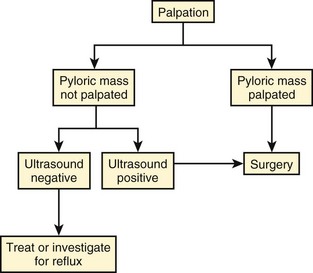
Hypertrophic Pyloric Stenosis
Overview
Hypertrophic pyloric stenosis (HPS) is the most common surgical entity affecting infants during the first 6 months of life.1 It has an incidence of approximately 2 to 5 per 1000 births among children of European descent, but its incidence is much lower in other populations—approximately 0.7 per 1000 births among children of African American or Asian extraction. The preponderance among male infants is well known, variably cited as 2.5 to 5.5 : 1.2 Its incidence is inversely proportional to birth order, with odds ratio to birth order cited as 1, 2, 3, 4+ being roughly equivalent to increased risk levels of 1.9, 1.5, 1.3, and 1.0.3 The condition demonstrates a familial predisposition, suggesting a polygenic inheritance with greater penetrance in males; thus among children of affected fathers, the risk is approximately 5% for sons and 2.5% for daughters, whereas among children of affected mothers, the corresponding risk is 20% for boys and 7% for daughters. Proband concordance in monozygotic twins is cited as between 0.25 and 0.44, whereas in dizygotic twins it is reduced to 0.05 to 0.10, which is very similar to that of nontwin siblings.3
Hirschsprung originally described the entity postmortem in two patients4 and incorrectly assumed that HPS is congenital. Although some exceptions may exist, as discussed later in this chapter, data indicate that HPS is not present at birth, and its symptoms and characteristic anatomic changes typically present between 3 and 12 weeks of age. Rollins et al.5 examined 1400 neonates who had normal ultrasound findings at birth; HPS later developed in nine of these subjects. These findings and others lend credence to the belief that the disease evolves over a course of days to weeks.6–8 Occasional case reports indicate that the diagnosis of HPS is made earlier in life, but documentary proof often is incomplete.9
Anatomy
The stomach is divided by the incisura angularis into the body and fundus proximally and the antrum distally. The latter is further subdivided by the sulcus intermedius into a pyloric vestibule proximally and a pyloric antrum or pyloric canal distally, which is approximately 2.5 cm in length and terminates at the pyloric sphincter and orifice, which lead into the duodenum10 (Fig. 101-1).
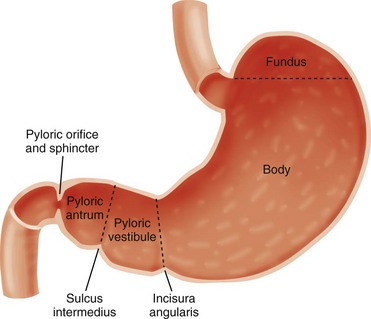
Figure 101-1 Schematic illustration of gastric segmental anatomy.
Abnormal
In infants with HPS, the pyloric antrum is no longer distensible and therefore is no longer clearly demarcated from the pyloric sphincter. The entire complex presents as one elongated canal with thickened muscular walls and filled with edematous mucosa. The thickened muscular walls are unable to relax, thus preventing the normal distensibility of the canal, and the lumen is obstructed by redundant, thickened, and hyperemic folds of mucosa (Fig. 101-2, A and B).6,11,12
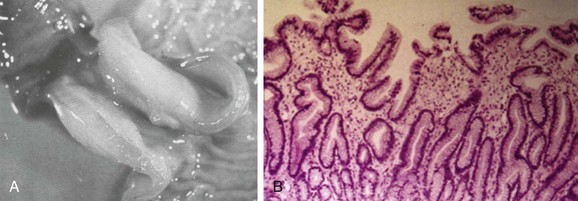
Figure 101-2 Histopathologic anatomy.
A, A gross postmortem specimen of hypertrophied pylorus in an infant who succumbed to this condition. Note the thickened muscle and the thickened mucosa, which would fill and obstruct the lumen of the unsectioned antropyloric canal. B, A histologic specimen of antropyloric mucosa in an infant with pyloric stenosis with typical presentation, operative findings, and postoperative recovery. The hematoxylin and eosin stain shows mucosal hyperplasia with elongated, branched, and mildly distorted pits and abundant edematous lamina propria.
