Hypertensive Crises
Introduction
Hypertension is a common clinical disorder. Estimates indicate that almost 30% of the U.S. adult population suffers from elevated blood pressure.1 Furthermore, one third of these patients are unaware of their diagnosis, and of those who are diagnosed and treated, only 34% have adequate control of their blood pressure.2 Severe elevations in blood pressure, hypertensive crises, will occur in about 1% of patients with chronic hypertension.1,3 Hypertensive crises constitute a clinical problem that the intensivist will encounter in the hospital setting. Unfortunately, a paucity of clinical studies evaluating optimal therapeutic strategies and a lack of consideration for key pathophysiologic aspects have led to common misunderstandings and pitfalls in the management of patients with hypertensive crises.
Definitions
According to the seventh report of the Joint National Committee (JNC) on Detection, Evaluation, and Treatment of High Blood Pressure, hypertension is classified into three stages: prehypertension, stage 1, and stage 2 (Table 34.1).3 The terms malignant hypertension and accelerated hypertension have been abandoned. These terms were utilized to describe severe elevations in blood pressure associated with advanced retinopathy (Keith-Wagener-Barker stages 3 and 4). Prognosis of these clinical entities has improved dramatically with the advent of effective drugs for hypertension. In addition, studies have demonstrated that retinopathy as measured by the Keith-Wagener-Barker classification does not correlate with severity of hypertension or outcomes.4
Table 34.1
Classification of Hypertension (Joint National Committee [JNC] 7)
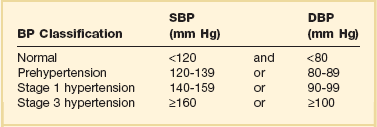
BP, blood pressure; SBP, systolic blood pressure; DPB, diastolic blood pressure.
A hypertensive emergency is a severe elevation in blood pressure associated with the presence of acute end-organ damage. Hypertensive emergencies require immediate control of blood pressure, within 1 to 2 hours, to prevent further organ damage. This will usually require the use of intravenous medications and invasive monitoring (arterial line) in a high-dependency unit such as the intensive care unit. The principal systems susceptible to acute end-organ damage from severe elevations in blood pressure include the central nervous, cardiovascular, and renal systems (Fig. 34.1). Several clinical situations are associated with hypertensive emergencies (Box 34.1). The absolute level of blood pressure and the time course of this elevation will determine the development of a hypertensive emergency. However, acute end-organ damage can occur at different blood pressure values in different patients. Therefore, it is more useful to define hypertensive emergencies with the presence of acute end-organ damage as opposed to specified numbers of systolic or diastolic blood pressure. In addition to initiating immediate therapeutic interventions, patients with a hypertensive emergency may require further diagnostic evaluation to determine the cause of their elevated blood pressure. Depending on the population studied, 20% to 50% of patients presenting with a hypertensive emergency will have a secondary cause of hypertension identified.5
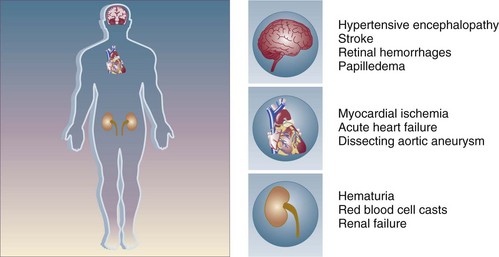
Figure 34.1 End-organ failure in hypertensive emergency.
Pathophysiology
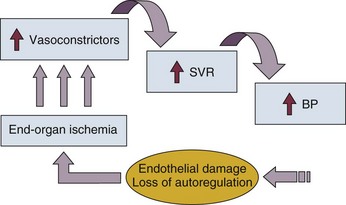
The underlying pathophysiology of hypertensive crises is still not fully understood. The transition of mild hypertension or normotension to a hypertensive crisis is usually caused by an event that leads to an abrupt increase in blood pressure. Situations associated with this event may include cessation of hypertensive medications with potential rebound effects, consumption of illicit drugs, and severe pain, as well as several clinical syndromes. Blood pressure is determined by the product of cardiac output and systemic vascular resistance (BP = CO × SVR). In most hypertensive crises, the initial rise in blood pressure is secondary to increased systemic vascular resistance. The rise in systemic vascular resistance is believed to be caused by humoral vasoconstrictors.6 With the increase in blood pressure, mechanical stress on the arteriolar wall leads to endothelial damage and fibrinoid necrosis of the arterioles.6,7 Vascular damage leads to loss of autoregulatory mechanisms, ischemia, and acute end-organ damage, which prompts further release of vasoconstrictors and initiates a vicious cycle (Fig. 34.2).6,7
Approach to Management
1. Should the blood pressure be lowered acutely?
2. How much should the blood pressure be lowered?
3. Which medication should be used to lower the blood pressure?
Should the Blood Pressure Be Lowered Acutely?
