Chapter 68 Hydatid disease of the liver
Overview
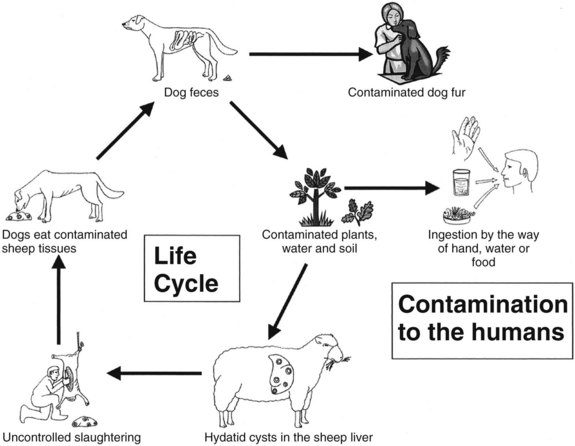
Hydatid disease, or echinococcosis, is a widespread zoonotic parasitic disease caused by a tapeworm that continues to be a clinical and public health problem worldwide, especially in areas where animal husbandry and subsistence farming form an integral part of community life. Hydatidosis infects a large number of wild and domestic animals and humans, and the larval stage of the disease develops into a hydatid cyst. Hydatid disease is most frequently caused by Echinococcus granulosus, and the liver is the most commonly involved organ in two thirds of patients, although it may affect any part of the body, either as a primary or secondary event (Shaw et al, 2006). The life cycle of Echinococcus requires a definitive host, which is often a dog, and an intermediate host, which is commonly sheep. Humans become accidental intermediate hosts when they become infected after ingesting ova passed in dog feces.
Pathogenesis and Etiology
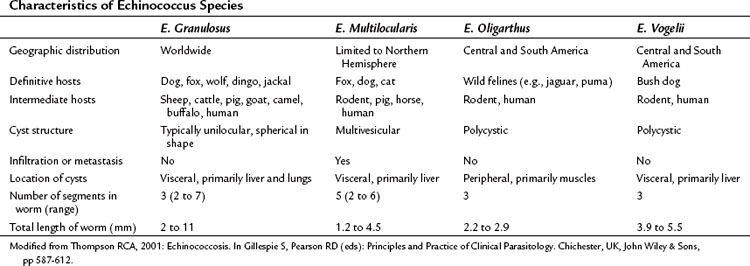
Although 16 species and 13 subspecies were originally described in the genus Echinococcus, molecular epidemiologic studies have led to the recognition of only four clinically important species: E. granulosus, E. multilocularis (E. alveolaris), E. oligarthrus, and E. vogeli (Thompson, 2001). Recently, a new strain, E. shiquicus, has been identified on the Tibetan plateau, but to date no human infection has been described (Shaw et al, 2006). The characteristics of the four Echinococcus species are summarized in Table 68.1. E. granulosus is the most common and is the main focus of this chapter. E. multilocularis, a rare and aggressive form of hydatid disease, is discussed separately at the end of the chapter.
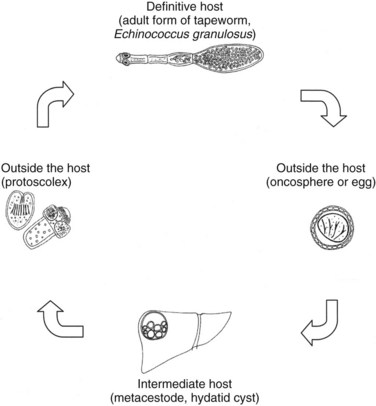
E. granulosus is a small, hermaphroditic tapeworm about 5 mm in length. The tapeworm consists of a head, or scolex, and a body, or strobila, with three or four proglottids (Fig. 68.1). The last one is the largest and bears the mature eggs. The eggs contain a hexacanth embryo that has three pairs of lancet-shaped hooklets. The life cycle of E. granulosus requires two hosts, a carnivore and an herbivore (Fig. 68.2). The adult tapeworm lives in the intestine of the dog, which is the most common definitive host for E. granulosus. Worms release large numbers of infected eggs that pass out in the dog feces and contaminate soil, water, and plants. The eggs are ingested by the intermediate host (humans are accidental intermediate hosts), the eggs hatch, and the embryo migrates through the intestinal wall into the portal system. Most embryos lodge in the liver, mainly in the right lobe because of preferential portal flow; there they develop into hydatid cysts within months to years. Embryos may escape this first filter and lodge in the capillaries of the lung, where they develop. A small percentage of embryos find their way into the systemic circulation and involve other organs, including the spleen, kidney, brain, bone, or any other site. Most patients have single organ involvement. In the liver, the parasite develops into the larval stage, the hydatid cyst, which is filled with fluid and contains hundreds of protoscolices. For the life cycle to be complete, a canine host must ingest the hydatid cyst or its contents, which commonly occurs when infected sheep are slaughtered and organs containing hydatid cysts are fed to dogs (Krige & Beckingham, 2001).
Epidemiology
The disease occurs principally in sheep-grazing areas, especially where dogs are allowed to stray and eat uncooked viscera. Echinococcosis is endemic in many Mediterranean countries, the Middle and Far East, South America, and South and East Africa. The incidence of disease in humans in endemic areas depends on the level of health care and veterinary control. The incidence of human hydatidosis is often established by the number of surgically treated patients. The yearly incidence of human hydatidosis per 100,000 population ranges from 0.4 in Switzerland and Wales to 196 in Turkana in the northwest of Kenya. This high incidence is due to the close relationship the Turkana people have with their dogs: they sleep with them for warmth in the desert nights, and dogs are kept as “nurses” to lick babies clean after they vomit or defecate (Richards, 1992; Watson-Jones & Macpherson, 1988). This domestic intimacy results in many of the population becoming infected when very young (Morris, 1992).
As a result of slow growth, cysts usually become symptomatic a few years after infection, in adolescence or early adulthood. Infected adults may become symptomatic later in life. Host immunity may overcome infection, resulting in a nonviable echinococcal cyst without the person ever becoming symptomatic. Humans are accidental hosts and play little part in the transmission of the disease, making them so-called dead-end hosts, and the disease is not transmitted from human to human (Shaw et al, 2006).
Development of a Hydatid Cyst
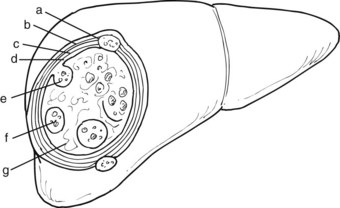
When the parasite reaches the liver parenchyma, it develops into a cystic larval phase, which is visible within 3 weeks and may measure up to 3 cm in diameter after 3 months. The mature E. granulosus cyst consists of three layers: a germinal layer, a laminated layer, and an ectocyst. The inner, germinal layer surrounds the fluid-filled central hydatid cavity and in turn is surrounded by the laminated layer. These two layers together form the endocyst. Compression of the host tissue around the endocyst produces a fibrous layer known as the ectocyst or pericyst (Figure 68.3).
The germinal layer, also called the germinative membrane, is the living component of the parasite. Undifferentiated cells in the germinal layer produce invaginations into the cyst cavity, forming brood capsules that contain protoscolices, which are released into the cyst fluid. The germinal membrane secretes fluid into the cyst and is the source of daughter cysts (Fig. 68.4). The presence of daughter cysts creates multivesicular cysts, which are more common in adults than in children. Daughter cysts have a structure similar to the mother cysts, including a laminated and germinative membrane, cyst fluid, brood capsules, and protoscolices. The only difference is the absence of an adventitial layer (Krige & Beckingham, 2001).
The ectocyst or pericyst is a fibrous capsule that develops from host tissue as an inflammatory reaction to E. granulosus. This thick fibrous layer is present in hydatid cysts in the liver and spleen but is absent in pulmonary and brain hydatid cysts. Vascular structures and bile ducts in the adventitial layer remain intact and patent despite enlargement of the cyst and may result in postoperative bleeding or bile leaks after partial pericystic resection. The blood supply of the adventitial layer is abundant and results in the appearance of a hypervascular rim or halo around the cystic cavity on computed tomography (CT) scans after contrast injection. No clear cleavage planes are apparent between the adventitial layer and the surrounding normal host tissue, and the cyst is not readily separable from the surrounding parenchyma. With time, the adventitial layer may calcify, either partially or totally (Krige & Beckingham, 2001).
An uncomplicated hydatid cyst typically contains a clear, colorless, odorless fluid secreted by the germinal membrane. Sodium, chloride, and bicarbonate concentrations are the same in the fluid as in the patient’s plasma, whereas potassium and calcium levels are lower. In uncomplicated cysts, hydatid fluid is sterile. Bile-stained cyst fluid indicates a cystobiliary communication. When superadded infection is present, the cyst fluid appears frankly purulent; in degenerated cysts, the fluid becomes turbid. Spillage of hydatid fluid content as a result of traumatic or iatrogenic rupture produces implantation of protoscolices and secondary cysts on surrounding viscera, known as secondary hydatidosis (Krige & Beckingham, 2001). Although any segment of the liver can be involved, the location of liver hydatid cysts seems to be related to the respective volume of each lobe of the liver; thus a higher involvement of the right lobe is observed, especially in segments VII and VIII (Kayaalp et al, 2003).
Complications
Compression
As the cyst grows, enlargement tends to occur toward the surface of the liver and Glisson’s capsule, compressing the surrounding parenchyma and leading to compensatory hypertrophy of the remaining liver tissue. Sometimes an entire liver lobe can be replaced by a large cyst without any symptoms. Depending on the location, large cysts can cause compression of the adjacent bile ducts, portal or hepatic veins, or vena cava that causes obstructive jaundice, portal hypertension, or Budd-Chiari syndrome (Moreno-Gonzalez et al, 1994).