Etiology
Although the diagnosis and surgical treatment of HPS have undergone a remarkable evolution during the past century, its cause remains unknown. The final common pathway seems to be one of work hypertrophy of the circular muscle of the pylorus, as if it were under constant stimulation, and it becomes unable to relax; the mucosa also becomes edematous and hypertrophied, sometimes markedly, simulating a polypoid mass.13,14
Because of a lack of an obvious etiologic agent or process, associated findings have been linked to etiology—for example, to a paucity of omentum (likely due to emaciation) and to “higher development of the nervous system” in children of the “intellectual classes”18 in the eighteenth and early twentieth centuries. More recently, attention has centered on the hypertrophied muscle. The thickened muscle is depleted of inhibitory peptides (such as vasoactive polypeptide); synaptic vesicles, presynaptic terminals, and neural cell adhesion molecules; markers for enteric glia; interstitial cell of Cajal; and nitric oxide synthetase activity at the messenger ribonucleic acid level, with increases in insulin-like and platelet-derived growth factors. Immunoreactivity studies evaluating desmin content suggest immaturity of the intermediate filaments in the hypertrophied pyloric muscle.19–29 The mucosa is thickened and edematous, and both muscle and mucosa are hyperemic when evaluated with color and spectral Doppler imaging.6,13 Despite the extensive anatomic and biochemical changes documented in persons with HPS, as early as 4 months after surgery, the anatomic changes and assays for nerve growth factor, interstitial cells of Cajal, and nitric oxide synthase activity revert to normal.27,30
Prostaglandin E2 generation in the gastric mucosa and increased concentration in the gastric secretions of patients with HPS have been reported.31 Prostaglandin E1 and E2 have been reported to induce proliferation of gastric mucosa32 and are related to muscle contraction in the human gastrointestinal tract23; prostaglandin therapy has preceded the development of HPS requiring surgery.33 The macrolide antibiotic erythromycin is a prokinetic agent and has been associated with increased risk of developing HPS in some series, particularly when the infants are exposed very early in life; however, further studies have determined that the link, if it exists, is weak and the potential risk is very small.3 More recently, genetic susceptibility loci have been identified, including chromosomes 16p12-p13 and 11q14-q22 and genes encoding neuronal nitric oxide synthase on chromosome 12q.15–17
Clinical Presentation
Babies with HPS typically present with a history of forceful nonbilious vomiting, often described as “projectile,” that may be relatively sudden in onset or preceded by initially mild symptoms consistent with gastroesophageal reflux. If the diagnosis is not made promptly, protracted vomiting leads to dehydration and to the development of hypochloremic metabolic alkalosis, with paradoxical aciduria as renal mechanisms designed to maintain intravascular volume supervene, conserving sodium at the expense of hydrogen ions. Starvation can exacerbate the effect of the diminished hepatic glucuronyl transferase activity, leading to indirect hyperbilirubinemia in a small number of infants. In patients who present after significant weight loss and who are thin and dehydrated, peristaltic waves may be seen on the wall of the scaphoid abdomen, progressing from the left upper quadrant across the epigastrium, flanked by a protuberant rib cage.11 Currently, most infants present earlier, with less severe physical findings and laboratory abnormalities. Investigators have emphasized the changing pattern of presentation of infants with HPS; most infants presenting today do not have evidence of metabolic abnormalities, and it has been suggested that the increased availability of ultrasound may be a factor in earlier diagnosis.34–36
Diagnosis and Imaging
Physical examination in experienced hands often is diagnostic through palpation of the “olive” or “pyloric tumor,” terms often used to designate the tactile findings in persons with HPS. This examination requires a calm infant and a committed examiner willing to dedicate 15 minutes or longer to the examination, which may require decompression of the overdistended stomach via a nasogastric tube.37 If the olive is palpable, the examination is diagnostic. The sensitivity of abdominal palpation varies with the experience of the examiner and ranges between 24% and 99%; specificity ranges between 92% and 99%.37–41 False-positive results do occur and have been reported to be as high as 14%, with anatomic variants such as unusual extensions of the left lobe of the liver and congenital abnormalities such as malrotation and duplication cysts.42,43
The documented accuracy of ultrasound diagnosis has generated a trend toward reliance on imaging rather than on the potentially more protracted physical examination. Although the loss of clinical skills may be lamentable in some respects, and some persons have argued that imaging increases the financial cost of the workup of HPS, other investigators have recognized that, despite “fading skills,” the rapidity and accuracy of ultrasound diagnosis can result in “better patients,” that is, better surgical candidates at the time of diagnosis.34,35
Radiographic Studies
Plain radiographs may strongly suggest the diagnosis, revealing a markedly distended stomach, particularly if several peristaltic waves are seen, with little gas distally (Fig. 101-3),44 although absence of these findings does not negate the diagnosis. In rare instances, HPS has been reported in association with isolated gastric pneumatosis that resolves after the stomach is decompressed (e-Fig. 101-4).45
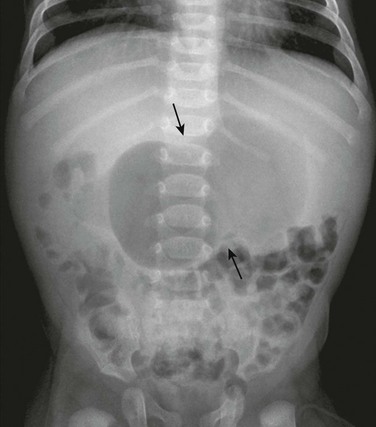
Figure 101-3 An abdominal radiograph in an infant with pyloric stenosis.
An abdominal radiograph demonstrates a distended stomach with a peristaltic wave (arrows), with normal to mildly decreased distal gas. In the appropriate clinical setting, these findings are highly suggestive of pyloric stenosis.
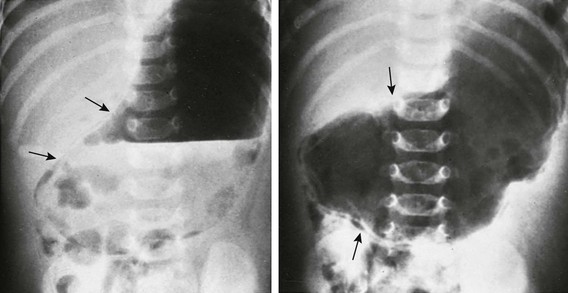
e-Figure 101-4 Gastric pneumatosis in an infant with pyloric stenosis.
Supine abdominal radiographs in a 6-week-old girl with pyloric stenosis shows a markedly dilated stomach with several peristaltic waves and decreased distal gas. Intramural gas is present (arrows), which resolved within 24 hours after decompression with gastric intubation. (Courtesy Dr J. Leonidas, New Hyde Park, NY.)
Upper Gastrointestinal Series
In addition to a delay in the passage of contrast material from the stomach, several other radiographic signs are present in infants with HPS. The pyloric channel is narrowed, and as contrast material begins to enter the narrowed channel, it may appear as a “beak” that evolves into a “string” or a “double tract” sign as contrast material courses between the interstices of the luminal mucosa, compressed by the thickened, unrelaxing antropyloric muscle, which typically is curved upward and posteriorly (Fig. 101-5). The enlarged muscle mass encroaches upon the lumen of the antrum proximally, resulting in the “shoulder sign”; when the duodenal base is also deformed by the thickened muscle, the appearance may resemble an “apple core” lesion. The “pyloric tit” occasionally can be seen along the lesser curve just proximal to the impression of the pyloric mass; it represents contrast material trapped within the lumen of the stomach, compressed between a peristaltic wave and the impression of the pyloric muscle upon the adjacent, more distal portion of the stomach. Virtually all of the aforementioned signs can be seen transiently in most infants. The study should document the persistence of the findings to ensure the diagnosis of HPS.
