8 Humidity and Aerosol Therapy
Note 1: This book is written to cover every item listed as testable on the Entry Level Examination (ELE), Written Registry Examination (WRE), and Clinical Simulation Examination (CSE).
The listed code for each item is taken from the National Board for Respiratory Care (NBRC) Summary Content Outline for CRT (Certified Respiratory Therapist) and Written RRT (Registered Respiratory Therapist) Examinations (http://evolve.elsevier.com/Sills/resptherapist/). For example, if an item is testable on both the ELE and the WRE, it will be shown simply as: (Code: …). If an item is testable only on the ELE, it will be shown as: (ELE code: …). If an item is testable only on the WRE, it will be shown as: (WRE code: …).
MODULE A
1. Maintain adequate humidification (ELE code: IIIB8) [Difficulty: ELE: R, Ap, An]
a. Indications for humidity therapy
1. Humidification of dry therapeutic medical gases in patients with a normal upper airway
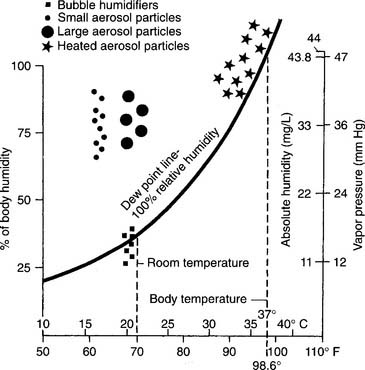
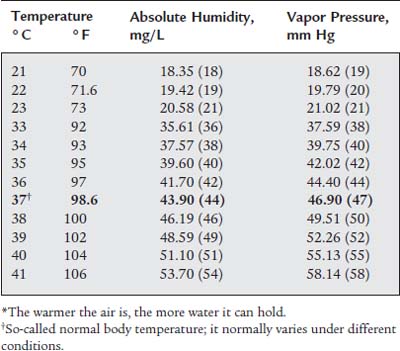
Body humidity is the water saturation condition of the gas in the lungs. Under normal conditions with air, it is 43.9 (44) mg/L absolute humidity and 46.90 (47) mm Hg at 37° C (98.6° F). In other words, air is always warmed to body temperature and saturated with water by the time it reaches the lungs. As can be seen in Table 8-1, both water content and vapor pressure in the lungs vary with the patient’s temperature.
TABLE 8-1 Saturated Air Values for Absolute Humidity and Vapor Pressure Under Room and Body Temperature Ranges*
The clinical practice guidelines of the American Association for Respiratory Care state that supplemental humidity is not needed for O2 at flows of 4 L/min or less. This includes nasal cannulas and some air entrainment mask settings. As long as the patient has a normal upper airway and the hospital has a relative humidity (RH) of about 40%, the patient should be able to fully saturate the gas with no adverse effects. Some clinicians believe that any O2 flow through a nasal cannula should be humidified to prevent the local mucosa from drying out. Usually, an unheated bubble-type humidifier is used to deliver about 40% RH at room temperature. The patient then is able to fully saturate the gas. All agree that dry O2 at flows of greater than 4 L/min by any device must be humidified.
b. Indications for aerosol therapy
3. Delivery of medications to the airways and lungs
Respiratory therapists give many medications to patients, and these medications obviously must reach the target area. Table 8-2 gives the particle size of each medicinal aerosol and its most likely deposition area.
TABLE 8-2 Aerosol Particle Sizes and Their Likely Deposition Points in the Airways and Lungs
| Location | MMAD* Particle Size, μm |
|---|---|
| Nose or mouth to larynx | 10 and larger |
| Trachea to terminal bronchioles | 9-5 |
| Respiratory bronchioles to alveoli | 5-2 |
| Lung parenchyma (alveoli) | 1-3 |
MMAD, Mass median aerodynamic diameter.
Note: Some controversy exists over the size of the particles that deposit in the airways and lungs. This table lists what seems to be a majority opinion. Aerosol particle diameter sizes are listed in units of micrometers, which are one-thousandth of a millimeter. As a point of clarification, most references list the symbol for a micrometer as μ; others use the international system unit of micrometer, which is symbolized as μm.
* MMAD is defined as the aerosol diameter around which the mass is equally divided, that is, 50% of the aerosol mass is found in particles smaller than the MMAD, and 50% of the aerosol mass is found in particles larger than the MMAD.
4. Increased clearance of secretions
Traditionally, many patients with a mild case of bronchitis were given breathing treatments with a bland aerosol to help them mobilize secretions. The aerosol was thought to add enough liquid to the secretions to enable the patient to cough them out. The patient, as it is now understood, can cough more effectively because the aerosol irritates the airway, and the patient’s own bronchial/submucosal glands pour out additional mucus. This reflex is mediated by the vagus nerve. This form of therapy probably is not indicated in most situations. It is clinically more effective to increase the patient’s oral or intravenous fluids so that the bronchial/submucosal glands can produce secretions that are not thick.
