152 Hydrocarbons
• Many hydrocarbons possess a characteristic odor similar to that of gasoline or lighter fluid. Solvents containing aldehyde or ketone groups smell sweet or fruity, and essential oils are characteristically pungent or aromatic. In acutely intoxicated patients, these odors are rarely missed.
• In assessing the patient with possible hydrocarbon exposure, important tasks are as follows:
• Many hydrocarbons are acutely cardiotoxic and have a propensity to induce tachyarrhythmias by sensitizing the myocardium to the arrhythmogenic effects of catecholamines.
• Gaseous or volatilized hydrocarbons are likely to cause toxicity through inhalation.
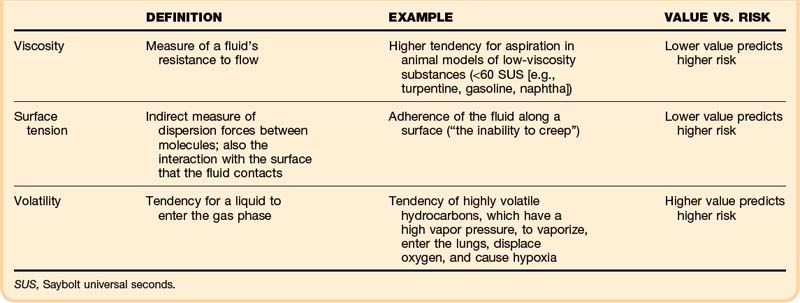
• Viscosity, surface tension, and volatility determine the aspiration potential and the risk of pulmonary toxicity.
Pathophysiology
Lipid-soluble solvents (aromatic, aliphatic, or halogenated hydrocarbons) are more likely than water-soluble hydrocarbons (alcohols, ketones, or esters) to cause acute central nervous system (CNS) effects. Clinicians are familiar with these effects from experience with inhaled anesthetic agents, which cause CNS sedation similar to that resulting from other hydrocarbons. The Meyer-Overton hypothesis suggests that inhaled anesthetics dissolve into some critical lipid compartment of the CNS and cause generalized inhibition of neuronal transmission. This mechanism is probably oversimplified, but it helps to explain partly the nonspecific inhibition of neuronal transmission that hydrocarbons produce in the CNS. Specific membrane interactions may also contribute to this process,1 and several receptor-mediated interactions are known to occur.
Specific physical properties of ingested hydrocarbons help to predict the risk of pulmonary aspiration (Table 152.1). In particular, viscosity, surface tension, and volatility determine aspiration potential and the contribution to pulmonary toxicity.2 Viscosity is a measure of a fluid’s resistance to flow, commonly described in units of Saybolt universal seconds (SUS). This property is not the same as the fluid’s density; in fact, these two properties correlate poorly.
Surface tension indirectly measures dispersion forces between molecules in a fluid, but it also characterizes the interaction with the surface that the fluid contacts. This property can be quantified on a modified Wilhelmy balance, which measures adherence of the fluid along a surface (“the inability to creep”). In theory, the lower the surface tension is, the higher is the aspiration risk.2 A lower surface tension value predicts a higher risk.