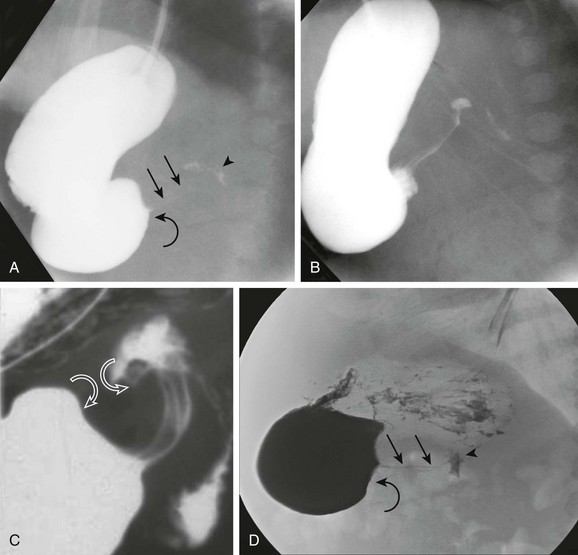
Figure 101-5 Upper gastrointestinal series in patients with pyloric stenosis.
A, An orogastric tube was used to evacuate the stomach, and barium was instilled under fluoroscopic control. The image shows a peristaltic wave and a small amount of contrast entering the narrowed canal, demonstrating both a beak (curved arrow) and string sign (straight arrows). The arrowhead points to the duodenal bulb. B, Shortly after the image shown in part A, additional contrast material egressed into the pyloric channel, now outlining a double tract proximally. C, An oblique radiograph in another infant with pyloric stenosis shows mass impression upon the proximal antrum and the distal duodenal base (curved arrows), spanned by the upwardly and posteriorly directed antropyloric canal with three contrast tracts coursing through the intraluminal mucosal folds. D, An oblique radiograph of the same infant shown in Fig. 101-8, C. Curved arrows point to the mass impression of the pyloric muscle on the gastric antrum, or “shoulder sign.”
Ultrasonography
Ultrasound was first applied to the diagnosis of HPS in five cases described in 1977 utilizing B-mode sonography.46 Since that time, ultrasound has become the modality of choice in the diagnosis of HPS in most pediatric centers and is considered the “gold standard” by many investigators.11,35,37 Unlike the upper GI test, this examination does not require contrast material to traverse the obstructed canal for diagnosis, and thus the diagnosis can be made quickly, without the need for additional distension of the stomach and without radiation exposure to the infant, which can be prolonged in infants with a high degree of obstruction.
The examination is begun with the patient in the supine position. The transducer is placed in the transverse plane over the gastroesophageal junction, which is constant in location anterior to the aortic hiatus, and the transducer is then swept along the length of the stomach until the distal antrum and duodenal cap are identified; once the duodenal bulb is identified, the immediately proximal antropyloric canal can be evaluated for morphology and relaxation, thus identifying either HPS or a normal antropyloric canal (Fig. 101-6). The abnormal pylorus is typically, but not invariably, found just medial to the gallbladder, anterior to the right kidney.
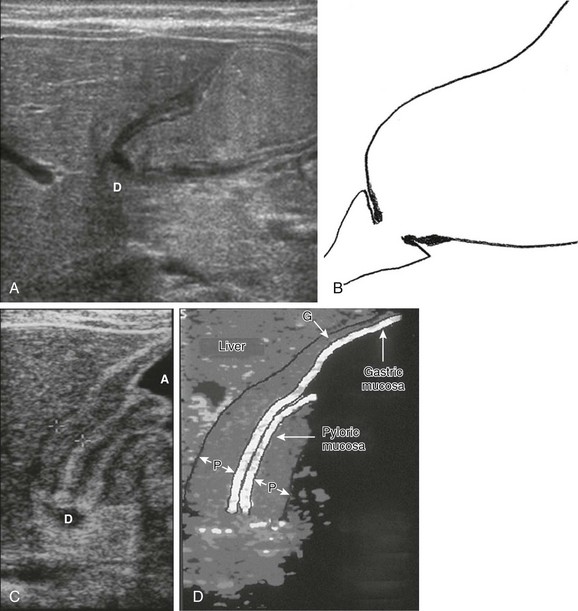
Figure 101-6 Antropyloric canals with schematic drawings.
A, Ultrasonogram in an infant with a normal pylorus. The stomach is distended with formula ingested just prior to the examination. The pylorus is normal. D, Duodenal bulb. B, A schematic drawing of findings in part A. C, Ultrasonogram in a child with pyloric stenosis. The pyloric muscle (between crosshairs) is thickened, measuring >3 mm; the mucosa is hypertrophied, filling the lumen and protruding into the fluid-filled antrum (A). D, Duodenal bulb. D, A schematic drawing of the findings in part C. G, Gastric wall muscle; P, pyloric muscle.
Much emphasis has been placed on the measurements used to diagnose HPS. Certainly, these measurements are helpful and necessary, but they can be confusing because they vary among different patients, in the same patient, and among various series published over three decades, paralleling an increase in operator experience and equipment resolution. The initial and seminal report of static B-mode ultrasound in HPS by Teele and Smith, published in the New England Journal of Medicine in 1977,46 used a pyloric diameter of 1.8 to 2.8 cm (mean, 2.5 cm) for diagnosis. Muscle thickness became more important in subsequent reports with real-time capability.
A 1986 prospective study of 200 infants with HPS who underwent scanning with a mechanical sector transducer operating at 7.5 MHz found a mean muscle thickness of 3.4 mm (range, 3 to 5 mm) and a mean pyloric length of 22.3 mm (range, 18 to 28 mm) that discriminated between normal and abnormal patients with a 100% success rate.43 A subsequent study of 323 patients found that mean muscle thickness was 4.8 mm (range, 3.5 to 6 mm) and mean pyloric length was 17.8 mm (range, 11 to 25 mm)47; these investigators found muscle thickness to be the most helpful discriminant, with an accuracy of 99.4%. In a 1991 series by O’Keeffe et al.,7 who reported on 145 consecutive patients, a muscle thickness of 3 mm or greater was determined to be diagnostic of HPS, with muscle thickness less than 1.5 mm in 98% of normal patients. These results were validated in a 1993 series by Hernanz-Schulman et al.,48 who evaluated 152 infants and found that a persistent muscle thickness of 3 mm or greater was diagnostic of HPS, with no false-positive examinations.