2. Interview the patient to determine sputum production (Code: IB5c) [Difficulty: ELE: R, Ap; WRE: An]
3. Observe the patient’s sputum production
a. Observe the patient for changes in sputum characteristics (ELE code: IIIE5) [Difficulty: ELE: R, Ap, An]
b. Use inspection to assess the patient’s cough, sputum amount, and character (Code: IB1c) [Difficulty: ELE: R, Ap; WRE: An]
The patient’s sputum characteristics (e.g., consistency, color, smell) and amount must be known if the effectiveness of humidity or aerosol therapy is to be assessed. Review the related discussion in Chapter 1, and see Table 1-12. Try to relate this information to breathing treatments, medications, activities, meals, and allergies.
4. Assess the patient’s overall cardiopulmonary status by auscultation to determine the presence of normal or abnormal breath sounds (Code: IB4a) [Difficulty: ELE: R, Ap; WRE: An] and (Code: IIIE11) [Difficulty: ELE: R, Ap; WRE: An]
Review the discussion in Chapter 1, if necessary. The presence of expiratory crackles (also called rhonchi) would indicate airway secretions. If the patient is able to cough out the secretions, abnormal breath sounds, such as crackles, should improve. If the patient’s secretions are thick (high viscosity), then aerosol therapy, as well as increased fluid intake, probably is indicated.
MODULE B
1. Humidity delivered through small-bore tubing
a. Manipulate bubble-type humidifiers by order or protocol (ELE code: IIA3) [Difficulty: ELE: R, Ap, An]
1. Get the necessary equipment for the procedure
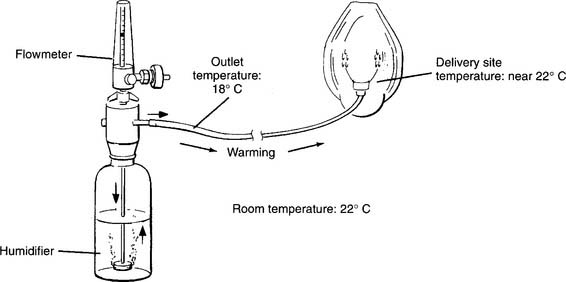
Bubble-type humidifiers are used on patients with a normal upper airway who need some supplemental humidity because of the dryness of medical O2. These devices usually are not heated and, in fact, deliver gas cooled to below room temperature. They provide around 40% RH at the delivered gas temperature. The rest of the humidity has to be made up by the patient (Figures 8-1 and 8-2). If clinically indicated, a wraparound type of heater can be added to raise the temperature of the delivered gas and reduce the patient’s humidity deficit.
Three different types of these humidifiers are designed to add some humidity to dry O2 delivered through small-bore tubing: traditional bubble humidifiers, jet humidifiers, and underwater jet humidifiers.
a. Bubble humidifiers.
Bubble humidifiers use a perforated capillary tube or a porous diffusion head to break the O2 into small bubbles (see Figure 8-2). This allows for greater surface area contact of the O2 with the water and raises the RH by evaporation. The water level in the reservoir must be kept within the manufacturers’ specifications and, if possible, as full as possible. The lower the water level, the lower is the RH because less time exists for evaporation. The faster the O2 flow, the lower is the RH.
b. Nasal cannula
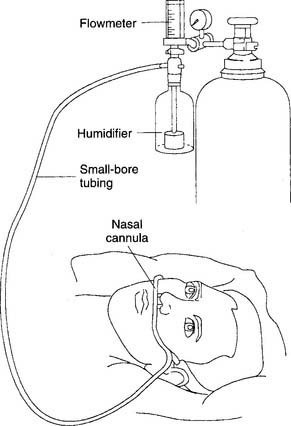
As has been discussed earlier, current guidelines state that humidity does not need to be added to these devices if the flow is 4 L/min or less. However, some patients complain of nasal dryness and discomfort if the cannula’s O2 is not humidified. The physician and the practitioner may believe that the patient’s discomfort warrants the addition of a bubble-type humidifier. Agreement has been reached on the addition of humidity to flows greater than 4 L/min. See Figure 8-3 for a humidified nasal cannula setup. See Chapter 6 for the discussion on a high-flow nasal cannula.
2. Humidity delivered through large-bore tubing
a. Manipulate large-volume humidifiers by order or protocol: cascade, wick, and passover types (ELE code: IIA3) [Difficulty: ELE: R, Ap, An]
1. Get the necessary equipment for the procedure
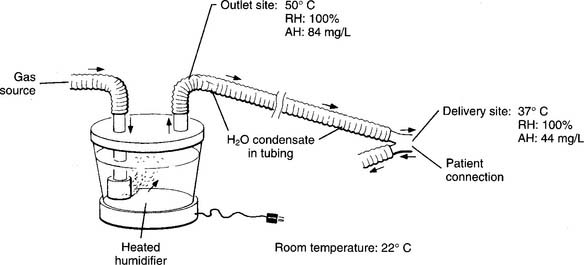
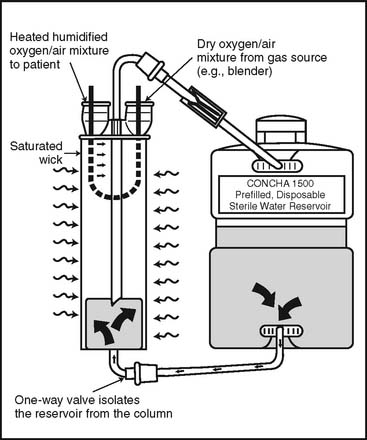
Cascade, wick, and passover-type humidifiers have an adjustable heater so the water in the reservoir is at or greater than body temperature. This enables them to provide up to 100% of the patient’s body humidity. With all of these units, the temperature of the inspired gas near the patient must be measured. Ideally, the gas temperature is kept the same as the patient’s to provide 100% relative humidity. One of these types of units is used to provide humidity when the patient’s upper airway is bypassed by an endotracheal or tracheostomy tube (Figure 8-4).
