Hyperparathyroidism
1. What is hyperparathyroidism?
Hyperparathyroidism (HPT) is a clinical syndrome causing specific symptoms and signs that result from excessive parathyroid hormone (PTH) secretion, PTH-induced bone resorption, and hypercalcemia. The three types of HPT are primary, secondary, and tertiary.
The prevalence of primary HPT in the United States is 0.1% to 0.3% of the general population. The female-to-male ratio is 2:1 to 3:1. The incidence increases with age, and the incidence in postmenopausal women is five times higher than that in the general population.
Primary HPT is characterized by abnormal regulation of PTH secretion by calcium, resulting in excessive PTH secretion and hypercalcemia. Although the cause of primary HPT is not known, increased PTH secretion is due in part to elevation of the calcium-suppressible PTH secretion set point and a change in the slope of the calcium-PTH curve that causes relatively nonsuppressible PTH secretion. Expression of the calcium sensor-receptor (CaSR) is reduced in parathyroid adenomas and hyperplasia and may be partly responsible for this relative PTH nonsuppressibility.
4. What anatomic alterations occur in primary HPT?
Most patients with primary HPT have a single parathyroid adenoma (85%), whereas four-gland hyperplasia (10%) and multiple adenomas (5%) are less common, and parathyroid carcinomas are rare (<5%). More than 95% of those with parathyroid adenomas have a single adenoma and fewer than 5% have two or more adenomas. Normal parathyroid glands weigh less than 50 mg each. The average weight of parathyroid adenomas is 500 mg to 5 g; however, some may weigh more than 25 g. The largest reported tumor weighed 120 g, and the largest number of glands reported in one patient was eight.
5. How do you diagnose primary HPT?
Persistent hypercalcemia with increased or high-normal serum PTH levels confirms the diagnosis of primary HPT. Associated low or low-normal serum phosphate makes this diagnosis more likely. Primary HPT should be suspected whenever a patient has documented hypercalcemia, which is the most common cause of hypercalcemia. Because symptoms of primary HPT are nonspecific or absent (see question 12), one must base the diagnosis primarily on laboratory studies. Furthermore, most patients with mild primary HPT have no specific symptoms or signs. Most cases are suspected after an elevated serum calcium value is found on routine laboratory screening.
6. How does age complicate the diagnosis of HPT?
The laboratory reference range for intact PTH (10-65 pg/mL) and calcium (8.5-10.5 mg/dL) may be different in the elderly and young individuals. However, the PTH reference range is not usually adjusted for age. PTH levels normally increase with age. Why PTH increases with age is unclear, but the change may be related to age-related declines in renal function and vitamin D levels. Thus, PTH levels in the upper normal range are more likely to represent HPT in patients who are younger than in those older than 50 years. Although serum calcium levels decline with age, the decline is usually related to decreasing albumin and does not generally affect serum PTH levels.
7. How might you make the diagnosis of primary HPT more certain before recommending parathyroidectomy?
Obtain at least three fasting serum calcium levels, ideally with no venous occlusion, and two PTH measurements at least several weeks apart. Ensure that the patient has normal renal function. Discontinue any thiazide diuretics for at least 1 week before measurement. Discontinue lithium if safe to do so. Measure serum total calcium and calculate the correction for albumin and total protein levels; order an ionized calcium measurement if there is any doubt (see Chapter 13). If calcium is elevated and the PTH value is high or high-normal, primary HPT is usually present. If calcium is normal and PTH is high, measure the serum 25-hydroxyvitamin D (25-OHD) level, because vitamin D deficiency is a common cause of secondary HPT. To exclude vitamin D deficiency, the 25-OHD level should be higher than 30 ng/mL. The immunoradiometric assay (IRMA) for intact PTH is standard and sufficient for diagnosis. However, if there is concern about the IRMA results, immunochemiluminometric assay (ICMA), which also measures intact PTH (1-84), can be used.
8. When lab results are not specific for primary HPT, what other classic laboratory changes may help with the diagnosis?
Increased serum chloride (Cl), decreased phosphate (PO4), a Cl/PO4 ratio greater than 33, elevated urinary pH (> 6.0), and increased alkaline phosphatase levels support the diagnosis of primary HPT but are not specific. If the PTH level is normal or low and the patient has suspected cancer, serum PTH-related peptide (PTHrP) should be measured. Ectopic PTH is rare and should be considered only if the patient has cancer or results of surgical neck exploration for HPT are negative.
