Hypersensitivity Reactions
At the conclusion of this chapter, the reader should be able to:
• Define the terms hypersensitivity, allergy, sensitization, and immunization.
• Identify and explain the three categories of antigens.
• Compare the basic differences among and give examples of types I, II, III, and IV hypersensitivity reactions.
• Describe the etiology, immunologic activity, signs and symptoms, laboratory evaluation, and treatment of type I hypersensitivity reactions.
• Discuss examples of type II hypersensitivity reactions, including laboratory evaluation.
• Describe the mechanism of tissue injury, clinical manifestations, and laboratory testing for type III hypersensitivity reactions.
• Describe the characteristics and laboratory evaluation of type IV hypersensitivity reactions.
• Discuss the acquisition and consequences of latex sensitivity.
• Analyze case studies related to hypersensitivity reactions.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principle, clinical applications, or sources of error of a food allergy test, and the direct antiglobulin test.
Types of Antigens and Reactions
Food Allergies
The NIAID guidelines separate diseases defined as FA that include both IgE-mediated reactions to food (food allergies), non–IgE-mediated reactions to certain foods (e.g., celiac disease), and mixed IgE and non-IgE disorders (Table 26-1).
Table 26-1
Classification of Hypersensitivity Reactions
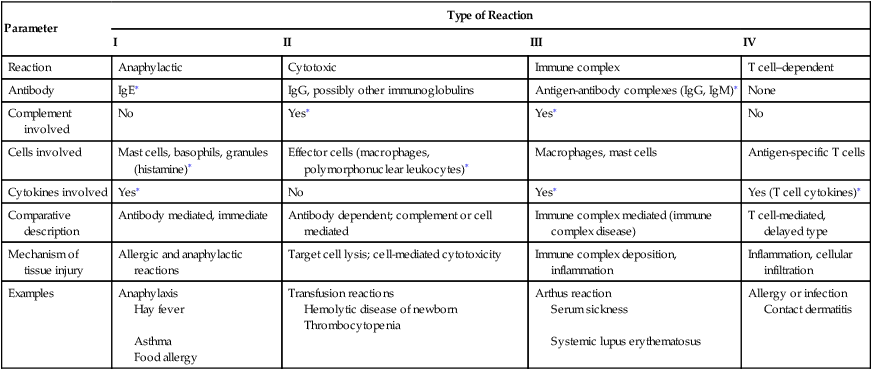
| Parameter | Type of Reaction | |||
| I | II | III | IV | |
| Reaction | Anaphylactic | Cytotoxic | Immune complex | T cell–dependent |
| Antibody | IgE∗ | IgG, possibly other immunoglobulins | Antigen-antibody complexes (IgG, IgM)∗ | None |
| Complement involved | No | Yes∗ | Yes∗ | No |
| Cells involved | Mast cells, basophils, granules (histamine)∗ | Effector cells (macrophages, polymorphonuclear leukocytes)∗ | Macrophages, mast cells | Antigen-specific T cells |
| Cytokines involved | Yes∗ | No | Yes∗ | Yes (T cell cytokines)∗ |
| Comparative description | Antibody mediated, immediate | Antibody dependent; complement or cell mediated | Immune complex mediated (immune complex disease) | T cell-mediated, delayed type |
| Mechanism of tissue injury | Allergic and anaphylactic reactions | Target cell lysis; cell-mediated cytotoxicity | Immune complex deposition, inflammation | Inflammation, cellular infiltration |
| Examples | Anaphylaxis Hay fever Asthma |
Transfusion reactions Hemolytic disease of newborn Thrombocytopenia |
Arthus reaction Serum sickness Systemic lupus erythematosus |
Allergy or infection Contact dermatitis |
Types of Hypersensitivity Reactions
The four types of hypersensitivity reaction (I to IV) are defined by the principal mechanism responsible for a specific cell or tissue injury that occurs during an immune response (Table 26-2). Types I, II, and III reactions are antibody dependent and type IV is cell mediated. Some overlapping occurs among the various types of hypersensitivity reactions, but there are major differences in how each type is diagnosed and treated.