The Bennett Cascade is a classic example of these types of humidifiers (Figure 8-5). It was used most commonly with a mechanical ventilator but also was used with other types of systems for delivering humidity with or without oxygen. Its basic principle of operation is an efficient bubble-type humidifier. The inspiratory gas must flow through the water before evaporation can occur. A variety of similar devices are now on the market. Note: Even though these units are no longer available, the NBRC refers to this basic type of device as a Cascade-type humidifier.
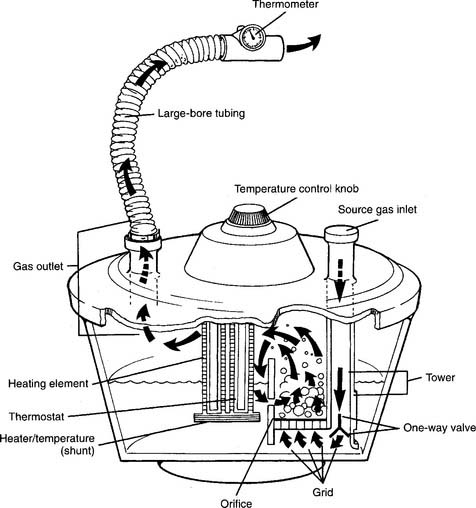
Figure 8-5 Cascade-type humidifier.
(From Scanlan CL: Humidity and aerosol therapy. In: Scanlan CL, Spearman CB, Sheldon RL, editors: Egan’s fundamentals of respiratory care, ed 5, St Louis, 1990, Mosby.)
The wick-type heated humidifier employs a wick, often made of sponge or paper, to soak up water for evaporation (Figure 8-6). The water, wick, or both are heated so that 100% RH can be delivered. These units also are used with mechanical ventilators or other systems, including air entrainment devices, because they have very little resistance to the gas flowing through them as evaporation occurs. Some units are designed for use with a heated wire ventilator circuit and feature an automatic water feed system and a servo-controlled thermostat to keep the water and the circuit at the same temperature. This minimizes condensation.
b. Manipulate a heat-moisture exchanger by order or protocol (ELE code: IIA3) [Difficulty: ELE: R, Ap, An]
1. Get the necessary equipment for the procedure
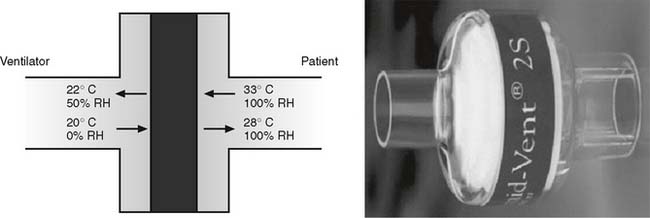
A heat-moisture exchanger (HME) is a passive humidifier that recycles the patient’s own exhaled water vapor. The HME contains a highly absorbent material that is warmed and moistened when a patient’s exhaled breath passes through it. The patient’s next inspiration is warmed and humidified by the water absorbed within the HME (Figure 8-7). These units are not as efficient as the humidifiers described earlier and are not able to provide 100% of body humidity to a patient. If possible, select an HME that has these characteristics: (1) is at least 70% efficient (provides at least 30 mg/L water vapor), (2) has a low compliance if used with a ventilator circuit, (3) is lightweight, (4) has little dead space, and (5) has little flow resistance.
2. Put the equipment together and make sure that it works properly (NBRC code: IIA3) [Difficulty: R, Ap]
3. Troubleshoot any problems with the equipment (NBRC code: IIA3) [Difficulty: R, Ap]
Secretions coughed into the HME can obstruct the flow of gas and thus make it difficult or impossible for the patient to breathe. The HME must be removed and discarded if it becomes obstructed, and it must be replaced by a new one. An HME should not be used with a patient known to cough out large quantities of secretions or blood.
3. Manipulate nebulizers and related delivery systems by order or protocol (ELE code: IIA4) [Difficulty: ELE: R, Ap, An]
a. Ultrasonic nebulizers
1. Get the necessary equipment for the procedure
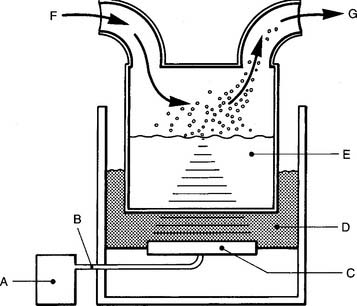
Large-volume ultrasonic units often are chosen for delivery of bland solutions to the lower airways because of the small particle size and high output. (So-called “bland aerosols” are composed of particles of water or saline rather than medicated aerosols.) Ultrasonic nebulizers (USNs) work by converting electrical energy into high-frequency sound energy, which creates aerosol particles. The frequency is vital because it results in a stable aerosol with a mean particle size of about 3 μm in diameter. This is an ideal size for penetrating deeply into the lungs to the smallest airways. The only control on these units is used for amplitude (power), and it controls the aerosol output. The range is usually up to 3 to 6 mL/min, depending on the model. This output is greater than that possible with most pneumatic nebulizers. The aerosol can be carried to the patient via a built-in fan or by an outside O2 source (Figure 8-8). The warm aerosol that is created minimizes the patient’s humidity deficit.