9. What differentiates familial hypocalciuric hypercalcemia from primary HPT?
If there is a family history of hypercalcemia and/or the serum calcium and PTH are mildly elevated chronically, consider familial hypocalciuric hypercalcemia (FHH). Calculate the fractional excretion of calcium (FECa) (see Chapter 13). The FECa is less than 1% in FHH and more than 2% in primary HPT. If the FECa is low, test family members to confirm the diagnosis. If they test positive, FHH is probably present. Avoid neck exploration, which will have no effect on reversing hypercalcemia in this genetic condition.
10. How does chronic kidney disease (CKD) complicate the diagnosis of primary HPT?
Renal failure increases serum PO4 and decreases serum 1,25(OH)2D (calcitriol). Because PO4 directly stimulates and calcitriol directly inhibits PTH secretion, serum PTH levels increase in renal failure (secondary HPT). High PO4 and low calcitriol levels also directly decrease serum calcium. The resulting absolute or relative hypocalcemia further increases PTH secretion. Symptoms and signs of renal insufficiency, such as lethargy, depression, anorexia, nausea, constipation, and weakness, may be identical to those of primary HPT. Thus, unless it is overt, the diagnosis of primary HPT may be more difficult in renal failure. Before parathyroidectomy for presumed primary HPT, tissue localization with ultrasound and with a technetium 99m sestamibi scan may be appropriate.
11. What changes occur in renal failure that may complicate the PTH assay result?
In renal failure, PTH rises above normal because of the stimulatory effects of high PO4 and low calcitriol levels. In addition, a molecular fragment of PTH (PTH 7-84), which has antagonistic actions to those of intact PTH, accumulates in renal failure and cross-reacts with intact PTH in the intact two-site assays. For this reason, measured levels of intact PTH in patients with renal failure may be more than 1.5 times those of normal subjects to maintain physiologic PTH concentrations.
12. What are the symptoms and signs of primary HPT?
More than 85% of patients with primary HPT are asymptomatic. However, vascular, musculoskeletal, gastrointestinal, and neurologic symptoms may occur in primary HPT. The classic phrase for many of these features is “stones, bones, abdominal groans, and psychic moans.” Because of earlier diagnosis today, the incidence of nephrolithiasis has decreased to less than 10% in patients with primary HPT. Proximal muscle weakness is also characteristic. Other characteristic symptoms and signs and their probable cause(s) are outlined in Table 14-1.
TABLE 14-1.
HYPERPARATHYROIDISM: SYMPTOMS AND SIGNS AND THEIR PROBABLE CAUSES
| SYMPTOMS AND SIGNS | PROBABLE CAUSE(S) |
| Renal: hypercalciuria, nephrolithiasis, nephrocalcinosis, polyuria, polydipsia, renal insufficiency | Parathyroid hormone (PTH) stimulates bone resorption, hypercalcemia, bicarbonaturia, and phosphaturia, causing decreased tubular responsiveness to antidiuretic hormone (ADH), polyuria, calcium oxalate and phosphate crystallization, nephrocalcinosis, and renal insufficiency |
| Neuromuscular: weakness, myalgia | Prolonged excessive PTH arguably causes direct neuropathy with abnormal nerve conduction velocities (NCVs) and characteristic electromyographic changes and myopathic features on muscle biopsy |
| Neurologic and psychiatric: memory loss, depression, psychoses, neuroses, confusion, lethargy, fatigue, paresthesias | PTH and calcium cause peripheral neuropathy with abnormal NCVs and central nervous system damage with abnormal electroencephalographic changes |
| Skeletal: bone pain, osteitis fibrosa, osteoporosis, and subperiosteal skeletal resorption | PTH increases bone resorption and acidosis with subsequent bone buffering and bone loss of calcium and phosphate |
| Gastrointestinal: abdominal pain, nausea, peptic ulcer, constipation, and pancreatitis | Hypercalcemia stimulates gastrin secretion, decreases peristalsis, and increases the calcium-phosphate product with calcium-phosphate deposition in and obstruction of pancreatic ducts |
| Hypertension | Hypercalcemia causes vasoconstriction, and parathyroid hypertensive factor (PHF) may raise blood pressure |
| Arthralgia, synovitis, arthritis | HPT is associated with increased crystal deposition from calcium phosphate (para-articular calcification), calcium pyrophosphate (pseudogout), and uric acid/urate (gout) |
| Band keratopathy | Calcium-phosphate precipitation in medial and limbic margins of cornea |
| Anemia | Unknown |
Band keratopathy is a classic but unusual sign of HPT characterized by an irregular region of calcium phosphate deposition at the medial and lateral limbic margins of the outer edges of the corneas. The location is believed to be a result of diffusion of carbon dioxide from air-exposed areas of the cornea, leaving an alkaline environment that favors precipitation of calcium phosphate crystals. Band keratopathy occurs only with a high calcium phosphate product. Diagnosis is made by ophthalmologic slit-lamp examination. The sign differs from arcus senilis, an age-related, linear, concentric gray crescent separated from the extreme periphery (limbus corneae) by a rim of clear cornea that with time completely encircles the cornea.
