Chapter 26 Hyperlipidaemias
Some pathophysiology
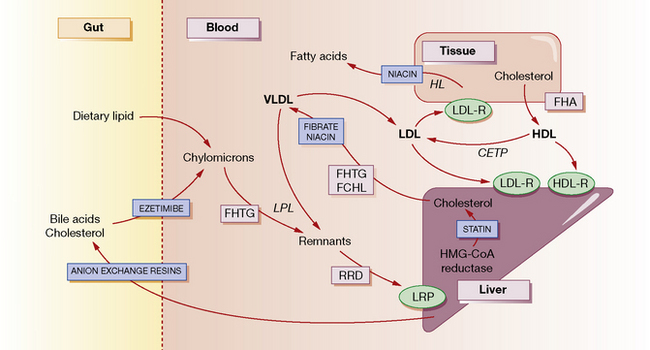
The normal function of lipoproteins is to distribute and recycle cholesterol. The pathways of lipid metabolism and transport and their primary (inherited) disorders are shown in Figure 26.1, and can be summarised thus:
• Cholesterol is absorbed from the intestine within chylomicrons. These are catabolised by lipoprotein lipase (LPL) to remnants, which are taken up by the hepatic low-density lipoprotein (LDL) receptor-related protein (LRP). A specific active transport mechanism also carries cholesterol across the gut mucosa (see below, ezetimibe).
• Cholesterol is synthesised de novo within the liver and peripheral tissues where, for example, it is converted to steroid hormones or incorporated into cell membranes. Much of the hepatic cholesterol enters the circulation as very-low-density lipoprotein (VLDL) and is metabolised to remnant lipoproteins after LPL removes triglyceride. The remnant lipoproteins are removed by the liver through apolipoprotein E receptors or LDL receptors (LDL-R), or further metabolised to LDL and then removed by peripheral tissues or the liver by LDL receptors.
• The quantity of cholesterol transported from the liver to peripheral tissues exceeds its catabolism there, and mechanisms exist to return about half of the cholesterol to the liver. Through this ‘reverse transport’, cholesterol is carried by high-density lipoprotein (HDL) from peripheral cells to the liver, where it is taken up by a process involving hepatic lipase. Cholesterol in the plasma is also recycled to LDL and VLDL by cholesterol-ester transport protein (CETP).
• Cholesterol in the liver is reassembled into lipoproteins, or secreted in bile and bile acids (essential for fat digestion and absorption), and then recycled by absorption or excreted in the faeces.
Lipid disorders
There are five primary inherited lipoprotein disorders that disturb lipid metabolism at the points indicated in Figure 26.1. These are:
• Familial hypertriglyceridaemia (FHTG) (rare), including lipoprotein lipase (LPL) deficiency, in which low LPL activity results in decreased removal, and thus increased levels of plasma triglyceride; there is increased hepatic secretion and thus a raised plasma concentration of triglyceride-rich VLDL. Patients are at risk of recurrent acute pancreatitis when plasma triglyceride exceeds 10 mmol/L, and especially when greater than 20 mmol/L.
• Familial combined hyperlipidaemia (FCHL) (common and most important) in which there is increased hepatic secretion of apolipoprotein B containing VLDL, and conversion to LDL; in consequence plasma LDL and VLDL levels are raised. Patients exhibit macrovascular disease (coronary heart, peripheral and cerebral).
• Remnant removal disease (RRD, also called remnant lipaemia, familial dysbetalipoproteinaemia) (uncommon) in which there is a defect of apolipoprotein E. This is the major ligand that allows internalisation and subsequent metabolism of remnant particles derived from VLDL and chylomicrons. The consequence is accumulation of VLDL remnants called intermediate-density lipoprotein (IDL), with cholesterol and triglycerides usually in the range 6–9 mmol/L. Patients experience severe macrovascular disease (as above).
• Familial hypoalphalipoproteinaemia (FHA, Tangier disease) (rare) in which the serum concentration of (protective) HDL is very low. Coronary heart and peripheral vascular disease result.
