Chapter 6 Hydrocephalus in Children and Adults
• Hydrocephalus is one of the most challenging and common conditions that a neurosurgeon encounters.
• This condition can be due to intraventricular hemorrhage in the preterm infant, and its many other causes include trauma, intracranial hemorrhage, tumor, infection, aqueductal stenosis, and idiopathic origin in both the infant or adult.
• For 50 years, the mainstay of hydrocephalus treatment for patient survival and improved quality of life has been cerebrospinal fluid (CSF) diversion by shunting the ventricle or lumbar subarachnoid space to the peritoneum or atrium. However, the long-term complications of placement of a CSF shunt apparatus include infection, overshunting, and failure from obstruction.
• A more recent advance or alternative to shunting in a selected population of patients with hydrocephalus is endoscopic third ventriculostomy (ETV), in which CSF diversion is achieved via minimally invasive endoscopic fenestration of the floor of the third ventricle. It appears safe and is most effective for children over 1 month of age, those with aqueductal stenosis, and those who have not had a shunt previously.
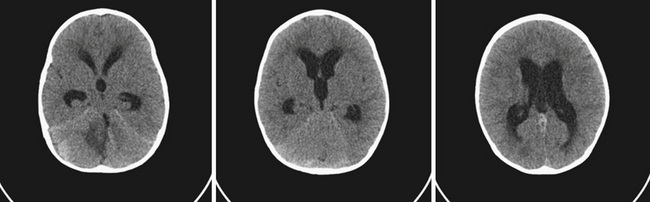
Hydrocephalus refers to the buildup of cerebrospinal fluid (CSF) within the intracranial compartments usually associated with clinical sequelae from an increase in intracranial pressure (ICP). It can develop at any time, from the fetal period into adulthood, and it can have a myriad of causes including, but not limited to, congenital defects, perinatal insults, acquired conditions such as infection, tumor, traumatic and nontraumatic hemorrhage, and rarely CSF overproduction (Fig. 6.1).
Hydrocephalus is one of the most commonly encountered conditions in neurosurgery. One recent study by Sipek and colleagues analyzed data from the Czech national registry from 1961 to 2000 retrospectively and found the mean incidence of congenital hydrocephalus diagnosed both pre- and postnatally to be 6.35 per 10,000 liveborn infants.1 Another series by Fernell and Hagberg from Sweden found that the prevalence of infantile hydrocephalus was 6.99 per 1000 in the 1970s, increasing to 25.37 in the 1980s.2 The increase was thought to be due to the increased survival of very preterm infants. In the 1990s the prevalence of infantile hydrocephalus in the Swedish population decreased to 13.69. Despite changes in prevalence rates, outcome in surviving children with hydrocephalus remained similar. Other studies have looked at the prevalence of hydrocephalus in the adult population. A recent Norwegian study by Brean and Eide found the prevalence of idiopathic normal-pressure hydrocephalus (NPH) to be 21.9 per 100,000 and the incidence to be 5.5 per 100,000, reflecting minimum prevalence/incidence rate estimates.3 The incidence of hydrocephalus in developing countries is unknown but may be higher.
History
The recognition of hydrocephalus as a clinical entity dates back to antiquity. In the fifth century BC Hippocrates described the clinical presentation of hydrocephalus secondary to water accumulation within the head.4,5 Later, Galen described the choroid plexus and the relationship of CSF to the brain, though his understanding of CSF physiology was lacking.4 In the seventeenth century, Willis proposed the secretion of CSF by the choroid plexus and its absorption into the venous system, although the pathways he described were less than accurate.4 In 1701 Pacchioni described the arachnoid granulations, though he misidentified their function as the site of CSF production rather than absorption, and it was not until the late nineteenth century that the current accepted physiology of CSF production and absorption was clarified.4,6,7
The evolution of the treatment of hydrocephalus can be described by three stages.7 The first stage, up to the Renaissance, was characterized by poor medical understanding of CSF dynamics and pathology; therefore, nonsurgical and surgical treatment was largely useless. The second stage encompasses the period between the nineteenth and mid-twentieth centuries, when CSF physiology and pathology were elucidated but treatment options were in their infancy. In 1891 Quincke described lumbar puncture as a diagnostic modality and a means of treating hydrocephalus. Keen drained the cerebral ventricles through a temporal approach.4 Various lumbar and ventricular cannulation attempts were described, some more successful than others. Cushing reported treating hydrocephalus by a lumbar peritoneal connection, which was encouraging. Lespinasse in 1910 was the first to describe choroid plexus coagulation and the use of an endoscope (cystoscope) for the cannulation of the cerebral ventricles.4,7 In 1922 Dandy was the first to perform a third ventriculostomy through a subfrontal approach, and a year later Mixter performed the first endoscopic third ventriculostomy for noncommunicating hydrocephalus using a urethroscope.4 In 1939 Torkildsen described the use of a valveless rubber catheter to connect the lateral ventricles with the cisterna magna for noncommunicating hydrocephalus.4
The third stage of evolution in the treatment of hydrocephalus started with the development of silicone shunts with unidirectional valves in the 1950s. Nulsen and Spitz used a stainless steel unidirectional ball valve connected to a rubber catheter to divert CSF from the ventricles to the jugular vein in a hydrocephalic child.8 This was a landmark operation that marked the beginning of a new and powerful way of treating hydrocephalus. Variations and improvements on the valve used by Nulsen and Spitz ensued. Eventually, ventriculoperitoneal shunting became the standard surgical treatment for hydrocephalus, although both historically and in current clinical practice various sites for CSF diversion have been, and are still, used. However, shunt systems represent the introduction of a foreign material in the body, and complications related to infection and plugging of the shunt system are often encountered in clinical practice. Currently advances in technology and endoscopy have prompted resurgence in the use of third ventriculostomy for treating noncommunicating hydrocephalus as a means of obviating the inherent complications associated with shunts.
Cerebrospinal Fluid and Pathophysiology of Hydrocephalus
CSF is a clear colorless fluid produced mostly by the choroid plexus of the lateral, third, and fourth ventricles, and to a lesser degree (<20%) by the interstitial space and ependymal lining of the ventricles. In the spinal compartments, the nerve sleeve dura is responsible for CSF production9 (Table 6.1). Ninety-five percent of CSF produced by the ventricular choroid plexus occurs at the level of the lateral ventricles. CSF is found within the ventricles and cisterns of the brain and subarachnoid space, and surrounds both the brain and spinal cord. The infant has an approximate total volume of about 50 mL of CSF, and adults average 150 mL, half in the cranial compartment and half in the spinal compartment. Newborns produce CSF at a rate of 25 mL/day, which increases to about 500 mL/day in the adult (0.3-0.35 mL/minute).9 Intracranial pressure ranges from 9 to 12 cm H2O in the newborn to less than 18 to 20 cm H2O in the adult population. Generally, the rate of CSF formation is not dependent on intracranial pressure (ICP); however, CSF absorption is pressure dependent, and it occurs at the level of the arachnoid villi that are found in proximity to the dural venous sinuses.
| Compartment | Site |
|---|---|
| Intracranial | Choroid plexus of the lateral, third, and fourth ventricles Ependymal lining Interstitial space |
| Spinal | Dura of the nerve root sleeves |
The circulation and physiology of CSF and its circulation can be quite complex. More than 2 centuries ago Alexander Monro applied principles of physics to the relationship of intracranial contents.10 This was supported by experiments conducted by Kellie.10 Today their work is known as the Monro-Kellie doctrine (Table 6.2), which states that within the rigid container of the skull, the sum of intracranial contents, including CSF, blood, and brain, is constant.10,11 Therefore, a change in one component (e.g., increase in CSF) requires a compensatory change in one or both of the other components. However, in very young children, usually less than 2 to 3 years of age, the fontanelles are still open and the system is no longer closed; in this scenario the hypothesis does not apply.
TABLE 6.2 The Monro-Kellie Doctrine
| The sum of intracranial contents to include blood, CSF, and brain should remain constant in a fixed container, such as the skull. |
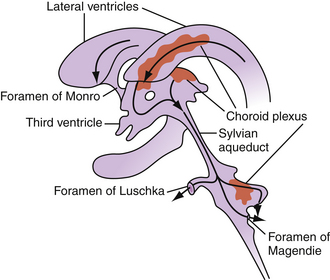
CSF flow through the ventricular system occurs in a progressive manner, from the lateral ventricles through the foramina of Monro to the third ventricle, through the sylvian aqueduct to the fourth ventricle, then through the foramina of Luschka and Magendie to the subarachnoid spaces (Fig. 6.2). From the subarachnoid spaces, CSF is absorbed into the venous circulation through the arachnoid granulations. A dysregulation in production, absorption, or circulation of CSF can lead to symptomatic hydrocephalus.
