CHAPTER 63 HYDROCEPHALUS, INCLUDING NORMAL-PRESSURE HYDROCEPHALUS
Hydrocephalus is an abnormal enlargement of the ventricles caused by an excessive accumulation of cerebrospinal fluid (CSF) that results from a disturbance of its flow, absorption, or, uncommonly, secretion.
CEREBROSPINAL FLUID PRODUCTION, FLOW, AND ABSORPTION
Hydrocephalus is a disturbance of CSF circulation. Average CSF production is 0.44 mL/minute, corresponding to a total of about 500 mL/day1 (see Gjerris, 2000). The normal volume of CSF is approximately 150 mL. CSF is secreted by the epithelial cells of the choroid plexus, through an energy-dependent process involving ion pumps and enzyme systems.2 Specifically, an adenosine triphosphate-dependent, ouabain-sensitive sodium-potassium exchange pump at the apical (CSF) surface of the choroidal cells drives secretion, with water entering via aquaporin 1 channels, after the accumulation of sodium and chloride. The (passive) import of sodium into the choroidal cells from the pericapillary space is necessarily coupled with that of chloride, itself exchanged for bicarbonate. Carbonic anhydrase inhibitors, by reducing available bicarbonate, indirectly reduce CSF formation.
CSF production is relatively independent of intracranial pressure (ICP), whereas, in patent CSF pathways and venous systems, CSF absorption is proportional to ICP. ICP is therefore determined by the pressure at which the rate of absorption rate balances the rate of production.1 CSF is also produced through transependymal bulk flow from the brain parenchyma itself.3,4 There is a marked diurnal variation in CSF production, with an increase at night to as high as twice diurnal values at 2:00 A.M.5 CSF flow and circulation are maintained by the arterial systolic pulsations of the brain and by continuous CSF secretion, as well as by changes in central venous pressure through respiration. These mechanisms combine as a “CSF pump.”
The arachnoid villi of the dural sinuses are traditionally regarded as the main site of CSF absorption.6 However, perineural lymphatic pathways, including those at the cribriform plate, and transependymal pathways, with absorption into capillaries or venules in the brain parenchyma, may also contribute.7,8 These alternative routes of CSF absorption may become increasingly important in hydrocephalus.
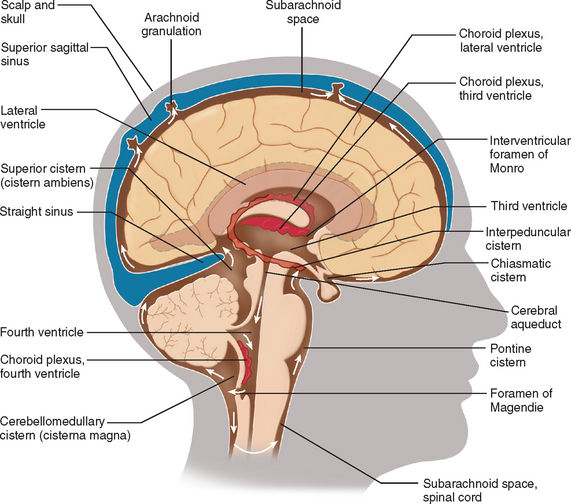
The CSF circulation is shown in Figure 63-1. Flow is from the lateral ventricles through the foramen of Monro into the third ventricle, via the aqueduct of Sylvius into the fourth ventricle and then via its outlets (foramina of Luschka and Magendie) into the subarachnoid space and basal cisterns. CSF then circulates throughout the spinal subarachnoid space and basal cisterns up through the tentorial hiatus. It flows over the cerebral hemispheres and is absorbed at the sites identified previously.
HYDROCEPHALUS
Classification and Etiology
Obstruction of Cerebrospinal Fluid Circulation
Reduction of Cerebrospinal Fluid Absorption
Reduced CSF absorption through the arachnoid granulations may be caused by
Overproduction of Cerebrospinal Fluid
Choroid plexus papillomas are rare ventricular tumors that can produce excessive volumes of CSF. In some cases, the hydrocephalus persists despite tumor resection, which is probably related to chronic pressure changes or proteinaceous debris within the spinal fluid. This tumor type is the only known cause of overproduction of CSF10,12 (see also Kaye, 2005.)
