Hydrocephalus
Hydrocephalus is one of the most common sequelae of any insult to a child’s central nervous system (CNS). Hydrocephalus occurs in 1 in 2000 live births and is associated with one third of all CNS malformations. Since the 1970s, the incidence of spinal dysraphism related to hydrocephalus has declined.1 Reasons include maternal folate therapy, which has resulted in fewer patients with spinal dysraphism, and vaccinations, which have diminished the number of patients with meningitis and its complications.
Physiology of Cerebrospinal Fluid
The sites of CSF absorption remain controversial. It is widely accepted that arachnoid villi are one of the major sites in adults and older children.2 The arachnoid villi (pacchionian granulations) are not developed in children until the closure of the fontanels. Various studies also have suggested that a portion of CSF drains through the perivascular and perineural spaces into the lymphatic system.3 In neonates, most CSF absorption may occur through the lymphatic and venous system.4
Mechanisms of Hydrocephalus
Hydrodynamic Model for Csf Circulation
The hydrodynamic model is based on the concept that the absorption of CSF occurs through the capillaries in the CNS rather than through the arachnoid granulations and villi.3 The skull is a nonelastic housing for brain tissue; blood, CSF, and brain tissue are almost incompressible. As stated by the Monro-Kelly doctrine, the total volume of arteries, veins, CSF, and brain confined within the skull cavity and dura mater is constant, and any increase in volume in one or more compartments causes a decrease in volume in the others. Skull and dura mater are more elastic. The elasticity of these structures plays a pivotal role in the hydrodynamic theory of hydrocephalus. During cardiac systole, the expansion of the intracranial arteries increases the ICP, causing CSF displacement into the spinal canal and an increase in the venous outflow. During cardiac diastole, inflow of CSF from the spinal canal occurs, which causes elevation of pressure in the subarachnoid space. Thus increased pressure is present in the CSF spaces during the entire cardiac cycle, which in turn compresses the venous outlets, causing an increase in outlet resistance and venous “counter” pressure. This pressure is necessary to keep the intracerebral veins sufficiently distended to accommodate the normal cerebral flow.
Imaging
Computed Tomography and Magnetic Resonance Imaging
Computed tomography (CT) and MRI are used as primary modalities to assess ventricular size. Ultrasound of the head is used as the initial study in infants with macrocephaly. Several parameters can help differentiate between hydrocephalus and ex vacuo dilatation of ventricles from cerebral atrophy in infants (Box 32-1).
The most reliable sign of hydrocephalus is enlargement of the anterior and posterior recesses of the third ventricle (Fig. 32-1); this phenomenon does not occur in ex vacuo ventricular enlargement. The disproportionate enlargement of the recesses occurs because the thin hypothalamus and cisterns surrounding these recesses provide relatively little resistance to expansion. In contrast, the body of the third ventricle is restricted by the rigid thalami, which provide more resistance to expansion. The anterior recesses (i.e., the chiasmal and infundibular recesses) expand earlier than the posterior recesses (i.e., the pineal and suprapineal recesses), which is best appreciated on midsagittal MRI.5 On axial CT, dilation of the anterior recesses of the third ventricle is detected when the third ventricle is larger at the level of the optic chiasm than at the middle of the ventricle.
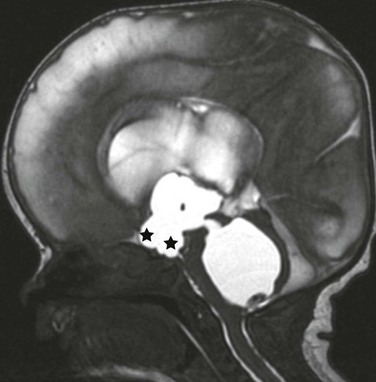
Figure 32-1 Obstructive hydrocephalus.
A midline sagittal balanced steady-state free precession image demonstrates the dilatation of the chiasmatic and infundibular recess of the third ventricle (stars). A dilated fourth ventricle also is noted in this patient with fourth ventricular outflow obstruction (an entrapped fourth ventricle) from neonatal hemorrhage.
Commensurate dilation of the temporal horns with the lateral ventricles also is a strong indicator of hydrocephalus. The dilation of the temporal horns is best viewed on coronal T2-weighted images. The choroidal fissure is enlarged, and the hippocampus is compressed and displaced inferomedially (Fig. 32-2). Studies have suggested that temporal horns dilate less than the bodies of the lateral ventricles in generalized atrophy.6 This finding may be related to the small size of the temporal lobes and to their relatively small volume of white matter.