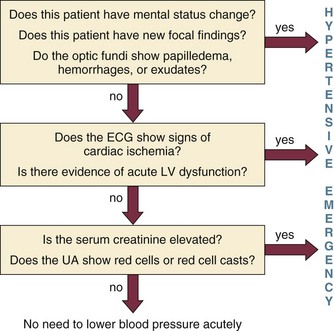
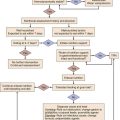
To answer this question, the clinician must determine if there is evidence of acute end-organ damage. In patients with hypertensive emergencies (the presence of acute end-organ damage), the blood pressure should be lowered acutely to a safe target to prevent further end-organ damage. An organized approach in the evaluation of the patient is necessary. A focused history should determine a previous diagnosis of hypertension, medication history, use of illicit drugs or over-the-counter agents with potential hypertensive effects, and the presence of symptoms consistent with neurologic, visual, cardiac, or renal dysfunction. Physical examination should confirm vital signs. It is important to measure blood pressure adequately and in both upper extremities. Pulses should also be checked in all extremities, as inequalities in blood pressure or pulses can exist with aortic dissection. In addition, a thorough neurologic and cardiopulmonary examination should evaluate possible signs of end-organ failure such as altered mentation, new focal neurologic deficits, or cardiogenic pulmonary edema. A funduscopic examination of the eyes should be done to look for signs of acute papillary edema or new retinal hemorrhages. A set of simple diagnostic tests can complete the evaluation for acute end-organ damage. An electrocardiogram to rule out active ischemia and a chest x-ray to assess for pulmonary edema or signs of aortic pathology can help the clinician to evaluate the cardiopulmonary system. Abnormalities in blood urea nitrogen (BUN), creatinine, and the urinalysis (red blood cell [RBC] casts) suggest renal involvement. Additional tests may be indicated based on the individual characteristics of each case. An algorithm to establish the presence of acute end-organ damage at the bedside when evaluating patients is presented in Figure 34.3.
How Much Should the Blood Pressure Be Lowered?
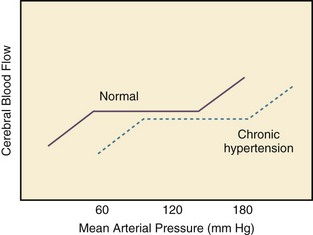
Different organs have the ability to autoregulate and maintain a constant blood flow through a range of mean arterial pressures (MAPs). Under normal conditions cerebral autoregulation will keep blood flow constant between MAPs of 60 to 150 mm Hg.8 When the MAP drops, cerebral arteries will dilate, and if the MAP rises, they will constrict to maintain constant blood flow to the brain. Drops in the MAP below the lower limit of autoregulation will lead to hypoperfusion resulting in brain ischemia. Rises in the MAP above the higher limit of autoregulation will lead to acute end-organ damage from hypertension (hypertensive emergency). In the case of the brain, this may result in hypertensive encephalopathy. With chronic hypertension, compensatory functional and structural changes will occur in the vasculature.9,10 These changes will shift the autoregulatory curve to the right.11 The autoregulatory curve for cerebral blood flow in healthy individuals and in patients with chronic hypertension is shown in Figure 34.4. Hence, patients with chronic hypertension will have a higher tolerance to elevated blood pressures, as their autoregulatory curve is shifted to the right. This explains why many patients present with severely elevated blood pressure and no evidence of acute end-organ damage. However, rapid reductions of blood pressure to “normal” levels can fall below the lower autoregulatory capacity of the circulation in a chronically hypertensive patient. This phenomenon explains the hypoperfusion of vital organs and the development of renal failure or cerebral ischemia often seen when blood pressure is lowered too far or too fast.12 Based on these principles, most experts would recommend that for most hypertensive emergencies the goal should be to lower MAP by 15% to 25% over a period of several minutes to hours, depending on the clinical situation.13 Reduction of blood pressure to normal levels may be warranted in special situations such as those involving patients with aortic dissection or previously normotensive patients with a postoperative hypertensive emergency.
Which Medication Should Be Used to Lower the Blood Pressure?
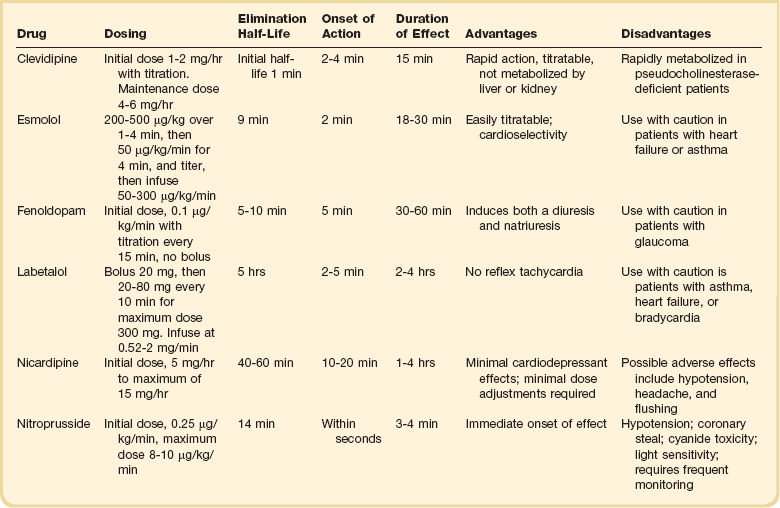
The ideal medication to treat a hypertensive emergency should have a rapid onset of action, high potency, immediate reversibility, no tachyphylaxis, and minimal or no adverse effects. Although there is no perfect medication, several agents with some of these characteristics are summarized in Table 34.2. There are a limited number of studies comparing agents in terms of clinical outcomes. With no clear outcome data the selection of a particular agent is based on the clinical scenario, pharmacologic characteristics of the drug, and availability. We will further discuss parenteral agents that are useful in treating hypertensive emergencies (in alphabetical order).