Clinical Presentation
Infantile Hydrocephalus
The incidence of infantile hydrocephalus is approximately 3 to 4 per 1000 births. Most cases arise from congenital abnormalities or from intraventricular hemorrhage associated with premature birth.10,13 The incidence of hydrocephalus occurring as an isolated congenital disorder is 1 to 1.5 per 1000 births. The most common congenital cause of hydrocephalus is stenosis of the aqueduct of Sylvius. The incidence of this abnormality is increased in children with a Chiari type II malformation. Hydrocephalus also occurs with spina bifida and myelomeningoceles. Its incidence in this context varies from 1.5 to 2.9 per 1000 births; however, this rate is decreasing with improved prevention through folate supplementation and prenatal screening for spina bifida (see Kaye, 2005).
Congenital atresia of the foramina of Luschka and Magendie (Dandy-Walker cyst) is a rare cause of congenital hydrocephalus.11,14,15 Hydrocephalus can also occur as a component of numerous genetic disorders (see Online Mendelian Inheritance in Man at www.ncbi.nlm.gov/entrez/query.fcgi?db=OMIM for an up-to-date listing of such syndromes). The best characterized is X-linked, with aqueduct stenosis, and accompanied by mental retardation, caused by mutations in the L1CAM gene.
The acquired forms of hydrocephalus occur most frequently after intracranial bleeding (particularly in premature infants), as a complication of meningitis, and secondary to tumors. The marked improvement in the survival of premature infants of very low birth weight has resulted in an increase in the numbers of infants with hydrocephalus resulting from perinatal intracranial hemorrhage.10
Major clinical features of hydrocephalus in infants include:
Radiological Investigations
The most important investigation is either a computed tomographic (CT) scan or magnetic resonance imaging (MRI) of the brain (Figs. 63-3 and 63-4), which demonstrate enlargement of the ventricles. The type and cause of the hydrocephalus may also be determined on imaging.12 For example, an enhanced CT scan or MRI may demonstrate a tumor obstructing the ventricles. In communicating hydrocephalus, all the ventricles are dilated, whereas in obstructive hydrocephalus caused by a lesion at or above the level of the aqueduct of Sylvius, the fourth ventricle is of normal size.
MRI is particularly helpful when performed in the sagittal plane, because it may demonstrate aqueduct stenosis and lesions around the third ventricle. Flow of CSF through the aqueduct can be determined through the use of dedicated flow sequences on MRI.16,17
In adult neurology, the question often arises as to whether dilated ventricles are a result of atrophy of the brain parenchyma (sometimes termed hydrocephalus ex vacuo) or are a result of acquired hydrocephalus (typically NPH, discussed later). A number of features are suggestive of a diagnosis of hydrocephalus rather than atrophy, including enlargement of the temporal horns (however, hippocampal and parahippocampal atrophy in Alzheimer’s disease can look similar), a convex third ventricle with dilated anterior recesses, an acute rather than obtuse angle between the frontal horns on axial scans, enlargement of the ventricles out of proportion to sulcal enlargement (or frank sulcal effacement), and periventricular smooth high signal on fluid-attenuated inversion recovery imaging (representing transependymal edema).18
Treatment
Surgical Management
External drainage
An external ventricular drain may be required as an urgent treatment for acute hydrocephalus associated with rapid deterioration of consciousness level, occurring with ventricular hemorrhage, acute infection, or a cerebral mass lesion.19
Because acute hydrocephalus is a surgical emergency, a temporary drain can be used. In addition to drainage of CSF, any intraventricular blood that is present can be drained, and ICP can be monitored as well.20,21
Internal shunting
The usual method of CSF diversion is a ventriculoperitoneal shunt, in which a catheter is placed into the lateral ventricle and is connected through a burr hole to a valve, which is itself attached to a catheter threaded subcutaneously down to the abdomen and inserted into the peritoneal cavity (Fig. 63-5).22 Alternative sites for CSF drainage such as the atrium, pleural cavity, or ureter have now been largely abandoned except in special circumstances: for example, when prior peritoneal scarring or multiple abdominal operations leads to reduced absorptive capacity. It is occasionally necessary to place the shunt into the pleural cavity.