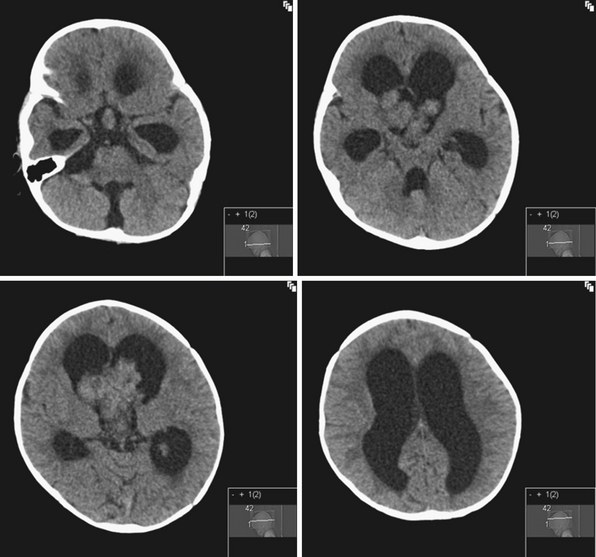
Rarely hydrocephalus can be associated with CSF overproduction, as in the case of choroid plexus tumors. However, in most instances hydrocephalus is due to an obstruction along the pathway of CSF flow. Tumors of the lateral ventricles can cause hydrocephalus by mass effect or CSF overproduction in the case of tumors derived from the choroid plexus. Common tumors of the lateral ventricles include meningiomas, gliomas, and choroid plexus tumors. Choroid plexus tumors are rare, seen more commonly in children younger than 2 years of age, and account for less than 1% of all intracranial tumors12 (Fig. 6.3). Even with complete surgical tumor resection, hydrocephalus may still persist and require treatment. This is thought to be due to more distal obstruction (i.e., aqueduct, arachnoid villi) either from preoperative microhemorrhages or scarring after surgery. Rarely choroid plexus villous hypertrophy can lead to CSF overproduction, which can be treated by choroid plexus coagulation.13 Obstruction at the foramina of Monro can also be caused by congenital atresia, membranes, or gliosis after hemorrhage, and can lead to unilateral ventriculomegaly.14
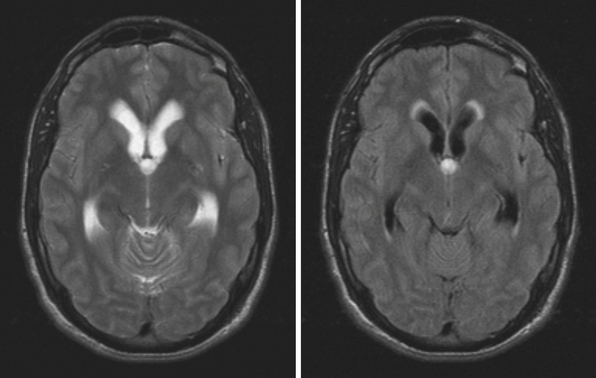
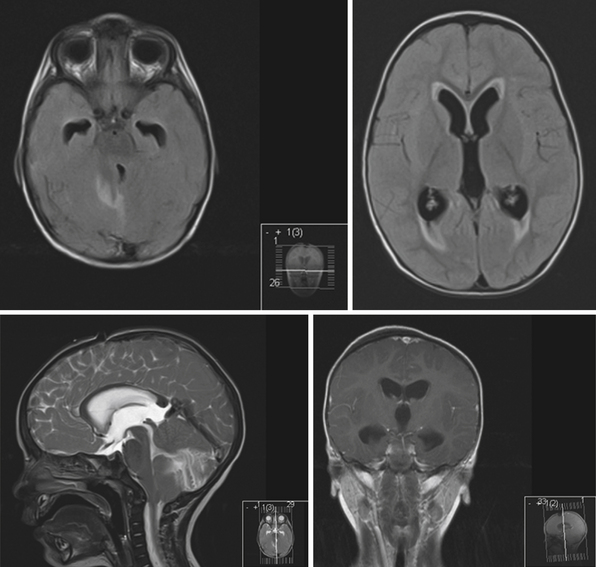
In the third ventricle, cysts and tumors can cause hydrocephalus by CSF obstruction. Colloid cysts, found at the anterior superior part of the third ventricle, generally represent less than 2% of intracranial tumors and are more commonly symptomatic in the adult population, with clinical presentations ranging from acute to chronic hydrocephalus due to obstruction of the foramina of Monro15 (Fig. 6.4). Treatment is aimed at stereotactic aspiration, endoscopic resection, or open microsurgery.16 Other cystic lesions include arachnoid and ependymal cysts, and rarely dermoid cysts. Third ventricular neoplasms include craniopharyngiomas and gliomas, including hypothalamic astrocytomas and subependymal giant cell astrocytomas (in association with tuberous sclerosis). Hydrocephalus may persist after glioma resection, necessitating shunt placement.
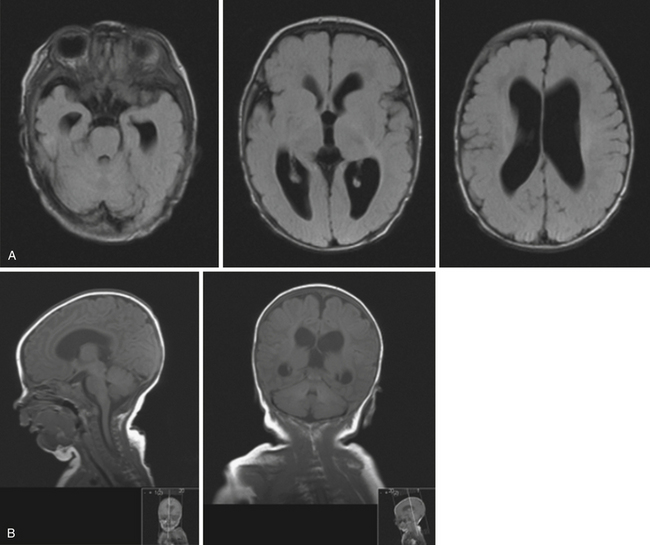
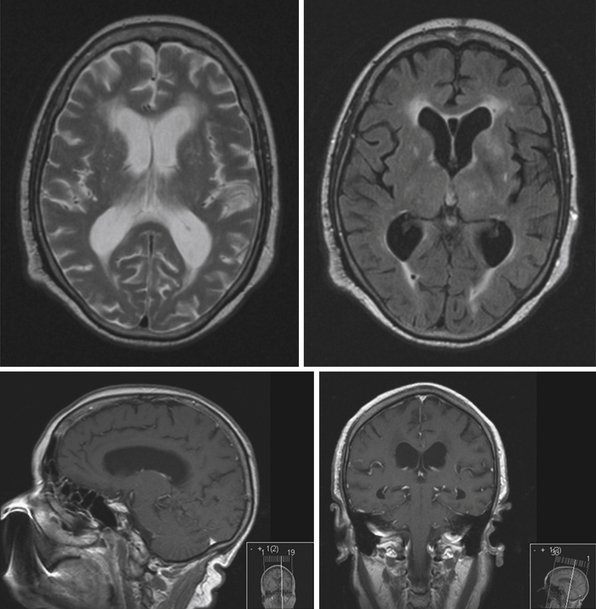
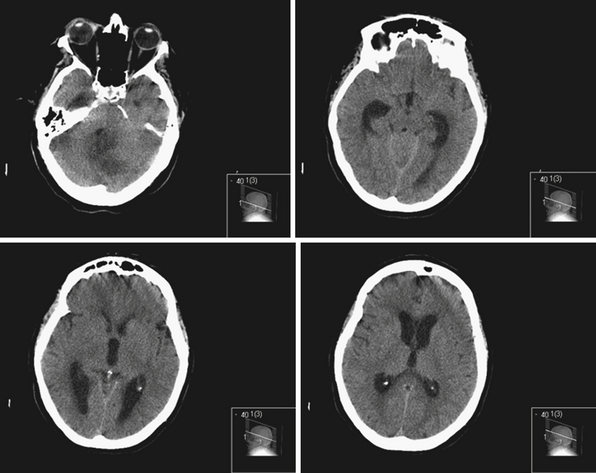
Obstruction may be seen at the level of the sylvian aqueduct, especially in neonates in whom the small diamter (0.2-0.5 mm) of the aqueduct puts it at risk for obstruction from congenital and acquired causes (Fig. 6.5). This leads to enlargement of the third and lateral ventricles. Congenital aqueductal malformations include stenosis, forking, septum formation, and subependymal gliosis due to in utero infections. True luminal stenosis is not as common. In addition, lesions such as arteriovenous malformations and periaqueductal tumors such as tectal gliomas and pineal region tumors can cause obstruction at or near the sylvian aqueduct, leading to triventricular obstructive hydrocephalus (Fig. 6.6). Often, resection of the lesion is sufficient to treat the hydrocephalus. Newer methods of endoscopic aqueductoplasty and stenting are showing promise.16
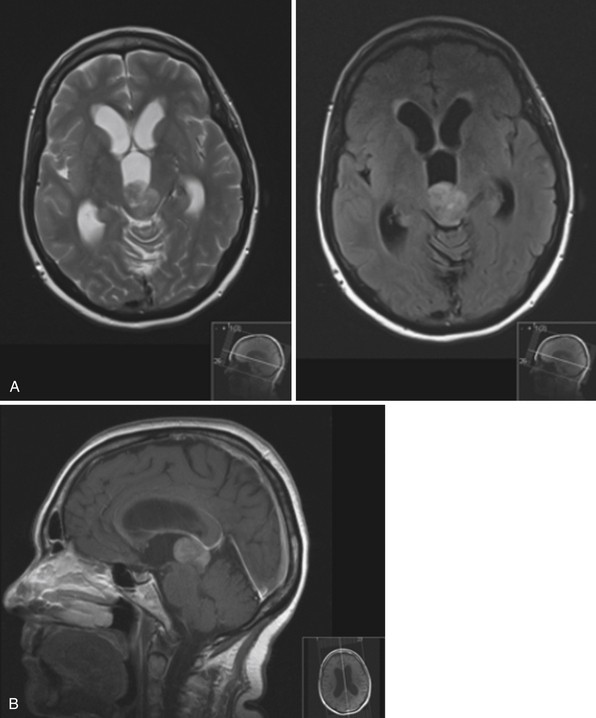
The fourth ventricle and basal foramina can also be sites of obstruction leading to hydrocephalus. Dandy-Walker malformations present in infants with a large posterior fossa cyst in the setting of cerebellar vermian hypoplasia and cerebellar atrophy, commonly associated with hydrocephalus as well as other congenital abnormalities (Fig. 6.7). Tumors of the posterior fossa and fourth ventricular region can present with acute or chronic hydrocephalus in addition to other associated posterior fossa symptoms. In the adult population common tumors include metastases, gliomas, meningiomas, neuromas, and hemangioblastomas. In the pediatric population, infratentorial tumors are common causes of hydrocephalus and common offenders include medulloblastomas, ependymomas, cerebellar astrocytomas, and brainstem gliomas. Across the age spectrum, infections and subarachnoid hemorrhage can lead to arachnoid scarring and hydrocephalus (Fig. 6.8). Congenital conditions such as Chiari malformations may cause hydrocephalus by obstruction of CSF flow around the base of the skull. Obstruction of CSF flow can also occur at the level of the arachnoid granulations with impairment of CSF absorption. This may be idiopathic or may be seen after infection, subarachnoid hemorrhage, trauma, or tumors and can lead to enlargement of CSF spaces over the convexities.
In models of adult hydrocephalus, conditions leading to scarring distal to the ventricular system (e.g., subarachnoid hemorrhage or infection) cause a resistance to normal CSF outflow. However, the pathophysiology of CSF circulation is quite complex. CSF pulsation variability and cerebral blood flow dynamics have been implicated in both idiopathic and secondary hydrocephalus, though their exact role is still a topic of investigation.17,18 In addition, the properties of the surrounding parenchyma, particularly its compressibility, has been proposed as a means of explaining the observation of hydrocephalic symptoms in the settings of lower ventricular pressures.19 For example, if the brain parenchyma is able to attenuate increases in intraventricular pressure, the overall intracranial pressure does not need to rise above normal levels in the setting of abnormal transventricular pressure gradients, leading to the entity commonly called normal-pressure hydrocephalus (NPH).19 It is not clear if parenchymal damage plays a primary or secondary role in the development of NPH.