Clevidipine
Clevidipine is relatively new agent approved for use in treating severe hypertension during surgery. It is an ultra-short-acting calcium-channel antagonist. Clevidipine has vasoselective properties with a rapid onset of action and a very short half-life (<1 minute).14 Clevidipine is metabolized by red blood cell esterases; therefore, its use is not affected in patients with renal and or hepatic failure. Clevidipine reduces blood pressure by a direct and selective effect on arterioles. It does not produce reflex tachycardia, and its effect on reducing afterload is often associated with increased cardiac output. Clevidipine is administered intravenously as a continuous drip. The initial dose is usually 1 to 2 mg/hour with adjustments as needed to obtain the desired response in blood pressure. The maintenance dose is usually 4 to 6 mg/hour; however, higher doses may be required in certain clinical situations. Small studies have compared clevidipine to nitroprusside for the treatment of severe hypertension in anesthetized patients undergoing surgery.15,16 These studies showed that clevidipine had similar effects on blood pressure control with less effect on cardiac filling and heart rate. Although clevidipine has not been studied extensively in other clinical situations, its characteristics make it an attractive option for the treatment of hypertensive emergencies outside of the operating room. More recently it seems to have received increased attention in neurologic hypertensive emergencies.17,18
Esmolol
Esmolol is an ultra-short-acting cardioselective, beta-adrenergic agent that can be administered intravenously for the treatment of hypertensive emergencies.19 Esmolol has a rapid onset of action (within 2 minutes), a short elimination half-life (approximately 9 minutes), and a rapid offset of action (within 15 to 30 minutes after stopping infusion).20 Esmolol is rapidly metabolized by red blood cells and is not dependent on renal or hepatic function.20 These properties enable easy titration of the drug and make it attractive in situations of a hypertensive emergency associated with intense adrenergic responses or tachycardia. This agent is available for intravenous (IV) use, both as a bolus and as a continuous infusion. The usual dose is 0.5 mg/kg as a loading dose, followed by a maintenance infusion of 25 to 300 µg/kg/min titrated to the patient’s individual response.20 Esmolol has been found to be effective in controlling postoperative hypertension and tachycardia in several clinical studies.21–23 Esmolol has also been utilized successfully to treat hypertensive emergencies under other various clinical situations.24,25 Esmolol seems to be more effective in situations where both blood pressure and tachycardia are present and there are no problematic issues with beta-blockade (severe systolic cardiac dysfunction or asthma). It is often used in conjunction with other agents to achieve a better response.
Fenoldopam
Fenoldopam is a selective dopamine agonist that causes systemic and renal vasodilation by stimulating dopamine-1 adrenergic receptors.26,27 Fenoldopam is administered intravenously and has a rapid onset of action (5 minutes) and a short duration of action (30 to 60 minutes).27 It is rapidly metabolized by conjugation in the liver to inactive metabolites that are excreted by the kidney. The plasma elimination half-life is approximately 5 to 10 minutes.27 Fenoldopam is administered as a continuous infusion (without a bolus dose), at an initial dose of 0.1 µg/kg/min; this dose is titrated by 0.05 to 0.1 µg/kg/min based on desired effect up to a maximum dose of 1.6 µg/kg/min.26 The most common adverse effects of the drug are related to its vasodilator properties and include hypotension, headache, reflex tachycardia, and flushing.26 Fenoldopam also increases intraocular pressure and should be used with caution in patients with glaucoma.28 Fenoldopam has been demonstrated to be safe and effective in postoperative hypertension.29 Two clinical studies in severely hypertensive patients found fenoldopam to be comparable in efficacy to sodium nitroprusside.30,31 Because of its effects on the renal vasculature and its ability to increase urine output, fenoldopam has been proposed as a renal protective drug. A number of small clinical studies report conflicting results in this respect. However, a meta-analysis found improved renal function and mortality in critically ill patients with acute kidney injury treated with fenoldopam.32 In the setting of a hypertensive emergency, protective effects of fenoldopam on renal function have not been confirmed. However, because it does not affect renal function adversely and does not have increased toxicity in patients with renal failure, it may be a useful alternative to sodium nitroprusside in patients with hypertensive emergency and renal failure.
Labetalol
Labetalol is a combined alpha- and beta-adrenergic receptor blocker that is currently approved for both oral and intravenous use in the treatment of hypertension. Labetalol lowers blood pressure by decreasing systemic vascular resistance by alpha1-blockade and at the same time counteracts the reflex tachycardia from vasodilation through its beta-blocker effect.33,34 Labetalol reduces peripheral vascular resistance while maintaining cerebral, renal, and coronary blood flow. Unlike other beta-blockers, labetalol does not reduce cardiac output. When administered intravenously it has a rapid onset of action (2 to 5 minutes) with peak hypotensive effect occurring within 5 to 10 minutes and lasting 2 to 4 hours.35 The drug is primarily metabolized by the liver and has a plasma elimination half-life of about 5 hours.33 Labetalol is usually administered intravenously at a loading dose of 20 mg, followed by incremental doses of 20 to 80 mg every 10 minutes until the target blood pressure is achieved or a maximal dose of 300 mg has been reached. An alternative regimen is a continuous infusion starting at 1 to 2 mg/min and titrated upward to achieve a desired blood pressure end point. Adverse effects of labetalol include orthostatic hypotension, bronchospasm (should be avoided in asthma patients), heart failure, and significant bradycardia (should be avoided in the presence of sinus bradycardia or heart block greater than first degree). Labetalol has been shown to be effective in a wide range of clinical situations associated with hypertensive emergencies.33,34,36–39
Nicardipine