A lumboperitoneal shunt involves drainage of the CSF from the lumbar theca rather than the ventricle. This type of shunt can be considered only in patients with communicating hydrocephalus. A catheter is threaded percutaneously into the lumbar theca and then tunneled subcutaneously to the anterior abdominal wall and placed into the peritoneal cavity. The practical advantage of this shunt system is that the brain is not manipulated. However, this shunt is not as reliable as a ventriculoperitoneal shunt and is more difficult to assess if the patient develops malfunction of a shunt.22,23
Shunt Complications
The risk of shunt failure is greatest within the first year of insertion, estimated to be 10% to 20%, depending on the underlying cause of the hydrocephalus. The subsequent failure rate is about 5% per year after this.24,25
Infection is a dreaded complication, especially in patients who are shunt dependent. The infection rate has significantly decreased as a result of improved surgical technique, more stringent asepsis, and possibly the use of antibiotic-impregnated shunts, as well as intraoperative prophylactic antibiotics,26 and the overall infection rate in children is now about 3%.27
Important risk factors associated with infection include suboptimal surgical technique, prolonged operating time,28,29 multiple surgical revisions,29 skin abrasion,30 and ventriculoatrial shunting.28,31 Although the treatment of shunt infection is complex, the most commonly accepted method involves immediate removal of the shunt device, along with ventriculostomy.19,28,29 One suggested protocol for the replacement of the shunt then requires a minimum of three negative CSF cultures and the absence of fever for at least 24 hours before reinsertion.19,28,32
Shunt occlusion may result from blockage of the ventricular catheter, malfunction or blockage of the valve, or obstruction of the peritoneal catheter. In the majority of cases, the site of occlusion is the ventricular portion.19 Shunt occlusion can arise from tissue debris, hematoma, or plugging by choroid plexus and ependymal cells.19,33
The slit-ventricle syndrome occurs when overdrainage leads to collapse of the ventricular walls surrounding the apertures at the ventricular end of the shunt.34 This then leads to repeated shunt blockage, because the CSF flow is intermittently obstructed through a cycle of ventricular collapse and dilatation. In this condition, raised ICP and marginally dilated ventricles are evident on CT scanning. This condition occurs predominantly but not exclusively in the pediatric population.
Intracranial hemorrhage after the insertion of a ventriculoperitoneal shunt may occur either in the brain parenchyma or in the subdural space. A subdural hematoma is particularly likely to occur in patients with long-standing severe hydrocephalus, in whom rapid decompression and collapse of the ventricles may result in tearing of the cortical bridging veins. Consequently, patients should remain initially supine after insertion of a shunt, to reduce the risk of sudden decompression occurring with elevation of the cerebral venous sinuses in relation to the right atrium. Attempts have been made to overcome this condition and that of shunt overdrainage with the use of an anti-siphon device that prevents excessive drainage with postural changes. However, antissiphon devices have also been associated with insufficient drainage and are not routinely used.35
Endoscopic surgery
Third ventriculostomy, originally performed by Walter Dandy in 1922, was one of the first surgical treatments for hydrocephalus. Endoscopic third ventriculostomy through the lamina terminalis or the ventricular floor establishes communication between the ventricular system and the subarachnoid space.19,36 Therefore, the procedure is useful for patients with obstructive hydrocephalus who maintain a normal subarachnoid space: that is, predominantly those with aqueduct stenosis.19,36
In patients who undergo endoscopic third ventriculostomy, ICP occasionally remains high immediately after the procedure, with substantial reduction around the fourth day after surgery. This time course is indicative of the physiological changes that adjust CSF circulation to new flow conditions.37,38
Hydrocephalus with ventriculomegaly in a single lateral ventricle may arise secondarily to obstruction of the foramen of Monro. This condition may also be treated through endoscopic procedures such as fenestration of the septum pellucidum. Such a technique may preclude the need for shunting.19,39
Medical Treatment
The placement of the head at 30 degrees in relation to the rest of the body helps venous drainage and downward displacement of the CSF pressure40; because there are no valves in the dural sinus/jugular system, a flat or head-down posture results in transmitted right atrial backward pressure, raising venous backward pressure to CSF absorption, as well as intracranial venous volume.
Restriction of fluid is indicated, because hypervolemia may contribute to raised ICP by elevating central venous pressure. Osmotic diuretics such as mannitol reduce ICP, with a maximum effect within the first 20 to 60 minutes. This effect results predominantly from the dehydration of brain parenchyma secondary to the increase of osmotic pressure in the vascular space (but not the brain),41 although hyperosmolality does reduce CSF formation to some extent.
Mannitol can also be used in combination with diuretics, such as furosemide, and/or carbonic anhydrase inhibitors, such as acetazolamide, which reduce CSF production22,42 (see also earlier section).