Classification of Hydrocephalus
There are different classifications and means of describing hydrocephalus. Often different schemes are employed for hydrocephalus in infants and children versus adults, delineating the difference in pathophysiology and clinical presentation across the age spectrum. In general, hydrocephalus can be defined as nonobstructive, associated with ventricular system enlargement (e.g., hydrocephalus ex vacuo), or obstructive, associated with defective CSF circulation or absorption (Table 6.3). A more clinically useful scheme divides obstructive hydrocephalus into communicating and noncommunicating types. Generally, communicating hydrocephalus refers to an obstruction outside the ventricular system, often at the level of the subarachnoid space or the arachnoid villi. Noncommunicating hydrocephalus refers to an impedance of CSF flow within the ventricular system, such as blockage at the aqueduct of Sylvius or the basal foramina of Luschka and Magendie. Less clinically useful schemes describe hydrocephalus as physiological (secondary to CSF overproduction) or nonphysiological, external or internal (obstruction outside or within the ventricular system, respectively).
| Type | Features |
|---|---|
| Nonobstructive | Ventricular enlargement (e.g., hydrocephalus ex vacuo) |
| Obstructive | |
| Communicating | Obstruction outside the ventricular system (e.g., subarachnoid space or arachnoid villi) |
| Noncommunicating | Obstruction within the ventricular system (e.g., aqueduct or basal foramina) |
A system proposed by Gowers in 1888, still useful today, divides hydrocephalus as either acute or chronic, primary or secondary.20 Generally, acute hydrocephalus implies a rapid decompensation usually associated with an underlying condition and presents with elevated intracranial pressure. Chronic hydrocephalus may be either idiopathic or secondary to a known pathological condition, and generally is associated with lower or normal intracranial pressures. Secondary hydrocephalus can be due to a number of clinical conditions, including but not limited to tumors, hemorrhage, trauma, and infection.
Etiology and Clinical Presentation
There are different etiologies for hydrocephalus depending on the age group of patients (Table 6.4). In infants, numerous causes are clinically identified. Particularly in premature infants, posthemorrhagic hydrocephalus (PHH) is often seen as a sequela of intraventicular or germinal matrix hemorrhage (Table 6.5). The intraventricular blood leads to fibrosing arachnoiditis, meningeal fibrosis, and subependymal gliosis, altering the physiology of CSF flow.21 In full-term infants hydrocephalus can be ascribed to a different etiological set, including but not limited to aqueductal stenosis, Dandy-Walker malformations, tumors, arachnoid cysts, vein of Galen malformations, Chiari malformations, and so on.22 Intrauterine infections can also lead to congenital hydrocephalus. In older children, common causes include various tumors that obstruct CSF flow along its ventricular path, as well as trauma and infection (e.g., meningitis).
TABLE 6.4 Pathologic Conditions Associated With Hydrocephalus
| Congenital | Acquired |
|---|---|
| Chiari I malformation | Postinfectious |
| Chiari II malformation (associated with myelomeningocele) | Posthemorrhagic (including subarachnoid and intraventricular hemorrhage) |
| Primary aqueductal stenosis (or gliosis secondary to intrauterine infection or germinal matrix hemorrhage) | Post-traumatic |
| Dandy-Walker malformation | Secondary to mass lesions (e.g., tumors, vascular malformations, cysts) |
| Hydranencephaly | Postoperative (e.g., after tumor resection including posterior fossa tumors) |
TABLE 6.5 Grading of Germinal Matrix Hemorrhage
| Grade | Description |
|---|---|
| I | Subependymal hemorrhage |
| II | Intraventricular hemorrhage with no ventricular dilatation |
| III | Intraventricular hemorrhage with ventricular dilatation |
| IV | Intraventricular hemorrhage with intracerebral hemorrhage |
Several studies have looked at trends in causes of hydrocephalus in specific groups. A retrospective review by Green and co-workers of 253 infants and children with hydrocephalus treated at a British tertiary care center over a 10-year period revealed interesting trends in the causes of this condition.23 In the first half of the decade, the predominant causes were posthemorrhagic hydrocephalus and hydrocephalus due to brain tumors, which decreased from over half of the children to one third by the end of the decade; the rate of neonatal intraventricular hemorrhage decreased by 45%.23
In older children, hydrocephalus may occur secondary to neoplasms or trauma (Fig. 6.9). Children can present with headaches (dull, typically upon awakening), vision changes (blurry or double vision), lethargy, vomiting, decreased food intake, behavioral disturbances, poor school performance, and endocrinopathies (e.g., short stature, precocious puberty). On examination, papilledema and lateral rectus palsies can be seen, as well as hyperreflexia and clonus. If progressive lethargy is noted, diagnosis and treatment become urgent. In severe cases of increased intracranial pressure and severe hydrocephalus, Cushing’s triad can be seen. Cushing’s triad consists of bradycardia, hypertension, and irregular breathing, and requires emergent evaluation and treatment.
NPH is a common diagnosis encountered by neurosurgeons in the adult population (Fig. 6.10). Patients with NPH present with the clinical triad of gait disturbances, dementia, and urinary incontinence, usually in the sixth to eight decades of life24 (Table 6.6). Generally, gait disturbances, described as apraxic or magnetic, are the first symptoms noted. Patients develop a slow, wide-based shuffling walking pattern. Urinary incontinence is a common presentation of NPH. Early in the course patients develop urinary frequency and urgency, which progress to incontinence owing to bladder hyperactivity.24 The cognitive decline must be differentiated from other causes of dementia (vascular, Alzheimer’s disease, dementia with Lewy bodies, etc.). Generally, the dementia associated with NPH is subcortical in nature. Often patients show apathy, psychomotor retardation, difficulty with executive function, and inattention; apraxia, aphasia, and agnosia are not seen.24 In the elderly, the symptoms associated with NPH must be distinguished from other common causes seen in this patient population, including vascular and neurodegenerative disorders.
TABLE 6.6 Symptomatic Triad of Normal-Pressure Hydrocephalus (NPH)
A nonhydrocephalic clinical entity presenting with increased intracranial pressure, seen in both the pediatric and adult populations, is the syndrome of idiopathic intracranial hypertension, also commonly known as pseudotumor cerebri. The ventricles are not enlarged and are usually small. Patients are generally overweight women who develop increased intracranial pressure without an identifiable source, and it remains a diagnosis of exclusion. The pathophysiology of this syndrome is still unclear, though venous stenosis and effects of the hormone leptin have been implicated.25 Symptoms include headaches and papilledema, and up to 25% of patients develop visual deterioration from optic nerve atrophy.
Diagnosis
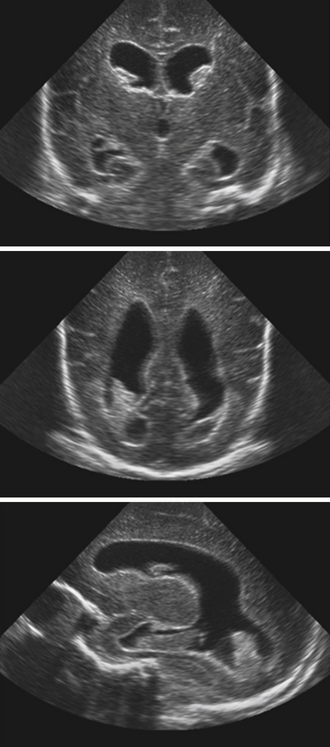
Neuroimaging studies are the mainstay for visualizing and understanding ventricular anatomy and diagnosing hydrocephalus in symptomatic cases. In utero diagnosis of hydrocephalus is often accomplished via ultrasound studies, though fetal MRIs are gaining popularity as diagnostic adjuncts.26 In infants with intraventricular hemmorrhage (IVH) and suspected hydrocephalus, sonography is often used to evaluate the ventricles because the anterior fontanelle provides a good window for ventricular visualization.27 Diagnosis of mono-, bi-, or triventricular hydrocephalus can be made by using ultrasound evaluation of the lateral and third ventricles; however, posterior fossa imaging is limited (Fig. 6.11).
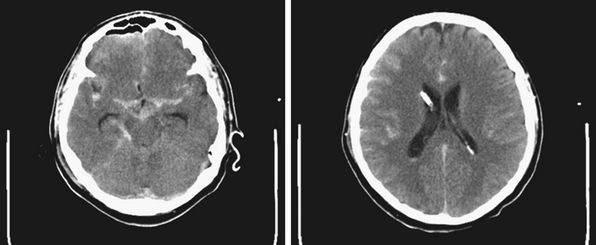
The current standard imaging method in the diagnosis of hydrocephalus is CT scanning. CT scans provide an effective way of visualizing ventricular morphology, blood products complicating the picture of hydrocephalus, transependymal edema, and signs of increased intracranial pressure such as sulcal/gyral effacement and obliteration of subarachnoid spaces. The Evans ratio describes the ratio of the lateral ventricular frontal horn width to the maximal biparietal diameter, and is abnormal and indicative of ventriculomegaly if it is greater than 0.3.28 Occasionally, in select cases, cisternography or ventriculography can be performed. This often entails the administration of a radiopaque contrast agent into the ventricular system to evaluate the compartmentalization of cysts and determine whether communication of CSF is present (i.e., posterior fossa cysts in Dandy-Walker malformations, or a trapped fourth ventricle).29 MRI studies are particularly useful in delineating disease responsible for CSF pathway obstruction, such as tumors compressing the ventricular system, and provide a better evaluation of the posterior fossa and foramen magnum. MRIs allow for the evaluation of ventricular anatomy in coronal, sagittal, and axial planes (Fig. 6.12). In addition, MRI CSF flow studies (e.g., cine flow studies) timed to the cardiac cycle can provide useful information in selected cases.
Though CT and MRI studies have made it significantly easier to evaluate ventricular morphology, it is important to note that morphology and symptomatology do not always correlate.30,31 Therefore, these studies have to be interpreted in the context of the appropriate clinical setting. Occasionally, in the setting of altered ventricular compliance, ventricular size may not change despite increases in intracranial pressure and clinical symptoms.30,31 Invasive intracranial pressure monitoring can be used in certain settings when symptoms and imaging do not correlate.