Mechanisms of Toxicity
Although organ-specific pathophysiology is often unique to individual agents, much of the toxicity of hydrocarbons results from their ability to dissolve fats or, similarly, to diffuse across hydrophobic barriers intended to protect anatomic structures (e.g., lipid bilayers, myelin). Hydrocarbon solvents cause irritation of skin and mucous membranes. Recurrent or prolonged contact results in “defatting” of skin, dissolving lipid components, and disrupting the normal architecture of the stratum corneum.3
Skin Effects
The skin is a common site of contact and a potential portal of entry for hydrocarbons. Skin is composed of both hydrophilic and hydrophobic elements. Agents that contain both hydrophobic and hydrophilic regions (glycol ethers, dimethylformamide, dimethylsulfoxide) are highly absorbed. Dermal absorption usually constitutes a small fraction of the hydrocarbon dose absorbed by other routes (e.g., inhalation), however. The absorbed dose depends on the surface area exposed, the duration of contact, and skin’s integrity (e.g., cut, abraded).5
Pulmonary Effects
Severe hydrocarbon pneumonitis also results from intravenous injection of a hydrocarbon. In animals, intravascular hydrocarbons injure the first capillary bed encountered. The clinical course after intravenous hydrocarbon exposure mirrors that of aspiration injury.5
Cardiac Effects
Endogenous or exogenous catecholamines (e.g., epinephrine) are proarrhythmic. Hydrocarbons enhance this potential and are said to sensitize the myocardium to the arrhythmogenic effects of catecholamines. Essentially every class of hydrocarbon compounds, including general anesthetic agents, can sensitize the heart. Some classes carry a high risk, however, and others sensitize the myocardium modestly, if at all. The ability of these substances to sensitize the heart constitutes an accepted system for grading halocarbon (e.g., Freon) toxicity. Unsaturated, aliphatic hydrocarbons (e.g., ethylene) and aliphatic ethers have been studied but do not appear to be sensitizers. Other unsaturated compounds (e.g., acetylene), are weak sensitizers. Aromatic hydrocarbons and, especially, halogenated hydrocarbons are often potent sensitizers.6
Sensitization appears to be mediated by slowed conduction velocity, possibly by chemical and functional changes in the membrane transport proteins at gap junctions. The major ventricular gap junction protein is composed of connexin 43. This protein is regulated by phosphorylation, such that the dephosphorylated state of the hexamers in the channel is associated with greater gap junction resistance. In the presence of epinephrine, halocarbons increase gap junction resistance in myocardial tissue and slow conduction velocity.7
Nervous System Effects
The mechanism by which hydrocarbons depress consciousness is unknown. Diffusion across the blood-brain barrier with neuronal membrane stabilization provides the foundation of the Meyer-Overton hypothesis. To date, no specific receptor wholly explains this generalized effect. In cases of pulmonary toxicity, hypoxemia may contribute to depressed consciousness.8
Chronic solvent abuse leads to irreversible CNS toxicity, best described in the setting of toluene abuse. Volitional abusers demonstrate loss of cerebral white matter, with a characteristic syndrome of cognitive and motor deficits. Autopsied brains of long-term toluene abusers show profound atrophy and mottling of the white matter, as though the lipid-based myelin had been dissolved away. Microscopic examination shows a consistent pattern of demyelination, with relative preservation of axons. These pathologic features correlate with the clinical syndrome of subcortical dementia.9 Mild cognitive deficits show improvement after 6 months of abstinence. In patients with advanced disease, regardless of the exposure history, full recovery is unlikely.10
Exposure to n-hexane or to methyl n-butyl ketone (MnBK) can cause peripheral neuropathy. This toxic axonopathy appears to result from 2,5-hexanedione, a metabolic intermediate common to both agents. The mechanism appears to involve decreased phosphorylation of neurofilament proteins, with disruption of the axonal cytoskeleton.11
Radiologically and histopathologically, prolonged and repeated exposures to toluene are associated with brain demyelination. Although the mechanism of this process is not fully understood, it is presumed to result from dissolution of myelin by the solvent. Toluene encephalopathy is characterized by a specific constellation of findings, alternatively described as “subcortical dementia,” “white matter dementia,” or “toxic leukoencephalopathy.” Findings include the following: loss of cortical gray matter–white matter differentiation; atrophic changes in the basal ganglia, cerebellum, pons, and thalamus; and thinning of the corpus callosum. Changes noted on magnetic resonance imaging appear to progress in a lifetime dose–dependent fashion.12 Unfortunately, toluene is an addictive substance, and progression of abnormalities is likely with persistent use. Resolution of neurologic abnormalities has not been documented once white matter loss becomes radiographically evident. Complete recovery from solvent encephalopathy is not considered likely, even with abstinence or removal from exposure.10
Renal Effects
Halogenated hydrocarbons may be nephrotoxic. Examples include chloroform, carbon tetrachloride, and ethylene dichloride. Furthermore, recurrent or prolonged toluene exposure causes distal renal tubular acidosis. Sodium loss in the urine may transform the initial nongap metabolic acidosis into anion gap–positive metabolic acidosis. Toluene’s principal metabolite, hippuric acid, contributes to the elevated anion gap. Renal potassium loss may be severe and can result in symptomatic hypokalemia. In one series, distal renal tubular acidosis was seen in 44% of hospitalized paint sniffers.13
Presenting Signs and Symptoms
The route of exposure considerably influences the organs affected. The principal organ systems affected by hydrocarbons are the skin (from dermal contact), the GI system (when ingested), the CNS, and the lungs. Some classes of hydrocarbons are cardiotoxic. Certain agents may cause organ-specific toxicity to cranial or peripheral nerves, the liver, or the kidneys. Typical presenting symptoms can be found in Table 152.2.