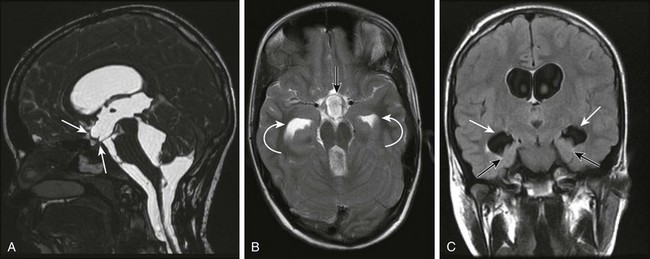
Figure 32-2 Magnetic resonance imaging findings in a patient with hydrocephalus.
A, A midsagittal T1-weighted image in a 10-year-old girl with obstructive hydrocephalus demonstrates dilation of the chiasmatic and infundibular recesses (arrows). B, An axial fluid-attenuated inversion recovery image shows dilated anterior recess of the third ventricle (straight arrow). The temporal horns also are dilated (curved arrows), with a surrounding increase in signal suggestive of increased transependymal cerebrospinal fluid resorption. C, A coronal T2-weighted image shows the characteristic dilation of the temporal horns (white arrows) with enlargement of the choroidal fissure and inferomedial displacement of the hippocampus (black arrows).
The mamillopontine distance is measured on MRI from the anterior root of the mamillary body to the top of the pons parallel to the anterior mesencephalon. The normal average distance is 3.8 mm.5 The floor of the third ventricle as seen on sagittal MRI is usually concave downward. With enlargement of the third ventricle, it becomes straightened or convex downward, resulting in reduction of the mamillopontine distance (e-Fig. 32-3).
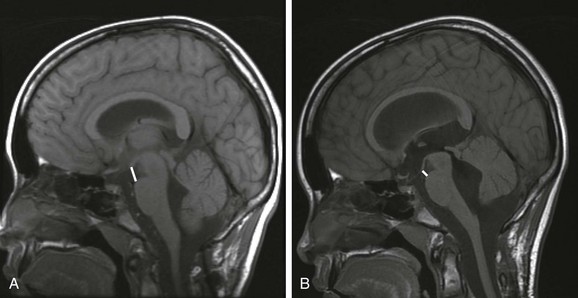
e-Figure 32-3 The mamillopontine distance.
A, A baseline T1-weighted, sagittal, midline, magnetic resonance image in this 11-year-old patient demonstrates normal mamillopontine distance. The distance (bar) is measured from the anterior base of the mamillary body to the top of the pons parallel to the anterior aspect of the mesencephalon. B, A reduction in the mamillopontine distance within 6 months as a result of interval development of hydrocephalus from an intraventricular hemorrhage. Note the stretching of the corpus callosum and the third ventricular dilation.
The ventricular angle (e-Fig. 32-4) measures the divergence of the frontal horns.6 Concentric enlargement of the frontal horns in a patient with hydrocephalus causes diminution of this angle, as seen on axial or coronal images. This concentric dilation produces an enlargement of the frontal horn radius with a rounded configuration of the frontal horns, or a “Mickey Mouse ears” appearance.
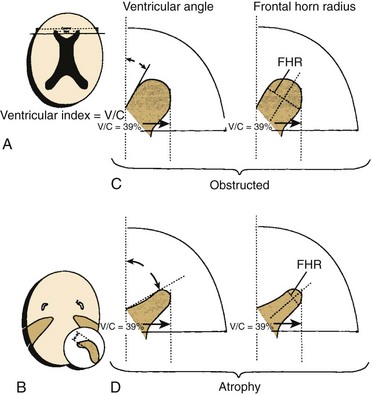
e-Figure 32-4 Various methods of radiographically diagnosing hydrocephalus.
