Chapter 562 Hyperthyroidism
Hyperthyroidism results from excessive secretion of thyroid hormone; during childhood, with few exceptions, it is due to Graves disease (Table 562-1). Graves disease is an autoimmune disorder; production of thyroid-stimulating immunoglobulin (TSI) results in diffuse toxic goiter. Germline mutations of the thyroid-stimulating hormone (TSH) receptor resulting in constitutively activating (gain-of-function) mutations are found in both familial (autosomal dominant) and sporadic cases of non-autoimmune hyperthyroidism. These patients, whose disease can appear in the neonatal period or in later childhood, have thyroid hyperplasia with goiter and suppressed levels of TSH. Different activating mutations have been identified in some cases of thyroid adenomas. Hyperthyroidism occurs in some patients with McCune-Albright syndrome as a result of an activating mutation of the α subunit of the G-protein; these patients tend to have a multinodular goiter. Other rare causes of hyperthyroidism that have been observed in children include toxic uninodular goiter (Plummer disease), hyperfunctioning thyroid carcinoma, thyrotoxicosis factitia, subacute thyroiditis, and acute suppurative thyroiditis.
Table 562-1 CAUSES OF HYPERTHYROIDISM
| CAUSES OF HYPERTHYROIDISM | PATHOPHYSIOLOGIC FEATURES | INCIDENCE |
|---|---|---|
| CIRCULATING THYROID STIMULATORS | ||
| Graves disease | Thyroid-stimulating immunoglobulins | Common |
| Neonatal Graves disease | Thyroid-stimulating immunoglobulins | Rare |
| Thyrotropin-secreting tumor | Pituitary adenoma | Very rare |
| Choriocarcinoma | Human chorionic gonadatropin secretion | Rare |
| THYROIDAL AUTONOMY | ||
| Toxic multinodular goiter | Activating mutations in thyrotropin receptor or G-protein | Common |
| Toxic solitary adenoma | Activating mutations in thyrotropin receptor or G-protein | Common |
| Congenital hyperthyroidism | Activating mutations in thyrotropin receptor | Very rare |
| Iodine-induced hyperthyroidism (Jod-Basedow) | Unknown; excess iodine results in unregulated thyroid hormone production | Uncommon in USA and other iodine-sufficient areas |
| DESTRUCTION OF THYROID FOLLICLES (THYROIDITIS) | ||
| Subacute thyroiditis | Probable viral infection | Uncommon |
| Painless or postpartum thyroiditis | Autoimmune | Common |
| Amiodarone-induced thyroiditis | Direct toxic drug effects | Uncommon |
| Acute (infectious) thyroiditis | Thyroid infection (e.g., bacterial, fungal) | Uncommon |
| EXOGENOUS THYROID HORMONE | ||
| Iatrogenic | Excess ingestion of thyroid hormone | Common |
| Factitious | Excess ingestion of thyroid hormone | Rare |
| Hamburger thyrotoxicosis | Thyroid gland included in ground beef | Probably rare |
| ECTOPIC THYROID TISSUE | ||
| Struma ovarii | Ovarian teratoma containing thyroid tissue | Rare |
| Metastatic follicular thyroid cancer | Large tumor mass capable of secreting thyroid hormone autonomously | Rare |
| Pituitary resistance to thyroid hormone | Mutated thyroid hormone receptor with greater expression in the pituitary compared with peripheral tissues | Rare |
Adapted from Cooper DS: Hyperthyroidism, Lancet 362:459–468, 2003.
Studies have examined the health and quality of life of subjects with subclinical hyperthyroidism (i.e., with TSH <0.1 mU/L) or who are euthyroid on antithyroid medication. These studies suggest that subclinical hyperthyroidism carries a risk of late-life atrial fibrillation and that treatment of hyperthyroidism with antithyroid medication does not reliably induce remission that is durable when medication is discontinued. There appears to be no difference in long-term quality of life among hyperthyroid patients treated with antithyroid medication, radioiodine ablation, or surgery. Quality of life was diminished relative to control subjects in all three cases (Chapter 562.1).