14. What are the classic radiographic findings in HPT?
Because most patients are diagnosed early, there are often no radiographic findings related to HPT. If HPT is prolonged, osteopenia or osteoporosis develops. However, the classic radiographic finding is subperiosteal bone resorption along the radial aspect of the middle and distal phalanges and distal clavicles. Salt-and-pepper skull is another classic finding. Because cortical bone loss is higher in HPT, bone densitometry of the distal radius is a good way to monitor for bone loss in patients who do not undergo parathyroidectomy.
15. What is the differential diagnosis of primary HPT?
Because the main abnormality in primary HPT is hypercalcemia, the differential diagnosis initially is that of hypercalcemia (see Chapter 13). A history and physical examination focused on symptoms and signs (question 12) may suggest one of the causes of hypercalcemia. If hypercalcemia is mild and the history and physical findings are nonspecific, primary HPT is likely. The two most common causes of hypercalcemia are primary HPT and malignancy. In humoral hypercalcemia of malignancy (HHM), the tumor usually produces a PTH-like hormone called PTH-related peptide.
16. What lab tests help to distinguish the three types of HPT?
TABLE 14-2.
PARATHYROID HORMONE (PTH) AND CALCIUM LEVELS IN HYPERPARATHYROIDISM
| TYPE OF HYPERPARATHYROIDISM | PTH | CALCIUM |
| Primary | Normal ↑ | ↑ |
| Secondary | ↑ | ↓ Normal |
| Tertiary | ↑↑ | ↑ |
17. What pathophysiologic changes occur in primary HPT?
Primary HPT is idiopathic and results from excessive secretion of PTH from parathyroid adenomas, hyperplasia, or rarely carcinoma. The increased PTH causes hypercalcemia. The PTH level is inappropriately normal or high.
18. What pathophysiologic changes occur in secondary HPT?
Secondary HPT is excessive PTH secretion occurring as a compensatory response to absolute or relative hyperphosphatemia, hypocalcemia, or low calcitriol levels. Renal failure is the most common cause of secondary HPT, often producing PTH hypersecretion by means of all three of these stimuli. In renal failure, phosphorus increases because of decreased renal function. The increased phosphorous stimulates PTH secretion and decreases calcium and 1,25(OH)2D levels. The lower calcium and vitamin D levels also increase PTH synthesis and secretion. Thus, controlling phosphorus levels with diet and phosphate binders and appropriate calcitriol supplementation may delay onset of the secondary HPT of renal failure. Other causes of hypocalcemia are renal calcium leak, dietary calcium malabsorption, and vitamin D deficiency. Secondary HPT is generally accompanied by parathyroid hyperplasia as the parathyroid glands enlarge to enhance their PTH-secretory capacity.
19. What pathophysiologic changes occur in tertiary HPT?
Tertiary HPT results from progression of secondary HPT. In tertiary HPT, prolonged hyperphosphatemia and/or hypocalcemia cause further parathyroid hyperplasia with eventual development of autonomous parathyroid function and hypercalcemia. Spontaneous change from low or normal calcium levels to hypercalcemia marks the transition from secondary HPT to tertiary HPT. In tertiary HPT, PTH levels are usually approximately 15 to 30 times normal. This finding most commonly occurs in chronic renal failure. These changes are associated with decreased parathyroid CaSR, vitamin D receptor, and fibroblast growth factor receptor function and numbers. PTH remains elevated despite vitamin D therapy and correction of hyperphosphatemia. Hypercalcemia remains despite discontinuation of vitamin D and calcium supplements. Tertiary HPT usually requires resection of at least three and one-half parathyroid glands to correct the hypercalcemia. However, discontinuing vitamin D and a trial of cinacalcet therapy may lower PTH and calcium levels toward acceptable ranges and delay or obviate surgery.