Recently, small-volume USN units have been designed to specifically nebulize medications into the circuit of a mechanical ventilator. Depending on the manufacturer, the USN can be powered electrically by the ventilator, by any electrical outlet, or by batteries. These units are designed to rapidly nebulize the small volume of medication (with or without diluent). The tidal volume breath carries the medication into the patient’s airways. Be aware that even though drugs have been given this way, pharmaceutical companies have not included in their dosing information the delivery of undiluted medications.
3. Troubleshoot any problems with the equipment
Always follow the manufacturer’s instructions when you are setting up the delivery system. Figure 8-8 shows the common features, and Table 8-3 describes how to troubleshoot many common problems. Many clinical difficulties seem to involve keeping the proper fluid levels in the couplant chamber and the solution cup. If the sterile water in the couplant chamber is too low, the vibration cannot reach the solution cup and no aerosol will be produced. If the saline level in the solution cup is too low or too high, the vibrational energy will not focus properly on the surface of the saline solution, and no aerosol will be produced. Water should not be allowed to condense and fill low points in the large-bore tubing; if it does, ultrasonic particles will liquefy as the carrier gas is forced to pass through the condensate. The exiting gas would be humidified through evaporation but would carry no aerosol particles. If the carrier gas is O2 blended with air through an air entrainment system, any backpressure could result in an increase in the O2 percentage and a decrease in the total flow. Remember to always measure the O2 percentage near the patient.
| Symptom | Possible Problem | Suggested Check |
|---|---|---|
| Unit installed and connected as specified, but pilot light does not turn on when switch is turned to the “on” position |
From Op’t Hole T: Aerosol generators and humidifiers. In: Barnes TA, editor: Respiratory care practice, Chicago, 1988; Mosby.
b. Manipulate large-volume pneumatic nebulizers by order or protocol (ELE code: IIA4) [Difficulty: ELE: R, Ap, An]
1. Get the necessary equipment for the procedure
3. Troubleshoot any problems with the equipment
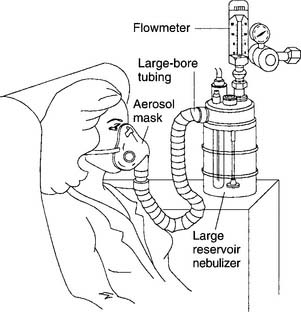
Most pneumatic nebulizers have an appearance that is similar to that of bubble-type humidifiers. Key components include a large reservoir jar and a top with a DISS O2 connector and a capillary tube to the jet. Make sure the nebulizer is screwed firmly onto the oxygen flowmeter and that the component parts are attached and work properly. Keep the capillary tube and jet clear of debris to keep the aerosol output from dropping. These units allow for variable O2 percentages. Keep the air entrainment ports open so that proper gas mixing occurs and the desired O2 percentage is provided (Figure 8-9). If water is present in the large-bore tubing or if the tubing is pinched, less room air will be entrained, and the delivered oxygen percentage will be higher than ordered. Keep the reservoir’s water level at the proper level. If the water level drops to below the refill line, no water will be drawn up the capillary tube to the jet. If the water is filled to above the maximum line, the jet may not operate properly. Heating of the water, aerosol, or both is accomplished in one of the following ways:
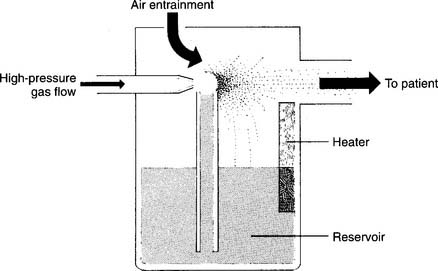
Figure 8-9 Large-volume air entrainment nebulizer.
(From Shapiro BA, Kacmarek RM, Cane RD, et al, editors: Clinical application of respiratory care, ed 4, St Louis, 1991, Mosby.)
Heating the water or aerosol reduces the patient’s humidity deficit and usually is done if the secretions are thick. See Figure 8-1 for the location of the aerosol particles and their relationship with the dew point and with the patient’s body humidity.
c. Aerosol masks
The aerosol mask looks similar to the simple O2 mask, except that it has larger side ports for exhalation and a 22-mm outer diameter (OD) adapter for attachment of the large-bore tubing (Figure 8-10). This often is considered to be a low-flow O2 mask because the ports are open to room air; therefore, it is difficult to ensure that the patient receives the set O2 percentage. If the flow is high enough that aerosol mist can be seen flowing out of the side ports during an inspiration, little or no room air is being inspired and the aerosol mask is now a high-flow device. Some clinicians have increased the total flow through an aerosol mask by using a Y adapter to combine the large-bore tubing coming from two large-volume nebulizers In any case, it is best to analyze the O2 percentage inside the mask to be sure of what the patient is inhaling. Any of the previously mentioned humidity or aerosol devices can be used and powered by compressed air or O2.
d. Face tents, tracheostomy masks, tracheostomy collars, and Brigg’s adapter/T-piece
A face tent, a tracheostomy mask and collar, and Brigg’s adapter (T-piece) are discussed in Chapter 6. All have a 22-mm ID adapter so that large-bore tubing can be added to them. Any of the previously mentioned humidity or aerosol devices can be used with these and are powered by compressed air or O2.