562.1 Graves Disease
Clinical Manifestations
The clinical course in children is highly variable but usually is not so fulminant as in many adults (Table 562-2). Symptoms develop gradually; the usual interval between onset and diagnosis is 6-12 mo and may be longer in prepubertal children compared with adolescents. The earliest signs in children may be emotional disturbances accompanied by motor hyperactivity. The children become irritable and excitable, and they cry easily because of emotional lability. They are restless sleepers and tend to kick their covers off. Their schoolwork suffers as a result of a short attention span and poor sleep. Tremor of the fingers can be noticed if the arm is extended. There may be a voracious appetite combined with loss of or no increase in weight. Recent height measurements might show an acceleration in growth velocity.
Table 562-2 MAJOR SYMPTOMS AND SIGNS OF HYPERTHYROIDISM AND OF GRAVES DISEASE AND CONDITIONS ASSOCIATED WITH GRAVES DISEASE
MANIFESTATIONS OF HYPERTHYROIDISM
Symptoms
Signs
MANIFESTATIONS OF GRAVES DISEASE
CONDITIONS ASSOCIATED WITH GRAVES DISEASE
Adapted from Weetman AP: Graves disease, N Engl J Med 343:1236–1248, 2000.
The size of the thyroid is variable. It may be so minimally enlarged that it initially escapes detection, but with careful examination, a diffuse goiter, soft with a smooth surface, is found in almost all patients. Exophthalmos is noticeable in most patients but is usually mild. Lagging of the upper eyelid as the eye looks downward, impairment of convergence, and retraction of the upper eyelid and infrequent blinking may be present (Figs. 562-1 and 562-2). Ocular manifestations can produce pain, lid erythema, chemosis, decreased extraocular muscle function, and decreased visual acuity (corneal or optic nerve involvement). The skin is smooth and flushed, with excessive sweating. Muscular weakness is uncommon but may be severe enough to result in clumsiness. Tachycardia, palpitations, dyspnea, and cardiac enlargement and insufficiency cause discomfort but rarely endanger the patient’s life. Atrial fibrillation is a rare complication. Mitral regurgitation, probably resulting from papillary muscle dysfunction, is the cause of the apical systolic murmur present in some patients. The systolic blood pressure and the pulse pressure are increased. Reflexes are brisk, especially the return phase of the Achilles reflex. Many of the findings in Graves disease result from hyperactivity of the sympathetic nervous system.
Differential Diagnosis
Diagnosis is rarely difficult once hyperthyroidism is considered. Elevated levels of T4 or free T4 and T3 in association with suppressed levels of TSH are usually diagnostic (see Table 562-1). The presence of TRSAb establishes the cause as Graves disease.
Treatment
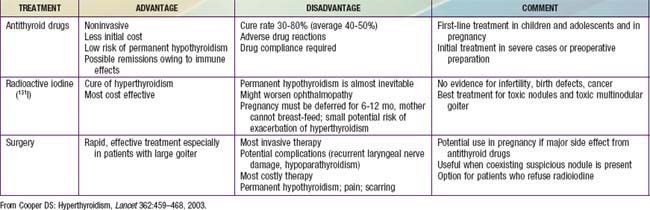
Most pediatric endocrinologists recommend initial medical therapy using antithyroid drugs rather than radioiodine or subtotal thyroidectomy, although radioiodine is gaining acceptance as initial treatment in children >10 yr of age. All therapeutic options have advantages and disadvantages (Table 562-3). The 2 antithyroid drugs used historically are propylthiouracil (PTU) and methimazole (Tapazole). Both compounds inhibit incorporation of trapped inorganic iodide into organic compounds, and they might also suppress TRSAb levels by directly affecting intrathyroidal autoimmunity. However, there are important differences between the drugs. Methimazole is at least 10 times more potent than PTU on a weight basis and has a much longer serum half-life (6-8 hr vs 0.5 hr); PTU generally is administered 3 times daily, but methimazole can be given once daily. Unlike methimazole, PTU is heavily protein bound and has a lesser ability to cross the placenta and to pass into breast milk; theoretically, PTU is the preferred drug during pregnancy and for nursing mothers. Due to reports of severe liver disease in patients treated with PTU, with some patients requiring liver transplant or potentially suffering a fatal outcome, the consensus is to use only methimazole to treat children with Graves disease.