There is no evidence that steroids are effective in the treatment of hydrocephalus, other than by reducing vasogenic edema associated with mass lesions that contribute to hydrocephalus.19
NORMAL-PRESSURE HYDROCEPHALUS
NPH, first described by Hakim and Adams in 1965,43 is characterized by the triad of gait disturbance, urinary incontinence, and cognitive changes that may occasionally progress to a subcortical-type dementia (for a more detailed review, see Vanneste, 2000).
Gait impairment and postural instability are usually the first symptoms of NPH and are also the most responsive to shunting.44 The gait is sometimes described as “apraxic,” but this constitutes very loose usage of the term; “frontal gait disorder” is probably preferable. The gait abnormalities themselves are not specific; slower velocity, shorter and more varying strides, reduced ground clearance and ankle dorsiflexion during the swing phase, and increased base of gait (step width) are all seen.45 There may be postural instability with frequent falls, gait ignition failure, or freezing (typically unresponsive to levodopa).44 Pyramidal signs, including extensor plantar responses, may be seen in the lower limbs.44
Urinary urgency, arising from impairment of frontal inhibitory pathways, is almost always present, but urinary incontinence is a relatively late feature.44 The detrusor muscle is unstable and hyperreflexic on urodynamic studies, but sphincteric function is normal.44,46 The urinary dysfunction may also improve on shunting.46
NPH is an uncommon cause of dementia.44 Indeed, dementia is an inapposite term for the cognitive impairment that may be seen in NPH, which often fails to meet any of the usual diagnostic criteria for dementia. Furthermore, some patients have no cognitive impairment at all. Dementia, if present, is the least likely of the three classic symptoms to improve with shunting,44 although cognitive improvement at 6 to 12 months, particularly in memory (word list acquisition) and psychomotor speed, has been convincingly demonstrated prospectively on careful testing.48 The pattern of cognitive impairment is typically that of subcortical dysfunction, with impaired working memory (the ability to manipulate data in consciousness) and slowed information processing and psychomotor speed.44 This is different from the profile in Alzheimer’s disease, and, indeed, visuoconstructional difficulties and executive dysfunction are predictive of a lower chance of cognitive improvement after shunting (and, by implication, a lower likelihood of actual NPH).48 The Mini-Mental State Examination is not particularly sensitive to the type of cognitive impairment observed in NPH,44 and measures such as the Trail Making Test or the HIV Dementia Scale,49,50 with their emphasis on aspects of cognition impaired by subcortical processes, are probably preferable screening tools.
Pathophysiology
Although the condition is named normal pressure because lumbar puncture typically demonstrates a CSF pressure within the normal range, there is experimental evidence that an initial stage of raised CSF pressure resulting from impaired CSF flow leads to ventricular enlargement.51 This disparity between ventricular and convexity CSF later diminishes, although episodic CSF pressure elevations persist, and continuous monitoring of the ICP frequently reveals abnormal ICP wave formation (predominantly so-called B waves), especially at night (see Edwards et al, 2004, and Kaye, 2005). Increased transmantle pressure and a “water hammer” effect from increased CSF pulse pressure waves have also been suggested as contributing factors.44 Neurological impairment in NPH is probably a result of progressive impairment of the periventricular microcirculation, with impaired perfusion secondary to compression and stretching of arterioles and venules.52 This may explain the increased prevalence of concomitant subcortical ischemic vascular disease and its etiological risk factors (such as hypertension) in NPH.45,54
Etiology
Perhaps as much as 50% of NPH may be secondary to causes of impaired extraventricular CSF flow.44 The commonest cause is traumatic subarachnoid hemorrhage,53 but spontaneous subarachnoid hemorrhage and meningitis may also be responsible. The cause or causes of the remaining idiopathic cases are unknown; there is no histological evidence for the leptomeningeal “fibrosis” sometimes suggested as an explanation.44 Although secondary NPH can develop at any age, idiopathic NPH typically manifests in the sixth or seventh decades.54
Diagnosis
The diagnosis of (shunt-responsive) NPH is difficult, because no single feature is reliably predictive of worthwhile improvement on shunting. Furthermore, although substantial improvement after shunting is seen in 30% to 50% of patients with idiopathic NPH, with 29% showing long-term improvement, there is a 6% to 8% chance of death or permanent neurological deficit.44,54 Factors associated with a greater likelihood of shunt responsiveness include a secondary cause (worthwhile improvement being seen in 50% to 70%), gait impairment preceding cognitive changes, and a short history of cognitive decline to only a mild or moderate level.44,54 Adverse factors include severe cognitive decline; cognitive decline as the first symptom; and MRI showing marked atrophy, extensive white matter involvement (subcortical ischemia), or both. Not surprisingly, in view of the difficulties of the diagnosis and the potential complications of shunting, numerous investigative techniques have been employed in an attempt to improve the accuracy of decision making in this area.