MODULE C
1. Manipulate incubators by order or protocol (WRE code: IIA12a) [Difficulty: WRE: R, Ap]
a. Get the necessary equipment for the procedure
An incubator is indicated in the care of a sick newborn who needs an enclosed space for an isolated, controlled environment. (See Figure 8-11.) (Some practitioners may refer to an incubator as an Isolette, which is a common brand of incubator.) Most incubators are used only in neonatal or pediatric care units and are powered electrically through standard electrical outlets. However, some incubators are designed to transport an infant between hospitals or within the hospital. They can make use of a standard electrical outlet but also feature self-contained batteries and oxygen tanks.
Because of these limits, it is not recommended that an incubator’s built-in oxygen delivery system be used. Instead, it is recommended that the following components be assembled for precise oxygen delivery and humidity control: (1) an oxygen blender with flowmeter and small-bore oxygen tubing adapter (nipple); (2) small-bore oxygen tubing and an adapter to connect the flowmeter to a cascade-type or wick-type heated humidifier; (3) sterile, distilled water to fill the humidifier reservoir; (4) large-bore (aerosol) tubing to direct the heated, humidified, high-percentage oxygen; (5) an oxygen hood (also called an oxyhood) to receive the humidified oxygen (see Chapter 6 for discussion and an illustration); (6) an oxygen analyzer to check the percentage inside the oxygen hood; and (7) a temperature probe to check the temperature of the heated humidified oxygen; place this into the large-bore tubing before it enters the incubator.
2. Radiant warmers
2. Manipulate aerosol (mist) tents by order or protocol (ELE code: IIA12b) [Difficulty: ELE: R, Ap]
a. Get the necessary equipment for the procedure
Aerosol or mist tents are essentially like the O2 tents discussed in Chapter 6. The main difference is that no supplemental O2 is used because the patient does not need it. The top of the canopy then can be left open for better flow-through ventilation. Aerosol tents sometimes are used to treat an active child with an upper respiratory tract problem such as laryngotracheobronchitis (LTB, or pediatric croup). The tent is used because a small, active child will not keep an aerosol mask in place. A tent is not indicated for an older child or an adult who will keep an aerosol mask in place.
c. Troubleshoot any problems with the equipment
Typically, some examination questions cover troubleshooting of problems with aerosol delivery equipment. Examples include but are not limited to (1) water in the large-bore tubing that prevents aerosol from traveling through it, (2) a plugged capillary line in a nebulizer so that no aerosol is produced, (3) a missing baffle in a nebulizer so that no aerosol is produced, (4) an incorrect water level in an ultrasonic nebulizer so that no aerosol is produced, and (5) replacement of a heat-moisture exchanger fouled with secretions or blood. Also, it is important to remember that with an oxygen-powered jet nebulizer system, the inspired oxygen percentage increases if condensate water fills the low point of the aerosol tubing. This is because the backpressure on the jet and air entrainment ports prevents room air from being drawn into the system. (See Figure 8-10.)
MODULE D
1. Manipulate small-volume pneumatic nebulizers by order or protocol (ELE code: IIA4) [Difficulty: ELE: R, Ap, An]
a. Get the necessary equipment for the procedure
Two different types of SVNs exist: mainstream and sidestream. Mainstream nebulizers are designed so the main flow of gas to the patient comes through the aerosol as it is produced. A second high-pressure gas flow is used to power the jet to create the aerosol (Figure 8-12). Sidestream nebulizers are designed so the aerosol is produced from the main flow of gas and is supplemented by the jet’s gas flow (Figure 8-13). Many manufacturers produce disposable medication SVNs, typically sidestream, for intermittent positive-pressure breathing circuits or hand-held circuits. Select the nebulizer that produces a particle size that matches the therapeutic target.
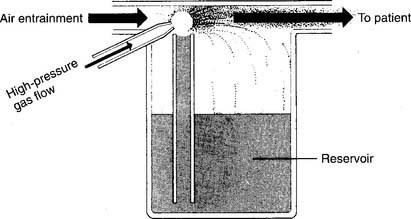
Figure 8-12 Mainstream-type small-volume nebulizer for medications.
(From Shapiro BA, Kacmarek RM, Cane RD, et al, editors: Clinical application of respiratory care, ed 4, St Louis, 1991, Mosby.)
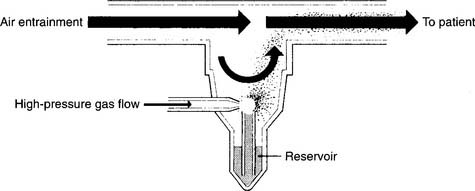
Figure 8-13 Sidestream-type small-volume nebulizer for medications.
(From Shapiro BA, Kacmarek RM, Cane RD, et al, editors: Clinical application of respiratory care, ed 4, St Louis, 1991, Mosby.)