A β-adrenergic blocking agent such as propranolol (0.5-2.0 mg/kg/24 hr orally, divided 3 times daily) or atenolol (1-2 mg/kg orally given once daily) is a useful supplement to antithyroid drugs in the management of severely toxic patients. Additional therapies for thyroid storm are listed in Table 562-4. Thyroid hormones potentiate the actions of catecholamines, including tachycardia, tremor, excessive sweating, lid lag, and stare. These symptoms abate with the use of propranolol, which does not, however, alter thyroid function or exophthalmos.
| GOAL | TREATMENT |
|---|---|
| Inhibition of thyroid hormone formation and secretion | Propylthiouracil (PTU), 400 mg every 8 hr PO or by nasogastric tube Sodium iodide, 1 g IV in 24 hr, or saturated solution of KI, 5 drops every 8 hr |
| Sympathetic blockade | Propranolol, 20-40 mg every 4-6 hr, or 1.mg IV slowly (repeat doses until heart rate slows); not indicated in patients with asthma or heart failure that is not rate related |
| Glucocorticoid therapy | Hydrocortisone, 50-100 mg IV every 6 hr |
| Supportive therapy | Intravenous fluids (depending on indication: glucose, electrolytes, multivitamins) Temperature control (cooling blankets, acetaminophen; avoid salicylates) O2 if required Digitalis for heart failure and to slow ventricular response; pentobarbital for sedation Treatment of precipitating event (e.g., infection) |
From Goldman L, Ausiello D: Cecil Textbook of Medicine, ed 22, Philadelphia, 2004, WB Saunders, p 1401.
Abraham-Nordling M, Torring O, Hamberger B, et al. Graves’ disease: a long-term quality-of-life follow up of patients randomized to treatment with antithyroid drugs, radioiodine, or surgery. Thyroid. 2005;15:1279-1286.
Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-736.
Barrio R, Lopez-Capape M, Martinez-Badas I, et al. Graves’ disease in children and adolescents: response to long-term treatment. Acta Paediatr. 2005;94:1583.
Brent GA. Graves’ disease. N Engl J Med. 2008;358:2594-2605.
Cassio A, Corrias A, Gualandi S, et al. Influence of gender and pubertal stage at diagnosis on growth outcome in childhood thyrotoxicosis: results of a collaborative study. Clin Endocrinol (Oxf). 2006;64:53-57.
Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
Food and Drug Administration. FDA Drug Safety Communication: New boxed warning on severe liver injury with propylthiouracil. (website) www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm209023.htm Accessed September 15, 2010
Glaser NS, Styne DM. Predicting the likelihood of remission in children with Graves’ disease: a prospective, multicenter study. Pediatrics. 2008;121:e481-e488.
Goldstein SM, Katoqitz WR, Moshang T, et al. Pediatric thyroid-associated orbitopathy: the Children’s Hospital of Philadelphia experience and literature review. Thyroid. 2008;18:997-999.
Haentjens P, Van Meerhaeghe A, Poppe K, et al. Subclinical thyroid dysfunction and mortality: an estimate of relative and absolute excess all-cause mortality based on time-to-event data from cohort studies. Eur J Endocrinol. 2008;159:329-341.
Iwama S, Ikezaki A, Kikuoka N, et al. Association of HLA-DR, DQ genotypes and CTLA-4 gene polymorphism with Graves’ disease in Japanese children. Horm Res. 2005;63:55-60.
Kaguelidou F, Alberti C, Castanet M, et al. Predictors of autoimmune hyperthyroidism relapse in children after discontinuation of antithyroid drug treatment. J Clin Endocrinol Metab. 2008;93:3817.
Kharlip J, Cooper DS. Recent developments in hyperthyroidism. Lancet. 2009;373:1930-1932.
Laurberg P, Wallin G, Tallstedt L, et al. TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol. 2008;158:69-75.
Ma C, Kuang A, Xie J, et al: Radioiodine treatment for pediactric Graves’ disease, Cochrane Database Syst Rev CD006294, 2008.
Mesto S, Auvinen A, Huhtala H, et al. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer. 2007;109:1972-1979.