Table 26-2
| Mediator | Primary Action |
| Histamine | Increases vascular permeability; promotes contraction of smooth muscle |
| Leukotrienes | Alter bronchial smooth muscle and enhance effects of histamine on target organs |
| Basophil kallikrein | Generates kinins |
| Serotonin | Contracts smooth muscle |
| Platelet-activating factor | Enhances the release of histamine and serotonin from platelets that affect smooth muscle tone and vascular permeability |
| Eosinophil chemotactic factor of anaphylaxis | Attracts eosinophils to area of activity; these cells release secondary mediators that may limit the effects of primary mediators |
| Prostaglandins | Affect smooth muscle tone and vascular permeability |
Type I Reactions
Etiology
Several groups of agents cause anaphylactic reactions. The two most common agents are drugs (e.g., systemic penicillin) and insect stings. Insects of the order Hymenoptera (e.g., common hornet, yellow jacket, yellow hornet, paper wasp) are examples of insects causing the most serious reactions. Immune-mediated IgE adverse food reactions (Box 26-1) can be fatal.
Immunologic Activity
Immediate hypersensitivity is the basis of acute allergic reactions caused by molecules released by mast cells when an allergen interacts with membrane-bound IgE (Fig. 26-1). Acute allergic reactions result from the release of preformed granule-associated mediators, membrane-derived lipids, cytokines, and chemokines when an allergen interacts with IgE that is bound to mast cells or basophils by the alpha chain of the high-affinity IgE receptor (FcεRI-α). This antigen receptor also occurs on antigen-presenting cells, where it can facilitate the IgE-dependent trapping and presentation of allergen to T cells.
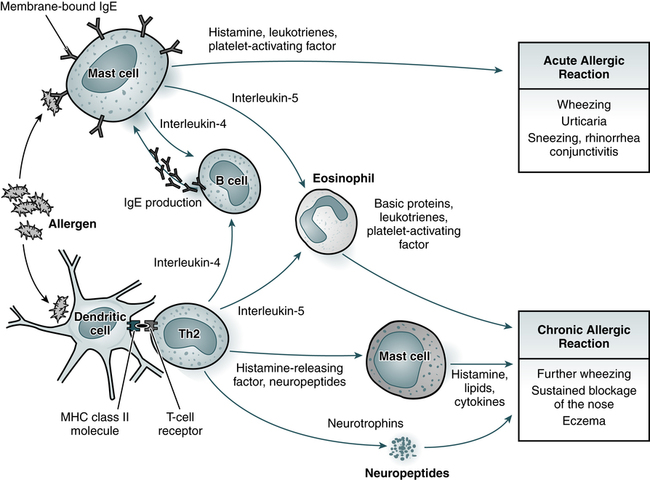
Acute allergic reactions are caused by the antigen-induced release of histamine and lipid mediators from mast cells. In the skin and upper airways, basophils (not shown) may also participate in allergic tissue reactions. Chronic allergic reactions, including the late-phase reaction, may depend on a combination of pathways, including recruitment of eosinophils, liberation of mast cell products by histamine-releasing factors, and neurogenic inflammation involving neurotrophins and neuropeptides. Th2, T-helper cell type 2; MHC, major histocompatibility complex. (Adapted from Kay AB: Allergy and allergic disease, N Engl J Med 344:30–38, 2001.)
Anaphylactic Reaction
1. The offending antigen attaches to the IgE antibody fixed to the surface membrane of mast cells and basophils. Cross-linking of two IgE molecules is necessary to initiate mediator release from mast cells.
2. Activated mast cells and basophils release various mediators.
3. The effects of mediator release produce vascular changes and activation of platelets, eosinophils, neutrophils, and the coagulation cascade.
Signs and Symptoms
Generalized Reaction
A generalized (anaphylactic) reaction is produced by mediators such as cytokines and vasoactive amines (e.g., histamine) from mast cells. Anaphylactic reactions are dramatic and rapid in onset. The physiologic effects of the primary and secondary mediators on the target organs, such as the cardiovascular or respiratory system, gastrointestinal (GI) tract, or the skin, define the signs and symptoms of anaphylaxis. Several important pharmacologically active compounds are discharged from mast cells and basophils during anaphylaxis (see Table 26-2).