Several studies have examined ICP monitoring for chronic NPH as a means of understanding responders to CSF shunting, though results are often inconclusive and not widely accepted at all institutions. The Dutch NPH study by Boon and associates concluded that positive predictors of outcome from shunting were observed with patients whose resistance to outflow of CSF was about 18 mm Hg/mL/minute; at lower levels outcomes became dependent on clinical and imaging findings to support shunting.32 Some studies, such as the one by Eide and Stanisic from Norway, suggest that CSF pulsatility, determined by ICP wave amplitude, can help identify clinical responders to shunting versus nonresponders.33 Another study by Eide and Sorteberg showed that in patients shunted for NPH whose intracranial pressure and waveforms were monitored invasively, 93% of those with increased CSF pulsatility (ICP waveform of >4 mm Hg amplitude on average) showed response to shunting as opposed to 10% of those without increased pulsatility.34
Normal-Pressure Hydrocephalus
The diagnosis of acute hydrocephalus can often be made effectively through a combination of clinical signs and imaging findings indicating increased intracranial pressure. However, the diagnosis of chronic hydrocephalus, particularly idiopathic NPH, has been a much studied and debated topic in the literature since the initial seminal work from the Massachusetts General Hospital in 1964. The true incidence is unknown and it is likely an underreported condition because it can be challenging to diagnoses in the patients who most commonly suffer from this condition, and are typically 60 to 80 years of age. In 2009, the incidence reported in Norway was estimated to be 1.09 per 100,000 per year.35 Currently several criteria are used for diagnosis and include history and clinical presentation, including the classic triad of gait disturbance, urinary incontinence, and dementia. These clinical findings are usually described in the setting of CT and MRI imaging showing ventriculomegaly with no evidence of extrinsic obstruction to CSF flow. Although not common, transependymal CSF flow occasionally can be seen in a patient’s MRI with NPH. Recent published idiopathic NPH consensus guidelines classify this entity as probable, possible, or unlikely based on the constellation of history, physical examination, and clinical findings.24,36 In addition, adjunctive prognostic tests can be used to help in diagnosis. Large volume lumbar punctures, in which 40 to 50 mL of CSF are withdrawn, are performed in conjunction with detailed examination before and after the procedure to document clinical response to the CSF removal24 in terms of intellectual function, memory, gait, and continence. Symptomatic improvement is correlated with response to shunting, with a positive predictive value of 73% to 100%.24 However, this test has a low sensitivity (26-61%).37 Prolonged external CSF drainage of 300 mL of CSF has a higher sensitivity (50-100%) and a high positive predictive value (80-100%), though it is a more invasive test with higher complication rates.37 However, many centers prefer to place a temporary indwelling lumbar catheter and drain CSF over the space of 24 to 72 hours, as part of their screening paradigm because improvements in gait, cognition, and continence can be more carefully compared to pretest analyses and carefully quantified during the hospital stay. The higher predictive value of this test also makes the need for placement of an unnecessary permanent CSF diversion device in an older, often more fragile patient less likely.
Treatment of Hydrocephalus
Nonsurgical Approaches
Pharmacological treatment is aimed at decreasing CSF production and increasing CSF absorption. The medications commonly used to decrease CSF production include acetazolamide and furosemide. Acetazolamide is a carbonic anhydrase inhibitor and furosemide is a loop diuretic. Their effect on hydrocephalus has been especially studied in infants with posthemorrhagic hydrocephalus. In a large randomized controlled trial, the International Posthaemorrhagic Ventricular Dilation Trial Group enrolled 177 patients to either standard therapy alone or treatment with acetazolamide (100 mg/kg/day) and furosemide (1 mg/kg/day) to determine if there is an advantage in preventing shunt dependence in infants with posthemorrhagic hydrocephalus.38 The study was stopped prematurely when the data showed increased neurological sequelae and increased shunt placement rate in the group receiving the medications.38 This emphasizes the conclusion that these drugs are not without side effects, including but not limited to metabolic acidosis, lethargy, poor feeding, electrolyte imbalances, tachypnea, and diarrhea. Other drugs have been used in the treatment of hydrocephalus with mixed success. Hyaluronidase promotes increase CSF absorption; however, its efficacy as a means of obviating shunting has not been shown.39 Medications used to decrease intracranial pressure include osmotic diuretics such as mannitol, urea, and glycerol.4 Usually these provide temporizing measures until definitive treatment is initiated.
Other nonsurgical means of treatment historically and currently used include head wrapping and intermittent CSF removal. Head wrapping has been used in the past to treat hydrocephalus, thought to be effective by creating a constant force high enough to promote increased CSF absorption in infants with unfused skulls.40 This is not a common practice, particularly due to complications including increased intracranial pressure. Intermittent CSF removal through lumbar or ventricular puncture has been used as a means of temporizing hydrocephalus until a more definitive treatment is undertaken. It has been particularly studied in infants with posthemorrhagic hydrocephalus, and has been used as a means of treatment until the infants’ CSF profiles and body weight increase permit shunt placement.41 Several studies looked at the efficacy of intermittent CSF removal and concluded that it is not effective in preventing hydrocephalus.42
Surgical Management
Surgical management of hydrocephalus has a long history, though ancient methods for treatment were not effective.43 Today surgical management of hydrocephalus can be divided into nonshunting versus CSF shunting options. Nonshunting options include endoscopic third ventriculostomy, resection of an obstructing lesion causing the hydrocephalus, when possible, and choroid plexus ablation. The success of choroid plexus coagulation has been mixed. Dandy described the procedure in 1918 with high morbidity and mortality rates.44 In the past it has been useful in temporarily decreasing the rate of CSF production, but does not completely halt hydrocephalus, because a portion of CSF is still produced by the ependymal lining. With the advent of neuroendoscopy there has been a recent resurgence in endoscopic choroid plexus ablation for hydrocephalus treatment.45
There are different types of valves currently in use. In general, the most commonly used system is a pressure-dependent valve, which allows CSF flow across the valve when the pressure differential exceeds its preset opening pressure. Flow-controlled valves, on the other hand, allow a constant flow of CSF across different pressure gradients with different patient positions. The choice of valve does not generally affect shunt failure rates. This was shown in a randomized study by Drake and colleagues in which 344 patients were shunted with one of three valve systems (a standard differential pressure valve, a valve with an antisiphon component, and a valve with a flow-limiting component).46 The study found no difference in shunt failure-free interval among the different kinds of valves. Newer advances in technology have introduced the variable pressure programmable valves. These are pressure-differential valves that can have their preset pressure changed by extrinsic devices without requiring surgery to change the valve itself. Studies show that both programmable and conventional valves have similar safety and efficacy profiles.47 Of note, since most programming devices work through a magnetic field interaction, exposing patients to MRIs may inadvertently change the valve setting.48 Therefore, patients with programmable valves should have their valve settings evaluated and reprogrammed appropriately after routine MRIs.
The most common type of CSF shunt currently performed is a ventriculoperitoneal shunt (Fig. 6.13). This procedure involves ventricular cannulation and tunneling of a distal subcutaneous catheter to the peritoneal cavity (Fig. 6.14). Cannulating the ventricle can be performed in several ways, which includes placing either a frontal, occipital or parietal catheter (Table 6.7). The frontal horn can be accessed at Kocher’s point, the occipital horn through Frazier’s point, and the trigone can less commonly be entered through a parietal approach at Keen’s point.9 Some surgeons prefer the parietal approach because it provides an easier pass from the scalp to the abdomen. Some choose the frontal approach both because of easier landmarks and because of migration of the catheter away from the choroid plexus with patient growth. Some studies suggest that regardless of the surgical approach, the most important factor in shunt failure is the final relationship of the ventricular catheter to the choroid plexus.49 Preoperative imaging can be used to optimize catheter length at insertion into the ventricle, though often infants outgrow their ventricular catheters and require revision to replace the ventricular catheter that tends to pull out of the ventricle with patient growth. In addition, frameless stereotactic guidance and endoscope-assisted ventricular catheter placement are gaining popularity when ventricular systems are difficult to cannulate.50,51
| Site | Entry | Trajectory and Course |
|---|---|---|
| Frontal (Kocher’s point) | Frontal horn, 2-3 cm from midline, midpupillary line, 1 cm anterior to coronal suture | Perpendicular to skull, medial canthus in the coronal plane, external auditory meatus in the sagittal plane |
| Parietal (Keen’s point) | Trigone, 2.5-3 cm posterior and superior to pinna | Perpendicular to skull |
| Occipital (Frazier’s point) | Occipital horn, 6-7 cm above inion, 3-4 cm from midline | Parallel to skull base, aiming for middle of forehead |
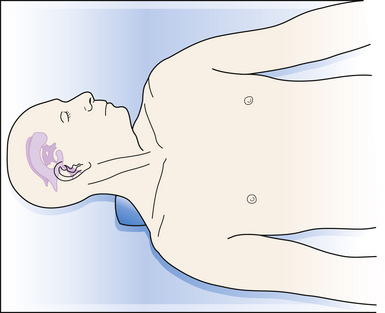
The proximal catheter is connected to a one-way valve and then to a distal catheter that is passed subcutaneously through a tunneling device to the final destination. Generally, patients are placed supine, with the head turned sharply to the opposite side (Fig. 6.15). A shoulder roll is placed to facilitate the passing of the distal catheter from the head, across the neck and clavicle, to the abdomen. The senior author performs a small ipsilateral paraumbilical incision, incises the anterior rectus sheath, bluntly dissects the rectus muscle, incises the posterior rectus sheath and the peritoneum, and uses a small dissector to verify peritoneal location. The catheter is then passed into the peritoneal cavity. Some surgeons prefer to use minimally invasive laparoscopic means of distal catheter placement into the abdomen.52 In infants, care must be taken to place sufficient distal catheter into the abdomen (generally >30-40 cm) to avoid the catheter’s pulling out of the abdomen with patient growth.
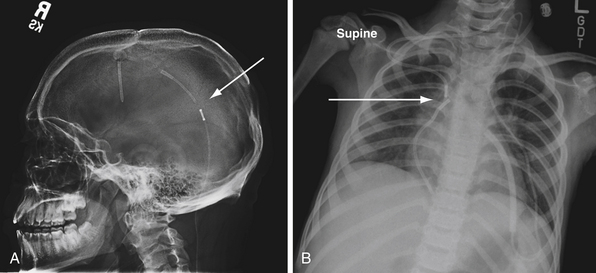
If the peritoneum is not a physiological option for shunting CSF (i.e., in the presence of peritonitis), an alternative site is the cardiac atrium or the pleura (Fig. 6.16). Techniques for placement of a ventriculoatrial shunt include an open cervical approach to cannulate the internal jugular vein or common facial vein, or a more commonly used modified Seldinger technique for percutaneous insertion of the distal tube through the subclavian vein, via a peel-away catheter, into the right atrium.53 Complications of ventriculoatrial shunts include migration out of the atrium, cardiac embolism, and immune-mediated glomerulonephritis. For ventriculopleural shunts, the second intercostal space is accessed on the superior aspect of the rib to prevent injury to the neurovascular bundle.4 The pleura is opened at end expiration, the catheter is inserted under direct visualization, and the wound is irrigated while the patient is ventilated.4 In children younger than 4 years of age pleural shunts may not be practical because of their association with pleural effusions and respiratory compromise.