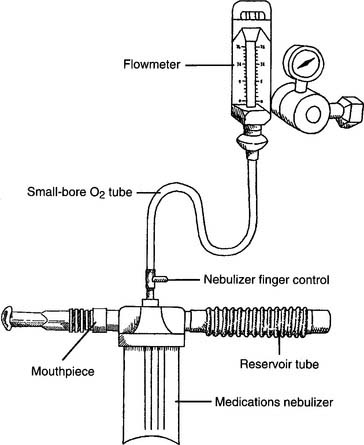
Figure 8-14 shows a typical small-volume nebulizer circuit. The nebulizer can be powered by compressed air or O2. Flows of 4 to 6 L/min typically are used to nebulize 3 to 5 mL of medication in about 10 minutes. The nebulizer finger control allows the patient to power the nebulizer by covering the open hole in the “T.” Uncovering the hole permits the gas to exit; thus, the medication is not nebulized and wasted. The reservoir tube serves to hold O2 and medication for the next inspiration.
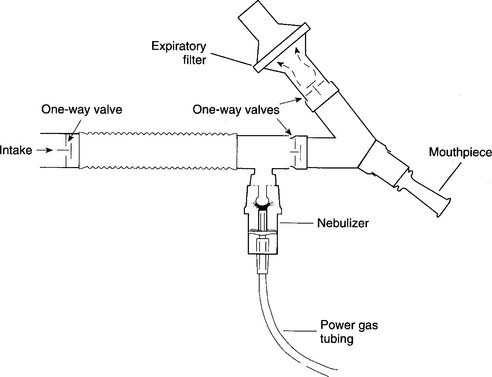
Practitioners face two possible risks when they use SVNs. First, any aerosolized medications that escape into the room air may be inhaled. It is possible that the practitioner, or anyone else nearby, may have an allergic or other adverse reaction. Second, nebulized secretions from the patient’s airway and lungs may be inhaled; this may place the practitioner or others at risk for acquiring a pulmonary infection from the patient. Although actual problems like these rarely occur, they are possible. If either of these situations is a concern, an SVN with one-way valves and a downstream particle filter should be used. This filter will trap any exhaled aerosol droplets (Figure 8-15). A filtered SVN is recommended when pentamidine isethionate (NebuPent) is nebulized. A filtered SVN should be used for any other antibiotic or medication that should not contaminate the room air.
c. Troubleshoot any problems with the equipment
If an SVN fails to generate aerosol, make sure that the pieces are properly assembled, the liquid medication is at the proper depth (typically 3 to 5 mL), and the capillary tube is not plugged with debris. If the capillary tube is plugged, liquid will not be drawn up to the jet. Sometimes, the capillary tube can be cleared by running it under water or pushing a needle through the channel. Do not use a nebulizer that does not generate an aerosol. The therapist must be sure that the patient can properly assemble, disassemble, and operate the SVN. If the patient cannot, another medication delivery system, such as a dry powder inhaler (DPI) or a metered-dose inhaler (MDI), should be recommended.
2. Manipulate metered-dose inhalers by order or protocol (ELE code: IIA23) [Difficulty: ELE: R, Ap, An]
a. Get the necessary equipment for the procedure
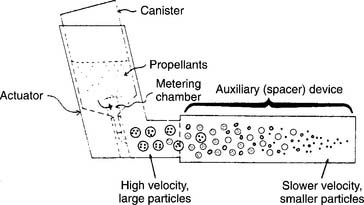
Metered-dose inhalers (MDIs) are designed to dispense a premeasured amount of medication into the airway. Each activation of the MDI delivers a dose of medication to the patient. Available medications include sympathomimetic and anticholinergic bronchodilators, corticosteroid drugs, and an antibiotic (see Chapter 9 for details on the medications). All MDIs operate in the same way. They contained several milliliters of medication and compressed hydrofluoroalkane (HFA) gas inside of a metal container with a built-in jet nozzle. (The older MDI units that contained the environmentally hazardous propellant chlorofluorocarbon [CFC] have been replaced by HFA gas units.)
Tipping the metering chamber over and back upright results in its filling with medication. A plastic actuator opens the jet when it is pressed into the container (Figure 8-16). The patient then can inhale the medication through the built-in mouthpiece or a spacer/holding chamber.