• Familial hypercholesterolaemia (FH) (common) is characterised by raised plasma levels of total and LDL cholesterol. It is caused by mutations in either the LDL receptor, Apo B-100 or PCSK9 genes. In the less severe heterozygous form, it affects about 1 in 500 of the population (who carry one copy of the mutant gene). LDL cholesterol levels are increased from childhood. Untreated, half the males will be dead by age 60 years, females 10 years later. The principal consequence is premature coronary heart disease, but occasionally also peripheral and cerebrovascular disease.
Sites of drug action
In general, drugs act to reduce the concentration of cholesterol within hepatocytes, producing a compensatory increase in LDL receptors on their surface, and increased uptake of cholesterol-rich LDL particles from the bloodstream (see Fig. 26.1). Statins decrease the synthesis of cholesterol and hence secretion of VLDL, and increase the surface expression of hepatic LDL receptors. Bile acid-binding (anion exchange) resins deplete the bile acid and thus the cholesterol pool. Fibrates decrease the secretion of VLDL and increase the activity of LPL, thereby increasing the removal of triglycerides. Nicotinic acid decreases fatty acid production in the tissues and the secretion of VLDL and clearance of HDL. It also enhances LPL. Ezetimibe blocks the uptake of cholesterol from the gut by targeting a specific cholesterol transporter.
Management
• Hyperlipidaemias are common; 66% of the adult UK population have a plasma cholesterol concentration in excess of 5.0 mmol/L, the lowest concentration generally associated with initiating drug treatment (in fact, statistical correlation with cardiovascular risk can be shown for cholesterol concentrations well below this value). The decision to treat an individual is made not just on the value for plasma lipids, but also on the absolute cardiovascular risk.
• Investigation of hyperlipidaemia must be directed initially at excluding contributory causes, i.e. secondary hyperlipidaemias (see above). None of these should be assumed to be the sole cause, even if present. Long-term decisions on management should be initiated only on the basis of at least two fasting blood samples.
• All patients (and their spouses/partners, if appropriate) should receive advice on lifestyle, diet and weight control, which are important components of overall macrovascular risk prevention. Dietary treatment of hypercholesterolaemia has a modest effect in the individual (at best an 8% reduction in LDL cholesterol is possible), but diet and weight reduction are more effective for hypertriglyceridaemia. Intake of total fat, especially saturated fat, should be reduced (and partially replaced with monounsaturated and polyunsaturated fats); spreads containing plant sterols and stanols, e.g. Benecol and Flora Proactiv, taken as part of a mixed meal are useful as they can reduce plasma cholesterol by up to 10%. Increasing attention is now paid the hydrogenated fat content of food (hydrogen bubbled through liquid oils improves texture, flavour and shelf-life). In some individuals, especially those with mixed hyperlipidaemia (raised cholesterol and triglyceride levels, often due to secondary factors on a polygenic background), successful adherence to dietary advice and weight loss produces very significant improvements. Patients with remnant lipaemia (RRD hyperlipidaemia) may respond excellently to diet and weight loss (and, possibly, the addition of a fibrate).
• Much of the work of lipid clinics is taken up with attending to multiple interacting risk factors such as hypertension, diabetes, thyroid disease and smoking, as well as to the lipid abnormality. The Joint British Societies Guidelines1 stress the importance of identifying the high-risk individuals who need intervention with diet and lifestyle changes and, if necessary, pharmacotherapy. Equal priority is given to: (1) patients with clinical atherosclerotic disease in any territory, (2) those with diabetes mellitus types 1 and 2 (the most numerous), and (3) those without symptomatic vascular disease but whose 10-year risk of developing it is greater than 20%.