Nicardipine is a short-acting calcium channel antagonist that produces selective arteriolar dilation. Nicardipine decreases systemic vascular resistance, without producing reflex tachycardia while maintaining or increasing cardiac output.40 Intravenous nicardipine has a rapid onset offset of action (the major effect lasts 10 to 15 minutes, and the plasma elimination half-life is 40 to 60 minutes), making it easily titratable when treating hypertensive emergencies.41,42 An initial dose of 5 mg/hour is recommended, increasing the infusion rate by 2.5 mg/hour every 5 to 15 minutes (to a maximum rate of 15 mg/hour) until the desired hemodynamic response is achieved. Once the target blood pressure is achieved, the infusion rate can be reduced to 3 mg/hour and adjusted to maintain the desired end point. The drug is rapidly distributed throughout the body and is metabolized by the liver into inactive metabolites. Nicardipine should be avoided or used cautiously in patients with aortic stenosis, in patients with cardiomyopathy receiving beta-blockers, and in patients with impaired hepatic function. Several studies have documented the utility and safety of nicardipine in patients with hypertensive emergencies. Randomized studies have demonstrated that nicardipine has similar efficacy when compared to nitroprusside in the management of postoperative hypertension.41,43
Nitroprusside
For many years sodium nitroprusside was the standard intravenous drug administered for hypertensive emergencies, and it remains a viable alternative today. Nitroprusside is a potent balanced arterial and venous vasodilator that decreases both cardiac afterload and preload. Nitroprusside has a rapid onset of action (2 to 3 minutes) and a short serum half-life (1 to 2 minutes) and may be easily titrated.44 Because of its potent effects on blood pressure, use of nitroprusside requires invasive hemodynamic monitoring (arterial line for continuous blood pressure monitoring). Nitroprusside is typically begun at 0.3 µg/kg/min and increased by 0.2 to 1 µg/kg/min every 3 to 5 minutes as needed until a maximum of 2 µg/kg/min. Cyanide and thiocyanate are metabolites of nitroprusside with potential toxic effects. Cyanide is released nonenzymatically from nitroprusside. Cyanide is converted to thiocyanate by the liver. Finally, the kidney excretes thiocyanate. The total dose of nitroprusside and the presence of liver or renal dysfunction increase the risk of toxicity. Cyanide toxicity can be associated with lactic acidosis, mental status changes, and hypotension. Signs of thiocyanate toxicity include delirium, headaches, nausea, abdominal pain, and muscular spasms.45 To reduce possible toxicity, the duration of treatment with nitroprusside should be limited and the maintenance rate of infusion should not exceed 2 µg/kg/min. In patients requiring higher doses of nitroprusside, an infusion of thiosulfate is recommended to decrease the risk of toxicity.46 Hydroxocobalamin has also been demonstrated to prevent and treat possible cyanide toxicity associated with the use of nitroprusside. Despite these concerns, nitroprusside has been utilized successfully in the treatment of hypertensive emergencies for many years, and with proper precautions toxicity is seldom encountered.
Other Agents
Several other agents have been used for treating severely elevated blood pressure. Many of them have been abandoned secondary to the emergence of safer and more efficacious alternatives. However, there are a few agents that despite some limitations compared to the drugs previously discussed may be useful in particular situations. Nitroglycerin directly interacts with nitrate receptors producing predominantly venous dilation. Because of its favorable effects on coronary perfusion and its ability to reduce preload, it is a drug well suited for treating hypertensive emergencies associated with myocardial ischemia or acute left ventricular failure.47 Several clinical studies have shown the safety and efficacy of nitroglycerin for the treatment of hypertension after cardiac surgery.48–50 Nitroglycerin is administered as a continuous infusion. The starting dose is 5 µg/min, and this dose can be titrated until a maximum of 200 µg/min is reached. Phentolamine is an α-adrenergic blocking agent that may be used for the management of catecholamine-induced hypertensive emergencies, such as pheocromocytoma.51 Phentolamine is administered intravenously in 1- to 5-mg boluses. The effects are immediate and may last up to 15 minutes. This drug may cause arrhythmias and angina. One the blood pressure is controlled, a long-acting α-adrenergic blocking agent such as oral phenoxybenzamine can be started. Enalaprilat is an angiotensin-converting enzyme inhibitor (ACE inhibitor) that can be administered intravenously. Its onset of action is within 15 minutes and its duration of action is 12 to 24 hours.52,53 The usual dose is of 1.25 mg IV every 6 hours, titrated by increments of 1.25 mg at 12- to 24-hour intervals to a maximum dose of 5 mg every 6 hours.54 The degree of blood pressure reduction with enalaprilat correlates directly with the pretreatment levels of angiotensin II and plasma renin activity.55 Enalaprilat is especially useful in hypertensive emergencies associated with scleroderma crises.56 Hydralazine is a vasodilator that has been used in pregnancy-related hypertensive crises for many years. However, it has unpredictable effects on blood pressure and a long half-life. Nicardipine and labetalol are better choices for treating pregnant patients with hypertensive emergencies in the intensive care unit.
Specific Clinical Considerations
Hypertensive Encephalopathy
When rises in mean arterial pressure exceed the upper limits of the cerebral blood flow, autoregulatory curve endothelial damage with extravasation of plasma proteins can lead to cerebral edema.57 Hypertensive encephalopathy, the clinical manifestation of this phenomenon, is characterized by headache, visual disturbances, confusion, and focal or generalized weakness. If untreated, hypertensive encephalopathy can lead to coma and death.58 Magnetic resonance imaging has demonstrated that the majority of the cases involves the cortical regions of the brain.59 However, hypertensive encephalopathy with brainstem involvement has also been described.60,61 The differential diagnosis of hypertensive encephalopathy includes several neurologic syndromes (Box 34.2). These should be quickly ruled out with the use of imaging of the brain and other pertinent diagnostic tests. Treatment should be instituted immediately. The goal is to reduce the mean arterial pressure by 15% to 20% within the first 1 to 2 hours.62 The hallmark of hypertensive encephalopathy is improvement of symptoms once the blood pressure is controlled. Caution should be taken not to cause worsening neurologic symptoms from hypoperfusion caused by lowering the mean arterial pressure excessively. Drugs suitable for treating hypertensive encephalopathy include nitroprusside, nicardipine, labetalol, and fenoldopam.