Lumboperitoneal shunts (Fig. 6.17) can also be placed in cases of communicating hydrocephalus, especially in instances when the ventricles cannot be easily accessed (e.g., slit-ventricle syndrome)54 (Fig. 6.18). The technique entails accessing the L4-L5 interspace, cannulating the subarachnoid space with a Tuohy needle, passing the catheter into the subarachnoid space, tunneling it to the peritoneal cavity, and securing it to the lumbar fascia with an anchor.4 Complications include sequelae of overdrainage, which are more difficult to assess and control, difficulties in pressure regulation, lumbar nerve root irritation, progressive cerebellar tonsillar herniation, arachnoiditis, and arachnoid adhesions.55 Other distal sites of CSF drainage that can be used if there are specific problems with diverting to the common locations (i.e., peritonitis, subacute bacterial endocarditis, pleural adhesions/effusions) include the gallbladder and the ureter or urinary bladder.
After shunt placement, patients are followed closely. An initial postoperative CT scan is obtained as a baseline evaluation of the ventricular system and catheter position after shunting. For programmable valves, radiographs can be obtained to confirm the valve setting. With ventriculoatrial shunts, radiographs postoperatively show the catheter’s position and evaluate for pneumothorax, which can occur after placement. In the first 1 to 2 years, there is more frequent radiographic surveillance, which may be performed less frequently afterward if no clinical or radiographic problems are noted.
Complications of Hydrocephalus Treatment
CSF shunts have become a powerful way to treat hydrocephalus. With the advent of CSF shunts, hydrocephalus and the sequelae of increased intracranial pressure can be successfully managed. However, shunting systems are troublesome devices. Common complications include shunt malfunction including underdrainage of CSF (usually from shunt system obstruction), overdrainge of CSF, or infection of the shunt system or of the CSF (Table 6.8).
CSF, cerebrospinal fluid.
Shunt Infections
Shunt infection is unfortunately still seen after shunt insertion, despite the best precautions taken at the time of surgery. Generally, shunt infection rates are in the 5% to 15% range, with more than 70% developing within 1 month of surgery, and 90% within 6 months.56 In some centers, the shunt infection rates are significantly lower or higher. Patients commonly present with low-grade fevers, headaches, malaise, elevation of inflammatory markers (e.g., erythrocyte sedimentation rate, C-reactive protein), erythema along the shunt tract, and symptoms of shunt malfunction if the shunt system becomes obstructed. In severe cases bacteremia, peritonitis, ventriculitis, bacterial endocarditis, and pleural empyema can occur. Peritoneal pseudocysts may be associated with CSF shunt infections. They may be diagnosed by ultrasound or CT evaluation of the abdomen.
CSF shunt catheters are foreign bodies that can be prone to bacterial infection. The most common organisms are coagulase-negative staphylococci followed by Staphylococcus aureus.57 Other organisms less commonly seen include other gram-positive (streptococci, enterococci), gram-negative, and anaerobic bacteria (Propionibacterium acnes).
Treatment of shunt infections always includes antibiotic therapy. Broad-spectrum antibiotics are started initially and tailored to the specific offending organism once it is isolated in culture. Some advocate administration of intrathecal antibiotics in addition to systemic treatment, though this is not routine practice in most centers.58 In addition, either externalization or removal of the shunt and external drainage are performed in suspected cases of infection. The management of infected CSF shunts varies greatly among clinicians. A recent study surveyed all active members of the American Society of Pediatric Neurosurgeons (ASPN) about their clinical practices.59 Most of the neurosurgeons who responded reported that they remove the infected shunt system and place an external ventricular drain.59 Another reported method involved distal externalization of the shunt system and drainage. There was considerable variation in the practices of the surveyed neurosurgeons as far as the duration of antibiotic therapy. In addition there is also substantial variation in how long to continue external ventricular drainage and how long to evaluate cultures before reinternalization of the shunt system.60 The senior author advocates waiting until the CSF cultures have been sterile for at least 72 hours before replacing the ventricular shunt.
In an attempt to improve shunt infection rates, antibiotic impregnated shunt catheters were introduced. Several series looked at the effectiveness of these catheters. One recent retrospective study of 353 shunt placements found a 2.4-fold decrease in infection rate with the use of antibiotic impregnated catheters.61 Other series show more modest effects.62,63 Most studies reported in the literature are retrospective, and large randomized controlled prospective studies comparing antibiotic impregnated versus standard shunt catheters are lacking.
Shunt Malfunction
A common complication of CSF shunting is malfunction from underdrainage of CSF (see Fig. 6.18). Generally, this is due to an obstruction or disconnection causing impedance of CSF drainage anywhere along the course of the shunt system.64 Patients present with symptoms of increased intracranial pressure, which differ depending on the age group. Often the presentation can be with symptoms similar to a previous malfunction, though patients can develop a different constellation of symptoms with subsequent obstructions, and such complaints should not be dismissed.
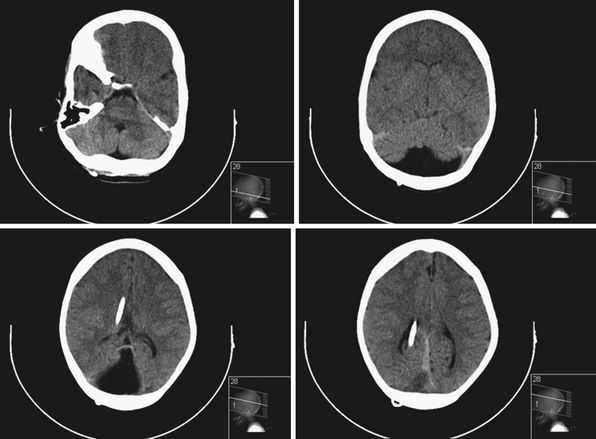
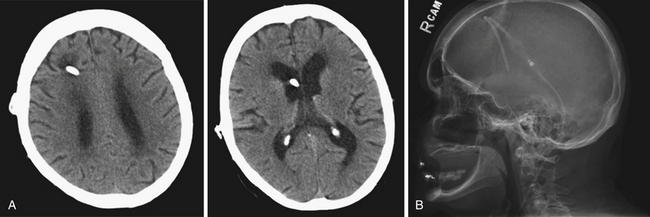
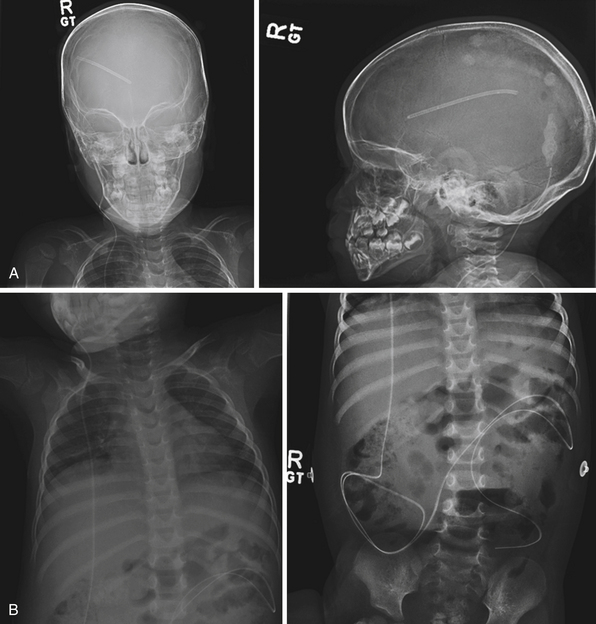
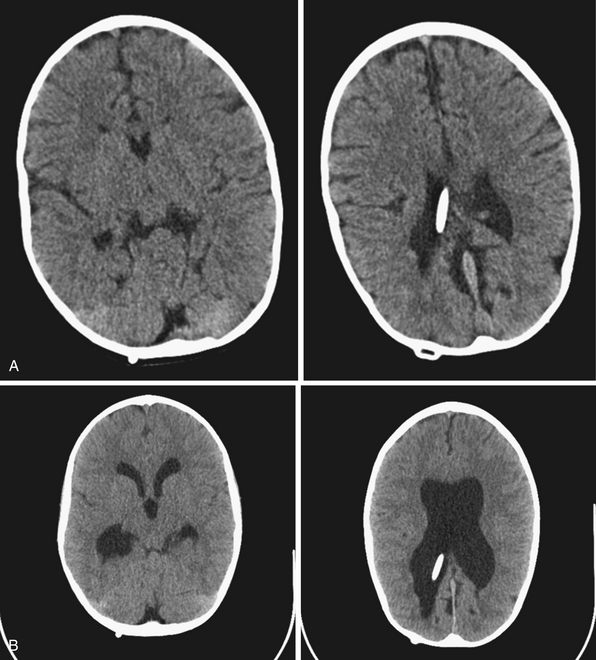
Shunt malfunctions can be proximal, from obstruction of the ventricular catheter, or distal, from a blockage in the extracranial components. Ventricular catheters that migrate toward the choroid plexus are more prone to occlusion. Disconnections can be seen along the distal tubing in areas with increased movement, generally occurring in children undergoing growth spurts in areas with increased movement. The presence of multiple tubing connectors puts the shunt at risk for disconnection (Fig. 6.19). With time distal tubing can calcify, predisposing the shunt system to fracture.
If a shunt obstruction is suspected, the initial evaluation includes imaging studies. A CT of the head allows for evaluation of the ventricles, especially when compared to previous scans. Though many shunted patients present with some degree of ventricular enlargement if the shunt becomes obstructed, those with altered ventricular compliance (e.g., slit-ventricle syndrome) may not show any change in ventricular size on imaging studies with a malfunction.30 A shunt series is also obtained, which includes radiographs of the skull and the body along the peripheral shunt tract, which allows for evaluation of the continuity of the distal tubing. Other adjunctive studies include radionuclide scans, which require injection of a tracer into the reservoir, which travels into the ventricle and then through the distal tubing.65 Failure of visualization of the tracer into the peritoneum indicates a shunt failure.
The valve can be examined at the bedside. The valve can be compressed against the skull. If the valve depresses but does not refill, it may indicate a shunt system obstruction. The shunt reservoir can also be accessed percutaneously with a small-gauge needle (23-25 gauge butterfly needle). Good flow of CSF indicates that the proximal part of the shunt is patent; in addition, ICP can be measured. In patients with symptoms concerning for shunt malfunction, but with imaging studies that are equivocal, CSF pressure can be measured by performing a lumbar puncture and checking the opening pressure, if there are no contraindications (e.g., mass lesion, history of myelomeningocele).