In younger and previously premature infants, the tendency exists for pyloric measurements to be smaller at presentation than in full-term, older infants.48,49 In a more recent review of these patients, a significant association was found between pyloric dimensions and patient age and weight; however, these changes did not have an impact on their existing diagnostic criteria for HPS: a muscle thickness of 3 mm or greater and length 15 mm or greater.50
A normal antropyloric channel has a sonographic appearance similar to that at upper GI, with a relaxed, open lumen directly adjacent to the duodenal bulb via the pyloric orifice (see Figs. 101-6, A, and 101-7).
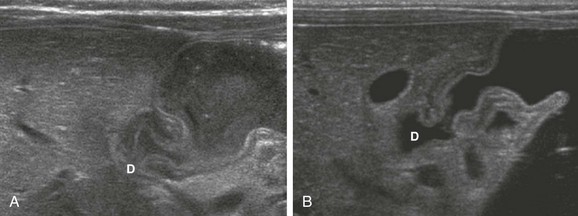
Figure 101-7 A normal antropyloric canal in two infants.
A, The stomach is filled with formula. Note that the antropyloric muscle is mildly thickened, but the mucosa is normal, and the antropyloric canal is distensible and filled with gastric contents. D, Duodenal bulb. B, The stomach is filled with Pedialyte. Again, the antropyloric muscle is mildly thickened, but the lumen is distensible and filled with gastric contents. D, Duodenal bulb.
The abnormal antropyloric canal, on the other hand, shows persistent thickening of the muscle and mucosa to variable degree (Fig. 101-8). The length of the hypertrophied canal is variable, ranging from as little as 14 mm to greater than 20 mm. The lower limit of persistent muscle thickness is 3 mm, without evidence of relaxation of the antropyloric canal throughout the examination.11 Evaluation of the patient over time is important. Increased but transient contractions of the pylorus, or pylorospasm, may mimic HPS in both appearance and measurement if the examination is performed quickly (Fig. 101-9).51 The hypertrophied pyloric muscle and mucosa typically show abundant flow as seen on color and spectral Doppler interrogation (e-Fig. 101-10). Although the patient might not have been fed for hours, the stomach typically is distended. However, it is important to realize that despite the lack of relaxation of the abnormal antropyloric canal, some gastric contents will pass through in many patients during the period of observation. HPS is not a complete obstruction; diagnosis by an upper GI examination is obviously based upon this fact (e-Fig. 101-11, Video 101-1). In some patients, for reasons that are not yet clear and with an unknown temporal relationship to the development of this condition, the muscle is very active, and visible contractions temporarily shorten and thicken, as well as lengthen and thin, the pyloric muscle as seen on real-time ultrasound. The appearance suggests that more proximal muscle that has not lost its ability to relax and return to normal is being actively recruited, an intriguing finding when considering the development and etiology of HPS (e-Fig. 101-12, Video 101-2).
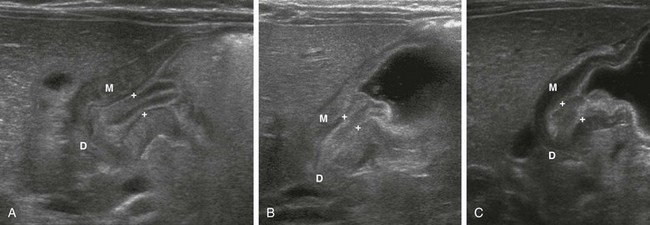
Figure 101-8 Pyloric stenosis in three infants.
A, An abnormal pylorus shows a double layer of edematous mucosa (between crosshairs) sandwiched within the thickened muscle (M), protruding into the gas and formula-filled antrum as the “nipple sign.” D, Duodenal bulb. B, An abnormal pylorus in another infant shows thickened muscle (M) and mucosa (between crosshairs) again with protrusion into the proximal antrum. D, Duodenal bulb. C, An infant with progressive vomiting and poor weight gain. The muscle (M) is thickened to >3 mm and the mucosa (between crosshairs) measured 8 mm in thickness, also protruding proximally into the fluid-filled antrum. D, Duodenal bulb. The same infant as in Figure 101-5, D.
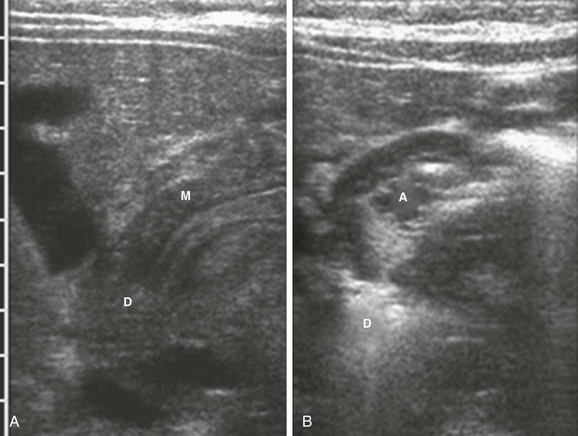
Figure 101-9 Transient ultrasound findings.
A, The antropyloric channel in an infant with vomiting shows narrowing with thickened muscle that measured up to 4 mm in thickness. M, Muscle; D, duodenal bulb. B, The antropyloric channel in the same infant as depicted in A, 4 minutes later. The canal is open. A, Open antropyloric canal; D, duodenal bulb.
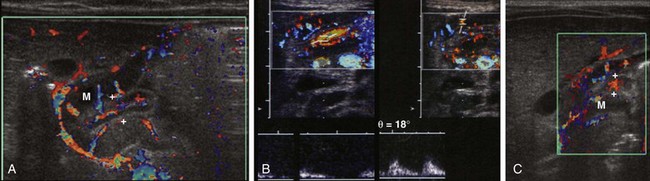
e-Figure 101-10 Doppler flow in pyloric stenosis.