3. Manipulate spacers and holding chambers for a metered-dose inhaler by order or protocol (ELE code: IIA23) [Difficulty: ELE: R, Ap, An]
c. Troubleshoot any problems with the equipment
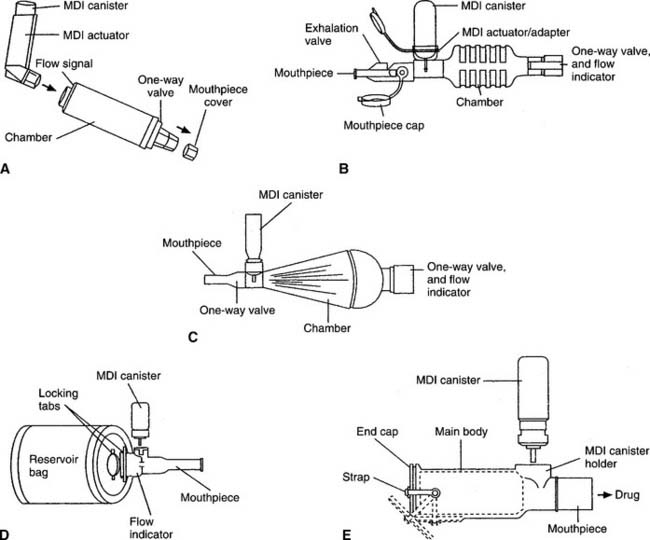
A spacer is a simple, open extension tube that is placed between the actuator and the patient. Its main advantage over direct inhalation from the MDI mouthpiece is that the aerosol plume expands and slows down so that more medication is inhaled (see Figure 8-16). The patient should be told to refrain from exhaling through the spacer because any remaining medication will be blown out and wasted. Some spacers are designed for use with a ventilator circuit when an MDI-based medication is to be given. The spacer should be placed into the inspiratory limb of the ventilator circuit, about 18 inches from the patient. Typically, the MDI is activated during the expiratory phase so the medication can fill the spacer and inspiratory tubing before the next inspiration.
A holding chamber holds the medication as does a spacer, but it also has valves. These holding chamber valves prevent the medicine from being exhaled out through the unit. The valves also allow the patient to inhale several times from the unit and get more medication than through a simple spacer. This is especially helpful with children or small adults with small tidal volume breaths. Some holding chambers have a built-in whistle that sounds if the patient is inhaling too quickly. Several types of spacers or holding chambers are available (Figure 8-17). Some spacers are designed to fit with only one actuator, whereas others adapt to fit with any actuator. A face mask comes attached to some holding chambers so pediatric patients or uncooperative adults can be given the medication.
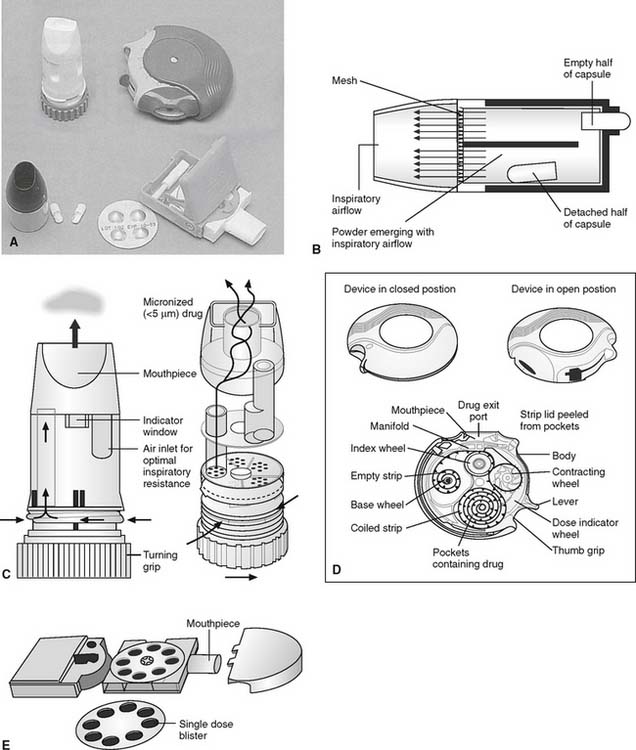
4. Manipulate dry powder inhalers by order or protocol (ELE Code: IIA24) [Difficulty: ELE: R, Ap, An]
a. Get the necessary equipment for the procedure
Dry powder inhalers (DPIs) dispense a dry medicinal powder into the patient’s airways and lungs when inhaled (Figure 8-18). The drug manufacturer sells both the medication and the dispenser to the patient. DPI inhalers that can provide the following classes of medications are now available: maintenance and fast-acting adrenergic (sympathomimetic) bronchodilators, anticholinergic (parasympathomimetic) bronchodilators, inhaled corticosteroids, an antiviral agent, and human insulin.
Other DPI units are multidose delivery systems that contain many doses of medication within a reservoir. Examples include the Turbuhaler for formoterol (Foradil), the Turbuhaler for terbutaline (Bricanyl), and the Pulmicort Turbuhaler (for budesonide), all of which contain 200 doses; the Diskus (for salmeterol [Serevent]), the Advair Diskus (combination of salmeterol and fluticasone propionate), and the Flovent Diskus [fluticasone propionate]), all of which contain 60 doses; and the Diskhaler (for zanamivir [Relenza]), which contains 4 or 8 doses. (See Chapter 9 for details on the medications.)
b. Put the equipment together and make sure that it works properly
Single-use DPI units require several steps to prepare the medication before inhalation. Steps typically include the following: (1) disassemble the device, (2) properly place the first medication capsule into the device, and (3) adjust the device to pierce the capsule. With all subsequent doses, the empty capsule from the previous treatment must be removed before the new capsule can be placed. The therapist must oversee that the patient is properly performing these steps. Patients who are too young to follow directions, who cannot physically perform the steps, or who do not have the mental capacity to understand the steps should not be using one of these DPI devices.