| Symptom | Notes |
|---|---|
| Coughing, choking, or vomiting | Heightens suspicion of pulmonary aspiration |
| Behavioral changes, impaired sense of smell, impaired concentration, and mildly unsteady movements or gait | Transient excitation initially possible after inhalation or ingestion, but early sedation more common |
| Elevated temperature | Initially noted in hydrocarbon aspiration Often spiking at 8 to 12 hours and then declining over several days, unless bacterial superinfection occurs |
| Drying, cracking, pitting, or eczematous lesions | Contact dermatitis or frostbite injury with intentional abuse |
| Muscle weakness | Renal tubular acidosis associated with hypokalemia, with consequent muscle weakness or arrhythmia, possibly the presenting complaint |
| Seizures | Uncommon, probably because of overwhelming anesthetic effects, and should raise suspicion of a coingestant Exceptions: (1) seizures that occur after large ingestion of pine oil or essential oils (e.g., oil of wormwood, fennel oil) and (2) anoxic seizures |
| Persistent hypoxia | Methemoglobinemia, carbon monoxide toxicity, and blood dyscrasias associated with specific ingestions |
| Peripheral neuropathy | Beginning in distal extremities and progressing proximally |
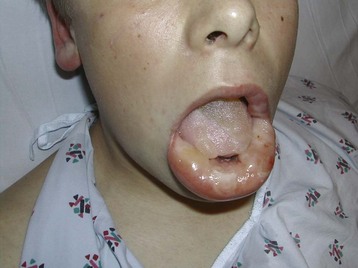
Cutaneous findings may be the chief complaints, with dermal exposure to solvents from work or hobbies. Drying, cracking, pitting, or eczematous lesions occur in up to 9% of workers who are repeatedly exposed. Allergic reactions are uncommon but may be seen with exposure to certain essential oils or to pine oil. Nonspecific skin irritation is the most common finding, and it may progress to blistering or contact dermatitis with recurrent, prolonged, or protracted VSA. With continued or recurring exposure, the skin changes may even progress to partial- or even full-thickness chemical burns.4 When the dermatitis involves the skin surrounding the nose and mouth, it has been dubbed “huffer’s rash” (Fig. 152.1). Abusers of volatile solvents may have telltale paint, shoeshine, or solvent stains on clothes or skin. Nonfreezing cold injury (frostbite) may occur on or about the face because of intentional release of liquid hydrocarbon propellant, which cools as it suddenly exits its container (Fig. 152.2).
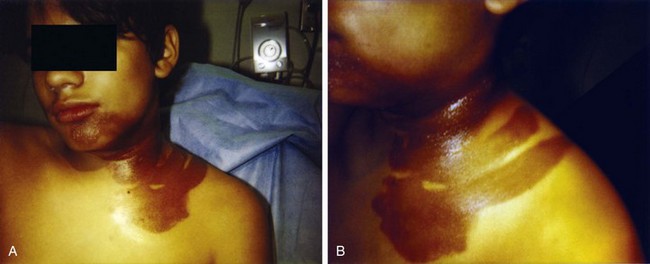
Fig. 152.1 A and B, “Huffer’s rash” resulting from repeated “sniffing” of liquid propane.
(Courtesy of E. O’Connell, MD.)