A, The antropyloric canal in the same infant shown in Fig. 101-8, A. Note flow within the hypertrophied muscle (M) and mucosa (between crosshairs). B, The spectral evaluation of color flow in the antropyloric canal of another infant demonstrates flow waveform spectra in mucosa (left) and muscle (right). C, The antropyloric canal in the same infant as shown in Fig. 101-8, C. Again note flow within the hypertrophied muscle (M) and mucosa (between crosshairs).
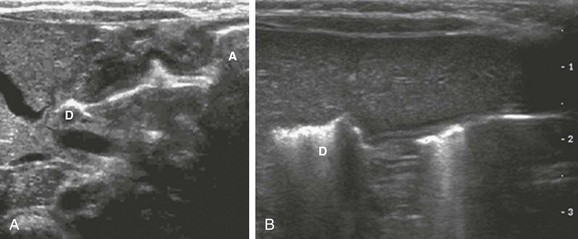
e-Figure 101-11 Air passing through both abnormal and normal pyloric canal (see Video 101-1).
A, A 3-week-old boy with pyloric stenosis. Hyperechoic air is seen along the anterior wall of the gastric antrum (A) and continues through the hypertrophied antropyloric canal into the duodenal cap. This finding was observed in real time. D, Duodenal bulb. B, In a 4-week-old boy with a normal pylorus, hyperechoic gas also is shown moving through the nearly empty but normal antropyloric canal, into the duodenal bulb. D, Duodenal bulb.
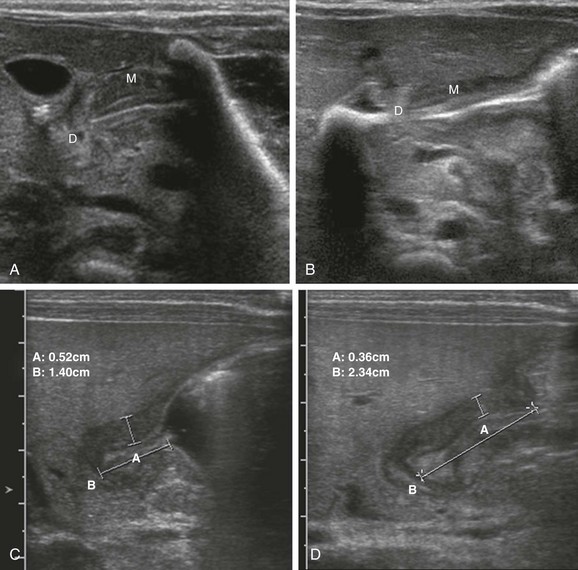
e-Figure 101-12 Changing dimensions (see Video 101-2).
A, A 3-week-old boy with pyloric stenosis. A longitudinal image of the antropyloric canal shows a thickened muscle, with a relatively short canal. M, Muscle; D, duodenal bulb. B, In the same patient during another portion of the study, a longer canal is seen with thinner muscle. M, Muscle; D, duodenal bulb. C, A 4-week-old boy with pyloric stenosis. A longitudinal image of the antropyloric canal shows a thickened muscle (between vertical calipers, measurement A) measuring approximately 5.2 mm and a relatively short canal (between horizontal calipers, measurement B), measuring 14 mm. The label B marking the horizontal calipers overlies the duodenal bulb. D, In the same patient as in C, 2 minutes later, a thinner muscle is seen (between vertical calipers, measurement A) measuring approximately 3.6 mm, and a longer canal is seen (between horizontal calipers, measurement B), measuring 23.4 mm. The label B marking the horizontal calipers overlies the duodenal bulb.
Pitfalls
The normal pylorus might be difficult to visualize because of excessive amounts of overlying bowel gas. In such cases, it is helpful to use the left lobe of the liver as an acoustic window and angle inferiorly to identify the pylorus. If the stomach is empty, the child is unlikely to have HPS; in such cases, the child may be given Pedialyte or glucose water, which will not only improve the ability to identify the pylorus and duodenal bulb, but also will allow definitive documentation of the normal anatomy (Fig. 101-13).11,48,52
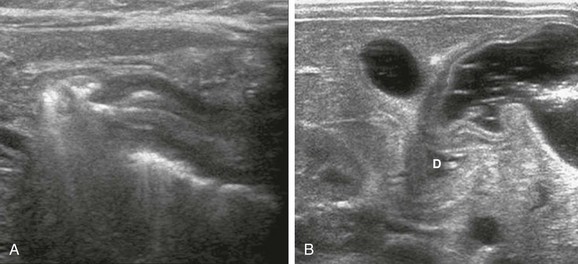
Figure 101-13 An empty stomach mimicking pyloric stenosis.
A, An 8-week-old girl with vomiting. The stomach initially was empty, which is initial evidence against the presence of pyloric stenosis in most infants. However, the collapsed distal antropyloric canal appears “elongated,” thus mimicking some of the findings in pyloric stenosis. The muscle measured between 1.7 and 2.8 mm. B, After the infant drank Pedialyte, the antrum distended with a completely normal appearance. D, Duodenal bulb.
In patients with HPS, the pylorus may be difficult to identify because a very distended stomach displaces it posterior to the antrum; this displacement is also the cause of one of the false-negative pitfalls in palpation (Fig. 101-14). Some investigators have advocated emptying the stomach with a nasogastric catheter,53 but we prefer to turn the supine infant toward his or her left side, thus displacing the bulk of the fluid toward the fundus and allowing the hypertrophied pylorus to rise anteriorly for optimal evaluation.
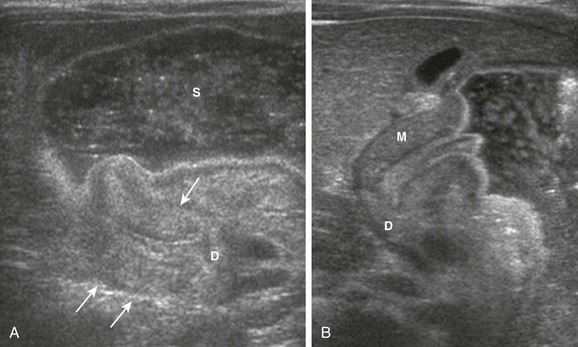
Figure 101-14 A pitfall when scanning an overdistended stomach in an infant with pyloric stenosis.