Hypertensive Crisis in Cerebrovascular Accidents
Hypertension is common after both ischemic and hemorrhagic strokes. Extreme elevations in blood pressure have been associated with poor outcomes after ischemic and hemorrhagic stroke.63,64 Significant elevations in blood pressure after strokes raise concerns for the potential development of reinfarction, cerebral edema, increased hemorrhage size, or hemorrhagic transformation of ischemic lesions. However, after acute stroke the cerebral vasculature’s ability to autoregulate blood flow is impaired.65 During this time period, flow to the brain is highly dependent on mean arterial pressure. Even modest reductions in blood pressure can compromise blood flow to the brain during this period with the potential for increased secondary neurologic damage. Optimal treatment of blood pressure during stroke is still not a settled debate. Current guidelines recommend withholding therapy for hypertension in the acute phase of ischemic strokes unless the patient will receive thrombolysis, there is evidence of concomitant acute end-organ damage, or excessive elevations in blood pressure are present (arbitrarily selected as systolic blood pressure > 220 or diastolic blood pressure >120 mm Hg).66 For hemorrhagic strokes, current recommendations are to maintain MAP ≤ 130 mm Hg in patients with a history of hypertension and a MAP ≤ 100 mm Hg in patients who underwent craniotomy.67 Current guidelines are summarized in Tables 34.3 and 34.4.
Table 34.3
Guidelines for Treatment of Hypertension in Ischemic Cerebrovascular Accidents
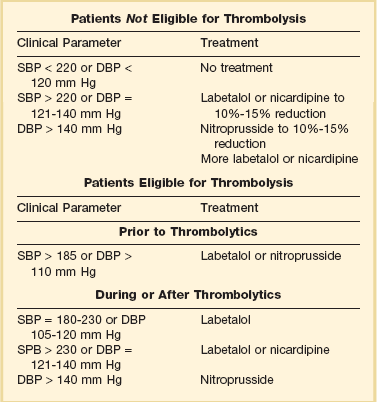
SBP, systolic blood pressure; DPB, diastolic blood pressure.
Table 34.4
Guidelines for Treatment of Hypertension in Hemorrhagic Cerebrovascular Accidents
| Clinical Parameter | Treatment |
| SBP < 180 and DBP < 105 mm Hg MAP < 130 mm Hg |
No treatment |
| SBP 180-230 or DBP 105-140 mm Hg MAP = 130-160 mm Hg |
Labetalol, esmolol, nicardipine, enalaprilat |
| SBP > 230 or DBP > 140 mm Hg Map > 160 mm Hg |
Nitroprusside Nicardipine + labetalol |
MAP, mean arterial pressure; SBP, systolic blood pressure; DPB, diastolic blood pressure.
Acute Aortic Dissection
Aortic dissection is a life-threatening complication of hypertension, caused by a tear in the intima of the aorta. This tear is then propagated by the aortic pulse wave. The aortic pulse wave (dP/dt) is dependent on myocardial contractility, heart rate, and blood pressure. The presenting symptom is usually severe sharp chest pain of abrupt onset. Chest x-ray may be associated with a widened mediastinum. The diagnosis is best made with contrast-enhanced computerized tomography or transesophageal echocardiography.68 Aortic dissections are classified as type A (proximal to the left subclavian artery, involving the ascending aorta) or type B (distal to the left subclavian artery, involving the descending aorta).69 The goal of treatment is rapid reduction of the pulsatile wave (dP/dt) and aortic stress. Both mean arterial pressure and cardiac output must be controlled in order to achieve this goal and prevent further propagation of the dissection in the aorta. In patients with aortic dissection, the mean arterial pressure and heart rate should be reduced to normal values as quickly as possible. Combining a vasodilator (nitroprusside, nicardipine, fenoldopam) with a beta-blocker (esmolol, metoprolol) is recommended.70 All patients with aortic dissection need emergent cardiovascular surgical evaluation. Type A dissections usually require emergent surgery to prevent serious complications such as acute aortic insufficiency, hemopericardium, and cardiac tamponade.71 Type B dissection is often managed medically. Indications for surgery in type B dissections include complications such as leak, rupture, and impaired flow to vital organs. See Chapter 33.
Hypertensive Crises in Pregnancy
Hypertension is a common complication of pregnancy and is responsible for 18% of maternal deaths in the United States.72 The spectrum of disease varies from mild increases in blood pressure to severe pregnancy-related syndromes with hypertensive emergencies such as preeclampsia and eclampsia.73 Hypertension in pregnancy is defined as a systolic blood pressure ≥ 140 mm Hg or diastolic pressure ≥ 90 mm Hg. Preeclampsia is a pregnancy-specific condition defined by new onset hypertension, proteinuria (>300 mg/24 hours), and pathologic edema during gestation. Eclampsia is defined by the development of seizures or coma in a pregnant patient with preeclampsia. The challenge in pregnant patients with hypertensive crises is to lower the blood pressure in order to prevent maternal end-organ damage while minimizing acute changes in placental perfusion that could negatively impact the well-being of the fetus. Treatment of severe preeclampsia and eclampsia includes delivery of the fetus, magnesium sulfate for the prevention and treatment of seizures, and appropriate blood pressure control. The goal is to reduce the diastolic blood pressure to 100 mm Hg or the mean arterial pressure by 20%. Historically, hydralazine has been preferred in pregnant patients for its safety profile from a fetal perspective. However, data suggest it may not be the most effective or safe agent for this patient population.74 For pregnant patients in need of acute lowering of blood pressure in the intensive care unit, drugs such as labetalol and nicardipine are probably better options.75,76 Nitroprusside is reserved for refractory cases because of concerns for potential fetal cyanide toxicity. Finally, ACE inhibitors such as enalaprilat are contraindicated in the second and third trimester because of the increase in fetal and neonatal morbidity and mortality.