Nabhan Z, Kreher NC, Eugster EA. Hashitoxicosis in children: clinical features and natural history. J Pediatr. 2005;146:533-536.
Pearce EN. Diagnosis and management of thyrotoxicosis. BMJ. 2006;332:1369-1373.
Pinto T, Cummings EA, Barnes D, et al. Clinical course of pediatric and adolescent Graves’ disease treated with radioactive iodine. J Pediatr Endocrinol Metab. 2007;20:973-980.
Rahhal SN, Eugster EA. Thyroid stimulating immunoglobulin is often negative in children with Graves’ disease. J Pediatr Endocrinol Metab. 2008;21:1085-1088.
Read CH, Tansey MJ, Menda Y. A 36-year retrospective analysis of the efficacy and safety of radioactive iodine in treating young Graves’ patients. J Clin Endocrinol Metab. 2004;89:4229-4233.
Rivkees SA, Dinauer C. An optimal treatment for pediatric graves’ disease is radioiodine. J Clin Endocrinol Metab. 2007;92:797.
Rivkees SA, Mattison DR. Ending propylthiouracil-induced liver failure in children. N Engl J Med. 2009;360:1574-1575.
Sugino K, Ito K, Mimura T, et al. Surgical treatment of Graves’ disease in children. Thyroid. 2004;14:447-452.
562.2 Congenital Hyperthyroidism
Clinical Manifestations
Many of the infants are premature and appear to have intrauterine growth restriction. Most have goiters. The infant is extremely restless, irritable, and hyperactive and appears anxious and unusually alert. Microcephaly and ventricular enlargement may be present. The eyes are opened widely and appear exophthalmic (Fig. 562-3). There may be extreme tachycardia and tachypnea, and the temperature is elevated. In severely affected infants, there is a progression of symptoms; weight loss occurs despite a ravenous appetite, hepatosplenomegaly increases, and jaundice can occur. Severe hypertension and cardiac decompensation can occur. The infant can die if therapy is not instituted promptly. The serum level of T4 or free T4 and T3 are markedly elevated, and TSH is suppressed. Advanced bone age, frontal bossing with triangular facies, and cranial synostosis are common, especially in infants with persistent clinical manifestations of hyperthyroidism.
Azizi F, Khamseh ME, Bahreynian M, et al. Thyroid function and intellectual development of children of mothers taking methimazole during pregnancy. J Endocrinol Invest. 2002;25:586-589.
Chester J, Rotenstein D, Ringkananont U, et al. Congenital neonatal hyperthyroidism caused by germline mutations in the TSH receptor gene. J Pediatr Endocrinol Metab. 2008;21:479-486.
Cohen O, Pinchas-Hamiel O, Sivan E, et al. Serial in utero ultrasonographic measurements of the fetal thyroid: a new complimentary tool in the management of maternal hyperthyroidism in pregnancy. Prenat Diagn. 2003;23:740-742.
Hashemipour M, Hasani N, Amini M, et al. Thyroid function abnormalities among first-degree relatives of Iranian congenital hypothyroidism neonates. Pediatr Int. 2010;52:467-471.
Kempers MJ, van Tijn DA, van Trotsenburg AS, et al. Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab. 2003;88:5851-5857.
Mastorakos G, Mitsiades NS, Doufas AG, et al. Hyperthyroidism in McCune-Albright syndrome with a review of thyroid abnormalities sixty years after the first report. Thyroid. 1997;7:433-439.
O’Connor MJ, Paget-Brown AO, Clarke WL. Premature twins of a mother with Graves’ disease with discordant thyroid function: a case report. J Perinatol. 2007;27:388-389.
Papendieck P, Chiesa A, Prieto L, et al. Thyroid disorders of neonates born to mothers with Graves’ disease. J Pediatr Endocrinol Metab. 2009;22:547-553.
Peleg D, Cada S, Peleg A, et al. The relationship between maternal serum thyroid-stimulating immunoglobulin and fetal and neonatal thyrotoxicosis. Obstet Gynecol. 2002;99:1040-1043.
Weber G, Ielo V, Vigone MC, et al. Neonatal hyperthyroidism: report of eight cases. Ital J Pediatr. 2001;27:757.