Laboratory Evaluation of Allergic Reactions
ImmunoCAP
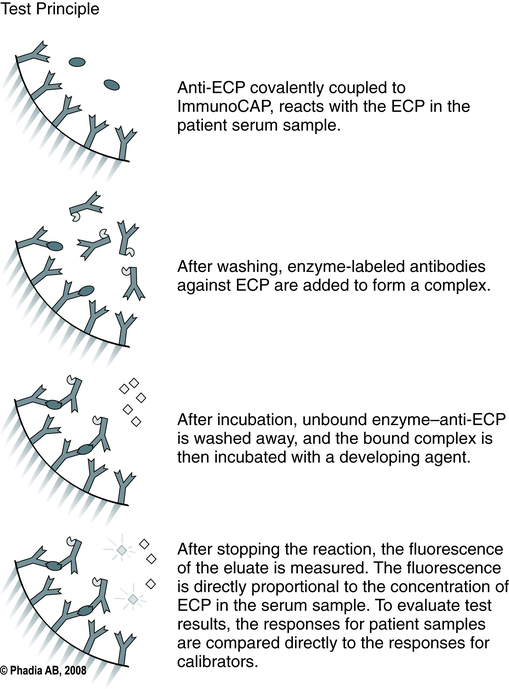
The U.S. Food and Drug Administration (FDA) has approved ImmunoCAP to provide an in vitro quantitative measurement of IgE in human serum (Fig. 26-2). It is considered to be the gold standard for the analysis of allergen-specific IgE. It is intended for in vitro use as an aid in the clinical diagnosis of IgE-mediated allergic disorders in conjunction with other clinical findings (Table 26-3) .
Table 26-3
Comparison of Tests for Specific IgE
| Parameter | Skin Prick Testing | Intradermal Testing | Blood Testing (ImmunoCAP) |
| Sensitivity (%) | 93.6 | 60.0 | 87.2 |
| Specificity (%) | 80.1 | 32.3 | 90.5 |
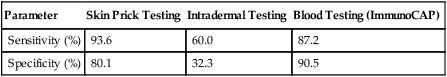
Adapted from Choo-Kang LR: Specific IgE testing: objective laboratory evidence supports allergy diagnosis and treatment, Med Lab Observer MLO 38:10–14, 2006.
Treatment
Drug Therapy
Drug treatments include the following:
• Epinephrine (adrenaline) can be lifesaving in anaphylaxis. Epinephrine stimulates both α-adrenergic and β-adrenergic receptors, decreases vascular permeability, increases blood pressure, and reverses airway obstruction.
• Antihistamines block specific histamine receptors and play an important role in allergies affecting the skin, nose, and mucous membranes. Antihistamines act much slower than epinephrine in treating anaphylaxis and are not very useful in asthma because histamine is not an important allergic mediator released by mast cells in the lung.
• Specific receptor antagonists block the effects of leukotrienes. One drug, montelukast, reduces the amount of airway inflammation in asthma.
• Corticosteroids, often given topically, are widely used in the prevention of symptoms in patients with allergy.
• Other drugs in development aim to block the Th2 cytokine pathway or prevent IgE binding to FcεRI-α.
Desensitization
Desensitization, or immunotherapy, is a well-established technique to improve allergy symptoms caused by specific allergens (e.g., hay fever; Fig. 26-3). If a patient has a history of life-threatening conditions, and if other treatment alternatives are unsatisfactory, desensitization is used to prevent anaphylaxis resulting from insect stings (e.g., yellow jackets). It is best if only one allergen is incriminated.