Rupture into the Biliary Tract
Intrabiliary rupture is the most common complication of liver hydatid cysts (Iscan & Duren, 1991; Yilmaz & Gokok, 1990). Cystobiliary communications that occur after rupture of a cyst into the bile ducts can be minor or major. Minor communications are usually asymptomatic and are revealed postoperatively by the presence of a bile leak, whereas major communications cause obstructive jaundice and cholangitis (Figure 68.5). In histologic studies of the pericyst wall, numerous biliary ducts of various sizes that communicate with the residual cavity have been demonstrated (Gahukamble et al, 2000), suggesting the existence of biliary communications in most hydatid cysts (Langer et al, 1984). The reported incidence of clinically evident cystobiliary communications rates vary from 2.6% to 28.6% (Langer et al, 1984; Ozmen et al, 1992). In a study by Kayaalp and colleagues (2003), the incidence of cystobiliary communications was 37% and the incidence of clinically apparent biliary leakage was 26%. The incidence of cystobiliary communications depends largely on the criteria used for defining the communications. Morel and colleagues (1988) showed a 36% rate of bile leaks when doing routine intraoperative cholangiography.
Preoperative and intraoperative determination of biliary communication is important. Kayaalp and colleagues (2002) found that the risk of biliary-cyst communication was higher in male patients (40.9% vs. 10.4%; P < .01) and in those with abnormal preoperative serum alkaline phosphatase and γ-glutamyltransferase (GGT); it was also higher in patients with multiple cysts, multilocular (23.8%) and degenerated cysts (24%) compared with unilocular cysts (12.5%); cysts near the biliary bifurcation, and in the presence of bile-stained or purulent cyst contents compared with others (61.9% vs. 2%; P < .001). Although the size of the cyst did not seem to be significant with regard to bile leakage in this study, Atli and colleagues (2001) found that a cyst diameter greater than 10 cm was an independent clinical predictor for the presence of intrabiliary rupture.
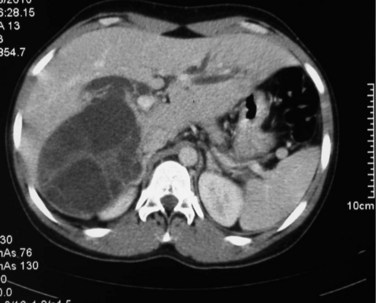
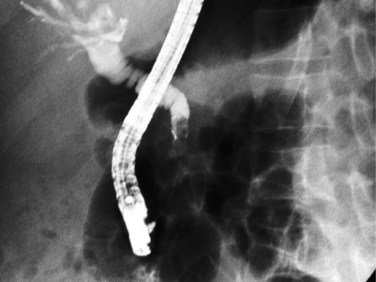
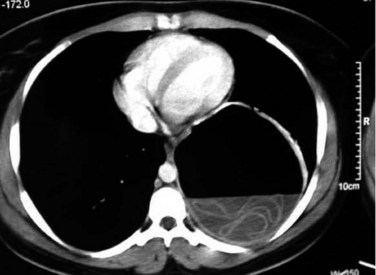
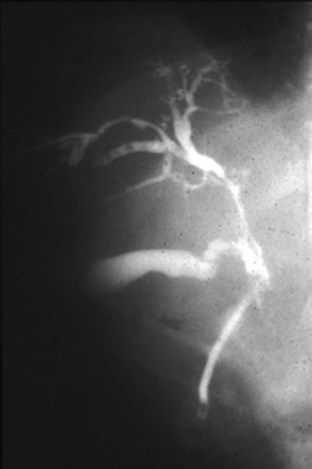
A major biliary communication has been defined as a fistula greater than 5 mm in diameter, a communication between the cyst and the main bile duct, or both (Bourgeon, 1985). The reported incidence of major biliary-cyst communications ranges from 5% to 10% (Zaouche et al, 2001). When large segmental ducts are involved, daughter cysts may enter the bile duct and cause obstructive jaundice or cholangitis or both. Ultrasound (US) and CT scans may show a detached membrane in the cyst cavity associated with dilated intrahepatic and extrahepatic bile ducts (see Fig. 68.5). Endosonography may also detect cystic material in the extrahepatic bile ducts. Endoscopic retrograde cholangiopancreatography (ERCP) is useful to confirm biliary obstruction that results from hydatid material and facilitate treatment with an endoscopic sphincterotomy and extraction of the hydatid debris with a balloon or basket (Fig. 68.6; Ozaslan & Bayraktar, 2002).
Rupture into the Bronchial Tree
A bile leak into large hydatid cysts in segments IVa, VII, and VIII of the liver with secondary infection results in inflammatory adhesions to the diaphragm and the pleurae, which may erode spontaneously into the pleural space, pulmonary parenchyma, and bronchi and lead to a bronchobiliary fistula. This classic presentation is confirmed when the patient reports coughing up “grape skins” (Krige & Beckingham, 2001).
Rupture into the Peritoneum
Intraperitoneal rupture of a hydatid cyst is an uncommon clinical presentation, even in endemic regions, with an incidence ranging from 1% to 8% (Sozuer et al, 2002). Rupture can occur spontaneously. Although this complication may be totally silent (Abdel Hameed & Abu Aisha, 1987), abdominal pain, nausea, vomiting, and urticaria are the most common symptoms, and acute abdominal signs—such as guarding, rebound, and tenderness—are generally present. This complication should be included in the differential diagnosis of an acute abdomen in endemic areas. The release of brood cystic fluid into the peritoneal cavity leads to multiple cysts throughout the peritoneal cavity, ultimately resulting in gross abdominal distension, ascites, and intestinal obstruction. US and CT may be helpful in defining the cyst with a detached membrane and intraabdominal fluid in patients with a ruptured hydatid cyst (Fig. 68.7).
Rupture into Other Cavities or Organs
Rupture into the gastrointestinal tract that involves the stomach and the duodenum has been reported (Diez Valladares et al, 1998). Isolated cases of rupture of liver hydatid cysts into the pericardium (Thameur et al, 2001) and into large vessels, including the inferior vena cava, have also been described (Karunajeewa et al, 2002).
Diagnosis
Symptoms
Small (<5 cm in diameter) and uncomplicated cysts usually are asymptomatic and detected incidentally during a radiologic examination of the upper abdomen or right upper quadrant. The expansion of larger cysts or the inflammatory reaction around a cyst causing irritation of the adjacent parietal peritoneum may cause moderate pain in the right upper quadrant or in the lower chest. Acute abdominal pain usually indicates an infected hydatid cyst or rupture into the peritoneal cavity. When antigenic cyst fluid is released into the circulation, especially after rupture into the peritoneal cavity, a variety of acute allergic manifestations may occur, such as urticaria, anaphylactic attacks, or episodes of asthma (Vuitton, 2004). Extrusion of cyst contents into the biliary tree may lead to absorption of the hydatid antigen in sensitized patients, resulting in similar allergic manifestations (Little, 1976). Clinical features of rupture into the biliary tree are recurrent colicky pain and jaundice, with or without resultant fevers and chills, mimicking obstructing bile duct stones. Bronchobilia resulting from a hepatobronchial fistula and ascites resulting from pressure on hepatic veins or inferior vena cava or both (Budd-Chiari syndrome) are rare clinical presentations.
Laboratory Tests
Even large hydatid cysts of the liver may not alter liver function tests, and transaminase levels are usually normal. Cholestatic enzymes, such as alkaline phosphatase and GGT, can be mildly elevated in about one third of patients, especially in patients with biliary compression (Kayaalp et al, 2002). Elevated bilirubin levels (>1 mg/dL) with elevated alkaline phosphatase and GGT levels are highly suggestive of a cystobiliary communication. White blood cell counts are elevated only if the cyst has become secondarily infected. Eosinophilia (>3%) occurs in 25% to 45% of patients with hydatid cysts in Western countries, but this is a nonspecific finding in endemic areas (Pitt et al, 1986). Serum immunoglobulin levels are elevated in 31% of patients with hydatid liver cysts (Kayaalp et al, 2002).
Radiology
Ultrasound and Computed Tomography
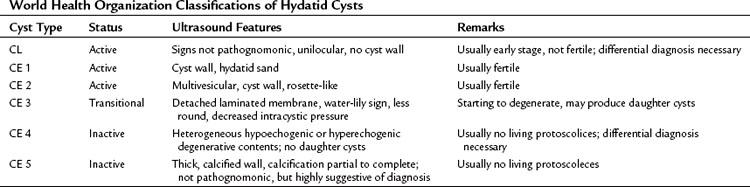
Hydatid cysts appear as well-defined, circumscribed cystic lesions with a clear membrane; they do not infiltrate surrounding liver tissue, and cysts are staged according to the content patterns. Staging is important for using a uniform nomenclature to allow a rational comparison of different management strategies. Although most staging protocols were based on US findings in the past, CT findings can be adapted easily to these systems. The World Health Organization (WHO) Informal Working Group on Echinococcosis (2003) described an ultrasound classification system that intended to follow the natural history of hydatid disease. Based on several studies and classifications, liver hydatid cysts can be divided into six types (Table 68.2; Beggs, 1983; Gharbi et al, 1981; McCorkel & Lewall, 1985):
1 CL type: a well-circumscribed liquid image with a clearly defined wall that is often difficult to differentiate from a simple biliary cyst and corresponds to an early stage of development
2 CE 1 type: a concentric, hyperechogenic halo around the cyst (Fig. 68.8), which may contain free-floating hyperechogenic foci called hydatid sand.