• The decision to use lipid-lowering drugs is made on the basis of the overall absolute cardiovascular disease risk (CVD; see below and NICE guidance in footnote 1), e.g. evidence of existing CVD, hypertension, diabetes mellitus and a positive family history. This will often be quantified using an appropriate risk equation as the patient’s 10-year risk of developing cardiovascular disease: the threshold is set at 20% in the current NICE guidance1. The justification is easiest in two cases. Firstly, for primary prevention in the relatively small number of patients who are asymptomatic but have familial forms of hyperlipdaemia, e.g. patients with FH and remnant lipaemia. Secondly, for secondary prevention in patients who have evidence of CVD (previous myocardial infarction (MI), angina pectoris, stroke or transient ischaemic attack (TIA)), peripheral vascular disease or diabetes mellitus: in the landmark Scandinavian ‘4 S’ Study,2 4444 patients with a total cholesterol concentration of 5.8–8.0 mmol/L after a MI were randomised to receive simvastatin (median dose 27 mg) or placebo. Those on active treatment showed a 30% reduction in the overall mortality rate, a 42% fall in deaths from coronary heart disease and a 34% fall in risk of recurrent MI. In terms of absolute risk, the authors estimated that addition of simvastatin to the treatment regimens of 100 patients with coronary heart disease would, over 6 years, preserve the lives of 4 of 9 patients who would otherwise die, and would prevent a non-fatal MI in 7 of an expected 21 cases. These benefits have been amply supported by a more recent meta-analysis of statin trials in secondary prevention.3
• The consensus minimum target for secondary prevention of CVD with statins is a total plasma cholesterol of less than 5 mmol/L. This is referred to as an ‘audit’ target and in high-risk patients, if the total cholesterol remains greater than 4 mmol/L or the LDL cholesterol greater than 2 mmol/L, the statin dose may be increased. The latest NICE guidelines do not recommend routine monitoring of cholesterol levels in primary prevention once the decision to initiate a statin has been taken. The exception to this is patients with FH where the statin-based therapy is intensified to achieve at least a 50% fall in the pre-treatment level of LDL cholesterol.4
• There is evidence that statins protect against stroke. The benefit is seen in patients with plasma cholesterol levels greater than 5.0 mmol/L (or LDL cholesterol above 3.0 mmol/L) who have a history of ischaemic stroke or TIA, or CVD or diabetes mellitus.
Evidence for primary prevention comes from a recent meta-analysis of 10 trials totalling some 70 338 subjects with a median follow up of 4.1 years. It showed that statin therapy reduced all-cause mortality by 12%, coronary artery events by 30% and strokes by 19%. These benefits occurred regardless of age, sex or diabetic status of the patients.5 Concerns that primary prevention could have a net adverse outcome (that cholesterol reduction increased the risk of cancer or violent deaths) were not supported by this meta-analysis. Clearly, meta-analysis of randomised trials amply confirms the value of statin therapy for both primary and secondary prevention.
The decision to offer a patient primary prophylaxis is influenced by the absolute risk for the individual, the potential risks from the statin therapy and costs to the health provider. As statins so far have an excellent safety record, costs will escalate with a decision to treat lower and lower levels of absolute risk. This will be partly offset by generic forms of atorvastatin becoming widely available. Current UK recommendations (NICE CG67) suggest treating all patients with a CVD risk of at least 20% over 10 years; all diabetics should be assumed to be in this highest risk category. In the UK the desire to expand primary prevention led to deregulation of simvastatin, so that its lowest dose can now be sold by pharmacists without a doctor’s prescription. The absolute CVD risk is computed using risk equations based on longitudinal cohorts such as those in the Framingham study; in reality this means consulting a simple colour-coded chart armed with data about the patient including age, sex, smoking status, pre-treatment blood pressure, and plasma levels of total and LDL cholesterol.6
Management may proceed as follows:
1. Any medical disorder that may be causing hyperlipidaemia, e.g. diabetes, hypothyroidism, should be treated first.
2. Dietary adjustment. The following applies to all patients:
 Those who are overweight should reduce their total calorie intake, ideally until they have returned to the weight that is appropriate for their height (i.e. body mass index, BMI), but realistically with an initial aim of reducing body-weight by 10% (see Appetite control, p. 585); this automatically assumes reduced intake of alcohol and total (especially animal) fat. Raised triglyceride concentrations may respond particularly well to alcohol withdrawal. Physical exercise assists weight reduction.