A, The ventricular index is the ratio of the ventricular diameter at the level of the frontal horns to the diameter of the brain measured at the same level. This method is not very sensitive because the ventricular index is enlarged in patients with cerebral atrophy, as well as in patients with hydrocephalus. B, The finding that the temporal horns have enlarged commensurately with the bodies of the lateral ventricles is probably the most sensitive and reliable sign in the differentiation of hydrocephalus from atrophy. There is significantly less dilation of the temporal horns than of the bodies of the lateral ventricles in cerebral atrophy. C, The ventricular angle measures the divergence of the frontal horn. In theory, the angle made by the anterior or superior margins of the frontal horn at the level of the foramina of Monro is diminished when concentric enlargement of the frontal horns occurs. Compare the illustration of hydrocephalus (top) with that of atrophy (bottom). The ventricular index in both cases is 39%, but the ventricular angle is markedly reduced in the presence of hydrocephalus. D, Concentric dilation produces an enlargement of the frontal horn radius (FHR) with a rounded configuration of the frontal horns, or a “Mickey Mouse ears” appearance. (From Barkovich AJ. Pediatric neuroimaging. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.)
The presence of periventricular interstitial edema is indicative of hydrocephalus (Fig. 32-5). With elevation of pressure within the ventricles, the normal centripetal flow toward the ventricles is reversed. The CSF is forced out through the ependyma into the surrounding extracellular spaces to be absorbed by alternative routes. This increase in periventricular fluid constitutes interstitial edema. It is best recognized on MRI with fluid-attenuated inversion recovery and proton density sequences. It is more difficult to appreciate on T2-weighted images because of the bright signal from the ventricles. Periventricular interstitial edema is difficult to appreciate in neonates and young infants because it is masked by a bright signal from immature myelin, with its high water content. On CT, periventricular interstitial edema is seen as hypoattenuation in the periventricular region, with indistinct ventricular margins.
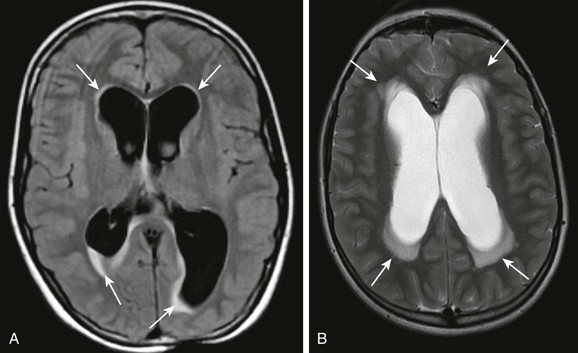
Figure 32-5 Hydrocephalus.
A, An axial fluid-attenuated inversion recovery sequence at the level of the lateral ventricles demonstrates characteristic transependymal flow of cerebrospinal fluid (CSF) and periventricular hyperintensities. B, An axial T2-weighted image in a different patient demonstrates increased transependymal flow of CSF characteristic of hydrocephalus.
A CSF “flow void” in the third ventricle, aqueduct of Sylvius, and fourth ventricle may be accentuated in persons with hydrocephalus as a result of hyperdynamic flow, although the specificity of this finding is unclear (e-Fig. 32-6).
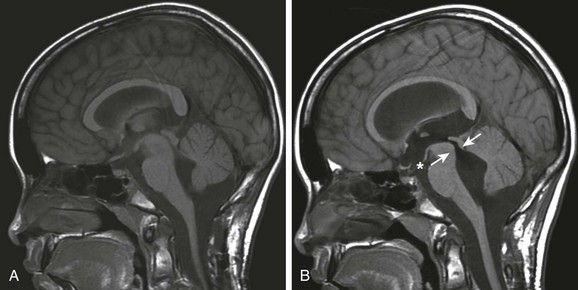
e-Figure 32-6 Rapid development of hydrocephalus.
A, Imaging at baseline: a T1-weighted midsagittal image shows normal anatomy. B, Imaging at 1 month follow-up: a flow void in the aqueduct of Sylvius indicating increased CSF velocity (arrows) is noted with enlarging lateral ventricles (not shown), indicative of hydrocephalus. Also note the dilatation of the anterior recesses of the third ventricle (asterisk).