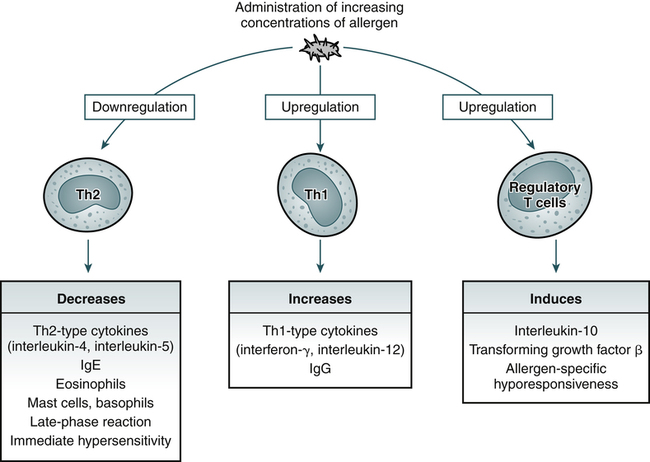
Specific immunotherapy is associated with downregulation of the cytokines produced by Th2 cells, upregulation of cytokines produced by Th1 cells, and induction of regulatory T cells. These changes in turn lead to inhibition of allergic inflammation, increases in cytokines that control production of IgE (interferon-g and interleukin-12) and blocking antibodies (IgG), and release of cytokines involved in allergen-specific hyporesponsiveness (IL-10 and transforming growth factor-β). (Adapted from Kay AB: Allergy and allergic disease, N Engl J Med 344:109–113, 2001.)
Type II Reactions
1. Antibody-dependent, complement-mediated cytotoxic reactions. These are characterized by the interaction of IgG or IgM antibody with cell-bound antigen. This binding of an antigen and antibody can result in the activation of complement and destruction of the cell (cytolysis) to which the antigen is bound. Erythrocytes, leukocytes, and platelets can be lysed by this process. Examples of antibody-dependent, complement-mediated cytotoxic reactions include immediate (acute) transfusion reactions and immune hemolytic anemias (e.g., hemolytic disease of the newborn).
2. Antibody-dependent, cell-mediated cytotoxicity. This depends on the initial binding of specific antibodies to target cell surface antigens. The antibody-coated cells are lysed by effector cells, such as natural killer (NK) cells and macrophages, expressing Fc receptors. The Fc receptors of these effector cells attach to the Fc portion of the antibody that is coating the target cell. Target cell destruction occurs when cytotoxic substances are released by the effector cells. This is the mechanism of injury in antibody-mediated glomerulonephritis and many other diseases. Antibody binding damages solid tissues, in which the antigen may be cellular or part of the extracellular matrix (e.g., basement membrane).
3. Antireceptor antibodies. These disturb the normal function of receptors. Less often, antibodies may modify the function of cells by binding to receptors for hormones (autoimmune hypersensitivity against solid tissue), as illustrated by autoimmune thyroid disease (see Chapter 28). Hyperacute graft rejection is also an example of type II hypersensitivity (see Chapter 31).
Type II Antibody-Dependent, Complement-Mediated Cytotoxic Reactions
Transfusion Reactions
Transfusion reactions can be divided into hemolytic and nonhemolytic types. Hemolytic reactions are associated with the infusion of incompatible erythrocytes. These reactions can be further classified into acute (immediate) or delayed in their manifestations (Box 26-2). Several factors influence whether a transfusion reaction will be acute or delayed, including the following:
• Number of incompatible erythrocytes infused
• Achievement of the optimal temperature for antibody binding
Type II Autoimmune Hypersensitivity Against Solid Tissue
Autoantibodies can also attack and damage components of solid tissues. These antibodies can simply stimulate the target organ function without causing much target organ damage, as in Graves’ disease. In other cases, stimulation of cells by autoantibody leads to tissue damage. As noted, Goodpasture’s syndrome involves IgG autoantibodies and a glycoprotein in the basement membrane of the lung and glomeruli. Anti–basement membrane antibody activates complement, which can trigger an inflammatory response. Goodpasture’s syndrome can be diagnosed by finding antibodies to glomerular basement membrane in patient serum on indirect immunofluorescence (Fig. 26-4) and autoantibodies in serum.