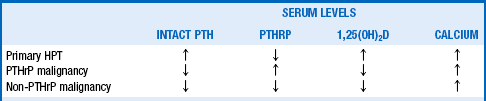
20. How is HHM distinguished from primary HPT?
The main distinguishing features of HHM are the levels of intact PTH and PTHrP. The classic and most common patterns of these hormones are shown in Table 14-3. The patient with primary HPT usually has elevated intact PTH; PTHrP, when measured, is low. Malignancy-associated hypercalcemia, in contrast, is associated with low intact PTH levels, but 80% of patients have increased PTHrP levels (PTHrP malignancy) and 20% have low PTHrP levels (non-PTHrP malignancy). Thus, measuring the two hormones distinguishes all three disorders (see question 21).
21. How do PTHrP and PTH differ?
PTHrP consists of three protein forms with 139, 141, and 173 amino acids. The first 139 amino acids are the same among the three forms. Eight of the first 13 N-terminal amino acids are identical to those of intact PTH (1-84), allowing PTHrP to bind to and stimulate the same receptors as PTH and to have similar hypercalcemic effects. But the two hormones have different effects on levels of 1,25(OH)2D, partly because of their different secretion patterns. Both PTH (in primary HPT) and PTHrP (in HHM) stimulate receptors that activate renal 1α-hydroxylase. However, although PTH secretion in primary HPT is intermittent, continuous secretion of PTHrP by malignant tumors probably downregulates these receptors, inhibiting 1α-hydroxylase activity and decreasing 1,25(OH)2D production. A continuous infusion of PTH causes similar decreases in 1,25(OH)2D. Other mechanisms may further decrease 1,25(OH)2D in PTHrP-associated HHM. HHM may have an associated five- to tenfold increase in the phosphaturic factor fibroblast growth factor-23 (FGF-23), which inhibits 1α-hydroxylase activity and decreases 1,25(OH)2D levels. The higher calcium levels typically encountered in HHM may also decrease 1α-hydroxylase activity and 1,25(OH)2D levels.
22. What hormonal and laboratory changes occur in primary HPT?
Secretion of PTH in primary HPT is intermittent; intermittent secretion avoids receptor downregulation and results in increased 1,25(OH)2D formation. Serum calcium levels are higher in HHM than in HPT, and higher calcium levels decrease 1,25(OH)2D production. Thus 1,25(OH)2D levels tend to be high in HPT and low in HHM (see Table 14-3). Traditional associations with primary HPT include hypophosphatemia, hyperchloremia, an increased chloride/phosphate ratio, and mild renal tubular acidosis. Unfortunately, such associations are nonspecific and too insensitive to be of diagnostic use. However, the triad consisting of hypercalcemia, elevated or high-normal PTH, and hypophosphatemia make the diagnosis of primary HPT likely.
23. What PTH assay is most useful in the workup of hypercalcemia?
Intact PTH has 84 amino acids, is 70% metabolized by the liver, is 20% metabolized by the kidneys, and has a half-life of 2 minutes. Less than 1% of the secreted intact hormone remains to interact physiologically with PTH receptors. Although the first 34 amino acids of the N-terminus contain the full biologic activity of the hormone, intact PTH (1-84) is the active hormone in vivo. The preferred assays for measurement are the ICMA and IRMA for intact PTH; both are highly sensitive and specific. Because of availability, the IRMA is more commonly used. The IRMA also measures the 7-84–amino acid fragment of intact PTH (1-84). The PTH assay from Scantibodies Laboratory Inc., Santee, CA, measures whole PTH (1-84) and is a measure of the true intact PTH hormone. This assay was initially believed to be potentially more useful in patients with renal failure; however, it has not proved clinically to be more useful than the IRMA or ICMA assays. Rapid PTH measurements are usually measures of intact PTH and are often performed both preoperatively and intraoperatively (10 minutes after parathyroidectomy). A reduction in PTH of at least 50% indicates a successful operation.
24. What methods best localize the parathyroid tumor in HPT?
Technetium 99m sestamibi single-proton emission computed tomography (SPECT) may be more than 85% to 90% sensitive, specific, and accurate and therefore is the procedure of choice. Sestamibi scanning is most accurate for localizing parathyroid adenomas but is much less useful for parathyroid hyperplasia. Ultrasonography is usually complementary to sestamibi scanning and, when combined with it, increases localization sensitivity to 95%. More frequent use is being made of combined sestamibi uptake and intraoperative gamma-probe localization (see next question). Less commonly, cervical computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), intravenous digital subtraction angiography (IVDSA), arteriography, and selective venous sampling are used.