Radiological investigations may demonstrate ventriculomegaly, and certain radiological features favor a diagnosis of hydrocephalus over atrophy (see “Radiological Investigations” section). However, in the presence of coexistent cortical atrophy, the diagnosis may be difficult to establish. Although marked atrophy is an adverse prognostic feature, moderate atrophy does not exclude the possibility of shunt responsiveness. CSF flow through the aqueduct is hyperdynamic in NPH, appearing as a flow void. This may be quantitated with phase-contrast cine-magnetic resonance flow imaging and has been claimed to be well correlated with findings of ICP monitoring and to be predictive of response to shunting with more than 80% accuracy,55,56 although other studies have failed to show this.44 Radioisotope cisternography may demonstrate disturbed flow, with stasis in the ventricles. However, the results are not successfully predictive of shunt outcome and do not add to the accuracy of clinical plus CT assessment.44,54
CSF drainage via single or repeated large-volume (40- to 50-mL) lumbar puncture or temporary external lumbar drainage catheter (for several days) may result in improvement in symptoms such as the gait disturbance and, more particularly, in gait velocity and stride length.45 The CSF tap test is widely used, probably because of its simplicity: It can be performed as an outpatient procedure. Although a positive result may be predictive of response to shunting, a negative result cannot be used to rule out the condition, because of its poor negative predictive value.44,54 Continuous lumbar CSF drainage may yield more accurately predictive results,57 but it has a number of potential complications44 and requires an inpatient stay.
A number of more complicated assessment procedures have been reported. Continuous CSF pressure monitoring is not widely available, but there is good evidence that the frequent occurrence of B waves is predictive of shunt responsiveness, whereas their absence is a poor prognostic factor.44 Resistance to CSF outflow, measured during lumbar infusion of artificial CSF, produces very good diagnostic information when performed by some, but not all, clinicians.44,54 The technique apparently requires considerable technical expertise.
Treatment
There are no randomized controlled trials of CSF shunting for NPH.58 A systematic review of the existing literature (largely small retrospective case series), published in 2001, revealed that 59% of patients (range, 24% to 100%) improved after shunting, with prolonged improvement in 29% (range, 10% to 100%), complications in 38% (range, 5% to 100%), a requirement for reoperation in 22% (range, 0% to 47%), and death or permanent neurological deficit in 6% (range, 0% to 35%).54 Vanneste reached similar conclusions in 2000.44 It is nevertheless apparent that symptoms may resolve almost completely if the correct diagnosis is made and shunting is successful. In such patients, the amount of CSF drainage and the valve pressure setting necessary to achieve therapeutic benefit vary considerably. Hence, it may be appropriate to use a valve that is adjustable, in order for the pressure and CSF drainage to be tailored to the patient’s physiological requirements. Such “programmable” valves have been used effectively in the treatment of NPH, but overshunting may lead to subdural hematoma. In some cases, a lumboperitoneal shunt may offer an effective solution.
Edwards RJ, Dombrowski SM, Luciano MG, et al. Chronic hydrocephalus in adults. Brain Pathol. 2004;14:325-336.
Gjerris F. Hydrocephalus in adults. In: Kaye AH, Black PMcL, editors. Operative Neurosurgery. London: Churchill Livingstone; 2000:1235-1247.
Kaye AH. Raised intracranial pressure and hydrocephalus. In: Essential Neurosurgery. Malden: Blackwell Publishing; 2005:27-39.
Pattisapu JV. Etiology and clinical course of hydrocephalus. Neurosurg Clin North Am. 2001;36:651-659.
Vanneste JA. Diagnosis and management of normal pressure hydrocephalus. J Neurol. 2000;247:5-14.
1 Lorenzo AV, Page LK, Watters GV. Relationship between cerebrospinal fluid formation, absorption and pressure in human hydrocephalus. Brain. 1970;93:679-692.