Marked hydrocephalus may lead to the formation of atrial diverticula, which is herniation of the ventricular wall through the choroidal fissure of the ventricular trigone into the supracerebellar and quadrigeminal cisterns. Diverticula may cause compression and distortion of the tectum and may mimic arachnoid cysts in the region of the quadrigeminal cistern.7
Qualitative and quantitative CSF analysis can be performed using phase-contrast cine MRI. The technique is useful in demonstrating pulsatile flow at the craniocervical junction (Fig. 32-7), aqueduct of Sylvius, and across a surgically created third ventriculostomy.18
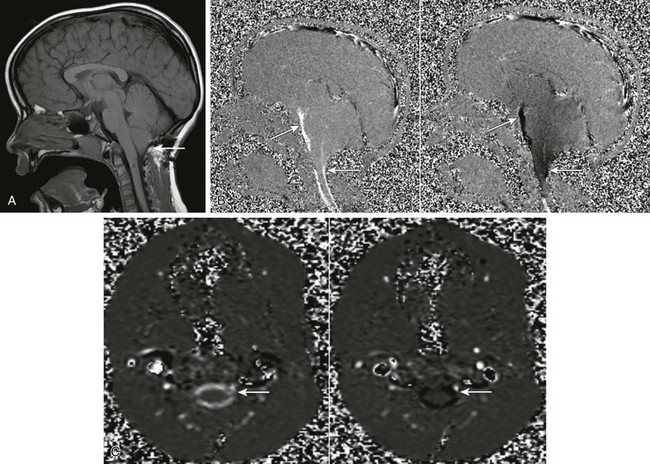
Figure 32-7 Phase-contrast cine magnetic resonance (MR) imaging. Images are acquired throughout the cardiac cycle and demonstrate pulsatile cerebrospinal fluid (CSF) motion.
A, A midsagittal T1-weighted image shows the presence of a Chiari 1 malformation (arrow). B, A sagittal cine phase-contrast MR image demonstrates pulsatile CSF flow in the prepontine cistern and craniocervical junction (arrows). C, An axial phase-contrast MR image demonstrates pulsatile CSF flow at the foramen magnum (arrows). CSF flow velocity can be measured using dedicated software.
Ultrasound
Ultrasound of the head is a useful initial examination for evaluation of macrocephaly in infants if the anterior fontanelle is open. Transcranial Doppler techniques may be helpful in identifying infants with raised ICP and may help determine the need for shunt placement. With elevated ICP, arterial flow tends to be reduced during diastole, resulting in elevated pulsatility of arterial flow. Many researchers have demonstrated elevated resistive indices in infants with raised ICP and a subsequent decrease on ventricular tapping. These results, however, have not been uniformly reproducible.8–10
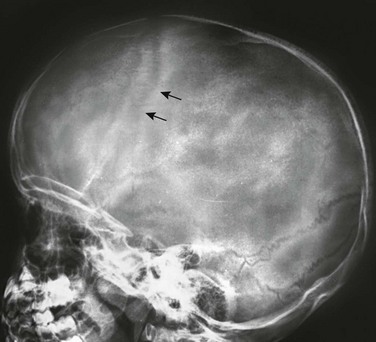
Plain Radiographs
The changes related to elevated ICP on a plain radiograph of the skull depend on the age of the child. In children up to 8 or 10 years of age, sutural diastasis may occur within a few days of elevated pressures (e-Fig. 32-8). After 12 to 13 years of age, sutural diastasis is uncommon early, and the first sign of long-standing hydrocephalus may be erosion of the sellar cortex caused by enlargement of the third ventricular anterior recesses. The anterior part of the base of the dorsum is the earliest to be eroded, but the erosion may spread to involve the sella floor.
Nuclear Medicine Cisternogram
1. The normal opening CSF pressure should be in the range appropriate for the age of the child (infants, 0 to 5 mm Hg; children, 5 to 10 mm Hg; and older children and adults, 10 to 15 mm Hg). Elevated pressures suggest hydrocephalus.
2. The percentage of isotope excretion is normally in the range of 40% to 50%. If the excretion measures below this level, the study result is abnormal.
3. In a normal cisternogram, the tracer enters the basal cisterns, and by 24 hours it is seen over the cerebral convexities, where it is absorbed by the arachnoid granulations. If the tracer enters the ventricular system and persists for more than 24 hours, it is indicative of communicating hydrocephalus.
Etiologies of Hydrocephalus
The common etiologies for ventriculomegaly are listed in Box 32-2.