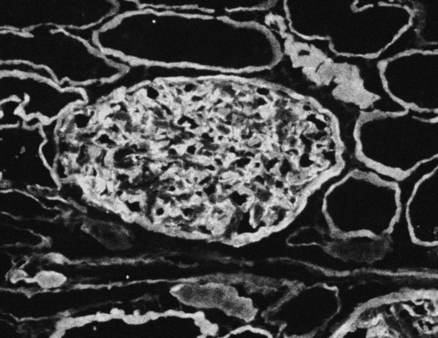
Kidney tissue is used as the target antigen for this test. Linear staining along the glomerular basement membrane appears to be lit up compared with the renal tubules in the background. (From Nairn R, Helbert M: Immunology for medical students, ed 2, St Louis, 2007, Mosby.)
Type III (Immune Complex) Reactions
Farmer’s lung and the Arthus reaction (Fig. 26-5) are examples of local immune complex diseases. Poststreptococcal glomerulonephritis is an example of a circulating immune complex disease, as in systemic lupus erythematosus (SLE; see Chapter 29). Immune complexes are lattices of antigen and antibody that may be localized to the site of antigen production or may circulate in the blood. Immune complexes are produced as part of the normal immune response and are usually cleared by mechanisms involving complement. However, they cause disease in various situations. Failure to clear immune complexes can result from the saturation of mechanisms involving excessive ongoing production of immune complexes, as well as antigenemia caused by chronic infection.
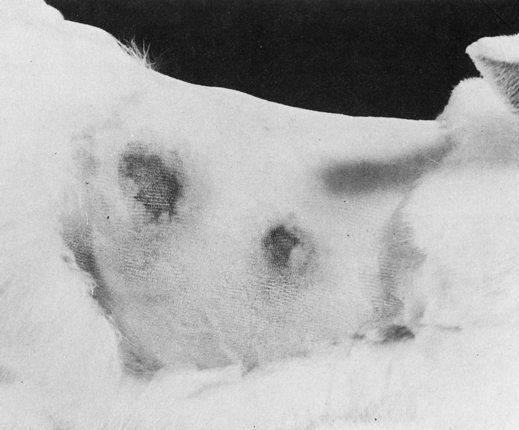
In these two reactions of the skin of a rabbit, the larger reaction has an extensive zone of erythema and edema surrounding its necrotic center. (From Markell EK, Voge M: Medical parasitology, ed 5, Philadelphia, 1981, WB Saunders.)
Other type III (immune complex) reactions include serum sickness and certain aspects of autoimmune diseases (e.g., glomerulonephritis in SLE). Circulating soluble immune complexes are responsible for or associated with various human diseases in which exogenous and endogenous antigens can trigger a pathogenic immune response and result in immune complex disease (Table 26-4).
Table 26-4
Diseases Associated With Immune Complexes
| Type | Examples |
| Autoimmune diseases | Rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, mixed connective tissue disease, systemic sclerosis, glomerulonephritis |
| Neoplastic disease | Solid and lymphoid tumors |
| Infectious disease | Bacterial infective endocarditis, streptococcal infection, viral hepatitis, infectious mononucleosis |
Mechanism of Tissue Injury
1. Antibody can react with soluble antigens in the circulation and form immune complexes that may disseminate and lodge in any tissue with a large filtration area and cause lesions of immune complex disease.
2. Antibody can react with antigen secreted or injected locally into the interstitial fluid. The classic example is the experimental Arthus reaction, the basic model of local immune complex disease (see Fig. 26-5).
3. Antibody can also react with structural antigens that form part of the cell surface membranes or with fixed intercellular structures such as the basement membranes. Systemic immune complex disease serum sickness is an example of soluble and tissue-fixed antigen involvement.
Clinical Manifestations
Autoimmune Disorders
SLE is an autoimmune disorder characterized by autoantibodies that form immune complexes with autoantigens, which are deposited in the renal glomeruli (see Chapter 29). As a consequence of this type III hypersensitivity reaction, glomerulonephritis (inflammation of capillary vessels in the glomeruli) develops.