Postoperative Hypertension
Postoperative hypertension deserving of immediate intravenous treatment consideration has been arbitrarily defined as a systolic blood pressure > 190 mm Hg or a diastolic blood pressure > 100 mm Hg on two consecutive readings after surgery. Previous history of hypertension, high body mass index, age, and the grade of surgical stress are recognized risk factors for developing postoperative hypertension.77 Severe increases in arterial blood pressure in the immediate postoperative period can result in serious complications such as heart failure, arrhythmia, myocardial ischemia, wound hemorrhage, and cerebral hemorrhage.78 Considering the deleterious effects of prolonged postoperative hypertension, many authors have recommended aggressive treatment.78 The goal of treatment is similar to other hypertensive emergencies: decrease blood pressure to safe levels and at the same time avoid complications related to hypotension. Although some clinicians feel that postoperative hypertension should be treated aggressively based on the potential for acute end-organ damage, others recommend evaluating for possible causes of hypertension such as pain, hypercarbia, hypoxemia, and urinary retention prior to initiating antihypertensive drugs. As most patients in the postoperative period are unable to take oral medications, even patients with no clear evidence of acute end-organ damage will receive intravenous medications. In patients with previous history of hypertension a reasonable goal is to reduce mean arterial pressure by 20%. In patients with no previous history of hypertension, the goal is to reduce blood pressure to normal levels. Clevidipine, nitroprusside, labetalol, and nicardipine have all been extensively studied in cardiac, vascular, and neurosurgical settings. Nitroglycerin is commonly used in postcoronary bypass surgery, and fenoldopam has been proposed for clinical settings with increased risk of renal ischemia.
Hypertensive Urgency
Hypertensive urgency refers to a clinical situation in which there is severe elevation of blood pressure without evidence of acute end-organ damage. This is a common clinical situation that is often mismanaged. Too often clinicians have the impulse to treat numbers and risk causing more damage to patients from precipitous drops in blood pressure. Despite markedly elevated blood pressure, patients with hypertensive urgency are at low risk of immediate complications. Morbidity from elevated blood pressure occurs over months to years. Therefore, it is more important to start patients with hypertensive urgency on a good long-term oral regimen and reduce their blood pressure gradually over 24 to 48 hours. One must avoid the use of medications that have the potential to produce abrupt drops in blood pressure and cause significant damage from hypoperfusion.12,79 In this respect, practices such as the use of sublingual nifedipine in hypertensive urgency have been abandoned secondary to the potential hazards to patients.80,81 Often, restarting a previously effective drug regimen is all that is needed to treat hypertensive urgencies. Physicians often feel compelled to treat elevated blood pressures immediately and feel a false sense of security if they see the numbers improve quickly. However, in the absence of acute end-organ damage this therapeutic strategy has a higher potential for causing damage and is not based on a clear scientific rationale.
References
1. Burt, VL, Whelton, P, Roccella, EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995; 25:305–313.
2. Hajjar, I, Kotchen, TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003; 290:199–206.
3. National Heart L, Blood Institute. Seventh report of the Joint National Committee on Prevention, Detection, Evalulation, and Treatment of High Blood Pressure (JNC VII). NIH. 2003.
4. Fuchs, FD, Maestri, MK, Bredemeier, M, et al. Study of the usefulness of optic fundi examination of patients with hypertension in a clinical setting. J Hum Hypertens. 1995; 9:547–551.
5. Houston, MC. Pathophysiology, clinical aspects, and treatment of hypertensive crises. Prog Cardiovasc Dis. 1989; 32:99–148.
6. Ault, MJ, Ellrodt, AG. Pathophysiological events leading to the end-organ effects of acute hypertension. Am J Emerg Med. 1985; 3:10–15.
7. Wallach, R, Karp, RB, Reves, JG, et al. Pathogenesis of paroxysmal hypertension developing during and after coronary bypass surgery: A study of hemodynamic and humoral factors. Am J Cardiol. 1980; 46:559–565.
8. Strandgaard, S, Paulson, OB. Cerebral autoregulation. Stroke. 1984; 15:413–416.
9. Johansson, B. Regional cerebral blood flow in acute experimental hypertension. Acta Neurol Scand. 1974; 50:366–372.
10. Johansson, BB, Nilsson, B. Cerebral vasomotor reactivity in normotensive and spontaneously hypertensive rats. Stroke. 1979; 10:572–576.
11. Strandgaard, S. Autoregulation of cerebral blood flow in hypertensive patients: The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 1976; 53:720–727.
12. Yanturali, S, Akay, S, Ayrik, C, Cevik, AA. Adverse events associated with aggressive treatment of increased blood pressure. Int J Clin Pract. 2004; 58:517–519.
13. Calhoun, DA, Oparil, S. Treatment of hypertensive crisis. N Engl J Med. 1990; 323:1177–1183.
14. Kieler-Jensen, N, Jolin-Mellgard, A, Nordlander, M, Ricksten, SE. Coronary and systemic hemodynamic effects of clevidipine, an ultra-short-acting calcium antagonist, for treatment of hypertension after coronary artery surgery. Acta Anaesthesiol Scand. 2000; 44:186–193.
15. Aronson, S. Clevidipine in the treatment of perioperative hypertension: Assessing safety events in the ECLIPSE trials. Expert Rev Cardiovasc Ther. 2009; 7:465–472.
16. Powroznyk, AV, Vuylsteke, A, Naughton, C, et al. Comparison of clevidipine with sodium nitroprusside in the control of blood pressure after coronary artery surgery. Eur J Anaesthesiol. 2003; 20:697–703.
17. Awad, AS, Goldberg, ME. Role of clevidipine butyrate in the treatment of acute hypertension in the critical care setting: A review. Vasc Health Risk Manag. 2010; 6:457–464.
18. Rivera, A, Montoya, E, Varon, J. Intravenous clevidipine for management of hypertension. Integr Blood Press Control. 2010; 3:105–111.