Hydrocarbons cause CNS depression. Transient excitation may initially occur after inhalation or ingestion, but early sedation is more common. Initial findings include behavioral changes, impaired sense of smell, impaired concentration, and mildly unsteady movements or gait. As with alcohol or other sedatives, mild exposures produce euphoria, likely contributing to abuse potential. Further acute exposure leads to slurred speech and progressive incoordination. Physical signs are nystagmus, tremor, spasticity with hyperreflexia, abnormal plantar reflexes, hearing loss, impaired vision, and a broad-based, staggering gait. Pain inhibition explains why hydrocarbons were chosen as general anesthetic agents. Stupor, lethargy, or obtundation is seen in overdose. Coma and seizures occur in up to 3% of cases.14
A typical case in the emergency department (ED) involves a young child who is suspected to have unintentionally ingested a hydrocarbon mixture. Infrequently, a parent may have witnessed the ingestion; more typically, the situation was discovered shortly thereafter. The caregiver often identifies the specific agent. A parent may report that the child was coughing, gagging, or vomiting, or that he or she noticed an odor of the suspected hydrocarbon. If the ingestion was unwitnessed, it may be difficult to quantify the amount ingested, but a “worst case scenario” can often be ascertained. A history of coughing, choking, or vomiting should heighten the ED’s suspicion of pulmonary aspiration. Respiratory findings include coughing, choking, gagging, grunting, tachypnea, retractions, fever, cyanosis or poor coloration, and abnormal sounds on chest auscultation. Mental status depression is common in patients with larger ingestions but may not occur for 30 to 60 minutes after ingestion. In a prospective, multicenter study of 760 pediatric kerosene ingestions, no association was found between the age of the patient and the amount ingested. The risks of pulmonary toxicity and of CNS depression were significantly higher in children who ingested more than 30 mL of kerosene (according to history). The incidence of pulmonary aspiration was higher in children who vomited after ingestion.15
![]() Red Flags
Red Flags
• History of coughing, choking, or vomiting should heighten the suspicion for pulmonary aspiration.
• Sudden sniffing death syndrome classically occurs after a sudden fright or physical exertion (e.g., running to avoid an authority), with a sudden catecholamine surge and a sensitized heart.
• Abusers of volatile solvents may have telltale paint, shoeshine, or solvent stains on clothes or skin.
• Nonfreezing cold injury (frostbite) may occur on or about the face; it is caused by intentional release of liquid hydrocarbon propellant, which cools as it suddenly exits its container.
• Hypokalemia, with consequent muscle weakness or arrhythmia, may be the presenting complaint in abusers of volatile substances in whom renal tubular acidosis develops from recurrent toluene exposure.
• An adolescent or teen who presents to the emergency department with sudden death or a malignant tachyarrhythmia (especially ventricular tachycardia or ventricular fibrillation) must be considered to have been abusing hydrocarbons unless the emergency physician is able to demonstrate otherwise.
• Hydrocarbons do not generally affect systemic vascular resistance, so coingestants should be considered in the persistently hypotensive patient.
• Hypotension may relate to excessive positive end-expiration pressure (PEEP), and reducing PEEP may improve hemodynamics.
Differential Diagnosis and Medical Decision Making
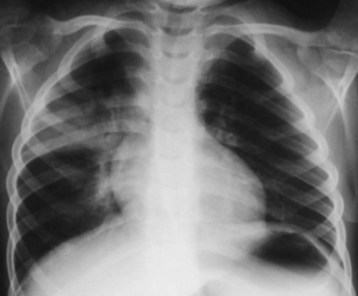
Early chest radiography may be indicated for severely symptomatic patients, to gauge the extent of pulmonary injury and guide the inpatient placement decision. Radiographic evidence of pneumonitis may develop as early as 15 minutes or as late as 24 hours after hydrocarbon aspiration. Ninety percent of patients who have radiographic abnormalities do so within 4 hours.16 After the initial episode of coughing, gagging, or choking, most patients with persistent respiratory signs and symptoms have pneumonitis and radiographic changes. Typical findings in hydrocarbon pneumonitis are audible abnormalities on chest auscultation, fever, leukocytosis, and abnormalities on chest radiograph (Fig. 152.3). These findings do not differ clinically from those of community-acquired pneumonia. Only the history differentiates the two entities. The elevated temperature initially noted in hydrocarbon aspiration often spikes at 8 to 12 hours, then declines over several days, unless bacterial superinfection intervenes. For an asymptomatic patient with hydrocarbon ingestion, however, early radiography does not help predict aspiration pneumonitis, and it is not cost-effective.