25. When should you use preoperative localization of a parathyroid adenoma?
More than 90% to 95% of the time, a skilled parathyroid surgeon can localize and remove a parathyroid adenoma without preoperative localization. For this reason, preoperative localization before standard bilateral neck exploration is not usually necessary. However, minimally invasive parathyroidectomy (MIP) using a small incision localized to one side of the neck is becoming the state-of-the-art surgical approach to treating primary HPT. Most surgeons require preoperative localization studies before MIP, reoperative parathyroid surgery, or surgery for suspected bilateral disease. The best preoperative parathyroid adenoma localization usually consists of combined parathyroid sestamibi scan and ultrasound. Minimally invasive radioguided parathyroidectomy (MIRP) uses radioactive sestamibi uptake with an intraoperative gamma probe and provides for the least invasive and most accurate tumor localization. With MIRP, the operative time is shorter, the procedure can be done on an outpatient basis with use of local anesthesia, accurate localization of multiple adenomas is possible, and the patient can be discharged within hours of the procedure. That being said, standard neck exploration, MIP, and MIRP all require an experienced parathyroid surgeon. Often finding an experienced parathyroid surgeon is the most important and most difficult task in localizing and treating a parathyroid adenoma.
26. Do all asymptomatic patients with HPT require surgical treatment?
No. Many asymptomatic patients with mild primary HPT do not require surgery (see question 27). However, the only definitive therapy for HPT is parathyroidectomy, and it is usually appropriate to recommend parathyroidectomy for patients with asymptomatic primary HPT if they have access to an experienced parathyroid surgeon. Advantages of parathyroid surgery are cure of HPT and hypercalcemia in most cases with a single operation, no need for regular prolonged follow-up, decreased fracture rate, increased bone mass in most patients, and decreased rate of cardiovascular disease.
27. What are the indications for parathyroidectomy in patients with asymptomatic primary HPT?
1. Serum calcium more than 1.0 mg/dL above the upper normal limit
2. Decreased estimated glomerular filtration rate (GFR) (to < 60 mL/min)
3. Reduced bone density as shown by dual-energy x-ray absorptiometry (DEXA) (T-score < −2.5) at any site and/or previous fragility fracture
4. Age less than 50 years with mild hypercalcemia
6. Patients for whom medical surveillance is neither desired nor possible
28. How should you monitor patients with asymptomatic HPT who have not had parathyroidectomy?
Initially, obtain serum calcium and PTH measurements, DEXA bone densitometry, and GFR estimation. Thereafter, serum calcium measurement and GFR estimation should be performed annually. Three-site DEXA bone densitometry (lumbar spine, hip, and forearm) should be performed every 1 to 2 years. Schedule office visits every 6 months and as needed. Evaluate for symptoms of HPT. Make sure patients maintain adequate hydration (at least 8 glasses of water daily), exercise, and a normal-calcium diet. Avoid thiazide diuretics, lithium, and excessive calcium input. Alert the primary physician to watch for any medical illness predisposing to dehydration.
29. How would you estimate GFR without performing a 24-hour urine collection?
There are multiple equations for estimating GFR; the easiest and most accurate way is to use a Web-based site or an automated GFR from your laboratory. A good Website with which to access most estimates for GFR is http://mdrd.comv. This site give you access to Cockcroft-Gault, MDRD, and CKD EPI estimates of GFR. At this time the CKD EPI estimate of GFR is believed to be the most accurate.
30. What therapeutic options are available for patients unable to undergo surgery for HPT?
Calcimimetics bind to the extracellular CaSRs on parathyroid cells and increase chief cell sensitivity to extracellular calcium. This effect shifts the calcium-PTH curve to the left, increasing parathyroid sensitivity to the suppressive effects of calcium at all concentrations. Cinacalcet is the only calcimimetic available for treatment of secondary HPT in end-stage renal disease and for parathyroid carcinoma. It has also been approved for primary HPT with associated severe elevations in calcium. Cinacalcet also decreases calcium reabsorption from the renal tubule and thereby increases urinary calcium excretion. Bisphosphonates inhibit osteoclast-mediated bone resorption and can increase bone mass in osteopenic and osteoporotic patients with primary HPT. Raloxifene may also preserve bone mass in patients who cannot tolerate bisphosphonates. Estrogens preserve bone mass, but their use remains controversial because of the associated potential risk of breast cancer and cardiovascular disease. Denosumab is a monoclonal antibody to receptor activator of nuclear factor-kappa β ligand (RANK-L) that decreases osteoclastic bone resorption. It is approved for treatment of osteoporosis but is not yet approved for hypercalcemia treatment. Angiographic ablation or percutaneous alcohol injection of parathyroid adenoma tissue can also be tried if a provider having adequate experience with this technique is available.