19. Barbier, GH, Shettigar, UR, Appunn, DO. Clinical rationale for the use of an ultra-short acting beta-blocker: Esmolol. Int J Clin Pharmacol Ther. 1995; 33:212–218.
20. Wiest, D. Esmolol: A review of its therapeutic efficacy and pharmacokinetic characteristics. Clin Pharmacokinet. 1995; 28:190–202.
21. Girard, D, Shulman, BJ, Thys, DM, et al. The safety and efficacy of esmolol during myocardial revascularization. Anesthesiology. 1986; 65:157–164.
22. Gold, MI, Sacks, DJ, Grosnoff, DB, et al. Use of esmolol during anesthesia to treat tachycardia and hypertension. Anesth Analg. 1989; 68:101–104.
23. Gray, RJ, Bateman, TM, Czer, LS, et al. Comparison of esmolol and nitroprusside for acute post-cardiac surgical hypertension. Am J Cardiol. 1987; 59:887–891.
24. Mooss, AN, Hilleman, DE, Mohiuddin, SM, Hunter, CB. Safety of esmolol in patients with acute myocardial infarction treated with thrombolytic therapy who had relative contraindications to beta-blocker therapy. Ann Pharmacother. 1994; 28:701–703.
25. Stumpf, JL. Drug therapy of hypertensive crises. Clin Pharm. 1988; 7:582–591.
26. Oparil, S, Aronson, S, Deeb, GM, et al. Fenoldopam: A new parenteral antihypertensive: Consensus roundtable on the management of perioperative hypertension and hypertensive crises. Am J Hypertens. 1999; 12:653–664.
27. Post, JB, 4th., Frishman, WH. Fenoldopam: A new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998; 38:2–13.
28. Elliott, WJ, Karnezis, TA, Silverman, RA, et al. Intraocular pressure increases with fenoldopam, but not nitroprusside, in hypertensive humans. Clin Pharmacol Ther. 1991; 49:285–293.
29. Goldberg, ME, Cantillo, J, Nemiroff, MS, et al. Fenoldopam infusion for the treatment of postoperative hypertension. J Clin Anesth. 1993; 5:386–391.
30. Panacek, EA, Bednarczyk, EM, Dunbar, LM, et al. Randomized, prospective trial of fenoldopam vs sodium nitroprusside in the treatment of acute severe hypertension. Fenoldopam Study Group. Acad Emerg Med. 1995; 2:959–965.
31. Pilmer, BL, Green, JA, Panacek, EA, et al. Fenoldopam mesylate versus sodium nitroprusside in the acute management of severe systemic hypertension. J Clin Pharmacol. 1993; 33:549–553.
32. Landoni, G, Biondi-Zoccai, GG, Tumlin, JA, et al. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: A meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007; 49:56–68.
33. Dimich, I, Lingham, R, Gabrielson, G, et al. Comparative hemodynamic effects of labetalol and hydralazine in the treatment of postoperative hypertension. J Clin Anesth. 1989; 1:201–206.
34. Leslie, JB, Kalayjian, RW, Sirgo, MA, et al. Intravenous labetalol for treatment of postoperative hypertension. Anesthesiology. 1987; 67:413–416.
35. Donnelly, R, Macphee, GJ. Clinical pharmacokinetics and kinetic-dynamic relationships of dilevalol and labetalol. Clin Pharmacokinet. 1991; 21:95–109.
36. Mabie, WC, Gonzalez, AR, Sibai, BM, Amon, E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet Gynecol. 1987; 70:328–333.
37. Michael, CA. Intravenous labetalol and intravenous diazoxide in severe hypertension complicating pregnancy. Aust N Z J Obstet Gynaecol. 1986; 26:26–29.
38. Morel, DR, Forster, A, Suter, PM. I. v. labetalol in the treatment of hypertension following coronary-artery surgery. Br J Anaesth. 1982; 54:1191–1196.
39. Orlowski, JP, Shiesley, D, Vidt, DG, et al. Labetalol to control blood pressure after cerebrovascular surgery. Crit Care Med. 1988; 16:765–768.
40. Lambert, CR, Hill, JA, Nichols, WW, et al. Coronary and systemic hemodynamic effects of nicardipine. Am J Cardiol. 1985; 55:652–656.
41. IV Nicardipine Study Group. Efficacy and safety of intravenous nicardipine in the control of postoperative hypertension. Chest. 1991; 99:393–398.
42. Wallin, JD, Fletcher, E, Ram, CV, et al. Intravenous nicardipine for the treatment of severe hypertension: A double-blind, placebo-controlled multicenter trial. Arch Intern Med. 1989; 149:2662–2669.
43. David, D, Dubois, C, Loria, Y. Comparison of nicardipine and sodium nitroprusside in the treatment of paroxysmal hypertension following aortocoronary bypass surgery. J Cardiothorac Vasc Anesth. 1991; 5:357–361.
44. Cohn, JN, Burke, LP. Nitroprusside. Ann Intern Med. 1979; 91:752–757.
45. Robin, ED, McCauley, R. Nitroprusside-related cyanide poisoning. Time (long past due) for urgent, effective interventions. Chest. 1992; 102:1842–1845.
46. Hall, VA, Guest, JM. Sodium nitroprusside-induced cyanide intoxication and prevention with sodium thiosulfate prophylaxis. Am J Crit Care. 1992; 1:19–25.
47. Yusuf, S, Collins, R, MacMahon, S, Peto, R. Effect of intravenous nitrates on mortality in acute myocardial infarction: An overview of the randomised trials. Lancet. 1988; 1:1088–1092.
48. Flaherty, JT, Magee, PA, Gardner, TL, et al. Comparison of intravenous nitroglycerin and sodium nitroprusside for treatment of acute hypertension developing after coronary artery bypass surgery. Circulation. 1982; 65:1072–1077.