Those who are overweight should reduce their total calorie intake, ideally until they have returned to the weight that is appropriate for their height (i.e. body mass index, BMI), but realistically with an initial aim of reducing body-weight by 10% (see Appetite control, p. 585); this automatically assumes reduced intake of alcohol and total (especially animal) fat. Raised triglyceride concentrations may respond particularly well to alcohol withdrawal. Physical exercise assists weight reduction. Those who fail to achieve adequate weight reduction or who are already at their ideal weight should reduce their total fat intake; polyunsaturated and monounsaturated fats or oils may be taken partially to substitute for the reduction in animal fats. Reduction in dietary cholesterol is a much less important element of the diet. Benecol or Flora Proactiv should be added.
Those who fail to achieve adequate weight reduction or who are already at their ideal weight should reduce their total fat intake; polyunsaturated and monounsaturated fats or oils may be taken partially to substitute for the reduction in animal fats. Reduction in dietary cholesterol is a much less important element of the diet. Benecol or Flora Proactiv should be added.3. Specific types of hyperlipidaemia are treated thus:
 Familial hypertriglyceridaemia responds best to dietary modification and weight reduction (above) together with a fibrate; nicotinic acid may be added.
Familial hypertriglyceridaemia responds best to dietary modification and weight reduction (above) together with a fibrate; nicotinic acid may be added. Familial combined hyperlipidaemia should be treated with dietary modification and weight reduction (above) together with a statin; nicotinic acid and/or a fibrate may be added in resistant cases.
Familial combined hyperlipidaemia should be treated with dietary modification and weight reduction (above) together with a statin; nicotinic acid and/or a fibrate may be added in resistant cases. Remnant removal disease (remnant lipaemia) responds to dietary modification and weight reduction (above) and a fibrate; a statin is an alternative and may be added where there is failure to respond.
Remnant removal disease (remnant lipaemia) responds to dietary modification and weight reduction (above) and a fibrate; a statin is an alternative and may be added where there is failure to respond.Drugs used IN TREATMENT
Statins
These drugs block the rate-limiting enzyme for endogenous hepatic cholesterol synthesis, hydroxymethylglutaryl coenzyme A (HMG CoA) reductase. This results in increased synthesis of LDL receptors (up-regulation) in the liver and increased clearance of LDL from the circulation; plasma total cholesterol and LDL cholesterol fall, with a maximum effect 1 month after commencing therapy. All statins cause a dose-dependent reduction in total and LDL cholesterol, although there are differences in their therapeutic efficacy; for example, at their starting doses LDL cholesterol falls after 1 month by an average of 17% with fluvastatin (20 mg/day), 28% with simvastatin (10 mg/day) and 38% with atorvastatin (10 mg/day). At higher doses, e.g. 80 mg/day atorvastatin or simvastatin, a 50% or greater reduction in LDL cholesterol is possible. The effects of pravastatin are similar and so-called superstatins (e.g. rosuvastatin) may achieve this level of reduction at lower doses.7
Statins are well absorbed by mouth, and are metabolised in the liver. Simvastatin is a prodrug, simvastatin lactone, which is itself inactive. Other statins are prescribed in their active form. Except for rosuvastatin, they are metabolised through CYP pathways (usually 3A4), which is an important source of drug interactions particularly involving simvastatin and atorvastatin (some examples of drugs appear in Table 20.2B, p. 315). They are well tolerated, the commonest adverse effect being a transient, and usually minor, increase in serum transaminases in some 1% of patients. Asymptomatic increases in muscle enzymes (creatine phosphokinase, CPK) and myositis (with a generalised muscle discomfort) occur more rarely,8 but are more frequent when statins are combined with other antihyperlidaemic drugs such as fibrates and nicotinic acid; patients should be counselled about myositis when these drugs are co-administered. There is also a genetic predisposition, as carriers of a common variant of the SLCO1B1 gene encoding the OATP1B1 anionic transporter on hepatocytes have a four-fold increased risk of myopathy on simvastatin; this increases to 16-fold if they are carrying two copies of the rs4149056 SNP.9 Myositis is also more likely with co-administered anti-HIV protease inhibitors, e.g. ritonavir, and with drugs that interfere with metabolism of some statins, e.g. ciclosporin.