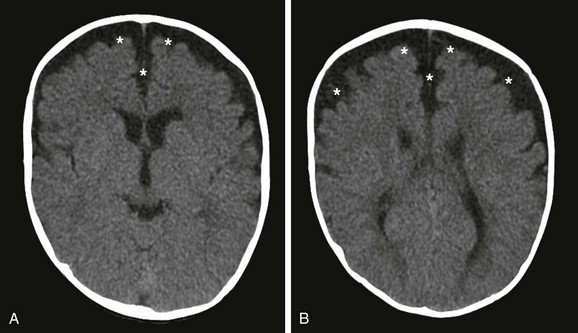
Benign Extraaxial Collections of Infancy
Imaging demonstrates mild prominence of the subarachnoid spaces along the frontoparietal convexities, the cortical sulci, the sylvian fissures, and the anterior interhemispheric fissures (Fig. 32-9). The ventricles typically are normal or mildly enlarged. The extraaxial fluid is most frequently symmetric (but may also be asymmetric), have the same signal intensity as CSF, and have no mass effect. If the fluid collection is of higher attenuation than CSF, is asymmetric, or exerts mass effect on adjacent structures, MRI should be performed to evaluate for blood products from a subdural hematoma. At our institution, we use proton density MRI to distinguish benign extraaxial collections and extraaxial hematomas. Extraaxial hematomas are brighter than CSF on proton density sequences. Both MRI and ultrasound can help differentiate between subarachnoid fluid and a subdural hematoma. With enlarged subarachnoid spaces, the cortical veins course through the fluid and lie adjacent to the inner table of the calvarium. If the subdural space is enlarged, the cortical veins should be displaced away from the inner table toward the cerebral cortex. The signal intensity of chronic subdural hematoma also differs from that of CSF on MRI.11 It has been questioned whether enlarged CSF spaces may make these patients more susceptible to subdural hemorrhage from minor trauma, which occurs in children with arachnoid cysts.
Older Children
Common causes of ventriculomegaly in older children are listed in Box 32-3. Because of the inability of their cranium to expand as quickly as in infants and young children, older children with hydrocephalus have a more acute presentation. They may have the classic triad of headache, vomiting, and lethargy. Children who have chronic hydrocephalus as a result of slowly expanding lesions typically present with persistent morning headaches and intermittent vomiting. Papilledema often is encountered. Focal neurologic deficits from the primary lesion and pyramidal tract signs, which are more marked in the lower extremities, may be present. Hypothalamic-pituitary dysfunction also may develop as a result of compression of these structures by enlarging anterior recesses of the third ventricle.
Assessment of Children with Ventricular Shunts
Shunt Malfunction
Hemorrhage from insertion of the proximal catheter occurs in approximately 1% of patients (Box 32-4). It is even more common when an old catheter is removed. Neuronal injury may result in focal deficits if the catheter traverses the internal capsule. Seizures can occur and are more common with catheters placed through a frontal approach. With lumbar catheters, there is a 5% reported risk of radiculopathy and a 1% risk of myelopathy.
Mechanical Shunt Failure
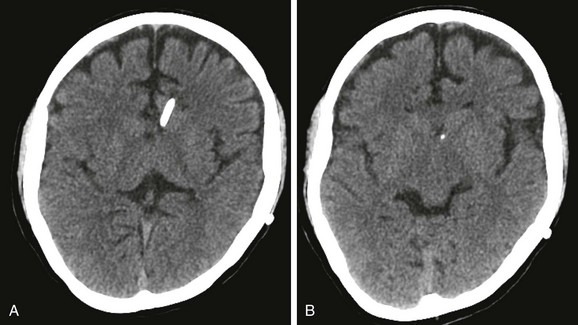
The leading cause of shunt malfunction is mechanical failure. Obstruction is most common at the proximal end and can result from occlusion by brain parenchyma, choroid plexus, a protein plug, or tumor cells.12 Disconnection may occur at any point in the shunt apparatus, but it is most frequent at the site of connection between the valve and the peritoneal catheter and at sites of increased mobility (i.e., the lateral neck) (e-Fig. 32-10). The shunt tubing may migrate to a variety of sites.
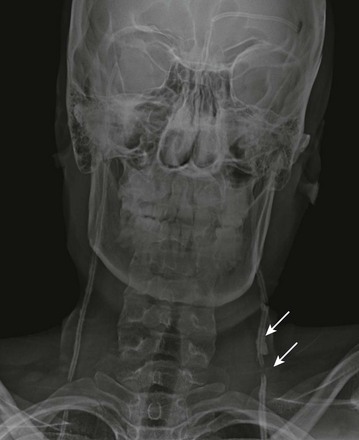
e-Figure 32-10 A broken shunt.
A frontal radiograph of the neck in a patient with new-onset irritation and vomiting shows pericatheter calcification and a break in the distal tubing (arrows).
The distal catheter has its own unique set of complications (Box 32-5), which are best evaluated with abdominal imaging. Pseudocysts may occur at the distal end, with or without infection, causing impairment of CSF absorption.