Treatment
Decontamination
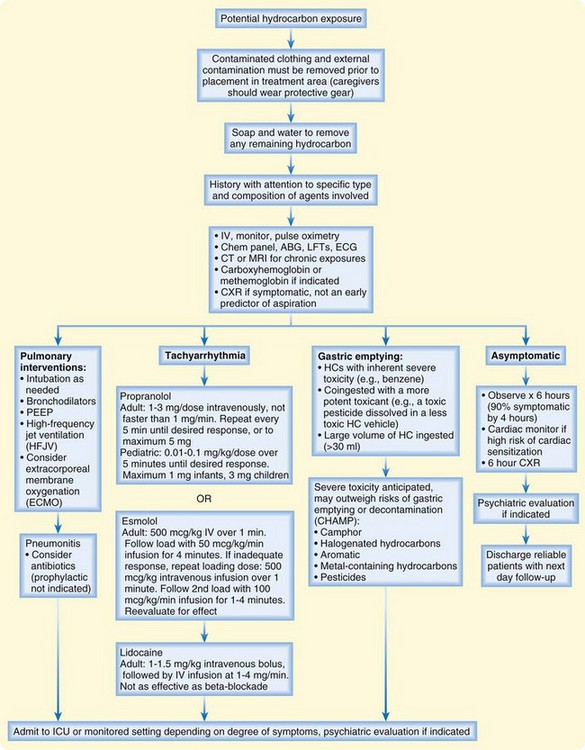
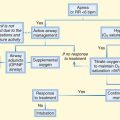
An algorithm for the treatment of hydrocarbon exposure is shown in Figure 152.4. The first priority in managing toxicity is to protect rescuers. Personal protection is paramount at each level of health care delivery. Second, the exposure should be removed from the patient, and the patient should be removed from the exposure. Contaminated clothing and external contamination must be removed before the patient enters any patient care or trafficked area. For most hydrocarbons, soap and water are all that are required for decontamination. Most hydrocarbons are flammable and pose a fire risk to the hospital and staff. Personal protective equipment should be worn by anyone who will touch the patient or any articles brought with the patient. Once the patient is externally decontaminated, standard precautions generally suffice in the ED.
Gastric emptying may be useful when the hydrocarbon is extremely toxic (e.g., carbon tetrachloride), when a large volume of hydrocarbon is ingested (more than 30 mL), or when severe toxicity is predicted (Box 152.1). If gastric lavage is performed, a small-bore (not large-bore) nasogastric tube should be used, to reduce the risk of vomiting and aspiration. Activated charcoal has little ability to reduce GI absorption of hydrocarbons and may cause gastric distention and vomiting. Any role for activated charcoal in isolated hydrocarbon ingestion is limited, at best.
Resuscitation
Dysrhythmias in the setting of hydrocarbon toxicity should prompt investigations into electrolyte and acid-base status, hypoxemia, hypotension, and hypothermia. Ventricular fibrillation is especially concerning because resuscitation algorithms recommend epinephrine to treat this rhythm. If the dysrhythmia can be ascertained to emanate from myocardial sensitization by a solvent, catecholamines should be avoided. Exogenous catecholamines (e.g., epinephrine) should be avoided. In this setting, lidocaine has been used successfully, as have beta-blockers.17,18
Management of hypotension may be precarious. Hydrocarbons do not generally affect systemic vascular resistance, so coingestants should be considered in the persistently hypotensive patient. Hypotension may relate to excessive PEEP, and reducing PEEP may improve hemodynamics. Rarely, some hydrocarbons can cause myocardial depression; in a patient with this finding, an inotrope (but not isoproterenol or dobutamine) should be considered. Pressors such as dopamine, epinephrine, isoproterenol, and norepinephrine must be avoided, if possible, because of the risk of myocardial sensitization.6
Tips and Tricks
• The emergency physician should be aware of the intentional abuse of volatile hydrocarbon inhalants by adolescents and young adults, a growing problem worldwide.
• Hydrocarbons enhance the proarrhythmic potential of endogenous or exogenous catecholamines (e.g., epinephrine) and are said to sensitize the myocardium to the arrhythmogenic effects of catecholamines, so these agents should be avoided in hydrocarbon exposure.
• Mental status depression is common in larger ingestions but may not occur for 30 to 60 minutes after ingestion.
• Typical findings in hydrocarbon pneumonitis are audible abnormalities on chest auscultation, fever, leukocytosis, and abnormalities seen on chest radiograph; these findings do not differ clinically from those of community-acquired pneumonia.