Shunt malfunction can also be observed from shunt overdrainage.66 This is can be seen with a variety of shunt valves, and is particularly augmented by the negative hydrostatic pressures generated when a patient is upright. Common symptoms include low-pressure headaches more pronounced in the upright position. A review of the literature by Pudenz and Foltz revealed that complications of overdrainage occur in at least 10% to 12% of patients with shunted hydrocephalus, usually within 6.5 years from the time of initial shunt placement.67 Overdrainage complication includes the formation of subdural hematomas, intracranial hypotension, craniosynostosis and microcephaly, and slit-ventricle syndrome.67 Valve pressure upgrades and the addition of antisiphoning devices may help with overdrainage symptoms.
Occasionally patients with known shunted hydrocephalus can present with concerning symptoms in the setting of small ventricles on imaging studies. Slit-ventricle syndrome refers to headaches lasting 10 to 90 minutes in the setting of imaging studies showing small ventricles and slow refill of pumping devices.68 However, consensus is still lacking as far as the exact definition of the condition, and evaluation and treatment paradigms. Overdrainage symptoms are treated accordingly, while patients with increased ICP without ventriculomegaly are treated with shunting procedures such as lumboperitoneal shunt placement.
Uncommon Complications
Less commonly encountered complications from CSF shunt placements include hemorrhage along the shunt tract or at the site of insertion, seizures, and shunt metastasis (Table 6.9). Although very uncommon, systemic metastasis of intracranial tumors in shunted patients has been described, particularly in patients with medulloblastoma and other types of malignant tumors.69,70
TABLE 6.9 Uncommon Complications of Cerebrospinal Fluid Shunts
| Cranial | Peripheral |
|---|---|
| Subdural hygroma Hemorrhage (subdural or intraparenchymal) Seizure Hemiparesis/new neurological deficits (due to misplaced catheters) |
Shunt tube migration Shunt tube disconnection/fracture Abdominal pseudocyst formation Bowel perforation Peritonitis Abdominal hernia Endocarditis (VA shunts) Immune-mediated glomerulonephritis (VA shunts) |
VA, ventriculoatrial.
Treatment Outcomes and Specific Problems Associated with Hydrocephalus
Clinical long-term outcomes after hydrocephalus treatment are largely dependent on the underlying pathology, response to shunting, and overall neurological comorbid factors. Despite frequency of ventricular shunting, data on long-term outcomes, particularly in the pediatric population, are scarce. Often the overall prognosis is dependent on the underlying congenital malformations. A study by Casey and co-workers followed a cohort of 155 children shunted for hydrocephalus over a 10-year period.71 Outcome measures were surgical morbidity and mortality rates, and academic achievement records. Fifty-nine percent of children attended public school; however, those shunted secondary to IVH more often required special schooling. Forty-four percent did not require shunt revision. The most common complications of shunting included obstruction and infection, most presenting within the first year after placement. An 11% mortality rate during the 10-year follow-up was noted. Another study by Billard and associates looking at IQ in shunted children found that 75% of patients had an IQ greater than 70. However, many children had a marked decrease in visual spatial skills.72 There was a trend toward lower IQ values in patients with hydrocephalus due to infection as opposed to other causes.
Hydrocephalus can be diagnosed in utero and can be detected often on prenatal ultrasonography and further evaluated with prenatal MRI (Fig. 6.20). In the 1980s fetal surgery for hydrocephalus was undertaken in an attempt to improve postnatal outcome in infants with hydrocephalus.73 However, outcomes were so poor that in utero treatment was largely abandoned.73
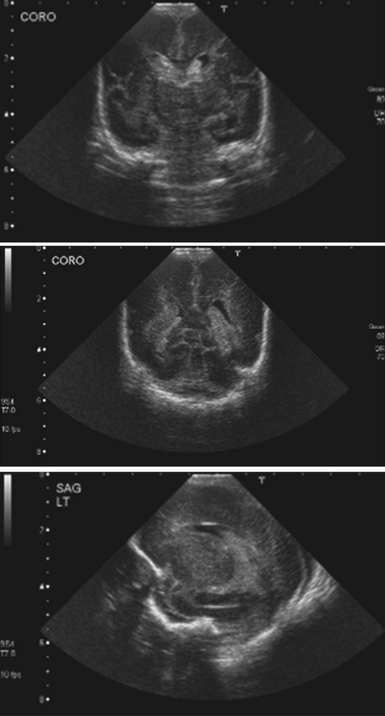
FIGURE 6.20 Transfontanelle sonogram of a premature infant with germinal matrix hemorrhage, coronal and sagittal views.
Premature infants weighing less than 1.5 kg are at risk for developing germinal matrix hemorrhages, leading to CSF obstruction and hydrocephalus (see Fig. 6.20). Generally these hemorrhages are thought to be due to fragility of the germinal matrix vasculature in the setting of dysautoregulation of cerebral blood flow.74 Prognosis depends on the extent of hemorrhage, which is described by four clinical grades (see Table 6.5). Several studies describe higher morbidity and mortality rates for higher grades, associated with more extensive hemorrhage.75 Treatment centers on a multimodal evaluation of all medical problems of the preterm infant. The infant is monitored closely with daily head circumferences, fontanelle evaluations, and serial transfontanelle ultrasound examinations. If hydrocephalus develops, CSF removal can be done by lumbar puncture, ventriculosubgaleal shunt, ventricular catheter and reservoir placement with percutaneous taps, or external ventricular drain placement. A ventriculoperitoneal shunt can be inserted at any point when necessary, although delaying shunt placement until the infant weighs more than 1.5 kg may reduce the chance of infection.
Other congenital conditions are associated with hydrocephalus. The Dandy-Walker malformation refers to a fourth ventricular cyst associated with cerebellar vermian hypoplasia, with a large posterior fossa, and associated hydrocephalus and systemic problems.76,77 Initial management is aimed at determining if there is communication of the cyst with the ventricular system. An infratentorial cystoperitoneal shunt can be used to decompress the cyst, and an additional ventricular shunt may or may not be needed as well.76 If both supra- and infratentorial shunts are placed, a Y-connector can be used to allow drainage from both compartments through the shunt.76 Alternatively, two separate shunt systems can be used, but if separate valves are used, they should have the same pressure settings. A high incidence of lower IQ scores has been observed in Dandy-Walker patients, thought to be due to associated congenital intracranial anomalies rather than initial hydrocephalus.77 Hydrocephalus associated with myelomeningocele is generally treated with ventriculoperitoneal shunting. The timing of shunt placement has been debated, but current literature suggests that shunt complications are not necessarily associated with the timing of surgery, and many practitioners advocate concurrent shunt placement at the time of myelomeningocele repair if there is significant hydrocephalus.78
Posterior fossa tumors can be associated with postresection hydrocephalus in up to 40% of patients (Fig. 6.21). Shunting is required for persistent ventricular dilatation and symptoms. Recently endoscopic third ventriculostomy has been described as an option to avoid the need for a shunt.79,80
Hydranencephaly is a developmental abnormality in which there is absence of the cerebral hemispheres (Fig. 6.22). Although life expectancy is markedly reduced, occasionally patients can survive into the teen or adult years.81 Shunt placement does not improve function. Patients with hydranencephaly and severe macrocephaly may benefit from ventricular shunt placement or endoscopic coagulation of the choroid plexus as palliative procedures.
For normal-pressure hydrocephalus there remains debate about the optimal valve pressure settings, the use of antisiphon devices, and whether to use ventriculoperitoneal or lumboperitoneal shunts. One study by Hebb and Cusimano reviewing the Medline literature found that 29% of patients had significant symptom improvement, with a complication rate of 6% after shunting.82 Some studies have also shown that patients who respond to intermittent CSF drainage and undergo shunt placement for NPH tend to show significant improvement in executive function and cognition on neuropsychological testing.83,84 A retrospective study by McGirt and colleagues of 132 patients undergoing 179 shunt placements showed that up to 75% had objective improvement in symptoms up to 24 months after shunting.85 Gait dysfunction was the first symptom to improve in 93% of patients, but dementia and urinary incontinence did not improve as dramatically as gait dysfunction.85
Endoscopic Treatment of Hydrocephalus
With advances in optics and computer technology, neuroendoscopic treatment of hydrocephalus has regained popularity since its introduction in the early 1900s. Endoscopic third ventriculostomy (ETV) is a particularly attractive option in selected patients with noncommunicating hydrocephalus, with aqueductal or fourth ventricular obstruction.86 It provides a means of bypassing a downstream obstruction and when successful, it obviates the need for extracranial CSF shunting and the long-term complications associated with this procedure.
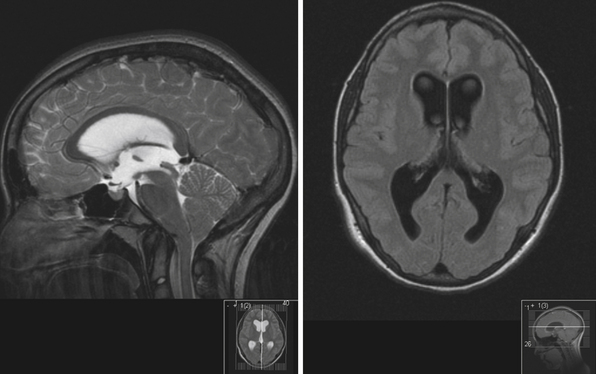
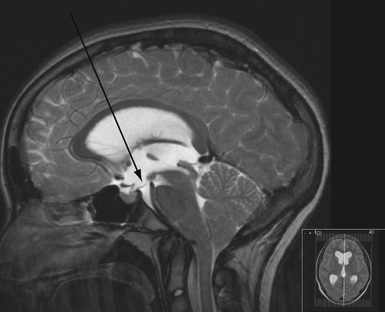
To determine which patients with hydrocephalus are good candidates for ETV, MRI findings become helpful in patient selection.4,86 In particular, careful consideration is given to the anatomy of the floor of the third ventricle, and generally the procedure becomes more challenging if the floor is thicker (Fig. 6.23). The relationship of the basilar artery to the floor of the third ventricle needs to be evaluated carefully. ETV is more likely to be successful in patients with acquired or late occlusion of the sylvian aqueduct, as well as patients with posterior fossa tumors causing ventricular obstruction.87 The results of ETV in young children are not as good as those in older children or adults.