Type IV Cell-Mediated Reactions
Characteristics
• Type IV delayed-type hypersensitivity (DTH) involves antigen-sensitized T cells or particles that remain phagocytized in a macrophage and are encountered by previously activated T cells for a second or subsequent time. T cells respond directly, or by the release of lymphokines, to exhibit contact dermatitis and allergies of infection (Figs. 26-6 and 26-7). One of the mechanisms of cell-mediated immunity is delayed hypersensitivity. Delayed hypersensitivity is a major mechanism of defense against various intracellular pathogens, including mycobacteria, fungi, and certain parasites. In addition, cell-l-mediated immunity is responsible for the immunologic mechanisms contact sensitivity
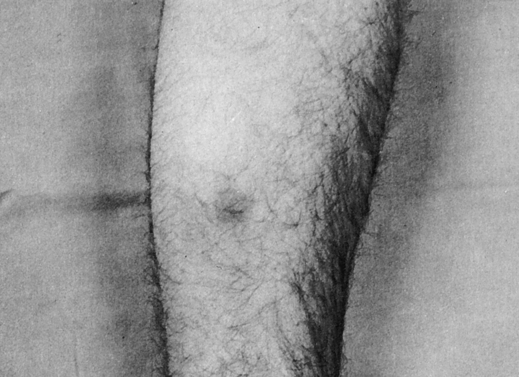
This reaction exhibited an erythematous but nonedematous zone 15 mm in diameter at 48 hours. A control site, inoculated higher on the forearm, showed no reaction at this time. (From Barrett JT: Textbook of immunology, ed 5, St Louis, 1988, Mosby.)
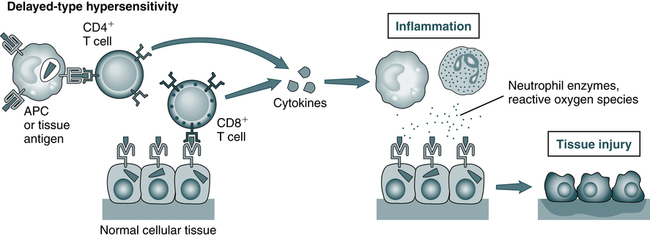
T cells may cause tissue injury and disease by two mechanisms: (1) delayed-type hypersensitivity reactions (A), which may be triggered by CD4+ and CD8+ T cells, and in which tissue injury is caused by activated macrophages and inflammatory cells; and (2) direct killing of target cells. (From Abbas AK, Lichtman AH: Basic immunology: functions and disorders of the immune system, ed 3, updated edition, Philadelphia, 2011, Saunders.)
• Rejection of foreign tissue grafts, elimination of tumor cells bearing neoantigens and
Chapter Highlights
• The term immunization, or sensitization, is used to describe an immunologic reaction dependent on the response of the host to a subsequent exposure of antigen.
• Hypersensitivity has traditionally been classified as immediate and delayed based on the time after exposure to an offending antigen.
• Type I hypersensitivity reactions can range from life-threatening anaphylactic reactions to milder manifestations associated with food allergies. This reaction is usually mediated by IgE antibody.
• In vitro evaluation of type I hypersensitivity reactions involves various methods. The advantages of in vitro testing include no risk of a systemic hypersensitivity reaction and no dependence on skin reactivity influenced by drugs, disease, or age.
• Type II cytotoxic reactions are characterized by the interaction of IgG or IgM antibody to cell-bound antigen. This binding of an antigen and antibody can result in activation of complement and destruction of the cell (cytolysis) to which the antigen is bound. Erythrocytes, leukocytes, and platelets can be lysed by this process.
• Examples of type III reactions include the Arthus reaction, serum sickness, and certain aspects of autoimmune disease.
Type IV cell-mediated immunity consists of immune activities that differ from antibody-mediated immunity. Cell-mediated immunity is moderated by the link between T lymphocytes and phagocytic cells. Delayed hypersensitivity is a major mechanism of defense against various intracellular pathogens, including mycobacteria, fungi, and certain parasites. In addition, cell-mediated immunity is responsible for the immunologic mechanisms of contact sensitivity, rejection of foreign tissue grafts, elimination of tumor cells bearing neoantigens and formation of chronic granulomas.