A, An image of the overdistended distal stomach in an infant with pyloric stenosis shows the hypertrophied pylorus (arrows) folded behind the stomach (S). D, Duodenal bulb. B, When the infant is turned toward a left posterior oblique position, the hypertrophied pylorus is allowed to rise anteriorly and is much more easily detected and detailed. Note the pyloric mucosa protruding into the fluid-filled antrum. M, Muscle; D, duodenal bulb.
Equivocal examinations occur when, after sufficient observation, the muscle measures between 2 and 3 mm in thickness. In such cases, follow-up examination is indicated if the patient continues to vomit. The rate of evolution of HPS is not known; also unknown are whether the process begins with pylorospasm, potential inciting event(s), and the time course. Therefore in equivocal cases, follow-up sonography is indicated.7,8,12
Differential Diagnosis
The most common antropyloric abnormality mimicking HPS on an upper GI series is pylorospasm. Although a spasm also may cause the pyloric muscle to appear thicker on ultrasound, a spasm is a transient phenomenon usually distinguishable from HPS, or which may necessitate a follow-up examination, as previously discussed. Focal foveolar hyperplasia, a polypoid mucosa in the antropyloric portion of the stomach, usually is associated with a history of congenital heart disease and prostaglandin therapy. The lobulated mucosal mass has been reported to cause gastric outlet obstruction and has developed into full-fledged HPS in some cases.32,33,54
Diagnostic Algorithm
Palpation of the pyloric mass is the first diagnostic examination by the physician. In further evaluation and referral, consideration can be given to the investment of time and relative invasiveness of inserting an orogastric tube to evacuate the stomach and for surgical or imaging referral. The cost and time delay of obtaining a surgical consultation/visit versus the cost, time delay, and accuracy of an imaging examination may differ across locations and clinical practices. If the pyloric mass is palpated by the surgeon, no diagnostic imaging is necessary; if it cannot be palpated by the surgeon, imaging should be considered. Waiting and reexamining the patient with prolongation of inpatient care likely is not cost-effective in most settings and may lead to delay in diagnosis and treatment. We do not advocate sedating an infant to increase sensitivity in palpating the pyloric mass, as has been suggested in the past.38
Some investigators have advocated use of the upper GI series as the first examination in patients with symptoms of HPS for two reasons: (1) although ultrasound may be quite accurate in the diagnosis of HPS, it is not always diagnostic in infants with vomiting from other causes, and (2) an upper GI series is deemed by some persons to be more cost effective as the first imaging examination compared with ultrasound. Because a follow-up upper GI series may be requested in patients with normal ultrasound findings, a cost savings could be realized if the upper GI series is performed instead of ultrasound.41,55 However, ultrasound images can be obtained beyond the lumen of the pylorus and can assess the thickness of the muscle, as well as any adjacent masses or other lesions, and there is no need to further distend the stomach with the addition of contrast media. More importantly, because we adopt the “as low as reasonably achievable” approach to pediatric imaging, we believe that ultrasound should be the first imaging examination in patients suspected of HPS, particularly in young infants in whom fluoroscopy could be prolonged.11,48,56 If the ultrasound shows that the pylorus is normal, vomiting is clearly nonbilious, and the duodenum is normal in caliber, the likelihood is high that the cause of vomiting is gastroesophageal reflux, which initially could be treated empirically or documented with scintigraphy (Fig. 101-15).
Treatment
Surgical pyloromyotomy, introduced by Ramstedt in 1912, is the traditional therapy for infants with HPS. In this procedure, the muscle is incised and the mucosa is allowed to protrude through the incision without resuturing (Fig. 101-16).
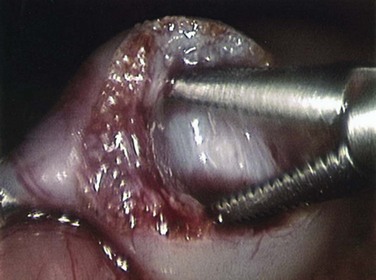
Figure 101-16 An intraoperative photograph of a muscle-splitting Ramstedt pyloromyotomy. (Courtesy Dr. Robert Cywes.)
Several publications have redirected attention to medical therapy, which is based on the theory that muscle spasm is a contributing factor to the muscular hypertrophy and obstruction. In a prospective trial of infants treated with surgical or medical therapy, the latter was successful in 85% of medical cases, with 2 of 14 requiring a pyloromyotomy; mean time to full feeds was 2.7 days in the surgical group and 5.3 days in the medically treated group.57 In a larger prospective trial including 52 medically treated infants, medical therapy had a 87% success rate; the mean hospital stay was 5 days in the surgical group and 13 days in the successfully medically treated group.58 Another trial reported a 75% success rate of medical therapy, with prolonged resolution of symptoms resulting in some of the parents in the medical treatment group opting for surgery before completion of medical treatment.59
Laparoscopic pyloromyotomy, which was first reported in 1991,60 has been advocated recently by some pediatric surgeons. Metaanalyses and prospective trials suggest that mucosal perforation, a complication of both open and laparoscopic procedures, may be more problematic with laparoscopy, because initially the perforation may not be recognized and thus require reoperation. Wound infection appears to be slightly less frequent with laparoscopy. Although cosmetic results are superior with laparoscopy, the data to date suggest that, once the learning curve for the laparoscopic approach is mastered, little difference exists in the overall outcome between these two procedures, with some prospective studies suggesting that the laparoscopic group shows less postoperative emesis and requires less postoperative analgesia.61–65
Balloon dilation has been attempted unsuccessfully in a limited number of patients; however, a potential role after failed pyloromyotomy or recurrent HPS has been investigated with partial success in a very limited number of patients.66–68
References
1. Oue, T, Puri, P. Smooth muscle cell hypertrophy versus hyperplasia in infantile hypertrophic pyloric stenosis. Pediatr Res. 1999;45(6):853–857.