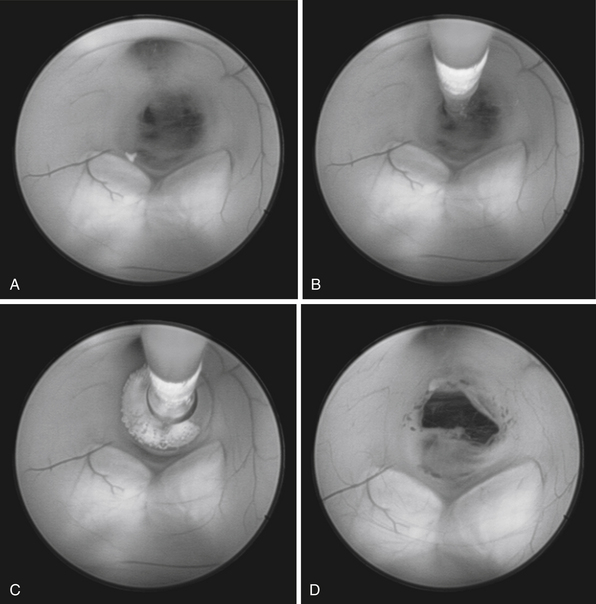
The technique for performing ETV is well established.4,88,89 The patient is positioned supine with the brow up. A coronal burr hole is placed just medial to the midpupillary line. The lateral ventricle is entered and then the endoscope is passed through the foramen of Monro into the third ventricle (Fig. 6.24). The fenestration is performed anterior to the mammillary bodies and posterior to the infundibular recess, through the thinned tuber cinereum using a blunt probe. The fenestration is then enlarged using a balloon catheter (Fig. 6.25). Image guidance can be used to facilitate the trajectory.
The outcome following ETV is good in properly selected cases. In a recent study by Sacko and co-workers from France evaluating 368 ETVs, the overall success rate was 68.5%.90 Factors related to increased failure of the ventriculostomy were age younger than 6 months, and hemorrhage-related or idiopathic chronic hydrocephalus. The associated morbidity rate was 10%, and 97% of failures occurred within 2 months. Another series by Gangemi and associates reported results in 125 patients who had undergone ETV, with an overall success rate of 86.4%.91 Amini and Schmidt reported their experience with 36 ETVs performed selectively in an adult population, with a success rate of 72%.92 Of those patients, 22% demonstrated delayed failure of the ventriculostomy at a mean of 3.75 years, emphasizing the need for longer follow-up of patients undergoing this procedure.92
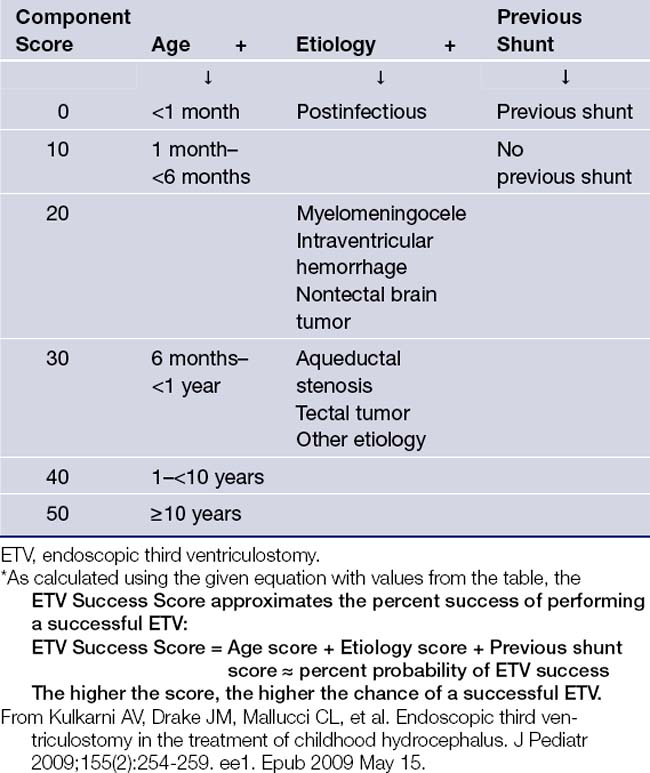
In 2009, a very important paper by Kulkarni and colleagues from the Canadian Pediatric Neurosurgery Study Group elucidated which children did best from an ETV. The researchers analyzed 618 patients undergoing an ETV at 12 international centers and followed up at 6 months.93 An ETV Success Score was validated and hovered around 64% at the time of follow-up, which is when most ETV failures become evident. The three most important parameters for ETV success appeared to be patient age at the time of ETV, etiology of hydrocephalus, and whether or not the patient was previously shunted. Table 6.10 shows the ETV success score, which approximates the percentage success of performing a successful ETV in a particular patient. The patients whose ETV was most successful were those over 10 years of age, those with aqueductal stenosis, and those who did not have a previous shunt. Those patients whose ETV fared the worst were patients under 1 month of age, those with postinfectious hydrocephalus, and those with a previous shunt.
It is unclear whether or not the success of an ETV is more sustainable than a shunt. Based on a 2010 paper from the same Canadian/International group, the relative risk of failure of an ETV is initially higher than that of a shunt but drops below the failure of a shunt after 3 months, and the long-term benefit of ETV may be realized in several years.94
Neuroendoscopic treatment of multiloculated hydrocephalus, including endoscopic cyst fenestration and fenestration of the septum pellucidum, is yet another effective alternative to multiple CSF shunt placements in selected patients.95–97 Recent studies have suggested a role for ETV for communicating hydrocephalus as well.98
Aronyk K.E. The history and classification of hydrocephalus. Neurosurg Clin North Am. 1993;4(4):599-609.
Aschoff A., Kremer P., Hashemi B., et al. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999;22(2-3):67-93.
Greitz D. Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst. 2007;23(5):487-489.
Kulkarni A.V., Drake J.M., Mallucci C.L., et al. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J. Pediatr. 2009;155(2):254-259.
Whitehead W.E., Kestle J.R. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatr Neurosurg. 2001;35(4):205-210.
Please go to expertconsult.com to view the complete list of references.
1. Sipek A., Gregor V., Horacek J., et al. Congenital hydrocephalus 1961-2000—incidence, prenatal diagnosis and prevalence based on maternal age. Ceska Gynekol. 2002;67(6):360-364.
2. Fernell E., Hagberg G. Infantile hydrocephalus: declining prevalence in preterm infants. Acta Paediatr. 1998;87(4):392-396.
3. Brean A., Eide P.K. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(1):48-53.
4. Roth P.A., Cohen A.R. The management of hydrocephalus in infants and children. In: Tindall G.T., Cooper P.R., Barrow D.L., editors. The Practice of Neurosurgery. Baltimore: Williams & Wilkins; 1996:2707-2728.
5. Ring-Mrozik E., Angerpointer T.A. Historical aspects of hydrocephalus. Prog Pediatr Surg. 1986;20:158-187.
6. Aronyk K.E. The history and classification of hydrocephalus. Neurosurg Clin North Am. 1993;4(4):599-609.
7. Hirsch J.F. Surgery of hydrocephalus: past, present and future. Acta Neurochir. 1992;116(2-4):115-160.
8. Nulsen F.E., Spitz E.B. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surg Forum. 1951:399-403.
9. Greenberg M.S. Handbook of Neurosurgery, 5th ed. Lakeland: Thieme; 2001.
10. Mokri B. The Monro-Kellie hypothesis: application in CSF volume depletion. Neurology. 2001;56(12):1746-1748.
11. Neff S., Subramaniam R.P. Monro-Kellie doctrine. J Neurosurg. 1996;85(6):1195.
12. McEvoy A.W., Harding B.N., Phipps K.P., et al. Management of choroid plexus tumors in children: 20 years experience at a single neurosurgical centre. Pediatr Neurosurg. 2000;32(4):192-199.
13. Hirano H., Hirahara K., Asakura T., et al. Hydrocephalus due to villous hypertrophy of the choroid plexus in the lateral ventricles. Case report. J Neurosurg. 1994;80(2):321-323.
14. Dastgir G., Awad A., Salam A., et al. Unilateral hydrocephalus due to foramen of Monro stenosis. Minimally Invasive Neurosurg. 2006;49(3):184-186.
15. Jeffree R.L., Besser M. Colloid cyst of the third ventricle: a clinical review of 39 cases. J Clin Neurosci. 2001;8(4):328-331.
16. Cinalli G., Spennato P., Savarese L., et al. Endoscopic aqueductoplasty and placement of a stent in the cerebral aqueduct in the management of isolated fourth ventricle in children. J Neurosurg. 2006;104(Suppl 1):21-27.
17. Penn R.D., Linninger A. The physics of hydrocephalus. Pediatr Neurosurg. 2009;45(3):161-174.
18. Linninger A.A., Sweetman B., Penn R. Normal and hydrocephalic brain dynamics: the role of reduced cerebrospinal fluid reabsorption in ventricular enlargement. Ann Biomed Engineering. 2009;37(7):1434-1447.
19. Levine D.N. Intracranial pressure and ventricular expansion in hydrocephalus: have we been asking the wrong question? J Neurol Sci. 2008;269(1-2):1-11.
20. Gowers W.R. A Manual of Diseases of the Nervous System. Philadelphia: Blakiston; 1888.
21. Cherian S., Whitelaw A., Thoresen M., et al. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14(3):305-311.
22. James H.E. Hydrocephalus in infancy and childhood. Am Fam Physician. 1992;45(2):733-742.
23. Green A.L., Pereira E.A., Kelly D., et al. The changing face of paediatric hydrocephalus: a decade’s experience. J Clin Neurosci. 2007;14(11):1049-1054.
24. Galia G.L., Rigamonti D., Williams M.A. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nature Clin Practice Neurol. 2006;2(7):375-381.
25. Dhungana S., Sharrack B., Woodroofe N. Idiopathic intracranial hypertension. Acta Neurol Scand. 2010;121(2):71-82.
26. Zimmerman R.A., Bilaniuk L.T. Magnetic resonance evaluation of fetal ventriculomegaly—associated congenital malformations and lesions. Semin Fetal Neonatal Med. 2005;10(5):429-443.
27. Taylor G.A. Sonographic assessment of posthemorrhagic ventricular dilation. Radiol Clin North Am. 2001;39(3):541-551.
28. Evans W.A. An encephalographic ratio for estimating ventricular and cerebral atrophy. Arch Neurol Psychiatry. 1942;47:931-937.
29. Draver B.P., Rosenbaum A.E. Pediatric metrizamide CT cisternography: cerebrospinal fluid circulation and hydrocephalus. Neurology. 1978;28(1):71-77.
30. McNatt S.A., Kim A., Hohuan D. Pediatric shunt malfunction without ventricular dilatation. Pediatr Neurosurg. 2008;44(2):128-132.
31. Winston K.R., Lopez J.A., Freeman J. CSF shunt failure with stable normal ventricular size. Pediatr Neurosurg. 2006;42(3):151-155.
32. Boon A.J., Trans J.T., Delwel E.J., et al. Dutch normal pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J Neurosurg. 1997;87(5):687-693.