• Volitional abusers demonstrate loss of cerebral white matter, with a characteristic syndrome of cognitive and motor deficits.
• Dysrhythmias in the setting of hydrocarbon toxicity should prompt investigations into electrolyte and acid-base status, hypoxemia, hypotension, and hypothermia.
• The possibility of methemoglobinemia should be evaluated in any patient who remains cyanotic once arterial oxygenation is normalized.
• Patients with hydrocarbon exposure who have no symptoms at home or on initial evaluation generally do not require gastric emptying.
Follow-Up, Next Steps of Care, and Patient Education
Patients who have been observed for 6 hours after an ingestion and who demonstrate no abnormal pulmonary findings, have adequate oxygenation, are not tachypneic, and have normal chest radiography findings have a good prognosis with a very low risk of subsequent deterioration.19 These patients can be safely discharged. Care may be individualized for asymptomatic patients with radiographic abnormalities, as well as for patients who have initial respiratory symptoms but who quickly become asymptomatic during medical evaluation. Reliable patients may be considered for discharge with next-day follow-up.
1 Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222.
2 Gerarde HW. Toxicological studies on hydrocarbons. IX. The aspiration hazard and toxicity of hydrocarbons and hydrocarbon mixtures. Arch Environ Health. 1963;6:329–341.
3 Lupulescu AP, Birmingham DJ. Effect of protective agent against lipid-solvent–induced damages: ultrastructural and scanning electron microscopical study of human epidermis. Arch Environ Health. 1976;31:33–36.
4 Hansbrough JF, Zapata-Sirvent R, Dominic W, et al. Hydrocarbon contact injuries. J Trauma. 1985;25:250–252.
5 Bratton L, Haddon JE. Ingestion of charcoal lighter fluid. J Pediatr. 1975;87:633–636.
6 Brock WJ, Rusch GM, Trochimowicz HJ. Cardiac sensitization: methodology and interpretation in risk assessment. Regul Toxicol Pharmacol. 2003;38:78–90.
7 Jiao Z, De Jesus VR, Iravanian S, et al. A possible mechanism of halocarbon-induced cardiac sensitization arrhythmias. J Mol Cell Cardiol. 2006;41:698–705.
8 Wolfsdorf J. Kerosene intoxication: an experimental approach to the etiology of the CNS manifestations in primates. J Pediatr. 1976;88:1037–1040.
9 Kornfeld M, Moser AB, Moser HW. Solvent vapor abuse leukoencephalopathy: comparison to adrenoleukodystrophy. J Neuropathol Exp Neurol. 1994;53:389–398.
10 Triebig G, Hallermann J. Survey of solvent related chronic encephalopathy as an occupational disease in European countries. Occup Environ Med. 2001;58:575–581.
11 Graham DG. Neurotoxicants and the cytoskeleton. Curr Opin Neurol. 1999;12:733–737.
12 Rosenberg NL, Grigsby J, Dreisbach J, et al. Neuropsychologic impairment and MRI abnormalities associated with chronic solvent abuse. J Toxicol Clin Toxicol. 2002;40:21–34.
13 Voigts A, Kaufman CE. Acidosis and other metabolic abnormalities associated with paint sniffing. South Med J. 1983;76:443–477. 452
14 Kulig K, Rumack B. Hydrocarbon ingestion. Curr Top Emerg Med. 1981;3:1–5.
15 Press E. Cooperative kerosene poisoning study: evaluation of gastric lavage and other factors in the treatment of accidental ingestion of petroleum distillate products. Pediatrics. 1962;29:648–674.
16 Daeschner CW, Blattner RJ, Collins VP. Hydrocarbon pneumonitis. Pediatr Clin North Am. 1957;4:243–253.
17 Moritz F, de La Chapelle A, Bauer F, et al. Esmolol in the treatment of severe arrhythmia after acute trichloroethylene poisoning [letter]. Intensive Care Med. 2000;26:256.
18 Zahedi A, Grant MH, Wong DT. Successful treatment of chloral hydrate cardiac toxicity with propranolol. Am J Emerg Med. 1999;17:490–491.
19 Anas N, Namasonthi V, Ginsburg CM. Criteria for hospitalizing children who have ingested products containing hydrocarbon. JAMA. 1981;246:840–843.