicobacter pylori infection [4,41,42].
Numerous reports have considered the role of socioeconomic status in IHPS. Most studies in the United States have no direct measures of family income or occupation and use maternal education as a proxy measure. In 2 of these studies [37,39], IHPS was inversely associated with maternal education; however, neither study reported a test for trend. A recent report from Scotland suggests an increasing prevalence of IHPS with higher levels of social deprivation [43].
Several researchers have examined the time trends in sudden infant death syndrome (SIDS) and IHPS. Persson and colleagues [44] found a correlation of +0.57 between the 2 conditions in Stockholm, Sweden in the period 1970 to 1997, and Sommerfield and colleagues [43] reported an even stronger correlation (r = +0.89) in Scotland in the period 1981 to 2004. Although these observations raise the possibility that public health interventions to promote safe sleeping practices may be associated with reductions in incidence of both conditions, the hypothesis that IHPS may be associated with infant sleep position requires more detailed study.
Several recent meta-analyses have examined associations between maternal health status and prenatal behaviors and birth defects. Among those that included IHPS as an outcome, Browne [45] found no association with maternal caffeine consumption in pregnancy, Goh and colleagues [46] found no association with use of prenatal vitamins with supplements, and a meta-analysis by Stothard and colleagues [47] found insufficient evidence to support an association between in the literature to support meta-analysis of the association with maternal overweight or obesity.
Genetics
IHPS is a complex condition, resulting from interaction of genetic and environmental factors. Genetic predisposition to IHPS was established by Carter and Powell [48] and Carter [49], who described the multifactorial sex-modified threshold model of inheritance of IHPS, with a male preponderance of 4 to 1. There is significant variation in incidence between populations, with IHPS being most common in white and Hispanic people, and less common in those of African and Asian heritage. The existence of a major dominant gene of low penetrance with a multifactorial background was considered likely in several families described in the literature [50,51]. Large, multigenerational families consistent with autosomal dominant transmission of IHPS have been reported [52,54]. The male sex bias tends to be less pronounced in familial cases [54]. Re-analysis of several previously published family studies led to the conclusion that the familial recurrence pattern of IHPS is compatible with multifactorial threshold inheritance or the effects of multiple interacting loci [55].
An analysis of cases of IHPS in Denmark occurring in a 31-year period confirmed strong familial aggregation of IHPS, with a nearly 200-fold increase among monozygotic twins and 20-fold increase among dizygotic twins and siblings [56]. The lack of increased risk among dizygotic twins compared with siblings suggests that intrauterine environmental factors are not significant in IHPS. High heritability of IHPS was further supported by strong aggregation of cases, even in third-degree and fourth-degree relatives. They observed similar rates of IHPS regardless of sex of the index case, sex of the affected relative, or presence of the affected relative in maternal or paternal side of the family. These findings are also at odds with the multifactorial threshold model, which predicts that male offspring of affected females would be at higher risk because of higher genetic and environmental liability for this trait. The investigators concluded that familial aggregation of IHPS is caused by shared genes that may affect responses to postnatal factors.
Five genetic loci associated with IHPS have been identified [57]. The gene for nitric oxide synthetase, NOS1 (IHPS1; chromosomal locus 12q24.2-q24.31) was identified as a candidate gene for IHPS because lack of neuronal nitric oxide in pyloric tissue seems to be responsible for pylorospasm and hypertrophy in IHPS [58,59]. Two loci, IHPS2 (16p13-p12) and IHPS5 (16q24.3), are associated with autosomal dominant forms of the disease. This finding is not surprising, because identification of monogenic subtypes of complex traits has become common. This genetic heterogeneity is consistent with the multiple variables and complex molecular pathways underlying the development of IHPS. IHPS3 (11q14-q22) and IHPS4 (Xq23-q24) were identified via genome-wide linkage analysis of families with 2 or more affected individuals [60]. It is notable that the X chromosome locus could provide a basis for the increased frequency of IHPS in boys. The IHPS3 and IHPS4 regions each include a candidate gene from the family of canonical transient receptor potential (TRPC) cation channels. Further analysis led to identification of an additional locus on chromosome 3q12-q25, a region including the candidate gene TRPC1. These genes, TRPC6 (IHPS3), TRPC5 (IHPS4), and TRPC1, are expressed in smooth muscle and have been implicated in smooth muscle hypertrophy. TRPC1 and TRPC6 are also believed to be mechanosensitive, responding to stimuli from food present in the stomach [61]. In this way, they may interact with environmental factors such as prone position for sleep.
IHPS has been described in a variety of syndromes including Cornelia de Lange, Apert, Opitz FG, Marden-Walker, Smith-Lemli-Opitz, Zellweger, trisomy 21, trisomy 18, duplication 1q, duplication 9q, deletion 11q, ring 12 [62], Denys-Drash [63], and paramyotonia congenita [64].
Environmental factors
A variety of environmental and mechanical factors have been implicated in the occurrence of IHPS. A study of isolated IHPS in Sicily by Bianca and colleagues [65] established the association of IHPS with primiparity and low parity status. There was a suggestion that the increased ratio of affected boys to affected girls may be associated with greater parity. There was no association with birth weight or maternal age. Increased incidence of IHPS has been reported in infants exposed prenatally to thalidomide, hydantoins, and trimethadione [62] and those exposed postnatally to erythromycin [66–69]. Possible postnatal environmental factors include feeding practices, maternal smoking, and infant sleeping position [56].
Prone sleeping may be an environmental risk factor for IHPS, which could account for lower occurrence of IHPS in Asian people, who routinely place infants in a supine position for sleep [57]. It has been speculated that pooling of a feed in the antrum, with prone sleep position, may lead to dysmotility of the stomach or pylorus caused by the effects on function of the stomach and/or pylorus via proteins sensitive to change in volume, pressure, or solute concentration.
The gene for motilin (MLN), a hormone that induces gastric contractions, was investigated as a candidate gene for IHPS because treatment with erythromycin, a motilin agonist, is associated with increased risk for IHPS. No association was found between MLN sequence variants and IHPS, although this does not exclude a role for motilin in the pathogenesis of this disorder [70]. Larger milk curds produced by artificial milk formulas may be more likely to cause obstruction in association with pylorospasm. A 30-year retrospective study in Nigeria suggests that exclusive breastfeeding was associated with reduced risk for IHPS [71]; however, this observation requires confirmation with a larger study that includes a control group.
Rogers [72] proposed that inheritance of high parietal cell mass results in hyperacidity, which causes repeated pyloric contraction, leading to pyloric hypertrophy and stenosis. Neonates normally have higher levels of gastrin, with higher acidity level, which can explain the development of IHPS within the first few weeks of life. Godwin and colleagues [73] found increased incidence of pyloric stenosis in Canada after grain products were fortified with folic acid for prevention of neural tube defects. However, no causal relationship was established and there was evidence that the rate of IHPS was increasing independently of folic acid supplementation.