Chronic shunt placement may alter CSF flow dynamics, resulting in isolated ventricles. An isolated fourth ventricle may occur with shunting of a noncommunicating hydrocephalus. Upon shunting, enlarged lateral ventricles collapse, resulting in obstruction of the aqueduct of Sylvius that becomes irreversible over time. The CSF being produced in the fourth ventricle cannot be drained from above (because of obstruction at the aqueduct) or below (because of the original outlet obstruction), causing progressive dilation of the fourth ventricle (e-Figs. 32-11 and 32-12).
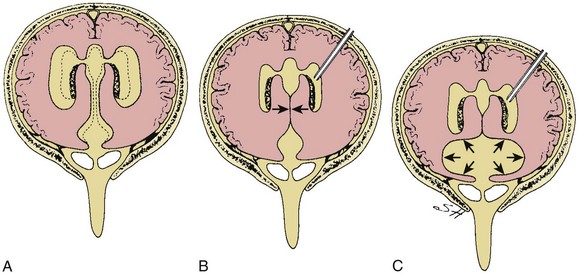
e-Figure 32-11 A diagram of the pathophysiology of the isolated fourth ventricle.
A, Panventricular hydrocephalus with dilation of the aqueduct of Sylvius and blockage of the basal cisterns. B, The shunting of the lateral ventricles leads to collapse of the aqueduct of Sylvius (arrows) that, with time, becomes irreversible. C, Because cerebrospinal fluid is still being produced in the fourth ventricle and cannot be drained from above or below, it causes progressive dilation of the fourth ventricle (arrows). (From Rekate HL. Treatment of hydrocephalus. In: McLone DG, ed. Pediatric neurosurgery: surgery of the developing nervous system. 4th ed. Philadelphia: WB Saunders; 2001.)
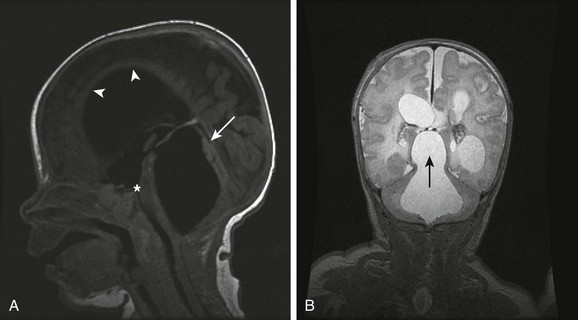
e-Figure 32-12 A trapped fourth ventricle.
A, A midsagittal T1-weighted image in a 12-week-old infant with neonatal ventriculitis and an intraventricular abscess shows marked dilatation and superior herniation of the enlarged fourth ventricle (arrow). Note also the dilated anterior recess of the third ventricle (asterisk) and thinning of the corpus callosum (arrowheads). B, A coronal T2-weighted image shows the fourth ventricular enlargement and supratentorial herniation (arrow) with cystic hydrocephalus as a result of a resolving intraventricular infection.
Evaluation of Shunt Malfunction
Plain radiographs of the entire shunt are obtained with frontal and lateral views of the skull and with frontal views of the chest and abdomen. These radiographs help assess shunt discontinuity or migration. Calcification along the tubing is common in old shunts, which are prone to fracture (see e-Fig. 32-10). Abdominal complications such as mass effect from pseudocyst formation, bowel perforation, and adhesions resulting in bowel obstruction also are evaluated on plain radiographs. If preperitoneal placement of distal tubing is suspected, a lateral radiograph should be obtained.13
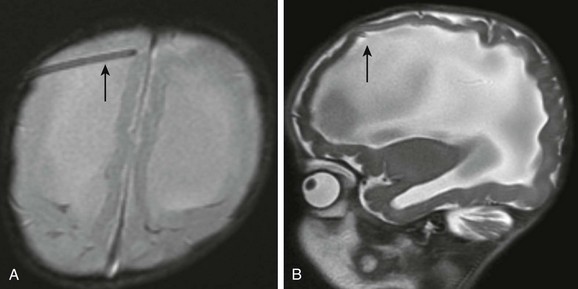
MRI is an alternative to CT for shunt evaluation with limited steady-state gradient-recalled sequence and balanced SSFP sequences (Fig. 32-13). Fast MRI sequences such as single-shot or half-Fourier T2-weighted sequences and T1-weighted spoiled gradient sequences reduce the scan time dramatically, decreasing or eliminating the need for sedation. These sequences provide reliable visualization of the catheter and superior anatomic detail.14
In general, small ventricles in a child with a shunt are good, and large ventricles are bad. Approximately 1% to 5% of patients with very small, or slit, ventricles become symptomatic with acute or chronic headaches, nausea, vomiting, and lethargy. These patients have been lumped together under the term “slit ventricle syndrome” (e-Fig. 32-14). This terminology is confusing because it has been used for multiple clinical entities.15 At one end of the spectrum is a child with small ventricles who is very sick as a result of intracranial hypertension. At the other end of the spectrum is an asymptomatic child with a harmless and inconsequential CT finding.