31. How would you evaluate and treat a patient with normocalcemic HPT?
Normocalcemic HPT (NCHPT) manifests as elevated PTH and normal corrected calcium level. Studies now suggest that NCHPT is more common than previously thought and may cause complications similar to those of hypercalcemic HPT. To diagnose NCHPT, one must evaluate and treat all secondary causes of HPT, such as vitamin D deficiency, CKD, and renal hypercalciuria. Ionized calcium should be measured to confirm the normocalcemia. Vitamin D deficiency should be corrected to a 25-OHD level higher than 30 ng/mL. After secondary HPT has been ruled out, the patient can be monitored and treated like a patient with hypercalcemic HPT. However, referral for parathyroid surgery should not be routine for the normocalcemic patient with HPT but instead should be based on symptoms and signs (see questions 27 and 28).
Ambrogini, E, Cetani, F, Cianferotti, L, et al, Surgery or surveillance for mild asymptomatic primary hyperparathyroidism. a prospective, randomized clinical trial. J Clin Endocrinol Metab 2007;92:3114–3121.
Augustine, MM, Bravo, PE, Zeiger, MA, Surgical treatment of primary hyperparathyroidism. Endocr Pract 2011;17(Suppl 1):75–82.
Bilezikian, JP, Khan, AA, Potts, JT, Guidelines for the management of asymptomatic primary hyperparathyroidism. summary statement from the third international workshop. J Clin Endocrinol 2009;94:335–339.
Bilezikian, JP, Silverberg, SJ. Asymptomatic primary hyperparathyroidism. N Engl J Med. 2004;350:1746–1756.
Bringhurst, FR, Demay, MB, Kronenberg, HM. Hormones and disorders of mineral metabolism. In: Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology. ed 12. Philadelphia: Elsevier Saunders; 2011:1237.
Farford, R, Presutti, J, Moraghan, TJ. Nonsurgical management of primary hyperparathyroidism. Mayo Clin Proc. 2007;82:351–355.
Fraser, WD. Seminar—hyperparathyroidism. Lancet. 2009;374:145–158.
Kukora, JS, Zeiger, MA, Clark, OH, et al. AACE/AAES position statement on diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49–54.
Lew, JI, Solorzano, C, Surgical management of primary hyperparathyroidism. state of the art. Surg Clin N Am 2009;89:1205–1225.
Lowe, H, McMahon, DJ, Rubin, MR, et al, Normocalcemic primary hyperparathyroidism. further characterization of a new clinical phenotype. J Clin Endocrinol Metab 2007;92:3001–3005.
Maruani, G, Hertiga, A, Paillard, M, et al, Normocalcemic primary hyperparathyroidism. evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab 2003;88:4641–4648.
Peacock, M, Bilezikian, JP, Klassen, PS, et al. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:135–141.
Quiros, RM, Alioto, J, Wilhelm, SM, et al. An algorithm to maximize use of minimally invasive parathyroidectomy. Arch Surg. 2004;139:501–507.
Rejnmark, L, Vestergtaard, P, Mosekilde, L. Clinical review—nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab. 2011;96(8):2377–2385.
Rodgers, SE, Lew, JI. Review article—the parathyroid hormone assay. Endocr Pract. 2011;17(Suppl 1):2–6.
Rubello, D, Al-Nahhas, A, Khan, S. Is there an ideal algorithm in preoperative localization of primary hyperparathyroidism. Nucl Med Rev. 2006;9:105–107.
Shin, JJ, Milas, M, Mitchell, J. Impact of localization studies and clinical scenario inpatients with hyperparathyroidism being evaluated for reoperative neck surgery. Arch Surg. 2011;146(12):1397–1430.
Siilin, H, Rastad, J, Ljunggren, Ö, et al. Disturbances of calcium homeostasis consistent with mild primary hyperparathyroidism in premenopausal women and associated morbidity. J Clin Endocrinol Metab. 2008;93:47–53.
Vaid, S, Pandelidis, S. Minimally invasive parathyroidectomy. Arch Surg. 2011;146(7):876–878.