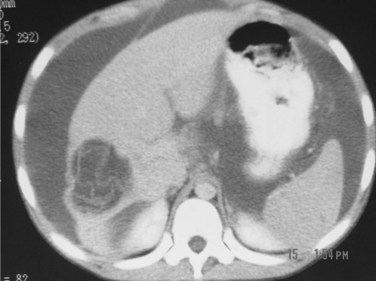
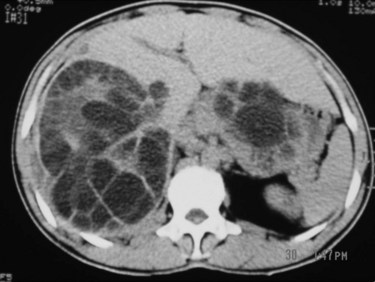
3 CE 2 type: multivesicular cysts that have the most characteristic appearance, with the “daughter” and “granddaughter” cysts identified by honeycomb, rosette, spoked-wheel, or cluster images; can also have some free cyst fluid within the main cavity or may be full of daughter cysts without any free fluid (Fig. 68.9).
4 CE 3 type: partial or total detachment of the laminated layer with floating and undulating hyperechogenic membranes showing the dual wall and “water lily,” and “water snake” signs.
5 CE 4 type: cysts that contain cystic and solid components together without visible daughter cysts.
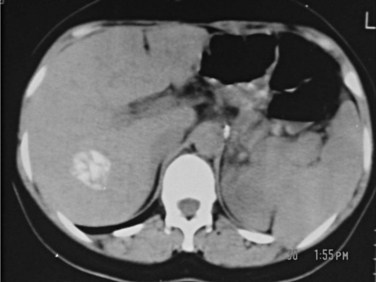
6 CE 5 type: cysts with a matrix or amorphous mass with a solid or semisolid appearance, often a limited amount of calcification in the rim of the host adventitial tissue. This is the least typical of the cysts and may pose a diagnostic problem; they can be mistaken for a tumor, hepatic abscess, or hemangioma. Calcification in the cyst wall and hypoechogenic lacunar structures in the matrix do not indicate that the cyst is dead; a completely calcified cyst (eggshell appearance) is accepted as an effete or dead cyst (Fig. 68.10).
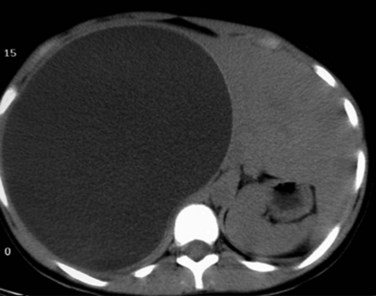
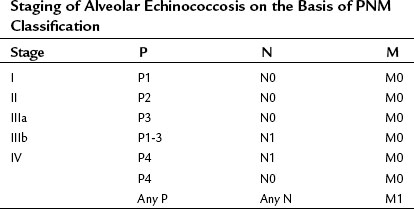
FIGURE 68.8 Computed tomographic scan of a univesicular hydatid cyst shows a single cyst with clear cyst contents.
CL, CE 1, and CE 2 are considered active, fertile cysts. CE 3 is a transitional cyst believed to have begun degeneration. CE 4 is a degenerated cyst and CE 5 is a calcified cyst. The degree of calcification varies from partial to complete. CE 4 and CE 5 are accepted as inactive cysts (Shaw et al, 2006).
Other Imaging Studies
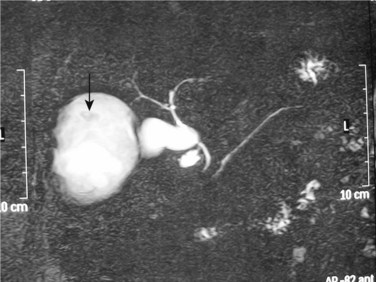
Plain radiography of the abdomen is of limited value in the diagnosis of hydatid liver cysts. A plain radiograph of the chest can reveal concurrent hydatid cysts of the lung. On T2-weighted magnetic resonance imaging (MRI), hydatid liver cysts may have a low signal intensity rim. This is a characteristic sign of hydatid disease that represents the outer, collagen-rich laminated membrane of the cyst. When present, daughter cysts are seen as cystic structures attached to the germinal layer that are hypointense relative to the intracystic fluid on T1-weighted images and hyperintense on T2-weighted images (Pedrosa et al, 2000). MRI is more specific than CT, especially if intracystic fat density is present, which suggests cystobiliary communication (Basaran et al, 2005). In cysts with biliary complications, MR cholangiography can provide good visualization of the intrahepatic and extrahepatic biliary tree and its relationship with the hydatid cyst and cystobiliary communications (Fig. 68.11; Little et al, 2002).
Serology
Immunoelectrophoresis
The diagnostic value of hydatidosis with immunoelectrophoresis ranges from 91% to 94% for hepatic cysts and 69% to 70% for pulmonary cysts (Varela-Diaz et al, 1983). Immunoelectrophoresis is not suitable for epidemiologic surveillance; rather, it is used for posttreatment follow-up.
Enzyme-Linked Immunosorbent Assay
Sensitivities for enzyme-linked immunosorbent assay (ELISA) vary from 64% to 100% depending on the antigens used (Coltorti, 1986; Iacona et al, 1980; Rickard, 1984). ELISA can also be automatized for large-scale epidemiologic studies. Selected test antibodies affect its value on posttreatment follow-up. Immunoglobulin (Ig) G assay may remain positive 4 years after successful treatment, so it is not a suitable test for posttreatment follow-up; IgM assay has been reported to be negative after 6 months of successful treatment (Zhang et al, 2003).
Blotting
Blotting allows molecular weight analysis of the antigens detected by the patient’s serum. Western blotting with purified antigens has proved to be very useful in the diagnosis and postsurgical monitoring of hydatidosis patients (Doiz et al, 2001). The Arc 5 antibody test is a specific precipitation during electrophoresis of blood of hydatid cyst patients, with a specificity of 91%. Purification of antigens strongly affects the diagnostic value of the tests. Purified fractions enriched in antigens 5 and B and in glycoprotein yield a sensitivity of 95% and specificity of 100% (Sbihi et al, 1996).
Treatment Indications and Methods
Three treatment options are currently available for hydatid disease of the liver: surgery, which remains the most efficient treatment and the therapy of choice; percutaneous aspiration; and medical treatment. In general, hydatidosis is a public health problem, especially in developing countries, and the specific treatment selected may depend on social circumstances and the medical expertise available (Shaw et al, 2006).
Since the 1990s, percutaneous treatment has been increasingly used. Surgery has the advantage of removing the parasitic content of the cyst and the cyst wall and dealing with any associated complications. Although surgery may be technically demanding in patients with large and complicated hydatid cysts, advances in liver surgery have made complex operations safer and have reduced morbidity (Krige & Beckingham, 2001).
Conservative Management
Asymptomatic and small (<5 cm) CL-type cysts can be followed up with a wait-and-see policy with serial US examinations (Buttenschoen & Buttenschoen, 2003). Similarly, densely calcified hydatid cysts are accepted as dead cysts and can be monitored without any specific therapy.
Surgical Treatment
The literature on the surgical treatment of hydatid liver disease describes a variety of different techniques, ranging from simple evacuation to major liver resection. These techniques can be divided into two broad groups, conservative and radical. The conservative method involves inactivation of protoscolices and removal of the cyst contents; radical methods include total excision of the cyst and pericyst layers along with a portion of surrounding liver. Although no prospective randomized data compare radical and conservative surgery, most surgeons, especially in endemic areas, prefer conservative surgery, but some experienced liver surgeons may at times use pericystectomy or hepatectomy to deal with liver hydatid cysts. The principles of liver hydatid surgery include inactivation of protoscolices within the cyst fluid, evacuation of the cyst contents and prevention of spillage of the cyst contents, secure closure of any cystobiliary communications, and management of the residual cyst cavity (Terblanche & Krige, 1998).
Prevention of intraoperative spillage of the hydatid cyst contents is an important step during the procedure. Several methods have been described to avoid spillage. First introduced by Saidi and Nazarian (1971) and later by Aarons and Kune (1983), cone techniques allow the cyst to be entered in a safe and controlled manner with a funnel-shaped cone attached to the hydatid surface by suction or by freezing the contact area—although such an approach is not suitable for posterosuperior cysts attached to the diaphragm.
Preoperative Preparation
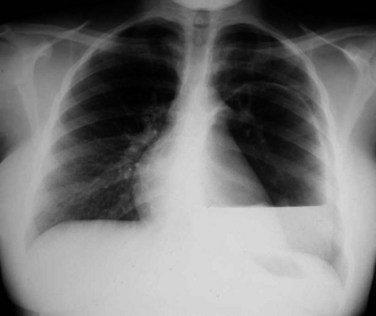
All patients undergoing surgery for hepatic hydatid cysts should have a detailed preoperative assessment that includes a chest radiograph to exclude pulmonary cysts (Figs. 68.12 and 68.13), an electrocardiogram, a complete blood count, international normalized ratio (INR), electrolyte and renal function tests, and liver function tests; in addition, blood should be grouped and screened. A recent CT scan of the abdomen should be reviewed before the operation to plan the surgical strategy and exclude hidden or occult pelvic, retroperitoneal, or hydatid cysts in other organs.
Surgical Incision
The position of the surgical incision depends on the location, size, and number of cysts in the liver and whether other extrahepatic intraabdominal cysts are present. Either a right subcostal incision with proximal midline extension or a bilateral subcostal incision will give adequate exposure to all liver hydatid cysts (Terblanche & Krige, 1998). A midline laparotomy is preferred in patients who have cysts in the left lobe of the liver and in those who have abdominal hydatidosis. Although thoracoabdominal incisions were used in the past for selected patients with large posterior liver hydatid cysts, a transthoracic approach is now used only for combined right lung and liver hydatid cysts, when a one-stage procedure can be done successfully for both cysts (Sahin et al, 2003).