Endoscopic Third Ventriculostomy
During the past decade, the treatment of hydrocephalus with endoscopic procedures has received renewed enthusiasm. The major attraction is to give children with hydrocephalus freedom from lifelong dependency on external shunts and their numerous inherent complications. Endoscopic third ventriculostomy is most often used for obstructive hydrocephalus, with a success rate of 60% to 70%. The technique involves use of an endoscope to perforate the floor of the third ventricle just anterior to the mammillary bodies, thereby establishing communication between the ventricles and cisterns. Endoscopic fourth ventricular aqueductoplasty, with or without stent placement, has been described as an alternative method when ETV is not feasible.16 This approach is especially useful in patients with a trapped fourth ventricle.
On postoperative imaging (Fig. 32-15), a gradual decrease is seen in the size of the ventricles over months to years. This finding is contrary to imaging with external shunts, when a rapid reduction in the size of the ventricles is seen. The third ventricle responds early and decreases in size over 3 months, whereas the lateral ventricles decrease over 2 years. Thus it is difficult to assess for third ventriculostomy patency on the basis of ventricular size.17
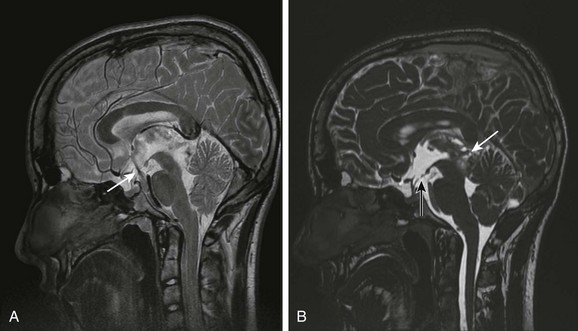
Figure 32-15 Third ventriculostomy.
A, A midsagittal T2-weighted image through the third ventricle at 2-mm section thickness and 512 × 512 matrix demonstrates the flow void through the ventriculostomy in a patient affected by a tectal tumor. B, A midsagittal balanced steady-state free precession image shows the ventriculostomy defect (dark arrow) and the tectal tumor (white arrow).
Phase contrast cine MRI is helpful to assess the patency of the ventriculostomy.18 With use of cardiac gating, phase contrast images can demonstrate the pulsatile flow of CSF through the ventriculostomy and determine flow velocity. A caveat of phase contrast cine MRI is the presence of turbulent or pulsatile flow as a result of third ventricular floor motion (e-Fig. 32-16).
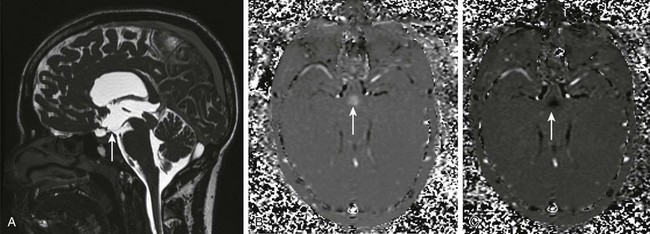
e-Figure 32-16 Failed third ventriculostomy.
A, A midsagittal T2-weighted image through the third ventricle floor demonstrates the absence of the third ventriculostomy defect. B and C, An axial phase contrast image may be deceiving because the signal noted is secondary to the on-off motion of the floor of the third ventricle and not flow of cerebrospinal fluid through the third ventricle floor.
Barkovich AJ, Raybaud C, eds. Pediatric neuroimaging, 5th ed, Philadelphia: Lippincott Williams & Wilkins, 2012.
Blount, JP, Campbell, JA, Haines, SJ. Complications in ventricular cerebrospinal fluid shunting. Neurosurg Clin N Am. 1993;4:633–656.