2. Mitchell, LE, Risch, N. The genetics of infantile hypertrophic pyloric stenosis. A reanalysis. Am J Dis Child. 1993;147(11):1203–1211.
3. MacMahon, B. The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology. 2006;17(2):195–201.
4. Hirschsprung, H. Falle von angeborener pylorusstenose, beobachtet bei Sauglingen. Jahrb d. Kinderh. 1888;27:61–68.
5. Rollins, MD, et al. Pyloric stenosis: congenital or acquired? Arch Dis Child. 1989;64(1):138–139.
6. Hernanz-Schulman, M, et al. Hypertrophic pyloric stenosis in infants: US evaluation of vascularity of the pyloric canal. Radiology. 2003;229(2):389–393.
7. O’Keeffe, FN, et al. Antropyloric muscle thickness at US in infants: what is normal? Radiology. 1991;178(3):827–830.
8. Keckler, SJ, et al. The progressive development of pyloric stenosis: a role for repeat ultrasound. Eur J Pediatr Surg. 2008;18(3):168–170.
9. Singh, SJ, et al. Antenatal prediction of hypertrophic pyloric stenosis. Pediatr Surg Int. 2001;17(7):560–562.
10. Gray, H. The digestive system. In: Goss C, ed. Anatomy of the human body. Philadelphia: Lea & Febiger; 1973:1220.
11. Hernanz-Schulman, M. Infantile hypertrophic pyloric stenosis. Radiology. 2003;227(2):319–331.
12. Hernanz-Schulman, M, et al. Hypertrophied pyloric mucosa in patients with hypertrophic pyloric stenosis. Pediatr Radiol. 1998;28(11):901.
13. Hernanz-Schulman, M, et al. In vivo visualization of pyloric mucosal hypertrophy in infants with hypertrophic pyloric stenosis: is there an etiologic role? AJR Am J Roentgenol. 2001;177(4):843–848.
14. Markowitz, RI, et al. Infantile hypertrophic pyloric stenosis—congenital or acquired? J Clin Gastroenterol. 1982;4(1):39–44.
15. Capon, F, et al. Linkage of monogenic infantile hypertrophic pyloric stenosis to chromosome 16p12-p13 and evidence for genetic heterogeneity. Am J Hum Genet. 2006;79(2):378–382.
16. Everett, KV, et al. Linkage of monogenic infantile hypertrophic pyloric stenosis to chromosome 16q24. Eur J Hum Genet. 2008;16(9):1151–1154.
17. Panteli, C. New insights into the pathogenesis of infantile pyloric stenosis. Pediatr Surg Int. 2009;25(12):1043–1052.
18. Mack, H. A history of hypertrophic pyloric stenosis and its treatment. Bull History Med. 1942;XII(3):465–689.
19. Kobayashi, H, O’Briain, DS, Puri, P. Immunochemical characterization of neural cell adhesion molecule (NCAM), nitric oxide synthase, and neurofilament protein expression in pyloric muscle of patients with pyloric stenosis. J Pediatr Gastroenterol Nutr. 1995;20(3):319–325.
20. Kusafuka, T, Puri, P. Altered messenger RNA expression of the neuronal nitric oxide synthase gene in infantile hypertrophic pyloric stenosis. Pediatr Surg Int. 1997;12(8):576–579.
21. Langer, JC, Berezin, I, Daniel, EE. Hypertrophic pyloric stenosis: ultrastructural abnormalities of enteric nerves and the interstitial cells of Cajal. J Pediatr Surg. 1995;30(11):1535–1543.
22. Malmfors, G, Sundler, F. Peptidergic innervation in infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1986;21(4):303–306.
23. Ohshiro, K, Puri, P. Increased insulin-like growth factor and platelet-derived growth factor system in the pyloric muscle in infantile hypertrophic pyloric stenosis. J Pediatr Surg. 1998;33(2):378–381.
24. Ohshiro, K, Puri, P. Increased insulin-like growth factor-I mRNA expression in pyloric muscle in infantile hypertrophic pyloric stenosis. Pediatr Surg Int. 1998;13:253–255.
25. Okazaki, T, et al. Abnormal distribution of nerve terminals in infantile hypertrophic pyloric stenosis. J Ped Surg. 1994;29:655–658.
26. Vanderwinden, J, et al. Nitric oxide synthase activity in infantile hypertrophic pyloric stenosis. N Engl J Med. 1992;327:511–515.
27. Vanderwinden, JM, et al. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis [published erratum appears in Gastroenterology 1996 Nov;111(5):1403]. Gastroenterology. 1996;111(2):279–288.
28. Wattchow, D, et al. Abnormalities of peptide-containing nerve fibers in infantile hypertrophic pyloric stenosis. Gastroenterology. 1987;92:443–448.
29. Guarino, N, Shima, H, Puri, P. Structural immaturity of the pylorus muscle in infantile hypertrophic pyloric stenosis. Pediatr Surg Int. 2000;16(4):282–284.
30. Yoshizawa, J, et al. Ultrasonographic features of normalization of the pylorus after pyloromyotomy for hypertrophic pyloric stenosis. J Pediatr Surg. 2001;36(4):582–586.
31. Shinohara, K, et al. Correlation of prostaglandin E2 production and gastric acid secretion in infants with hypertrophic pyloric stenosis. J Pediatr Surg. 1998;33(10):1483–1485.
32. Peled, N, et al. Gastric-outlet obstruction induced by prostaglandin therapy in neonates. N Engl J Med. 1992;327(8):505–510.
33. Callahan, MJ, et al. The development of hypertrophic pyloric stenosis in a patient with prostaglandin-induced foveolar hyperplasia. Pediatr Radiol. 1999;29(10):748–751.
34. Chen, E, et al. Pyloric stenosis in the age of ultrasonography: fading skills, better patients? J Pediatr Surg. 1996;31(6):829–830.