Conservative Surgery
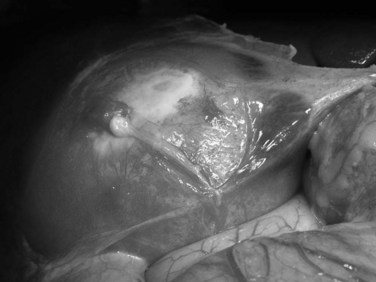
Cystectomy is the safest and simplest surgical approach (Morris, 1992). After entering the abdomen, the skin wound is carefully protected with a plastic drape or a commercially available ring-shaped wound protector. A full laparotomy is performed, paying particular attention to potential sites of dissemination, including the omentum and pelvis (Morris, 1992). The characterisitic shiny white adventitial surface of a hydatid cyst is usually easily identified (Fig. 68.14). The position, size, and number of cysts in the liver are noted, as are the presence of complications and other extrahepatic intraabdominal cysts. It is important to assess the relationship of the cyst to the inferior vena cava, hepatic veins, and porta hepatis structures because large or multiple cysts frequently distort normal liver anatomy. For deeper cysts, palpation and intraoperative US help determine the most superficial part of the cyst suitable for aspiration and evacuation. Mobilization of the liver and the cyst should be minimal to avoid iatrogenic perforation of thin-walled cysts.
The area around the cyst is carefully isolated by gauze packs: the first layer is soaked with normal saline, and the second layer is soaked with a scolicidal solution; these act as a mechanical and a chemical barrier (Besim et al, 1998). An area 2 cm in diameter on the most prominent part of the exposed pericyst is left uncovered by the packs for insertion and evacuation. The cyst should be handled carefully because the contents of a viable cyst are usually under pressure. The point where the cyst is to be entered is identified, and the smallest possible working area is delineated by additional packing. At least two drains with powerful suction should be available, and one should have a sump cannula. The suction reservoir should have a large capacity, so that suction is not interrupted while changing reservoirs.
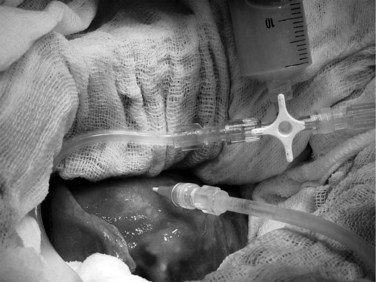
The cyst wall is pierced with a large-gauge needle connected to a 50-mL syringe and a three-way tap, and large-bore transparent plastic tubing is connected to a drain. The cyst is aspirated, and the volume and the color of the hydatid fluid are carefully noted (Fig. 68.15). As much fluid as possible is aspirated. If the cyst fluid is completely clear and not bile stained, turbid, or infected, scolicidal solution can be safely injected as long as the volume injected is less than what has been aspirated; the fluid is injected gently and not under pressure (Terblanche & Krige, 1998). A suction nozzle is kept at the needle puncture site at all times by the assistant to avoid any hydatid cyst fluid leaking out alongside the needle. The scolicidal fluid is left in the cavity for several minutes and then is reaspirated; this process is repeated twice. The cyst is decompressed again by aspiration; when the fluid level is sufficiently lowered, two stay sutures are placed close to the needle with upward traction on the stay sutures, which is maintained to allow the needle to be removed safely without spillage of residual cyst contents.
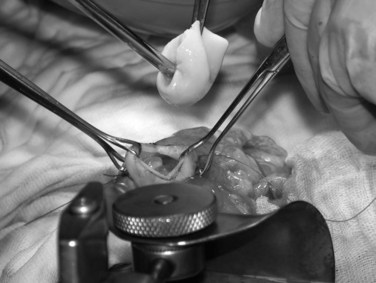
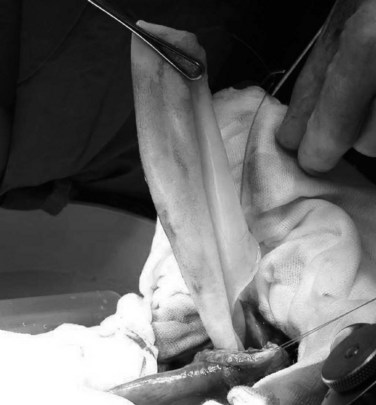
Evaluation of the cyst content is important. The typical content of a viable cyst is clear fluid containing hydatid sand, daughter cysts, and the debris of brood capsules. Bile staining of the fluid implies a communication with the biliary tree and should warn against injection of scolicidal agents that may damage the biliary tree. Once the liquid has been drained, the laminated membrane collapses into the cavity, and the cyst contents can be evacuated. The incision is enlarged further, a kidney dish is brought close to the incision, and the evacuation of the daughter cysts begins (Fig. 68.16). Laminated membrane is best extracted with a ring forceps (Fig. 68.17), and small daughter cysts are best removed with a suction drain.
When all visible daughter cysts have been evacuated, the cavity is rinsed with warm saline, and the redundant portion of the cyst roof is excised. The adventitia and thinned-out liver are cut with electrocautery, and the cut edges are oversewn with a running mattress suture with an absorbable suture material; this is an important component of the operation because the cut edges contain blood vessels and small bile ducts. It is important to inspect the cavity for small daughter cysts that may be hidden in recesses of the main cavity. After removing the laminated membrane, the cavity is flushed with saline and inspected for bile leakage (Fig. 68.18). The cavity is packed with dry, white packs that are left in place for a few minutes and then removed. Bile stains are indicative of a bile communication.
If the aspirated contents are clear, scolicidal solutions are injected into the cyst, but never if the cyst has bile-stained or purulent content, which indicates a bile communication and hence the risk of causing caustic sclerosing cholangitis. Although some debate surrounds the efficacy and safety of protoscolicidal solutions injected into the cyst cavity, many consider injection of scolicidal solutions a prudent step when applying conservative surgery to reduce recurrence. Widely used scolicidal agents include hypertonic saline, fresh half-strength Eusol, 0.5% silver nitrate, and hydrogen peroxide. Hypertonic saline has been widely used in the past because of its availability and effective scolicidal properties (Kayaalp et al, 1999). Studies on different concentrations of hypertonic saline, ranging from 3% to 30%, have shown that the higher the concentration, the higher the scolicidal properties with a shorter contact time. In practice, 20% hypertonic saline is preferable, which has 100% scolicidal effect with an ideal contact time of 6 minutes (Besim et al, 1998). A danger of this practice is excessive absorption, which may result in hypernatremia, and the solution should be used with caution (Krige et al, 2002). A cetrimide (0.5%) and chlorhexidine (0.05%) combination (Savlex; Drogsan, Ankara, Turkey) used for 5 minutes is an effective scolicidal solution in laboratory and clinical studies (Besim et al, 1998). However, metabolic acidosis and methemoglobinemia have been reported as side effects (Baraka et al, 1980; Momblano et al, 1984).
Absolute alcohol has been used by interventional radiologists for the percutaneous treatment of hydatid cysts to produce sclerosis of the cyst cavity (Akhan & Ozmen, 1999), but it is not effective on protoscolices inside daughter cysts (Karayalcin et al, 1999). Disadvantages of absolute alcohol are the potential danger of caustic cholangitis and its flammable nature during surgical procedures when using electrocautery.
Povidone-iodine has been criticized because of an inappropriate animal model (Kolbakir & Erk, 1992). Gokce and colleagues (1991) reported that 1% povidone-iodine was superior to 20% saline. Landa Garcia and associates (1997) reported that 10% povidone-iodine was an effective agent for prevention of secondary hydatidosis in their in vivo study. The disadvantages of povidone–iodine are concentration dependency (Besim et al, 1998), induction of caustic cholangitis (Castellano et al, 1994), and coloring of the cyst cavity, which makes it difficult to identify the cystobiliary communications. Formalin is no longer used because of a high propensity to induce caustic sclerosing cholangitis. If a possible bile leak is suspected, the cyst is carefully packed off and emptied by aspiration, and the contents are removed without injecting any scolicidal solutions to avoid the danger of inducing caustic sclerosing cholangitis (Shaw et al 2006).
Intraoperative Management of Biliary-Cyst Communication
Management of the Residual Cavity
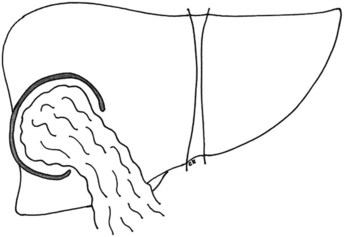
Although a variety of techniques have been described to prevent complications related to the residual cavity, depending on its size and shape and site, the safest way is to perform an omentoplasty. The omentum is mobilized from the transverse colon with sufficient mobility to pack the cavity and obliterate the dead space, and it is sutured to tether the omentum in place (Fig. 68.19). If the cavity has a large volume, a temporary drain is placed into the cavity alongside the omentum. Some put in two drains (Shaw et al, 2006). A closed, silastic suction drainage system is preferable; the tubes are removed as soon as drainage ceases. Omentum has a natural absorptive capacity that decreases the risk of infection and minimizes fistula formation. Older techniques, including capsulorrhaphy and capitonnage, are no longer used.
Postoperative Complications
Biliary Fistula
The incidence of biliary fistula after hydatid liver surgery varies from 1% to 10% (Abu Zeid et al, 1998; Barros, 1978). Endoscopic treatment is the main approach, and the aim of endoscopic drainage for biliary fistulas is to reduce the bilioduodenal pressure difference to 0. The optimal endoscopic approach for managing external biliary fistulas resulting from hydatid liver disease has not been established. Sphincterotomy alone, stent or nasobiliary drain placement alone, and the combination of sphincterotomy and stenting or nasobiliary drainage have been used successfully for fistula healing. The overall success rate is 83.3% to 100%. Although closure time has been reported to be as short as 2 to 6 days, average duration of bile drainage after stent placement is generally 2 to 4 weeks (Ozaslan & Bayraktar, 2002; Simsek et al, 2003).