2 Garton H, Piatt J. Hydrocephalus. Pediatr Clin North Am. 2004;51:305-325.
3 Milhorat TH, Hammock MK, Fenstermacher JD, et al. Cerebrospinal fluid production by the choroid plexus and brain. Science. 1971;173:330-332.
4 Pollay M, Stevens A, Estrada E, et al. Extracorporeal perfusion of choroid plexus. J Appl Physiol. 1972;32:612-617.
5 Nilsson C, Stahlberg F, Gideon P, et al. The nocturnal increase in CSF production is inhibited by a β1-receptor antagonist. Am J Physiol. 1994;267:R1445-R1448.
6 Weed LH. Forces concerned in the absorption of the cerebrospinal fluid. Am J Physiol. 1935;114:40-45.
7 Mollanji R, Bozanovic-Sosic R, Silver I, et al. Intracranial pressure accommodation is impaired by blocking pathways leading to extracranial lymphatics. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1573-R1581.
8 Greitz D. Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography. Acta Radiol Suppl. 1993;386:1-23.
9 Dodge PR, Swartz MN. Bacterial meningitis—a review of selective aspects. II. Special neurologic problems, postmeningitic complications and clinicopathological correlations. N Engl J Med. 1965;272:954.
10 Pattisapu JV. Etiology and clinical course of hydrocephalus. Neurosurg Clin North Am. 2001;36:651-659.
11 Squier MV. Pathological approach to the diagnosis of hydrocephalus. J Clin Pathol. 1997;50:181-186.
12 Bradley WG. Diagnostic tools in hydrocephalus. Neurosurg Clin North Am. 2001;36:661-684.
13 Rekate H. Treatment of Hydrocephalus. Principles and Practice of Pediatric Neurosurgery. New York: Thieme, 1999.
14 Raimondi AJ. Pediatric Neurosurgery: Theoretical Principles—Art of Surgical Techniques. New York: Springer Verlag, 1987.
15 Chahlavi A, El-Babaa SK, Luciano MG. Adult-onset hydrocephalus. Neurosurg Clin North Am. 2001;36:753-760.
16 Bradley WG, Kortman KE, Burgoyne B. Flowing cerebrospinal fluid in normal and hydrocephalic states: appearance on MR images. Radiology. 1986;159:611-616.
17 Citrin CM, Sherman JL, Gangarose RE, et al. Physiology of the CSF flow-void sign: modification by cardiac gating. AJNR Am J Neuroradiol. 1987;148:205-208.
18 Grossman RI, Yousem DM. Neurodegenerative diseases and hydrocephalus. In Neuroradiology: The Requisites, 2nd ed., St. Louis: Mosby; 2003:369-409.
19 Arriada N, Sotelo J. Review: treatment of hydrocephalus in adults. Surg Neurol. 2002;58:377-384.
20 Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid haemorrhage and acute aneurysm surgery. Neurosurgery. 1990;26:804-809.
21 Bogdahn U, Lau W, Hassel W, et al. Continuous-pressure controlled external ventricular draining for treatment of acute hydrocephalus—evaluation of risk factors. Neurosurgery. 1992;31:898-903.
22 Kanev OM, Park TS. The treatment of hydrocephalus. Neurosurg Clin North Am. 1993;4:611-624.
23 Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg. 1995;23:254-258.
24 Drake JM, Saint-Rose C. The Shunt Book. New York: Blackwell Science, 1995.
25 Drake JM. Ventriculostomy for treatment of hydrocephalus. Neurosurg Clin North Am. 1993;4:657-666.
26 Choux M, Genitori L, Lang D, et al. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg. 1992;77:875-880.
27 Davis SE, Levy ML, McComb JG, et al. Does age or other factors influence the incidence of ventriculoperitoneal shunt infections? Pediatr Neurosurg. 1999;30:253-257.
28 Moores LE, Ellenbogen RG. Cerebrospinal fluid shunt infections. In: Osenbach RK, Zeidman SM, editors. Infections in Neurological Surgery. Diagnosis and Management. Philadelphia: Lippincott-Raven; 1999:199-207.
29 Morrissette I, Gourdeau M, Francoeur J. CSF shunt infections: a fifteen-year experience with emphasis on management and outcome. Can J Neurol Sci. 1993;20:118-122.
30 Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg. 1992;77:29-36.