Fibric acid derivatives (fibrates)
The class includes bezafibrate, ciprofibrate, fenofibrate and gemfibrozil; the original fibrate, clofibrate, is obsolete. The drugs partly resemble short-chain fatty acids and increase the oxidation of these acids in both liver and muscle. In the liver, secretion of triglyceride-rich lipoproteins falls, and in muscle the activity of lipoprotein lipase and fatty acid uptake from plasma are both increased. Fibrates act through a nuclear transcription factor (peroxisome proliferator-activated receptor (PPAR-α), which up-regulates expression of LPL and apolipoprotein A-1 genes, and down-regulates expression of the apolipoprotein C-11 gene. The result is that plasma levels of triglyceride decline by 20–30% and those of cholesterol by 10–15%; associated with this is a rise in the ‘protective’ HDL cholesterol. The latter effect may have contributed to the reduction in non-fatal myocardial infarction with gemfibrozil in both the Helsinki Heart Study10 and more recent Veterans Affairs HDL Intervention Trials (VA-HIT).11
Anion-exchange resins (bile acid sequestrants)
Colestyramine
is an oral anion-exchange resin;12 it binds bile acids in the intestine and depletion of the bile acid pool stimulates conversion of cholesterol to bile acid. The result is a fall in intracellular cholesterol in hepatocytes, and an increase (up-regulation) in both LDL receptors and cholesterol synthesis. Plasma LDL cholesterol concentration falls by 20–25%. In many patients there is some compensatory increase in hepatic triglyceride output. Anion exchange resins may be used for hypercholesterolaemia but not when there is significant hypertriglyceridaemia.
Ezetimibe
The real potential of this drug is increased efficacy in combination with statins. Ezetimibe with a low dose of a statin, e.g. simvastatin 10 mg/day, produces a fall in plasma cholesterol similar to that with simvastatin 80 mg/day, providing a possible alternative treatment for patients unable to tolerate high-dose statin monotherapy. Ezetimibe may challenge resins as the agent of choice for patients with hypercholesterolaemia that is not controlled by a statin alone. It remains unclear after a decade of clinical use whether an ezetimibe–statin combination offers any benefit over high-dose statin monotherapy alone in the quest for aggressive reduction in LDL cholesterol levels.13
Other drugs
Orlistat,
a weight-reducing agent, lowers the glycaemia of diabetes mellitus to a degree that accords with the weight loss, and improves hyperlipidaemia to an extent greater than would be expected (see p. 444).
α-Tocopherol acetate
• The commonest and most important hyperlipidaemia is hypercholesterolaemia, which is one of the major risk factors for coronary heart disease.
• Most treatments work by reducing the intracellular concentration of cholesterol in hepatocytes, leading to a compensatory increase in low-density lipoprotein (LDL) receptors on their surface, and increased uptake of cholesterol-rich LDL particles from the bloodstream.
• The most effective cholesterol-reducing drugs are the statins, which inhibit the rate-limiting step in cholesterol synthesis.
• Additional agents may be required for mixed or severe hyperlipidaemia.
• In outcome trials with these drugs, reductions in blood cholesterol by 25–35% are associated with a 35–45% reduction in the risk of coronary heart disease.
• The main indications for their use are patients with even slightly increased levels of cholesterol (> 5 mmol/L) after a myocardial infarction or any other macrovascular event, in patients with familial hypercholesterolaemia or diabetes, and in patients with a significant absolute CVD risk, especially where there is a family history of premature CVD.
Amarenco P., Labreuche J., Lavallée P., Touboul P.J. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35(12):2902–2909.
Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790.
Ashen M.D., Blumenthal R.S. Low HDL-cholesterol levels. N. Engl. J. Med.. 2005;353:1252–1269.
Durrington P. Dyslipidaemia. Lancet. 2003;362:717–731.
Khera A.V., Rader D.J. Discovery and validation of new molecular targets in treating dyslipidemia: the role of human genetics. Trends Cardiovasc. Med.. 2009;19:195–201.
Mozaffarian D., Katan M.B., Ascherio A., et al. Trans fatty acids and cardiovascular disease. N. Engl. J. Med.. 2006;354:1601–1613.
von Bergmann K., Sudhop T., Lutjohann D., et al. Cholesterol and plant sterol absorption: recent insights. Am. J. Cardiol.. 2005;96:10–14.
Wang C.Y., Liu P.Y., Liao J.K. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol. Med.. 2008;14:37–44.
1 NICE (National Institute for Health and Clinical Excellence) guidance on lipid modification (2008) is contained in document CG67: http://www.nice.org.uk/CG067 (accessed November 2011).
2 Scandinavian Simvastatin Survival Study Group 1994 Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4 S). Lancet 344:1383–1389.
3 Keech A, Pollicino C, Kirby A, Sourjina T, Simes J on behalf of the Cholesterol Treatment Trialists’ (CTT) Collaborators 2005 Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 366:1267–1278.
4 NICE guidance on familial hypercholesterolaemia (2008) is available at: http://guidance.nice.org.uk/CG71 (accessed November 2011).
5 Brugts J J, Yetgin T, Hoeks S E et al 2009 The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. British Medical Journal 338:b2376.
6 The cardiac risk programme (as an Excel spreadsheet) and risk assessment charts can be downloaded from the British Hypertension Society website at: http://www.bhsoc.org/. They may also be found in the British National Formulary. Note that they are not for use in patients with established CVD or renal failure, and an additional loading must be made for non-caucasians and those with a positive family history of premature CVD. The small print ought to be read with care. See also the footnote on the JBS 2 guidelines.
7 The Heart Protection Study, which remains the largest statin intervention trial to date, randomised 20 536 patients to either placebo or simvastatin 40 mg for the secondary prophylactic effect on patients with coronary artery disease, occlusive arterial disease or diabetes. The simvastatin group had some 25% reduction in fatal/non-fatal MI, fatal/non-fatal stroke or revascularisation. This effect was seen across all groups and did not depend on cholesterol levels at entry or the reduction seen within the trial (averaging as a 1 mmol/L fall in LDL cholesterol). See: HPS Collaborative Group 2002 MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360:7–22.
8 In 30 641 patients in five major statin trials, myositis (serum creatinine kinase × 10 normal) occurred in 30 (control 29) and rhabdomyolysis in 2 (control 2) (Farmer J A 2001 Learning from the cerivastatin experience. Lancet 358: 1383–1385). There was also no significant excess of these events in the simvastatin arm of the Heart Protection Study.
9 SEARCH 2008 SLCO1B1 variants and statin-induced myopathy – a genomewide study. New England Journal of Medicine. 359:789–799.
10 Frick M H, Elo O, Haapa K et al 1987 Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidaemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. New England Journal of Medicine 317:1237–1245.
11 Rubins H B, Robins S J, Collins D et al for The Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group 1999 Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. New England Journal of Medicine 341:410–418.
12 The resins consist of aggregations of large molecules carrying a fixed positive charge, which therefore bind negatively charged ions (anions).
13 A recent review of the literature of statin–ezetimibe combination therapy has highlighted the limitations of the available data, including its short duration, use of surrogate endpoints (such as intimal media thickness) and lack of power in terms of vascular mortality to deliver clear guidance on ezetimibe’s use (Sharma M, Ansari M T, Abou-Setta A M et al 2009 Systematic review: comparative effectiveness and harms of combinations of lipid-modifying agents and high-dose statin monotherapy. Annals of Internal Medicine 151:622–30.