Biliary Stricture
Postoperative biliary strictures after surgical treatment of hepatic hydatid disease are uncommon. The most dramatic form is caustic sclerosing cholangitis, which is caused by the scolicidal solution entering the biliary tree from the cyst cavity (Belghiti et al, 1986). Diffuse caustic sclerosing cholangitis may result in secondary biliary cirrhosis, portal hypertension, and liver decompensation with ascites and bleeding esophageal varices, which may ultimately require liver transplantation (Loinaz et al, 2001). Minor passage of the scolicidal solution may cause a localized biliary stricture, which may be asymptomatic if the bile duct confluence is not involved (Belghiti et al, 1986). A bile duct stricture at the biliary confluence may result from a large biliary fistula treated by conservative surgery. Surgical repair is usually not feasible (Fig. 68.20), but long-term endoscopic stenting is a safe and effective method in these patients (Eickhoff et al, 2003; Yilmaz et al, 1998).
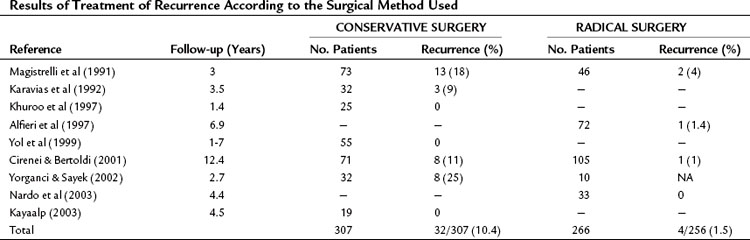
Recurrence
Recurrent disease is defined as the appearance of new active cysts after therapy of intrahepatic or extrahepatic disease (Sielaff et al, 2001). Failure to achieve permanent control of the primarily treated cyst is considered local recurrence, and the appearance of new cysts in the peritoneal cavity is regarded as disseminated disease. The incidence of local recurrence is approximately 10% after conservative surgery (Table 68.3). Intraoperative spillage of cyst contents, reduced effect of protoscolicidal agents, residual cyst content, and overlooked cysts lead to recurrence. All patients initially require close follow-up at 6-month intervals. Evaluation should include radiologic and serologic studies; complement fixation test, immunoelectrophoresis, counterimmunoelectrophoresis, ELISA, and blotting are used to detect recurrences. Even with complete removal of disease, blood titers may decrease slowly over months to years; therefore a positive serologic test during follow-up is not diagnostic of recurrence, but a rising titer is (Sielaff et al, 2001).
Pericystectomy
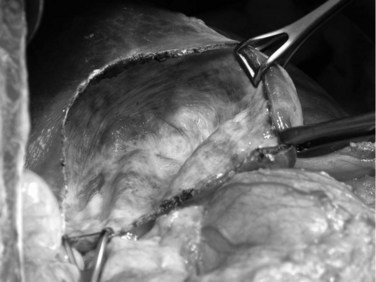
Also called radical cystectomy, capsulectomy, total pericystectomy, and cystopericystectomy, pericystectomy involves complete removal of the hydatid cyst. By creating a surgical plane just outside the pericyst layer without opening the cyst, the parasite and the adventitial layer are excised en bloc (Fig. 68.21). No clear anatomic plane exists, although the surgical approach of this plane does not differ from a classic liver parenchymal transection. The Cavitron ultrasonic aspirator (DentSply International, York, PA) is used to isolate the vessels and biliary ducts that are deviated and compressed by the cyst, and the parenchymal transection allows the suture of these vessels and bile ducts within the liver parenchyma. The aspirator should be used away from the pericyst to avoid fracture of the cyst, which can be responsible for spillage of the cyst contents.
Liver Resection
The indications for a formal hepatic resection for liver hydatid cysts are infrequent. Hepatic resection is the only surgical therapy for E. multilocularis, but it is inappropriately radical for E. granulosus (Morris, 1992). Other rare indications for liver resection are when the remaining parenchyma of a liver lobe is atrophic as a result of biliary obstruction, or when a large bile leak that cannot be safely managed with a Roux loop is present. Resection should be reserved for peripherally placed cysts, usually in the left lateral segment; for pedunculated lesions; or for extrahepatic intraabdominal cysts. Resection of small, pedunculated, and peripherally placed cysts is simple and safe, but in the majority of cases, cystectomy involves a major liver resection with its attendant increase in operative risk. Correct judgement is crucial because the operation may be complex as a result of distorted anatomy.
Radical surgery should not be standard procedure in the management of E. granulosus (Terblanche & Krige, 1998). In addition, a standard hepatic resection should not be done in patients with large cysts that occupy the greater portion of the right lobe because the medial wall of such a cyst often involves the inferior vena cava and displaces the hepatic veins. Attempts to dissect outside the cyst are hazardous and unnecessary. Surgery for hydatid disease is done predominantly by general surgeons, frequently with limited resources. Surgeons who do not have extensive training and experience in liver resections should not be tempted to resect for hydatid disease. Meticulous and careful conservative surgery for this benign disease gives good results, and unnecessary operative mortality will certainly outweigh the merits of totally removing the cyst.
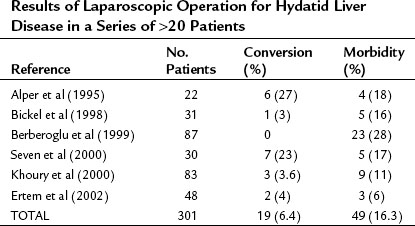
Laparoscopy
Although the laparoscopic approach to this disease offers some advantages, laparoscopic hydatid surgery has not gained a wide acceptance. Disadvantages of the laparoscopic approach are the limited area for manipulation, intricacy in controlling spillage during puncture, and difficulty in aspirating the thick, degenerated cyst contents. No prospective randomized clinical trials have compared laparoscopic treatment with conventional open treatment, and no reliable data are available on recurrence rates after laparoscopic treatment (Table 68.4).
Theoretically, a pneumoperitoneum can exert pressure on the hydatid cyst and increase the risk of hydatid fluid contamination during laparoscopic intervention. Bickel and colleagues (1998) examined the risk of spillage and found that laparoscopy had no disadvantages in the treatment of hydatid liver cysts. Berberoglu and colleagues (1999) used gasless laparoscopy for hydatid cyst surgery but found no significant advantage over the pneumoperitoneum method.
Some groups have reported the use of a perforator-grinder and aspirator apparatus for insertion and evacuation (Alper et al, 1995; Saglam, 1996). An umbrella-shaped laparoscopic trocar for evacuation of the cysts has been used with a locking mechanism that enables the surgeon to suspend the cyst wall against the abdominal wall (Seven et al, 2000). Bickel and Eitan (1995) reported the use of a large, transparent cannula (12 mm) with a beveled tip for safe laparoscopic management of hydatid cysts of the liver. Suction is applied through the cannula onto the surface of the cyst, enabling oblique contact, and the cyst is aspirated through the cannula under a “vacuum.” It is claimed that spillage was less with this method (Bickel & Eitan, 1995). Some investigators recommend washing the peritoneum with a scolicidal solution or creating a perihepatic scolicidal pool during laparoscopic hydatid surgery (Berberoglu et al, 1999; Palacios-Ruiz et al, 2002).
Percutaneous Treatment
Percutaneous aspiration and treatment of hydatid cysts has previously been discouraged because of the risk of anaphylaxis and intraperitoneal seeding. However, unintended percutaneous aspiration in sporadic cases were not associated with the expected side effects (McCorkell, 1984). These findings encouraged interventional radiologists to aspirate and treat hydatid cysts of the liver using US guidance. Mueller and associates (1985) were the first to report successful percutaneous drainage of hydatid cysts; since then, several authors have reported successful percutaneous treatment of hydatid liver cysts. With increased experience, successful percutaneous treatment of hydatid cysts for lung, kidney, peritoneum, and orbital cavity have also been reported (Akhan & Ozmen, 1999).
Three different techniques are used in percutaneous treatment of liver hydatid cysts. The first is the PAIR technique described by Ben Amor and associates (1986). PAIR is an acronym for puncture, aspiration of cyst content, injection of protoscolicidal solution, and reaspiration of the fluid. Patients are given albendazole before and after the procedure for prophylaxis. Under local anesthesia, a fine needle is inserted into the cystic cavity through normal liver tissue with US or CT guidance. As much fluid as possible is aspirated and, on completion, a protoscolicidal agent is injected into the cavity. After 15 minutes, as much fluid as possible is reaspirated and the needle is withdrawn. The most characteristic sonographic signs of involution at follow-up are heterogeneous reflections of cyst content (3 months), obliteration and pseudotumor appearance (5 months), and loss of echogenicity and disappearance of the cyst (9 months) (Bret et al, 1988; Khuroo et al, 1997). Direct microscopic examination of the aspirated fluid is used to identify protoscolices (Pelaez et al, 2000).
The second technique is a catheterization technique described by Akhan and associates (1993). A catheter is placed into the cavity by the Seldinger technique and, similar to the PAIR technique, the cyst is aspirated, injected with a protoscolicidal agent, and reaspirated, but the catheter is not removed at the end of the procedure and is instead left to facilitate drainage for 24 hours. If there is no biliary fistula within 24 hours, it is accepted that there is no communication between the biliary system and the cavity, which is also confirmed by obtaining a cystogram under fluoroscopic guidance. If the amount of drainage in 24 hours is less than 10 mL and free of bile, absolute alcohol (95%) is injected into the cavity (approximately 25% to 35% of the volume); after waiting 20 minutes, all the fluid is reaspirated and the catheter is withdrawn. If the amount of drainage in 24 hours is more than 10 mL or contains bile, the catheter is kept in place until the daily amount of drainage decreases to less than 10 mL. After that, the cystogram and sclerosis are performed as already mentioned. Alcohol instillation in cysts that have biliary communications is contraindicated. Some researchers suggest that all the liver hydatid cysts suitable for percutaneous technique should be treated by the PAIR technique. Others suggest that the cysts smaller than 6 cm in diameter (volume <100 mL) should be treated by the PAIR technique, and cysts larger than 6 cm in diameter (volume >100 mL) should be treated by the catheterization technique (Akhan & Ozmen, 1999).