31 Walters BC, Hoffman HJ, Hendrick EB, et al. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60:1014-1021.
32 Venes JL. Infections of CSF shunt and intracranial pressure monitoring devices. Infect Dis Clin North Am. 1989;3:289-299.
33 Del Bigio MR. Biological reactions to cerebrospinal fluid shunt devices: a review of cellular pathology. Neurosurgery. 1998;42:319-326.
34 Blount JP, Campbell JA, Haynes SJ. Complications in ventricular cerebrospinal fluid shunting. Neurosurg Clin North Am. 1993;4:633-656.
35 Czosnyka Z, Czosnyka M, Richards HK, et al. Posture-related overdrainage: comparison of the performance of 10 hydrocephalus shunts in vitro. Neurosurgery. 1998;42:327-333.
36 Grant JA, McLone DG. Third ventriculostomy: a review. Surg Neurol. 1997;47:210-212.
37 Schwartz TH, Ho B, Prestigiacomo CJ, et al. Ventricular volume following third ventriculostomy. J Neurosurg. 1999;91:20-25.
38 Schwartz TH, Yoon SS, Cutruzzola FW, et al. Third ventriculostomy: post-operative ventricular size and outcome. Minim Invasive Neurosurg. 1996;39:122-129.
39 Gangemi M, Maiuri F, Donati PA, et al. Endoscopic surgery for monoventricular hydrocephalus. Surg Neurol. 1999;52:246-251.
40 Sotelo J, Rubalcava MA, Gomez-Llata S. A new shunt for hydrocephalus that relies on CSF production rather than on ventricular pressure: initial clinical experiences. Surg Neurol. 1995;43:324-332.
41 James HE. Methodology for the control of intracranial pressure with hypertonic mannitol. Acta Neurochir (Wein). 1980;51:161-172.
42 Gilmore H. Medical treatment of hydrocephalus. In: Scott RM, editor. Hydrocephalus. Baltimore: William & Wilkins; 1990:37-46.
43 Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2:307-372.
44 Vanneste JAL. Diagnosis and management of normal-pressure hydrocephalus. J Neurol. 2000;247:5-14.
45 Stolze H, Kuhtz-Buschbeck JP, Drücke H, et al. Gait analysis in idiopathic normal pressure hydrocephalus—which parameters respond to the CSF tap test? Clin Neurophysiol. 2000;111:1678-1686.
46 Ahlberg J, Norlén L, Blomstrand C, et al. Outcome of shunt operation on urinary incontinence in normal pressure hydrocephalus predicted by lumbar puncture. J Neurol Neurosurg Psychiatry. 1988;51:105-108.
47 Duinkerke A, Williams MA, Rigamonti D, et al. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol. 2004;17:179-184.
48 Thomas G, McGirt MJ, Woodworth G, et al. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2005;20:163-168.
49 Power C, Selnes OA, Grim JA, et al. The HIV Dementia Scale: a rapid screening test. J AIDS. 1995;8:273-278.
50 van Harten B, Courant MN, Scheltens P, et al. Validation of the HIV Dementia Scale in an elderly cohort of patients with subcortical cognitive impairment caused by subcortical ischaemic vascular disease or a normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2004;18:109-114.
51 James AE, Burns B, Flor WF, et al. Pathophysiology of chronic communicating hydrocephalus in dogs (Canis familiaris): experimental studies. J Neurol Sci. 1975;24:151-178.
52 Del Bigio MR, Bruni JE. Changes in periventricular vasculature of rabbit brain following induction of hydrocephalus and after shunting. J Neurosurg. 1988;69:115-120.
53 Krauss JK, Regel JP, Vach W, et al. Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. Stroke. 1996;27:24-29.
54 Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49:1166-1186.
55 Poca MA, Sahuquillo J, Busto M, et al. Agreement between CSF flow dynamics in MRI and ICP monitoring in the diagnosis of normal pressure hydrocephalus: sensitivity and specificity of CSF dynamics to predict outcome. Acta Neurochir Suppl. 2002;81:7-10.
56 Egeler-Peerdeman SM, Barkhof F, Walchenbach R, et al. Cine phase-contrast MR imaging in normal pressure hydrocephalus patients: relation to surgical outcome. Acta Neurochir (Wien) Suppl. 1998;71:340-342.
57 Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102:987-997.
58 Esmonde T, Cooke S. Shunting for normal pressure hydrocephalus (NPH). Cochrane Database Syst Rev. (3):2005. CD003157