Li, V. Methods and complications in surgical cerebrospinal fluid shunting. Neurosurg Clin N Am. 2001;12:685–693.
Martin, AE, Gaskill, SJ. Cerebrospinal fluid shunts: complications and results. In Cheek WR, ed.: Pediatric neurosurgery: surgery of the developing nervous system, 3rd ed, Philadelphia: WB Saunders, 1994.
Maytal, J, Alvarez, LA, Elkin, CM, et al. External hydrocephalus: radiologic spectrum and differentiation from cerebral atrophy. AJR Am J Roentgenol. 1987;148:1223–1230.
Schmidek HH, ed. Schmidek & Sweet operative neurosurgical techniques: indications, methods and results, 4th ed, Philadelphia: WB Saunders, 2000.
References
1. Chi, JH, Fullerton, HJ, Gupta, N. Time trends and demographics of deaths from congenital hydrocephalus in children in the United States: National Center for Health Statistics data, 1979 to 1998. J Neurosurg. 2005;103:113–118.
2. Bergsneider, M. Evolving concepts of cerebrospinal fluid physiology. Neurosurg Clin N Am. 2001;36:631–638.
3. Greitz, D, Greitz, T, Hindmarsh, T. A new view on the CSF circulation with the potential for pharmacological treatment of childhood hydrocephalus. Acta Pediatr. 1997;86:125–132.
4. Papaiconomou, C, Zakharov, A, Azizi, N, et al. Reassessment of the pathways responsible for cerebrospinal fluid absorption in the neonate. Childs Nerv Syst. 2004;20:29–36.
5. El Gammal, TE, Allen, MB, Jr., Brooks, BS, et al. MR evaluation of hydrocephalus. Am J Roentgenol. 1987;149:807–813.
6. Heinz, ER, Ward, A, Drayer, BP, et al. Distinction between obstructive and atrophic dilatation of ventricles in children. J Comput Assist Tomogr. 1980;4:320–325.
7. Naidich, T, McLone, D, Hahn, Y, et al. Atrial diverticula in severe hydrocephalus. AJNR Am J Neuroradiol. 1982;3:257–266.
8. Chadduck, WM, Seibert, JJ, Adametz, J, et al. Cranial Doppler ultrasonography correlates with criteria for ventriculoperitoneal shunting. Surg Neurol. 1989;31:122–128.
9. Goh, D, Minns, RA, Pye, SD, et al. Cerebral blood flow velocity changes after ventricular taps and ventriculoperitoneal shunting. Childs Nerv Syst. 1991;7:452.
10. Taylor, GA, Madsen, JR. Hemodynamic response to fontanelle compression in neonatal hydrocephalus: correlation with intracranial pressure and need for shunt placement. Radiology. 1996;201:685–689.
11. Wilms, G, Vanderschueren, G, Demaerel, PH, et al. CT and MR in infants with pericerebral collections and macrocephaly: benign enlargement of the subarachnoid spaces versus subdural collections. AJNR Am J Neuroradiol. 1993;14:855–860.
12. Di Rocco, C, Marchese, E, Verladi, F. A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus: cooperative survey of the 1991–1992 Education Committee on the ISPN. Childs Nerv Syst. 1994;10:321–327.
13. Iskandar, BJ, Sansone, JM, Medow, J, et al. The use of quick magnetic resonance imaging in the evaluation of shunt-treated hydrocephalus. J Neurosurg. 2004;101:147–151.
14. Desai, KR, Babb, JS, Amodio, JB. The utility of the plain radiograph “shunt series” in the evaluation of suspected ventriculoperitoneal shunt failure in pediatric patients. Pediatr Radiol. 2007;37:452–456.
15. Bruce, DA, Weprin, B. The slit ventricle syndrome. Neurosurg Clin N Am. 2001;12:709–717.
16. Sansone, JM, Iskandar, BJ. Endoscopic cerebral aqueductoplasty: a trans- fourth ventricle approach. J Neurosurg. 2005;103:388–392.
17. Schwartz, TH, Yoon, SS, Cutruzzola, FW, et al. Third ventriculostomy: postoperative ventricular size and outcome. Minim Invasive Neurosurg. 1996;39:122–129.
18. Lev, S, Bhadelia, RA, Estin, D, et al. Functional analysis of third ventriculostomy patency with phase-contrast MRI velocity measurements. Neuroradiology. 1997;39:175–179.