Saremi and McNamara (1995) developed an alternative method called percutaneous evacuation of cyst content (PEVAC). As in the PAIR catheterization technique, the cyst is first aspirated as much as possible and the catheter is left in place for drainage. In a second session, the catheter is replaced with a 14- to 18-Fr stiff sheath. A suction catheter is introduced into the cyst cavity through the sheath, and the cyst contents are evacuated by applying suction and directing the catheter toward the daughter cysts, endocyst, and undrainable material. A special cutting instrument is used to fragment and evacuate daughter cysts and laminated membrane, while the cavity is continuously irrigated with a protoscolicidal solution. After removal of the sheath, a catheter of the same size as the sheath is placed into the cavity, similar to the PAIR catheterization technique (Schipper et al, 2002). In the absence of bile leakage or any discharge, the catheter is removed.
The effectiveness and safety of percutaneous treatment are supported by the results of more than 2500 procedures carried out in several countries by different teams, with a low morbidity (4.1%) and mortality (0.08%) (Filice et al, 1999). The major risk of percutaneous drainage of hydatid cysts is spillage of hydatid fluid during the placement of the needle. With US or CT guidance, the position of the needle can be precisely monitored, and a transhepatic approach to the cyst, rather than a direct transperitoneal approach, can be used to minimize the possibility of spillage. Urticaria, itching, and hypotension are minor complications that may occur during or several hours after the procedure; these can be treated with antihistamines. In some patients, fever (>38.5° C) may occur, but this generally resolves spontaneously. Cavity complications, such as biliary fistula and infections, have been reported in 10% of patients (Akhan & Ozmen, 1999).
Chemotherapy
Benzimidazole carbamates (mebendazole and albendazole) are antihelmintic drugs that kill the parasite by impairing its glucose uptake. The first report of the successful treatment of four patients with hydatid liver cysts was in 1977 (Bekhti et al, 1977). Mebendazole was introduced first, but albendazole became the drug of choice because of its superior absorption in the gastrointestinal tract and better clinical results (Morris, 1992; Saimot et al, 2001). Benzimidazole treatment is contraindicated for patients with chronic liver diseases and in early pregnancy (Saimot, 2001). Albendazole absorption varies from person to person, and variability may be substantial even in the same individual. In clinical practice, albendazole should be administered in a dose of 10 mg/kg twice daily with a meal; it is wise not to give albendazole with medication that reduces gastric acidity. Adverse events including headache, nausea, anorexia, vomiting, abdominal pain, and itching have been reported in 5% to 10% of patients (Schipper et al, 2000). In the first weeks of treatment, a transient increase in liver enzymes may occur, and leukopenia is rare. Complete hair loss, which is reversible when albendazole is stopped, may also occur.
Although most studies were not well designed, prospective, or randomized, it has been shown that a success rate of 74% can be expected in patients with single cysts treated for 3 to 6 months (Franchi et al, 1999). Most relapses occur within 2 years after cessation of treatment, but more prolonged monitoring has shown that a significant number of relapses occur 2 to 8 years after completing initial treatment. A controlled study of 29 patients who received albendazole (400 mg twice a day in three cycles of 6 weeks with 2 weeks between cycles) or placebo showed that 82% of the patients in the treatment group had cure or improvement compared with 14% in the placebo group (Keshmiri et al, 2001).
Factors that influence the efficacy of benzimidazoles have not been well defined, but it is known that penetration of the drug across the cyst wall depends on several factors. Young cysts without pericystic fibrosis are more sensitive to drugs than thick, calcified cysts. Chemotherapy is less effective in daughter cysts within a mother cyst and in cysts with infection or a biliary communication. Small cysts (<8 cm) and secondary cysts are mostly sensitive to chemotherapy, and chemotherapy seems to be more effective in young patients (Teggi et al, 1993; Todorov et al, 1990).
The efficacy of preoperative albendazole treatment was established by two studies. In the first study (Wen et al, 1994), cyst viability after 3 months of albendazole pretreatment was 8% at the time of surgery, significantly lower than in the control group (100%). In the other study, 1-month and 3-month preoperative courses of albendazole significantly reduced cyst viability to 28% and 6%. Although no published data are available on the efficacy of perioperative prophylaxis, it is generally advised that albendazole is given for 1 week before surgery. Similarly, postoperative treatment is recommended for 6 months in cases of intraoperative hydatid spillage.
Treatment is usually administered in three or four courses lasting 4 weeks separated by a 2-week interval. Three courses are routinely recommended, in agreement with viability data suggesting that a maximum benefit is not reached with less than 3 months of therapy, and more than 6 months of treatment is seldom necessary (WHO Informal Working Group on Echinococcosis, 1996). This cyclical method of treatment is recommended because of the limited toxicology data available at the time of the first treatment attempts, but more recent data on uninterrupted treatment show that this approach could have better efficacy (Saimot, 2001). The use of continuous treatment has been followed without evidence of increased adverse events, but controlled comparisons with intermittent treatment have not been carried out. Preoperative treatment with a benzimidazole should begin 1 week before surgery (WHO Informal Working Group on Echinococcosis, 1996). A new albendazole formulation has shown a fivefold to tenfold enhancement of bioavailability compared with classic albendazole (Rigter et al, 2004).
Alveolar Hydatid Disease
All four species of Echinococcus are potentially zoonotic, but E. alveolaris and E. multilocularis are the most pathogenic; both represent potentially fatal, chronically progressive hepatic infections, which is unusual for a parasitic helmintic infection. The fatal outcome may occur in 95% of untreated patients within 10 years after the diagnosis. These parasites occur within a large belt between the fortieth and forty-fifth degree of northern latitude (Ammann & Eckert, 1996). Surveys in central Europe have shown an increase in the known distribution of E. multilocularis from four countries at the end of 1980 to at least 11 countries in 1999 (Eckert & Deplazes, 1999). There is evidence that parasites have spread from endemic areas to previously nonendemic areas in North America, Japan, and China (Deplazes & Eckert, 2001).
Control of transmission of E. alveolaris is difficult because the definitive and intermediate hosts involved are usually wild animals. Risk factors for alveolar hydatid disease in endemic regions are complex and include seasonal fluctuations in the size of the fox population, the susceptibility and immunity of definitive hosts, and resistance of eggs to environmental factors (McManus et al, 2003).
Humans can come into contact with the eggs accidentally, such as when skinning foxes, and ingestion of the eggs results in alveolar hydatid disease. The laminated membrane of an alveolar hydatid cyst is very thin, and brood capsules and protoscolices rarely are formed in the human host (<10%). In most cases, the germinal membrane is undetectable, or it appears as an isolated, thin form. This tissue still has the potential for proliferation, however, and it produces protoscolices when inoculated into a viable intermediate host (rodent). The growth rate of the alveolar hydatid disease in the liver is usually slow; calculated median growth rate is 14.8 mL/year (Luder et al, 1985).
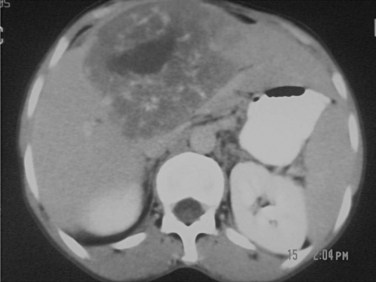
Routine laboratory tests do not yield specific findings. ESR is elevated in most cases, and eosinophilia is usually mild or absent (Ammann & Eckert, 1996). Alkaline phosphatase and GGT levels are usually increased, especially in advanced disease. US and CT demonstrate the lesions that appear as heterogenous, hypodense masses with irregular contours (Fig. 68.22). A necrotic cavity in the center of the mass without a well-defined wall is a frequent finding. Pressure on the portal or hepatic veins, or inferior vena cava may also occur, and calcification is common and appears as irregularly distributed clusters of microcalcifications or plaquelike foci (Fig. 68.23; Czermak et al, 2001). MRI demonstrates central necrosis better than CT but is less effective in showing microcalcification and small lesions. In most cases, T1-weighted images show the margins of the lesions more clearly (Harman et al, 2003). Alveolar hydatid disease is characterized clinically by a discrepancy between the often large, tumorlike lesions of the liver and the usually well general condition of the patient, which is in contrast to advanced malignancy.
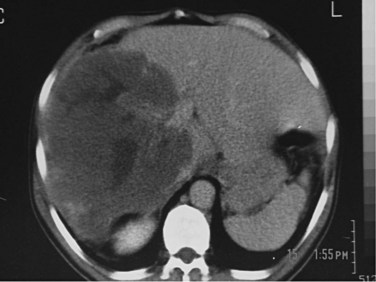
FIGURE 68.22 Heterogenous mass with irregular contours and necrotic cavity in the center in alveolar hydatid cyst.
Immunodiagnosis of alveolar hydatid disease is useful, effective, and more reliable than the diagnosis of cystic hydatid disease. The sensitivity in alveolar hydatid disease is 95% to 100%, and the specificity is high if purified and specific antigens are used. Native Em2 antigen, alone or mixed with recombinant Em10, is highly specific for alveolar hydatid disease using ELISA, and only a few cystic hydatid disease cases are cross-reactive (Silas-Lucas & Gottstein, 2001). Immunodiagnosis has a diagnostic sensitivity of 97.1% and overall specificity of 98.9%. The reported sensitivity of the recombinant Em18-ELISA was 87% with 97% specificity (Ito et al, 2002; Sako et al, 2002). The stage of the disease is the main prognostic factor, similar to malignant tumors. The so-called PNM system (parasitic mass, involvement of neighboring organs, metastasis) is a clinical staging system for alveolar hydatid disease (Box 68.1 and Table 68.5; Pawlowski et al, 2001).