MODULE E
MODULE F
1. Analyze the available information to determine the patient’s pathophysiologic state (Code: IIIH1) [Difficulty: ELE: R, Ap; WRE: An]
2. Determine the appropriateness of the prescribed respiratory care plan and recommend modifications when indicated
a. Recommend changes in the therapeutic plan when indicated (Code: IIIH4) [Difficulty: ELE: R, Ap; WRE: An]
Changes in the patient’s secretion volume and consistency may result in a change in the type of humidity and/or aerosol therapy needed. Be prepared to decrease humidity and/or aerosol therapy if the patient’s secretions are decreased in volume and easy for the patient to cough out. In contrast, be prepared to increase humidity and/or aerosol therapy if the patient’s secretions are thick and difficult to cough out.
a. Change the output of aerosol by the equipment (ELE code: IIIF2c2) [Difficulty: ELE: R, Ap, An]
1. Recommend discontinuing the treatment or procedure based on the patient’s response (Code: IIIG1i) [Difficulty: ELE: R, Ap; WRE: An]
1. Record and evaluate the patient’s response to the treatment(s) or procedure(s), including the following:
a. Record and interpret the following: heart rate and rhythm, respiratory rate, blood pressure, body temperature, and pain level (Code: IIIA1b4) [Difficulty: ELE: R, Ap; WRE: An]
Bland aerosol therapy should not cause a significant change in the patient’s vital signs.
b. Record and interpret the patient’s breath sounds (Code: IIIA1b3) [Difficulty: ELE: R, Ap; WRE: An]
Adams DA. Humidity and aerosol therapy. In: Wyka KA, Mathews PJ, Clark WF, editors. Foundations of respiratory care. Albany: Delmar, 2002.
American Association for Respiratory Care. Aerosol consensus statement. Respir Care. 1991;36:916.
American Association for Respiratory Care. Clinical practice guideline: bland aerosol administration. Respir Care. 1993;38:1196.
American Association for Respiratory Care. Clinical practice guideline: bland aerosol administration—2003 revision & update. Respir Care. 2003;48:529.
American Association for Respiratory Care. Clinical practice guideline: care of the ventilator circuit and its relation to ventilator associated pneumonia. Respir Care. 2003;48:869.
American Association for Respiratory Care. Clinical practice guideline: delivery of aerosols to the upper airway. Respir Care. 1994;39:803.
American Association for Respiratory Care. Clinical practice guideline: humidification during mechanical ventilation. Respir Care. 1992;37:877.
American Association for Respiratory Care. Clinical practice guideline: selection of aerosol delivery device. Respir Care. 1992;37:891.
American Association for Respiratory Care. Clinical practice guideline: selection of aerosol delivery device for neonatal and pediatric patients. Respir Care. 1995;40:1325.
Barnes TA, editor. Core textbook of respiratory care practice, ed 2, St Louis: Mosby, 1994.
Branson RD, Hess DR, Chatburn RL, editors. Respiratory care equipment, ed 2, Philadelphia: Lippincott Williams & Wilkins, 1999.
Fink J., Aerosol drug therapy. Scanlan CL, Wilkins RL, Kacmarek. Egan’s fundamentals of respiratory care, ed 9, St Louis: Mosby, 2009.
Fink J. Humidity and aerosol therapy. In Cairo JM, Pilbeam SP, editors: Mosby’s respiratory care equipment, ed 8, St Louis: Mosby, 2010.
Fink J. Humidity and bland aerosol therapy. In Wilkins RL, Stoller JK, Kacmarek RM, editors: Egan’s fundamentals of respiratory care, ed 9, St Louis: Mosby, 2009.
Fink JB. Humidity. In: Fink JB, Hunt GE, editors. Clinical practice in respiratory care. Philadelphia: Lippincott-Raven, 1999.
Fink JB, Dhand R. Aerosol drug therapy. In: Fink JB, Hunt GE, editors. Clinical practice in respiratory care. Philadelphia: Lippincott-Raven, 1999.
Fink JB, Hess DR. Humidity and aerosol therapy. In: Hess DR, MacIntyre NR, Mishoe SC, et al, editors. Respiratory care principles & practices. Philadelphia: WB Saunders, 2002.
Gardenhire DS. Rau’s respiratory care pharmacology, ed 7. St Louis: Mosby, 2008.
Hess D. Aerosol therapy. In: Dantzker DR, MacIntyre NR, Bakow ED, editors. Comprehensive respiratory care. Philadelphia: WB Saunders, 1995.
Hess D. The delivery of aerosolized bronchodilator to mechanically ventilated intubated adult patients. Respir Care. 1990;35:399.
Shapiro BA, Kacmarek RM, Cane RD, et al, editors. Clinical application of respiratory care, ed 4, St Louis: Mosby, 1991.
Ward JJ, Hess D, Helmholz HFJr. Humidity and aerosol therapy. In Burton GC, Hodgkin JE, Ward JJ, editors: Respiratory care: a guide to clinical practice, ed 4, Philadelphia: Lippincott-Raven, 1997.
Whitaker K. Comprehensive perinatal & pediatric respiratory care, ed 2. Albany, NY: Delmar, 1997.
White GC. Equipment theory for respiratory care, ed 3. Albany, NY: Delmar, 1999.
SELF-STUDY QUESTIONS FOR THE ENTRY LEVEL EXAM See page 589 for answers
SELF-STUDY QUESTIONS FOR THE WRITTEN REGISTRY EXAM See page 614 for answers