35. Glatstein, M, et al. The changing clinical presentation of hypertrophic pyloric stenosis: The experience of a large, tertiary care pediatric hospital. Clin Pediatr (Phila). 2011;50(3):192–195.
36. Papadakis, K, et al. The changing presentation of pyloric stenosis. Am J Emerg Med. 1999;17(1):67–69.
37. Schwartz, MZ. Hypertrophic pyloric stenosis. In: Grosfeld J, et al, eds. Grosfeld: pediatric surgery. Philadelphia: Mosby Elsevier, 2006.
38. Freund, H, et al. Diagnosis of pyloric stenosis. Lancet. 1976;2(7983):473.
39. Godbole, P, et al. Ultrasound compared with clinical examination in infantile hypertrophic pyloric stenosis. Arch Dis Child. 1996;75(4):335–337.
40. Macdessi, J, Oates, R. Clinical diagnosis of pyloric stenosis: a declining art. BMJ. 1993;306:553–555.
41. White, MC, et al. Sensitivity and cost minimization analysis of radiology versus olive palpation for the diagnosis of hypertrophic pyloric stenosis. J Pediatr Surg. 1998;33(6):913–917.
42. Scharli, A, Sieber, WK, Kiesewetter, WB. Hypertrophic pyloric stenosis at the Children’s Hospital of Pittsburgh from 1912 to 1967. A critical review of current problems and complications. J Pediatr Surg. 1969;4(1):108–114.
43. Stunden, RJ, LeQuesne, GW, Little, KE. The improved ultrasound diagnosis of hypertrophic pyloric stenosis. Pediatr Radiol. 1986;16(3):200–205.
44. Riggs, W, Jr., Long, L. The value of the plain film roentgenogram in pyloric stenosis. Am J Roentgenol Radium Ther Nucl Med. 1971;112(1):77–82.
45. Lim, R, et al. Massive gastric pneumatosis from pyloric stenosis. CMAJ. 2010;182(5):E227.
46. Teele, RL, Smith, EH. Ultrasound in the diagnosis of idiopathic hypertrophic pyloric stenosis. N Engl J Med. 1977;296(20):1149–1150.
47. Blumhagen, JD, et al. Sonographic diagnosis of hypertrophic pyloric stenosis. AJR Am J Roentgenol. 1988;150(6):1367–1370.
48. Hernanz-Schulman, M, et al. Hypertrophic pyloric stenosis in the infant without a palpable olive: accuracy of sonographic diagnosis. Radiology. 1994;193(3):771–776.
49. McKeown, T, MacMahon, B, Record, R. Size of tumor in infantile pyloric stenosis related to age at operation. Lancet. 1951;1:556–558.
50. Iqbal, C, et al. Evaluation of ultrasonographic parameters in the diagnosis of pyloric stenosis relative to patient age and size. J Pediatr Surg. 2012;47(8):1542–1547.
51. Cohen, HL, et al. Ultrasonography of pylorospasm: findings may simulate hypertrophic pyloric stenosis. J Ultrasound Med. 1998;17(11):705–711.
52. Blumhagen, JD. The role of ultrasonography in the evaluation of vomiting in infants. Pediatr Radiol. 1986;16(4):267–270.
53. Mandell, GA, et al. Cost-effective imaging approach to the nonbilious vomiting infant. Pediatrics. 1999;103(6 pt 1):1198–1202.
54. Babyn, P, et al. Radiologic features of gastric outlet obstruction in infants after long-term prostaglandin administration. Pediatr Radiol. 1995;25(1):41–43. [discussion 44].
55. Olson, AD, Hernandez, R, Hirschl, RB. The role of ultrasonography in the diagnosis of pyloric stenosis: a decision analysis. J Pediatr Surg. 1998;33(5):676–681.
56. Leonidas, JC. Letter to the Editor: Commentary on Olson AD, et al: The role of ultrasonography in the diagnosis of pyloric stenosis: a decision analysis. J Pediatr Surg. 1999;34(10):1583–1584.
57. Yamataka, A, et al. Pyloromyotomy versus atropine sulfate for infantile hypertrophic pyloric stenosis. J Pediatr Surg. 2000;35(2):338–341. [discussion 342].
58. Kawahara, H, et al. Medical treatment of infantile hypertrophic pyloric stenosis: should we always slice the “olive”? J Pediatr Surg. 2005;40(12):1848–1851.
59. Meissner, PE, et al. Conservative treatment of infantile hypertrophic pyloric stenosis with intravenous atropine sulfate does not replace pyloromyotomy. Pediatr Surg Int. 2006;22(12):1021–1024.
60. Alain, JL, Grousseau, D, Terrier, G. Extramucosal pyloromyotomy by laparoscopy. Surg Endosc. 1991;5(4):174–175.
61. Campbell, BT, et al. Ghosts in the machine: a multi-institutional comparison of laparoscopic and open pyloromyotomy. J Pediatr Surg. 2007;42(12):2026–2029.
62. Fujimoto, T, et al. Laparoscopic extramucosal pyloromyotomy versus open pyloromyotomy for infantile hypertrophic pyloric stenosis: which is better? J Pediatr Surg. 1999;34(2):370–372.
63. Hall, NJ, et al. Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet. 2009;373(9661):390–398.
64. Leclair, MD, et al. Laparoscopic pyloromyotomy for hypertrophic pyloric stenosis: a prospective, randomized controlled trial. J Pediatr Surg. 2007;42(4):692–698.
65. St Peter, SD, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg. 2006;244(3):363–370.
66. Hayashi, AH, et al. Balloon catheter dilatation for hypertrophic pyloric stenosis. J Pediatr Surg. 1990;25(11):1119–1121.
67. Khoshoo, V, et al. Endoscopic balloon dilatation of failed pyloromyotomy in young infants. J Pediatr Gastroenterol Nutr. 1996;23(4):447–451.
68. Nasr, A, Ein, SH, Connolly, B. Recurrent pyloric stenosis: to dilate or operate? A preliminary report. J Pediatr Surg. 2008;43(2):e17–e20.