Box 68.1
PNM Classification of Human Alveolar Echinococcosis
P: Hepatic Localization of Metacestode
PX Primary lesion unable to be assessed
P0 No detectable hepatic lesion
P1 Peripheral lesion without biliary or proximal vascular involvement
P2 Central lesions with biliary or proximal involvement of one lobe
P3 Central lesions with biliary or proximal vascular involvement of both lobes or two hepatic veins or both
P4 Any lesion with extension along the portal vein, inferior vena cava, or hepatic arteries
From Pawlowski I, et al, 2001: Echinococcosis in humans: clinical aspects, diagnosis and treatment. In Eckert J, et al (eds): WHO/OI Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris, World Organisation for Animal Health, pp 20-71.
Curative liver resection is the treatment of choice for localized lesions. Because of the frequent involvement of the biliary bifurcation, liver resection often requires a biliary reconstruction using a Roux-en-Y jejunal loop. Most of the lesions occur in the right lobe of the liver; if adherent to the diaphragm, such lesions may require an associated diaphragmatic resection. Palliative resection has been proposed in patients with large bilobar involvement with extension to the portal vein or to the vena cava. This approach, combined with medical treatment, can result in long-term survival. In contrast, surgical external biliary drainage of cyst cavities is associated with poor outcome. Liver transplantation should be considered in patients who have unresectable disease, especially with massive involvement of the pedicle or coexisting chronic liver failure (Bresson-Hadni et al, 2003). The frequent extrahepatic extension of the disease—involving the hepatoduodenal ligament, vena cava, diaphragm, and pericardium—makes total hepatectomy technically difficult. In addition, hydatid disease may recur even after liver transplantation (Bresson-Hadni et al, 1999). Adjuvant chemotherapy with albendazole should be added to posttransplant medication regimens to prevent recurrence (WHO Informal Working Group on Echinococcosis, 1996).
Albendazole (15 mg/kg/day) is probably the best drug currently available for alveolar hydatid disease. It is generally accepted that long-term albendazole therapy is primarily parasitostatic. Evidence of a parasiticidal effect of long-term albendazole has been reported, however, in a few cases (Ammann et al, 1998). More recent studies provide clear evidence that long-term chemotherapy of alveolar hydatid disease is effective. Oral albendazole resulted in an improved 10-year survival rate of 80% to 83% compared with 6% to 25% for untreated historic controls (Craig, 2003). Nitazoxanide, a broad-spectrum antihelmintic drug, has been tested for experimental alveolar hydatid disease. Nitazoxanide is better absorbed than albendazole after oral administration, and it provides an attractive potential alternative for medical treatment (Stettler et al, 2003).
Aarons B, Kune GA. A suction cone to prevent spillage during hydatid surgery. Aust N Z J Surg. 1983;53:471-472.
Abdel Hameed AA, Abu Aisha H. Uneventful intraperitoneal rupture of a hepatic hydatid cyst: a case report. Trop Geogr Med. 1987;39:80-82.
Abu Zeid M, et al. Surgical treatment of hepatic hydatid cysts. Hepatogastroenterology. 1998;45:1802-1806.
Akhan O, Ozmen MN. Percutaneous treatment of liver hydatid cysts. Eur J Radiol. 1999;32:76-85.
Akhan O, et al. Percutaneous treatment of abdominal hydatid cysts with hypertonic saline and alcohol: an experimental study in sheep. Invest Radiol. 1993;28:121-127.
Alfieri S, et al. Radical surgery for liver hydatid disease: a study of 89 consecutive patients. Hepatogastroenterology. 1997;44:496-500.
Alper A, et al. Laparoscopic surgery of hepatic hydatid disease: initial results and early follow-up of 16 patients. World J Surg. 1995;19:725-728.
Ammann RW, Eckert J. Cestodes, Echinococcus. Gastroenterol Clin North Am. 1996;25:655-689.
Ammann RW, et al. Long-term mebendazole therapy may be parasitocidal in alveolar echinococcosis. J Hepatol. 1998;29:994-998.
Atli M, et al. Intrabiliary rupture of a hepatic hydatid cyst: associated clinical factors and proper management. Arch Surg. 2001;136:1249-1255.
Baraka A, et al. Cetrimide-induced methaemoglobinaemia after surgical excision of hydatid cyst. Lancet. 1980;2:88-89.
Barros JL. Hydatid disease of the liver. Am J Surg. 1978;135:597-600.
Basaran C, et al. Fat-containing lesions of the liver: cross-sectional imaging findings with emphasis on MRI. AJR Am J Roentgenol. 2005;184:1103-1110.
Beggs I. The radiological appearances of hydatid disease of the liver. Clin Radiol. 1983;34:555-563.
Bekhti A, et al. Treatment of hepatic hydatid disease with mebendazole: preliminary results in four cases. Br Med J. 1977;2:1047-1051.
Belghiti J, et al. Caustic sclerosing cholangitis: a complication of the surgical treatment of hydatid disease of the liver. Arch Surg. 1986;121:1162-1165.
Ben Amor N, et al. Trial therapy of inoperable abdominal hydatid cysts by puncture. Ann Parasitol Hum Comp. 1986;61:689-692.
Berberoglu M, et al. Gasless vs gaseous laparoscopy in the treatment of hepatic hydatid disease. Surg Endosc. 1999;13:1195-1198.
Besim H, et al. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10:347-351.
Bickel A, Eitan A. The use of a large, transparent cannula, with a beveled tip, for safe laparoscopic management of hydatid cysts of liver. Surg Endosc. 1995;9:1304-1305.
Bickel A, et al. Laparoscopic approach to hydatid liver cysts: is it logical? Physical, experimental, and practical aspects. Surg Endosc. 1998;12:1073-1077.
Bourgeon R. L’ouverture des kystes hydatiques aux voies biliares intra-hépatiques. Lyon Chir. 1985;81:161.
Bresson-Hadni S, et al. Primary disease recurrence after liver transplantation for alveolar echinococcosis: long-term evaluation in 15 patients. Hepatology. 1999;30:857-864.
Bresson-Hadni S, et al. Indications and results of liver transplantation for Echinococcus alveolar infection: an overview. Langenbecks Arch Surg. 2003;388:231-238.
Bret PM, et al. Percutaneous aspiration and drainage of hydatid cysts in the liver. Radiology. 1988;168:617-620.
Buttenschoen K, Buttenschoen D. Echinococcus granulosus infection: the challenge of surgical treatment. Langenbecks Arch Surg. 2003;388:218-230.
Castellano G, et al. Sclerosing cholangitis: report of four cases and a cumulative review of the literature. Hepatogastroenterology. 1994;41:458-470.
Cirenei A, Bertoldi I. Evolution of surgery for liver hydatidosis from 1950 to today: analysis of a personal experience. World J Surg. 2001;25:87-92.
Coltorti EA. Standardization and evaluation of an enzyme immunoassay as a screening test for the seroepidemiology of human hydatidosis. Am J Trop Med Hyg. 1986;35:1000-1005.
Craig P. Echinococcus multilocularis. Curr Opin Infect Dis. 2003;16:437-444.
Czermak BV, et al. Echinococcus multilocularis revisited. AJR Am J Roentgenol. 2001;176:1207-1212.
Deplazes P, Eckert J. Veterinary aspects of alveolar echinococcosis: a zoonosis of public health significance. Vet Parasitol. 2001;98:65-87.
Diez Valladares L, et al. Hydatid liver cyst perforation into the digestive tract. Hepatogastroenterology. 1998;45:2110-2114.
Doiz O, et al. Western Blot applied to the diagnosis and post-treatment monitoring of human hydatidosis. Diagn Microbiol Infect Dis. 2001;41:139-142.
Eckert J, Deplazes P. Alveolar echinococcosis in humans: the current situation in Central Europe and the need for countermeasures. Parasitol Today. 1999;15:315-319.
Eickhoff A, et al. Endoscopic stenting for postoperative biliary strictures due to hepatic hydatid disease: effectiveness and long-term outcome. J Clin Gastroenterol. 2003;37:74-78.
Ertem M, et al. Laparoscopically treated liver hydatid cysts. Arch Surg. 2002;137:1170-1173.
Filice C, et al. Treatment of echinococcal cysts. Ultrasound Q. 1999;15:223-233.
Franchi C, et al. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin Infect Dis. 1999;29:304-309.
Gahukamble DB, et al. Outcome of minimal surgery for hydatid cysts of the liver in children with reference to post-operative biliary leakage. Ann Trop Paediatr. 2000;20:147-151.
Gharbi HA, et al. Ultrasound examination of the hydatic liver. Radiology. 1981;139:459-463.
Gokce O, et al. Povidone–iodine in experimental peritoneal hydatidosis. Br J Surg. 1991;78:495-496.
Harman M, et al. MRI findings of hepatic alveolar echinococcosis. Clin Imaging. 2003;27:411-416.
Iacona A, et al. Enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of hydatid disease. Am J Trop Med Hyg. 1980;29:95-102.
Iscan M, Duren M. Endoscopic sphincterotomy in the management of postoperative complications of hepatic hydatid disease. Endoscopy. 1991;23:282-283.
Ito A, et al. Evaluation of an enzymed-linked immunosorbent assay (ELISA) with affinity-purified Em18 and an ELISA with recombinant Em18 for differential diagnosis of alveolar echinococcosis: results of a blind test. J Clin Microbiol. 2002;40:4161-4165.
Karavias DD, et al. Improved techniques in the surgical treatment of hepatic hydatidosis. Surg Gynecol Obstet. 1992;174:176-180.
Karayalcin K, et al. Effect of hypertonic saline and alcohol on viability of daughter cysts in hepatic hydatid disease. Eur J Surg. 1999;165:1043-1044.
Karunajeewa HA, et al. Hydatid disease invading the inferior vena cava: successful combined medical and surgical treatment. Aust N Z J Surg. 2002;72:159-160.