33. Eide P.K., Stanisic M. Cerebral microdialysis and intracranial pressure monitoring in patients with idiopathic normal-pressure hydrocephalus: association with clinical response to extended lumbar drainage and shunt surgery. J Neurosurg. 2010;112(2):414-424.
34. Eide P.K., Sorteberg W. Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: a 6-year review of 214 patients. Neurosurgery. 2010;66(1):80-91.
35. Brean A., Fredø H.L., Sollid S., et al. Five-year incidence of surgery for idiopathic normal pressure hydrocephalus in Norway. Acta Neurol Scand. 2009;120(5):314-316.
36. Marmarou A., Bergsneider M., Relkin N., et al. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(Suppl 3):S1-S3.
37. Marmarou A., Bergsneider M., Klinge P., et al. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(Suppl 3):S17-S28.
38. International PHVD Drug Trial Group. International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. International PHVD Drug Trial Group. Lancet. 1998;352(9126):433-440.
39. Bhagwati S.N., George K. Use of intrathecal hyaluronidase in the management of tuberculous meningitis with hydrocephalus. Childs Nerv Syst. 1986;2(1):20-25.
40. Epstein F., Hochwald G.M., Ransohoff J. Neonatal hydrocephalus treated by compressive head wrapping. Lancet. 1973;1(7804):643-646.
41. Anwar M., Kadam S., Hiatt I.M., et al. Serial lumbar punctures in prevention of post-hemorrhagic hydrocephalus in preterm infants. J Pediatr. 1985;107(3):446-450.
42. Whitelaw A. Repeated lumbar or ventricular punctures for preventing disability or shunt dependence in newborn infants with intraventricular hemorrhage. Cochrane Database Syst Rev. 2000(2):CD000216.
43. Aschoff A., Kremer P., Hashemi B., et al. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999;22(2-3):67-93.
44. Dandy W.E. Extirpation of the choroid plexus of the lateral ventricle in communicating hydrocephalus. Ann Surg. 1918;68:569-579.
45. Philips M.F., Shanno G., Duhaime A.C. Treatment of villous hypertrophy of the choroid plexus by endoscopic contact coagulation. Pediatr Neurosurg. 1998;28(5):252-256.
46. Drake J.M., Keetle J.R., Milner R., et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43(2):294-303.
47. Pollack I.F., Albright A.L., Adelson P.D. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery. 1999;45(6):1399-1408.
48. Lavinio A., Harding S., Van Der Boogaard F., et al. Magnetic field interactions in adjustable hydrocephalus shunts. J Neurosurg Pediatr. 2008;2(3):222-228.
49. Dickermn R.D., McConathy W.J., Morgn J., et al. Failure rate of frontal versus parietal approaches for proximal catheter placement in ventriculoperitoneal shunts: revisited. J Clin Neurosci. 2005;12(7):781-783.
50. Reig A.S., Stevenson C.B., Tulipan N.B. CT-based, feducial-free frameless stereotaxy for difficult ventriculoperitoneal shunt insertion: experience in 26 consecutive patients. Stereotactic Funct Neurosurg. 2010;88(2):75-80.
51. Villavicencio A.T., Levegue J.C., McGirt M.J., et al. Comparison of revision rates following endoscopically versus nonendoscopically placed ventricular shunt catheters. Surg Neurol. 2003;59(5):375-379.
52. Cutico W., Vannix D. Laparoscopically guided peritoneal insertion in ventriculoperitoneal shunts. J Laparaoendoscopic Surg. 1995;5(5):309-311.
53. Britz G.W., Avellino A.M., Schaller R., et al. Percutaneous placement of ventriculoatrial shunts in the pediatric population. Pediatr Neurosurg. 1998;29(3):161-163.
54. Brazis P.W. Clinical review: the surgical treatment of idiopathic pseudotumor cerebri (idiopathic intracranial hypertension). Cephalalgia. 2008;28(12):1361-1373.
55. Wang V.Y., Barbaro N.M., Lawton M.T., et al. Complications of lumboperitoneal shunts. Neurosurgery. 2007;60(6):1045-1048.
56. Choux M., Genitori L., Lang D., et al. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg. 1992;77(6):875-880.
57. Campbell J.W. Shunt infections. In: Albright A.L., Pollack I.F., Adelson P.D., editors. Principles and Practice of Pediatric Neurosurgery. 2nd ed. New York: Thieme Medical Publishers; 2008:1141-1147.
58. James H.E., Bradley J.S. Aggressive management of shunt infection: combined intravenous and intraventricular antibiotic therapy for twelve or less days. Pediatr Neurosurg. 2008;44(2):104-111.
59. Whitehead W.E., Kestle J.R. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatr Neurosurg. 2001;35(4):205-210.
60. Desai A., Lollis S.S., Missios S., et al. How long should cerebrospinal fluid cultures be held to detect shunt infections? Clinical article. J Neurosurg Pediatr. 2009;4(2):184-189.
61. Sciubba D.M., Stuart R.M., McGirt M.J., et al. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg. 2005;103(Suppl 2):131-136.
62. Richards H.K., Seeley H.M., Pickard J.D. Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatr. 2009;4(4):389-393.
63. Ritz R., Roser F., Morgalla M., et al. Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 2007;8:38.
64. Browd S.R., Ragel B.T., Gottfried O.N., et al. Failure of cerebrospinal fluid shunts: Part I: obstruction and mechanical failure. Pediatr Neurol. 2006;34(2):83-92.
65. Vernet O., Farmer J.P., Lambert R., et al. Radionuclide shuntogram: adjunct to manage hydrocephalic patients. J Nucl Med. 1996;37(3):406-410.
66. Browd S.R., Gottfried O.N., Ragel B.T., et al. Failure of cerebrospinal fluid shunts: Part II: overdrainage, loculation, and abdominal complications. Pediatr Neurol. 2006;34(3):171-176.
67. Pudenz R.H., Foltz E.L. Hydrocephalus: overdrainge by ventricular shunts. A review and recommendataions. Surg Neurol. 1991;35(3):200-212.
68. Rekate H.L. The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg. 2004;40:259-263.
69. Magtibay P.M., Friedman J.A., Rao R.D., et al. Unusual presentation of adult metastatic peritoneal medulloblastoma associated with ventriculoperitoneal shunt: a case study and review of the literature. Neuro-Oncol. 2003;5(3):217-220.
70. Donovan D.J., Prauner R.D. Shunt-related abdominal metastases in a child with choroid plexus carcinoma: case report. Neurosurgery. 2005;56(2):E412.
71. Casey A.T., Kimmings E.J., Kleinlugtebeld A.D., et al. The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg. 1997;27(2):63-70.
72. Billard C., Santini J.J., Gillet P., et al. Long-term intellectual prognosis of hydrocephalus with reference to 77 children. Pediatr Neurosci. 1985;12(4-5):219-225.
73. von Koch C.S., Gupta N., Sutton L.N., et al. In utero surgery for hydrocephalus. Childs Nerv Syst. 2003;19(7-8):574-586.
74. Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Resolutions. 2010;67(1):1-8.
75. Levy M.L., Masri L.S., McComb J.G. Outcome for preterm infant with germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery. 1997;41(5):1111-1117.
76. Alexiou G.A., Sfakianos G., Prodromou N. Dandy-Walker malformation: analysis of 19 cases. J Child Neurol. 2010;25(2):188-191.
77. Pascual-Castroviejo I., Velez A., Pascual-Pascual S.I., et al. Dandy-Walker malformations: analysis of 38 cases. Childs Nerv Syst. 1991;7(2):88-97.
78. Radmanesh F., Nejat F., El Khashab M., et al. Shunt complications in children with myelomeningocele: effect of timing of shunt placement. Clinical article. J Neurosurg Pediatr. 2009;3(6):516-520.
79. Bhatia R., Tahir M., Chandler C.L. The management of hydrocephalus in children with posterior fossa tumors: the role of pre-resectional endoscopic third ventriculostomy. Pediatr Neurosurg. 2009;45(3):186-191.
80. Tamburrini G., Pettorini B.L., Massimi L., et al. Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst. 2008;24(12):1405-1412.
81. McAbee G.N., Chan A., Erde E.L. Prolonged survival with hydranencephaly: report of two patients and literature review. Pediatr Neurol. 2000;23(1):80-84.
82. Hebb A.O., Cusimano M.D. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166-1184.
83. Gleichgerrcht E., Cervio A., Salvat J., et al. Executive function improvement in normal pressure hydrocephalus following shunt surgery. Behav Neurol. 2009;21(3):181-185.
84. Thomas G., McGirt M.J., Woodworth G., et al. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dementing Geriatr Cognitive Disord. 2005;20(2-3):163-168.
85. McGirt M.J., Woodworth G., Coon A.L., et al. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(4):699-705.
86. Rekate H.L. Selecting patient for endoscopic third ventriculostomy. Neurosurg Clin North Am. 2004;15(1):39-49.
87. Feng H., Huang G., Liao X., et al. Endoscopic third ventriculostomy in the management of obstructive hydrocephalus: an outcome analysis. J Neurosurg. 2004;100(4):626-633.
88. Vries J.K. An endoscopic technique for third ventriculostomy. Surg Neurol. 1978;9(3):165-168.
89. Wellons J.C.3rd, Bagley C.A., George T.M. A simple and safe technique for endoscopic third ventriculocisternostomy. Pediatr Neurosurg. 1999;30(4):219-223.
90. Sacko O., Boetto S., Lauwers-Cances V., et al. Endoscopic third ventriculostomy: outcome analysis in 368 procedures. J Neurosurg Pediatr. 2010;5(1):68-74.
91. Gangemi M., Donati P., Maiuri F., et al. Endoscopic third ventriculostomy for hydrocephalus. Minimally Invasive Neurosurg. 1999;42(3):128-132.
92. Amini A., Schmidt R.H. Endoscopic third ventriculostomy in a series of 36 adult patients. Neurosurg Focus. 2005;19(6):E9.
93. Kulkarni A.V., Drake J.M., Mallucci C.L., et al. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 2009;155(2):254-259.
94. Kulkarni A.V., Drake J.M., Kestle J.R. Endoscopic third ventriculostomy vs. cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery. 2010;67(3):588.
95. Spennato P., Cinalli G., Ruggiero C., et al. Neuroendoscopic treatment of multiloculated hydrocephalus in children. J Neurosurg. 2007;106(suppl 1):29-35.
96. El-Chandour N.M. Endoscopic cyst fenestration in the treatment of multiloculated hydrocephalus in children. J Neurosurg Pediatr. 2008;1(3):217-222.
97. Oertel J.M., Schroeder H.W., Gaab M.R. Endoscopic stomy of the septum pellucidum: indications, technique, and results. Neurosurgery. 2009;64(3):482-491.
98. Greitz D. Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst. 2007;23(5):487-489.