49. Kaplan, JA, Dunbar, RW, Jones, EL. Nitroglycerin infusion during coronary-artery surgery. Anesthesiology. 1976; 45:14–21.
50. Tobias, MA. Comparison of nitroprusside and nitroglycerine for controlling hypertension during coronary artery surgery. Br J Anaesth. 1981; 53:891–897.
51. Vaughan, CJ, Delanty, N. Hypertensive emergencies. Lancet. 2000; 356:411–417.
52. De Marco, T, Daly, PA, Liu, M, et al. Enalaprilat, a new parenteral angiotensin-converting enzyme inhibitor: Rapid changes in systemic and coronary hemodynamics and humoral profile in chronic heart failure. J Am Coll Cardiol. 1987; 9:1131–1138.
53. DiPette, DJ, Ferraro, JC, Evans, RR, Martin, M. Enalaprilat, an intravenous angiotensin-converting enzyme inhibitor, in hypertensive crises. Clin Pharmacol Ther. 1985; 38:199–204.
54. Rutledge, J, Ayers, C, Davidson, R, et al. Effect of intravenous enalaprilat in moderate and severe systemic hypertension. Am J Cardiol. 1988; 62:1062–1067.
55. Hirschl, MM, Binder, M, Bur, A, et al. Impact of the renin-angiotensin-aldosterone system on blood pressure response to intravenous enalaprilat in patients with hypertensive crises. J Hum Hypertens. 1997; 11:177–183.
56. Strauss, R, Gavras, I, Vlahakos, D, Gavras, H. Enalaprilat in hypertensive emergencies. J Clin Pharmacol. 1986; 26:39–43.
57. Gifford, RW, Jr., Westbrook, E. Hypertensive encephalopathy: Mechanisms, clinical features, and treatment. Prog Cardiovasc Dis. 1974; 17:115–124.
58. Chester, EM, Agamanolis, DP, Banker, BQ, Victor, M. Hypertensive encephalopathy: A clinicopathologic study of 20 cases. Neurology. 1978; 28:928–939.
59. Schilling, S, Hartel, C, Gehl, HB, Sperner, J. MRI findings in acute hypertensive encephalopathy. Eur J Neurol. 2003; 10:329–330.
60. Uchino, M, Haga, D, Nomoto, J, et al. Brainstem involvement in hypertensive encephalopathy: A report of two cases and literature review. Eur Neurol. 2007; 57:223–226.
61. Biousse, V, Newman, NJ, Chang, GY. Brainstem involvement in hypertensive encephalopathy: Clinical and radiological findings. Neurology. 2004; 63:1759–1760.
62. Williams, O, Brust, JC. Hypertensive encephalopathy. Curr Treat Options Cardiovasc Med. 2004; 6:209–216.
63. Aslanyan, S, Weir, CJ, Lees, KR. Elevated pulse pressure during the acute period of ischemic stroke is associated with poor stroke outcome. Stroke. 2004; 35:e153–e155.
64. Dandapani, BK, Suzuki, S, Kelley, RE, et al. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke. 1995; 26:21–24.
65. Powers, WJ. Acute hypertension after stroke: The scientific basis for treatment decisions. Neurology. 1993; 43:461–467.
66. Adams, HP, Jr., del Zoppo, G, Alberts, MJ, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007; 115:e478–e534.
67. Morgenstern, LB, Hemphill, JC, 3rd., Anderson, C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010; 41:2108–2129.
68. Sommer, T, Fehske, W, Holzknecht, N, et al. Aortic dissection: A comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996; 199:347–352.
69. Prisant, LM, Nalamolu, VR. Aortic dissection. J Clin Hypertens. 2005; 7:367–371.
70. Braverman, AC. Acute aortic dissection: Clinician update. Circulation. 2010; 122:184–188.
71. DeBakey, ME, McCollum, CH, Crawford, ES, et al. Dissection and dissecting aneurysms of the aorta: Twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery. 1982; 92:1118–1134.
72. Koonin, LM, MacKay, AP, Berg, CJ, et al. Pregnancy-related mortality surveillance–United States, 1987-1990. MMWR CDC Surveill Summ. 1997; 46:17–36.
73. Vidaeff, AC, Carroll, MA, Ramin, SM. Acute hypertensive emergencies in pregnancy. Crit Care Med. 2005; 33:S307–S312.
74. Magee, LA, Cham, C, Waterman, EJ, et al. Hydralazine for treatment of severe hypertension in pregnancy: Meta-analysis. BMJ. 2003; 327:955–960.
75. Awad, K, Ali, P, Frishman, WH, Tejani, N. Pharmacologic approaches for the management of systemic hypertension in pregnancy. Heart Dis. 2000; 2:124–132.
76. Jannet, D, Carbonne, B, Sebban, E, Milliez, J. Nicardipine versus metoprolol in the treatment of hypertension during pregnancy: A randomized comparative trial. Obstet Gynecol. 1994; 84:354–359.
77. Nishigaki, R, Ito, A, Kamei, J, Takahashi, T, Fujii, E. Risk factors for development of postoperative hypertension. Methods and findings in experimental and clinical pharmacology. 2001; 23:203–207.
78. Gal, TJ, Cooperman, LH. Hypertension in the immediate postoperative period. Br J Anaesth. 1975; 47:70–74.
79. Zeller, KR, Von Kuhnert, L, Matthews, C. Rapid reduction of severe asymptomatic hypertension: A prospective, controlled trial. Arch Intern Med. 1989; 149:2186–2189.
80. Grossman, E, Messerli, FH, Grodzicki, T, Kowey, P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996; 276:1328–1331.
81. Rehman, F, Mansoor, GA, White, WB. “Inappropriate” physician habits in prescribing oral nifedipine capsules in hospitalized patients. Am J Hypertens. 1996; 9:1035–1039.