Kayaalp C, et al. Türkiye’de kist hidatik cerrahisinde skolisidal ve perioperatif benzimidazol kullanimi. Ankara Cerrahi Dergisi. 1999;4:201-207.
Kayaalp C, et al. Biliary complications after hydatid liver surgery: incidence and risk factors. J Gastrointest Surg. 2002;6:706-712.
Kayaalp C, et al. Distribution of hydatid cysts into the liver with reference to cystobiliary communications and cavity-related complications. Am J Surg. 2003;185:175-179.
Keshmiri M, et al. Albendazole versus placebo in treatment of echinococcosis. Trans R Soc Trop Med Hyg. 2001;95:190-194.
Khoury G, et al. Laparoscopic treatment of hydatid cysts of the liver and spleen. Surg Endosc. 2000;14:243-245.
Khuroo MS, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997;337:881-887.
Kolbakir F, Erk MK. Povidone–iodine in experimental peritoneal hydatidosis. Br J Surg. 1992;79:373-375.
Krige JEJ, Beckingham IJ. Liver abscesses and hydatid disease. In: Beckingham, IJ, editor. ABC of Liver, Pancreas, and Gallbladder. London: British Medical Journal Publishing Group; 2001:29-32.
Krige JEJ, et al. Fatal hypernatraemia after hypertonic saline irrigation of hepatic hydatid cysts. Pediatr Surg Int. 2002;18:64-65.
Landa Garcia JI, et al. Evaluation of scolicidal agents in an experimental hydatid disease model. Eur Surg Res. 1997;29:202-208.
Langer JC, et al. Diagnosis and management of hydatid disease of the liver: a 15-year North American experience. Ann Surg. 1984;199:412-417.
Little AF, et al. MR cholangiography in the evaluation of suspected intrabiliary rupture of hepatic hydatid cyst. Abdom Imaging. 2002;27:333-335.
Little JM. Hydatid disease at Royal Prince Alfred Hospital, 1964 to 1974. Med J Aust. 1976;1:903-908.
Loinaz C, et al. Long-term biliary complications after liver surgery leading to liver transplantation. World J Surg. 2001;25:1260-1263.
Luder PJ, et al. High oral doses of mebendazole interfere with growth of larval Echinococcus multilocularis lesions. J Hepatol. 1985;1:369-377.
Magistrelli P, et al. Surgical treatment of hydatid disease of the liver: a 20-year experience. Arch Surg. 1991;126:518-522.
McCorkell SJ. Unintended percutaneous aspiration of pulmonary echinococcal cysts. Am J Radiol. 1984;143:123-126.
McCorkell SJ, Lewall DB. Hepatic echinococcal cysts: sonographic appearance and classification. Radiology. 1985;155:773-775.
McManus DP, et al. Echinococcosis. Lancet. 2003;362:1295-1304.
Momblano P, et al. Metabolic acidosis induced by cetrimonium bromide. Lancet. 1984;2:1045.
Morel P, et al. Surgical treatment of hydatid disease of the liver: a survey of 69 patients. Surgery. 1988;104:859-862.
Moreno-Gonzalez E, et al. Liver transplantation for Echinococcus granulosus hydatid disease. Transplantation. 1994;58:797-800.
Morris DL. Surgical management of hepatic hydatid cyst. In: Morris, DL, Richards, KS. Hydatid Disease: Current Medical and Surgical Management. Oxford: Butterworth Heinemann; 1992:57-75.
Mueller PR, et al. Hepatic echinococcal cyst: successful percutaneous drainage. Radiology. 1985;155:627-628.
Nardo B, et al. Radical surgical treatment of recurrent hepatic hydatidosis. Hepatogastroenterology. 2003;50:1478-1481.
Ozaslan E, Bayraktar Y. Endoscopic therapy in the management of hepatobiliary hydatid disease. J Clin Gastroenterol. 2002;35:160-174.
Ozmen V, et al. Surgical treatment of hepatic hydatid disease. Can J Surg. 1992;35:423-427.
Palacios-Ruiz JA, et al. Hypertonic saline in hydatid disease. World J Surg. 2002;26:1398-1399.
Pawlowski I, et al. Echinococcosis in humans: clinical aspects, diagnosis and treatment. In: Eckert, J, et al. WHO/OI Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Paris: World Organisation for Animal Health; 2001:20-71.
Pedrosa I, et al. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000;20:795-817.
Pelaez V, et al. PAIR as percutaneous treatment of hydatid liver cysts. Acta Trop. 2000;75:197-202.
Pitt HA, et al. Management of hepatic echinococcosis in Southern California. Am J Surg. 1986;152:110-115.
Richards KS. Biology of Echinococcus and diagnosis of hydatid disease. In: Morris, DL, Richards, KS. Hydatid Disease: Current Medical and Surgical Management. Oxford: UK, Butterworth Heinemann; 1992:1-24.
Rickard MD. Serological diagnosis and post-operative surveillance of human hydatid disease: I. Latex agglutination and immunoelectrophoresis using crude cyst fluid antigen. Pathology. 1984;16:207-210.
Rigter IM, et al. Relative bioavailability of three newly developed albendazole formulations: a randomized crossover study with healthy volunteers. Antimicrob Agents Chemother. 2004;48:1051-1054.
Saglam A. Laparoscopic treatment of liver hydatid cysts. Surg Laparosc Endosc. 1996;6:16-21.
Sahin E, et al. Single stage transthoracic approach for right lung and liver hydatid disease. J Thorac Cardiovasc Surg. 2003;126:769-773.
Saidi F, Nazarian I. Surgical treatment of hydatid cysts by freezing of cyst wall and instillation of 0.5 per cent silver nitrate solution. N Engl J Med. 1971;284:1346-1350.
Saimot AG. Medical treatment of liver hydatidosis. World J Surg. 2001;25:15-20.
Sako Y, et al. Alveolar echinococcosis: characterisation of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J Clin Microbiol. 2002;40:2760-2765.
Saremi F, McNamara TO. Hydatid cysts of the liver: long-term results of percutaneous treatment using a cutting instrument. AJR Am J Roentgenol. 1995;165:1163-1167.
Sbihi Y, et al. Serologic recognition of hydatid cyst antigens using different purification methods. Diagn Microbiol Infect Dis. 1996;24:205-211.
Schipper HG, et al. Effect of dose increase or cimetidine co-administration on albendazole bioavailability. Am J Trop Med Hyg. 2000;63:270-273.
Schipper HG, et al. Percutaneous evacuation (PEVAC) of multivesicular echinococcal cysts with or without cystobiliary fistulas which contain non-drainable material: first results of a modified PAIR method. Gut. 2002;50:718-723.
Seven R, et al. Laparoscopic treatment of hepatic hydatid cysts. Surgery. 2000;128:36-40.
Shaw JM, Bornman PC, Krige JEJ. Hydatid disease of the liver. S Afr J Surg. 2006;44:70-77.
Sielaff TD, et al. Recurrence of hydatid disease. World J Surg. 2001;25:83-86.
Silas-Lucas M, Gottstein B. Molecular tools for the diagnosis of cystic and alveolar echinococcosis. Trop Med Int Health. 2001;6:463-475.
Simsek H, et al. Diagnostic and therapeutic ERCP in hepatic hydatid disease. Gastrointest Endosc. 2003;58:384-389.
Sozuer EM, et al. The perforation problem in hydatid disease. Am J Trop Med Hyg. 2002;66:575-577.
Stettler M, et al. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob Agents Chemother. 2003;47:467-474.
Teggi A, et al. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother. 1993;37:1679-1684.
Terblanche J, Krige JEJ. The management of hepatic Echinococcus. In: Cameron, JL, editor. Current Surgical Therapy. 6th ed. Baltimore: Mosby; 1998:326-330.
Thameur H, et al. Cardiopericardial hydatid cysts. World J Surg. 2001;25:58-67.
Thompson RCA. Echinococcosis. In: Gillespie, S, Pearson, RD. Principles and Practice of Clinical Parasitology. Chichester, England: John Wiley & Sons; 2001:587-612.
Todorov T, et al. Evaluation of response to chemotherapy of human cystic echinococcosis. Br J Radiol. 1990;63:523-531.
Varela-Diaz VM, et al. Immunodiagnosis of human hydatid disease: applications and contributions to a control program in Argentina. Am J Trop Med Hyg. 1983;32:1079-1087.
Vuitton DA. Echinococcosis and allergy. Clin Rev Allergy Immunol. 2004;26:93-104.
Watson-Jones DL, Macpherson CN. Hydatid disease in the Turkana district of Kenya. VI. Man–dog contact and its role in the transmission and control of hydatidosis amongst the Turkana. Ann Trop Med Parasitol. 1988;82:343-356.
Wen H, et al. Albendazole chemotherapy for human cystic and alveolar echinococcosis in north-western China. Trans R Soc Trop Med Hyg. 1994;88:340-343.
WHO Informal Working Group on Echinococcosis. Guidelines for treatment of cystic and alveolar echinococcosis in humans. Bull World Health Organ. 1996;74:231-242.
WHO Informal Working Group on Echinococcosis. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253-261.
Yilmaz E, Gokok N. Hydatid disease of the liver: current surgical management. Br J Clin. 1990;44:612-615.
Yilmaz U, et al. Management of postoperative biliary strictures secondary to hepatic hydatid disease by endoscopic stenting. Hepatogastroenterology. 1998;45:65-69.
Yol S, et al. Open drainage versus overlapping method in the treatment of hepatic hydatid cyst cavities. Int Surg. 1999;84:139-143.
Yorganci K, Sayek I. Surgical treatment of hydatid cysts of the liver in the era of percutaneous treatment. Am J Surg. 2002;184:63-69.
Zaouche A, et al. Management of liver hydatid cysts with a large biliocystic fistula: multicenter retrospective study. Tunisian Surgical Association. World J Surg. 2001;25:28-39.
Zhang W, et al. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18-36.